Abstract
The aim of the current study was to determine nutrient digestibility, VFA production, N metabolism, and CH4 production of canola (Brassica napus L.), rapeseed (B. napus L.), turnip (Brassica rapa L.), and annual ryegrass (Lolium multiflorum Lam.) fed with orchardgrass (Dactylis glomerata L.) in continuous culture. Diets were randomly assigned to fermentors in a 4 × 4 Latin square design using 7 d for adaptation and 3 d for collection. Diets were: 1) 50% orchardgrass + 50% annual ryegrass (ARG); 2) 50% orchardgrass + 50% canola (CAN); 3) 50% orchardgrass + 50% rapeseed (RAP); and 4) 50% orchardgrass + 50% turnip (TUR). Feedings (82 g DM/d) occurred four times daily throughout 4, 10-d periods at 730, 1030, 1400, and 1900 h. Methane samples were collected every 10 min using a photoacoustic gas analyzer (LumaSense Technologies, Inc.; Santa Clara, CA) during the last 3 d of the experiment. Effluent samples were collected on d 8, 9, and 10, composited by fermentor, and analyzed for VFA and pH as well as DM, OM, CP, and fiber fractions for determination of nutrient digestibility. Forage samples were analyzed for CP, NDF, ADF, minerals, and glucosinolate (GLS) concentrations. Data were analyzed using the GLIMMIX procedure of SAS. Apparent DM, OM, and NDF digestibilities and true DM and OM digestibilities were similar (P > 0.28) among diets (45.1, 63.2, 44.1, 67.1, and 87.2%, respectively). Total VFA (87.2 mol/100 mol), pH (6.47), and acetate (A: 44.6 mol/100 mol) were also not different (P > 0.20) among diets. The A:P (P = propionate) ratio was greater (P < 0.01) in ARG and CAN than RAP and TUR. Daily CH4 production was greater (P < 0.01) in ARG than all other diets (68.9 vs. 11.2 mg/d). Methane, whether expressed as g per g of OM, NDF, digestible OM, or digestible NDF fed was greatest (P < 0.01) in ARG but similar (P > 0.18) among brassica diets. A significant negative correlation was observed between total GLS and CH4 production. However, when multiple regression analysis on CH4 production was completed, neither total GLS nor individual GLS were a significant component of the model. Addition of brassicas provided similar nutrient digestibility to ARG while reducing daily CH4 production, potentially making brassicas an alternative for ARG in pasture-based ruminant diets.
Keywords: brassica, continuous culture, methane, ruminal fermentation
INTRODUCTION
Forage brassicas are cool-season annual forages that can be used for grazing during the summer in the Mid-Atlantic and Northeast United States and late fall throughout the temperate zone when cool-season perennial pastures may not be as productive (Hall and Jung, 2008). Many brassica varieties exist, including vegetable, oilseed, and forage varieties; however, forage varieties have been bred to mature more quickly and produce greater above-ground biomass than their vegetable and oilseed counterparts. Brassica species include rapeseed (Brassica napus L.), turnip (Brassica rapa L.), kale (Brassica oleracea L.), radish (Raphanus sativus L.), and swede (Brassica napobrassica (L.) Mill). Forage variety trials throughout the eastern United States have shown the high biomass potential of forage brassicas (1,500–5,000 kg DM/ha; Darby et al., 2013, 2015; Simon et al., 2014). Further studies reported high CP concentrations (>20%), low NDF (20–35%), and high DM digestibility (>85%; Darby et al., 2013; Villalobos and Brummer, 2015). The combination of greater biomass during periods of perennial forage shortages and greater forage nutritive value has increased producer interest in using brassicas for pasture-based ruminants with high nutrient requirements (i.e., beef stockers, finishing lambs, and lactating dairy cows).
Brassicas contain a class of sulfur-containing plant secondary metabolites called glucosinolates (GLS). The type and quantity of GLS in each brassica varies with cultivar, agronomic management, and climatic conditions (Gustine and Jung, 1985; Tripathi and Mishra, 2007). Ingestion of substantial amounts of GLS can have adverse effects on animal performance and health. These include reduced DMI, ADG, I and Cu deficiencies, and kale anemia (Gustine and Jung, 1985). For this reason, nutritionists typically suggest limiting brassicas to ≤50% of daily DMI (Hall and Jung, 2008).
Multiple studies (Brask et al., 2013a, 2013b; Storlien et al., 2017) have shown that feeding a diet of 10–49% whole rapeseed or rapeseed meal reduces enteric methane emissions in cattle by 9–23%. Additionally, Storlien et al. (2017) determined that rapeseed concentrate was still effective at reducing enteric CH4 39 d after the initial addition of brassicas into the diet, suggesting that brassicas have the potential for long-term CH4 reduction. However, to date only a few studies (Sun et al., 2012, 2015; Williams et al., 2016) have reported CH4 emissions from ruminants consuming forage brassicas. Animals used in these studies were consuming diets that were either 100% forage brassica (Sun et al., 2012, 2015) or that were a brassica/legume mixture (Williams et al., 2016). Furthermore, these studies have been unable to report a causal relationship between individual and total GLS concentration and reduced CH4 emissions. However, research has shown that the GLS concentration of feeds [i.e., rapeseed and canola (B. napus L.) meals] is largely responsible for the reduction in CH4 emissions in ruminants supplemented with these by-products (Busquet et al., 2005; Patra et al., 2010; Storlien et al., 2017). Although Sun et al. (2012, 2015) did attempt to determine the relationship between GLS in forage brassicas and reduced enteric CH4 emissions in lambs, they were unable to prove a causal relationship. Furthermore, no studies have reported CH4 emissions or rumen fermentation parameters in ruminant systems when a brassica/grass diet has been fed and attempted to establish a causal relationship between total and individual GLS and enteric CH4 production in brassica/grass mixtures. The objectives of the current study were to 1) determine the effects of three forage brassicas fed in a 50:50 with orchardgrass on ruminal fermentation, N metabolism, and enteric CH4 production in continuous culture; and 2) determine if a causal link exists between total GLS concentration and CH4 production. We hypothesized that feeding forage brassicas in a 50:50 mixture with orchardgrass would decrease enteric methane production, while not altering rumen fermentation parameters compared to an annual ryegrass (Lolium multiflorum Lam.)/orchardgrass mixture and that multiple regression analysis will reveal a causal relationship between total GLS concentration and CH4 production.
MATERIALS AND METHODS
Site, Experimental Design, and Diets
On August 26, 2015, “KB Supreme” annual ryegrass (King’s Agriseed, Inc., Ronks, PA), “Inspiration” canola (Rubisco Seed, LLC., Philpot, KY), “Barisca” rapeseed (King’s Agriseed, Inc.), and “Appin” turnip (King’s Agriseed, Inc.) were planted at the Pennsylvania State University Russell E. Larson Agricultural Research Center in Rock Springs, PA. Seeds were drilled into a prepared seedbed using a no-till drill (HEGE 1000; Wintersteiger AG, Waldenburg, Germany). Forage was harvested on November 3, 2015, when forage was ready for grazing (approximately 70 d after planting; Hall and Jung, 2008). Orchardgrass (D. glomerata L.) was harvested from a 3-yr-old pure stand on April 27, 2016. Orchardgrass was harvested in a vegetative stage of growth, typical of high-quality pastures used for grazing in temperate regions of the United States (25 to 30 cm tall; Dillard et al., 2017). A plot harvester (HEGE 212; Wintersteifger AG; 1.5-m-wide swath), set to a 10-cm stubble height was used to harvest all forage. Within 30 min of harvest, forage was placed in cloth bags and frozen (−4°C) until being freeze-dried (Ultra 35 Super ES; Virtis Co. Inc., Gardiner, NY). Freeze-dried forage was ground to pass through a 1-mm sieve (Wiley mill; Thomson Scientific Inc., Philadelphia, PA). Freeze-dried forages are not nutritionally identical to fresh forages; however, in order to be preserved and ground for use in the experiment, drying was necessary (Soder et al., 2016), and Jones and Bailey (1972) reported that oven-drying forages could denature protein in plant material and decrease digestibility.
The continuous culture fermentor study was conducted at the USDA-Agricultural Research Service, Pasture Systems and Watershed Management Research Unit (University Park, PA) from May to July 2016. Total DM fed to all fermentors was kept constant at 82 g/d for the duration of the experiment. Diets were as follows: 1) 50% orchardgrass + 50% annual ryegrass (ARG); (2) 50% orchardgrass + 50% canola (CAN); (3) 50% orchardgrass + 50% rapeseed (RAP); and (4) 50% orchardgrass + 50% turnip (TUR). Fermentors were fed forage four times daily (0730, 1030, 1400, and 1900 h) in equal proportions of the diet. Representative samples of freeze-dried forage were collected from each diet at the beginning of the study and analyzed for nutrient concentrations at a commercial laboratory (Dairy One Laboratories, Ithaca, NY; Table 1).
Table 1.
Chemical composition (% of DM) of individual forages and diets fed during continuous culture fermentation
| Item | Ingredient | Diet* | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Annual ryegrass | Canola | Rapeseed | Turnip | Orchard-grass | ARG | CAN | RAP | TUR | |
| OM | 88.1 | 89.3 | 88.6 | 85.8 | 90.2 | 89.2 | 89.7 | 89.4 | 88.0 |
| CP | 30.2 | 28.2 | 23.2 | 22.2 | 34.0 | 32.1 | 31.1 | 28.6 | 28.1 |
| RDP, % of CP | 83 | 87 | 89 | 87 | 80 | 82 | 84 | 85 | 84 |
| NDF | 29.7 | 16.1 | 16.6 | 17.2 | 41.2 | 35.5 | 28.7 | 28.9 | 29.2 |
| ADF | 21.2 | 10.8 | 11.8 | 12.0 | 22.8 | 21.2 | 16.8 | 17.3 | 17.4 |
| Lignin | 5.3 | 0.8 | 1.3 | 1.3 | 2.5 | 3.9 | 1.7 | 1.9 | 1.9 |
| NFC† | 21.9 | 39.2 | 44.3 | 42.1 | 10.5 | 16.2 | 24.9 | 27.4 | 26.3 |
| WSC‡ | 21.6 | 31.4 | 34.1 | 33.2 | 11.9 | 16.8 | 21.7 | 23.0 | 22.6 |
| ESC§ | 19.6 | 24.7 | 24.6 | 26.9 | 7.9 | 13.8 | 16.3 | 16.3 | 17.4 |
| Starch | 0.2 | 0.5 | 1.7 | 0.2 | 0.3 | 0.3 | 0.4 | 1.0 | 0.3 |
| Ether extract | 6.2 | 5.7 | 4.5 | 4.3 | 4.5 | 5.4 | 5.1 | 4.5 | 4.4 |
| NEM&, Mcal/kg | 1.63 | 1.96 | 1.83 | 1.74 | 1.61 | 1.62 | 1.79 | 1.72 | 1.68 |
| NEG&, Mcal/kg | 1.03 | 1.30 | 1.21 | 1.12 | 0.99 | 1.01 | 1.15 | 1.10 | 1.06 |
| NEL&, Mcal/kg | 1.68 | 1.98 | 1.87 | 1.79 | 1.61 | 1.65 | 1.81 | 1.74 | 1.70 |
| Ca | 0.64 | 1.78 | 1.98 | 2.47 | 0.42 | 0.53 | 1.10 | 1.20 | 1.45 |
| P | 0.28 | 0.40 | 0.36 | 0.35 | 0.43 | 0.36 | 0.42 | 0.40 | 0.39 |
| Mg | 0.18 | 0.25 | 0.22 | 0.24 | 0.28 | 0.23 | 0.42 | 0.25 | 0.26 |
| K | 3.62 | 2.63 | 2.64 | 3.44 | 3.03 | 3.33 | 2.83 | 2.84 | 3.24 |
| Na | 0.04 | 0.08 | 0.10 | 0.06 | 0.19 | 0.11 | 0.13 | 0.14 | 0.12 |
| S | 0.40 | 0.72 | 0.73 | 0.88 | 0.43 | 0.42 | 0.58 | 0.58 | 0.66 |
| Cu, ppm | 7 | 4 | 4 | 5 | 9 | 8 | 7 | 7 | 7 |
*Diets calculated using actual nutrient composition and proportion of individual forages (DM basis); ARG = 50% orchardgrass + 50% annual ryegrass; CAN = 50% orchardgrass + 50% canola; RAP = 50% orchardgrass + 50% rapeseed; TUR = 50% orchardgrass + 50% turnip.
†Calculated as NFC = 100 − (CP% + NDF% + ether extract% + ash%).
‡WSC = water-soluble carbohydrate.
§ESC = ethanol-soluble carbohydrate.
&Estimated by the NRC (2001) model.
Continuous Culture System and Operation
The experiment was designed as a 4 × 4 Latin square. Diets were fermented in a four-unit, continuous culture fermentor system (Applikon Biotechnology, B.V., Schideam, The Netherlands). Fermentors were fed equal portions of herbage four times daily (0730, 1030, 1400, and 1900 h). Solid mean retention time and liquid dilution rate of the fermentors were adjusted daily to 24 h and 10%/h, respectively, and were achieved by regulation of buffer input and effluent volume removal (Bargo et al., 2003; Rico et al., 2012; Soder et al., 2016). Mineral buffer was prepared using the method of Hoover et al. (1976), except that urea was added at a rate of 0.4 g/L to simulate recycled N (Weller and Pilgrim, 1974). To achieve the desired retention and dilution rates, the buffer pump was adjusted to 20.6 ± 0.2 mL buffer per 7.5 min. The buffer pumps were on (pulsing) for approximately 4.6 min, at which point they automatically stopped pulsing and did not deliver any buffer into the vessel for the remaining 2.9 min of the 7.5 min cycle. Adjustments to the pulse time were made daily to ensure that 20.6 mL of buffer were delivered every cycle. The daily estimated effluent volume was determined by the daily buffer and feed volume. The effluent pump was set to run for 6 s every 7.5 min and daily effluent volume was adjusted by altering the pump speed. The total volume of effluent that must be removed from the system was calculated using the following equation: VFluid = FLD × VFerm × tr where VFluid = total volume of fluid removed for given retention time (mL), FLD = liquid dilution factor (%), VFerm = volume of the fermenter (mL), and tr = retention time (hr). The resulting volume is equivalent to the daily effluent fluid volume needed to maintain proper turnover in the system. In order to determine the total effluent volume, the fluid volume needed to be adjusted for the solids (feed) entering the system. Therefore, total volume removed per day is calculated as: VTotal = VFluid + VSolids where VTotal = total volume of effluent removed daily (mL) and VSolids = total volume of feed added to the system per day (mL). The amount of effluent to be removed per sample can be determined using VSample = VTotal/ns where VSample = volume of effluent to be removed per sample (mL) and ns = number of samples per day. The chosen number of daily samples for this set-up was 192 samples per day, correlating to a 7 min and 30 s cycle running continuously for 24 h.
A ruminally fistulated, nonpregnant, nonlactating Holstein cow (BW = 794 kg) was used for ruminal fluid and digesta collections in accordance with the Pennsylvania State University Animal Care and Use guidelines (IACUC #46212). The donor cow was group housed and fed a diet of mixed-grass hay and grain (75:25 forage-to-concentrate ratio) in a feed bunk for a total of 13.8 kg DM of available feed per cow per day at the Pennsylvania State University Dairy Research Farm (University Park, PA). A vitamin–mineral premix was fed at 1.8% of total DMI in order to meet NRC (2001) recommendations. Approximately 3 h after feeding, 7 L of ruminal fluid was collected with a hand pump into a prewarmed container and maintained at 39°C. Hand-grab samples of solid digesta were taken from the ventral, central, and dorsal areas of the rumen. Liquid and solid digesta samples were transported to the laboratory in separate containers. Within 15 min of collection, fluid was strained through four layers of cheesecloth and 1.5 L poured into each prewarmed, fermentation vat. Each fermentor vat was then sealed and flushed with 20 mL/min CO2 for 1 h. After 1 h, CO2 was reduced to 1 mL/min and vats were continuously purged with 1 mL/min CO2 for the remainder of the experimental period. Temperature was maintained at 39°C. Fermentor pH and temperature were recorded every 2 min using an automated sensor (Applikon Biotechnology, B.V.).
The experiment was operated for four consecutive, 10-d periods and re-inoculated with fresh ruminal fluid at the beginning of each period. Each 10-d period consisted of a 7-d adaptation period followed by a 3-d collection period. Effluent was collected in 4-L plastic containers located inside a freezer to maintain an effluent temperature of 4°C and inhibit microbial fermentation. During the adaptation period, effluent was weighed daily, volume recorded, and then contents were discarded. During the collection period, daily effluent collections were mixed in a blender (model 38LL52 Waring, Torrington, CT) for 30 s on the lowest setting and speed controlled further with a PowerStat variable transformer (model 3PN126, Superior Electric, Bristol, CT) set at 35 to create a 1-cm vortex at the top of the blender contents in order to provide adequate mixing without too much abrasive agitation. After mixing, a 100-mL sample was taken and composited over the collection period to determine effluent DM. A 50-mL sample was strained through eight layers of cheesecloth and a 15-mL subsample was added to 3-mL 25% (vol/vol) m-phosphoric acid for determination of VFA [Varian 3300 Gas Chromatograph (FID detector), Varian 4290 Integrator; Rumen Fermentation Profiling Laboratory, West Virginia University, Morgantown, WV; Supelco, 1975, modified to use a 80/120 Carbopack B-DA/4% Carbowax 20M column; Erwin et al., 1961] and NH3-N (Yang and Varga, 1989). The GC parameters used were 2 m × 2 mm i.d. glass column packed with 80/120 Carbopack B-DA/4% Carbowax 20M (catalog no. 1-1889, Supelco, Inc.), column temperature of 175°C, injector temperature of 200°C, detector temperature of 200°C, N gas used as a carrier at a flow rate of 24 mL/min, and an injection volume of 1 µL. An additional 1.00-L sample was composited from each of the three collection days, freeze-dried, ground to 1 mm, and stored in a sealed plastic bag at ambient temperature for analyses of DM, OM, NDF, CP, and total purines. In addition to the previously described sampling, on day 10 the contents of each fermentor were processed according to the methods of Griswold et al. (1996), except during the initial centrifugation when contents were mixed in the above-described blender for 30 s and strained through two layers of 53-µm Nitex fabric (Wildco, Bufflao, NY) into a 2-L plastic container with 5 mL of 50% sulfuric acid added to inhibit microbial growth. Contents were centrifuged three times at 20,000 × g for 20 min at −4°C and then the pellet was re-suspended in 0.9% saline during the first two centrifugations and with 50% methanol during the third centrifugation (Griswold et al., 1996). The final pellet was stored at −4°C until freeze–drying and analyses for DM, OM, and CP [per AOAC (2006) procedures described in Nutrient Analyses section below] and purine (Zinn and Owens, 1986). Purine data were used to calculate bacterial efficiencies using the equations described by Soder et al. (2016).
Methane Collection and Measurements
Methane measurements were taken every 10 min using a photoacoustic gas monitor (LumaSense Technologies, Inc., Santa Clara, CA) connected to a multiport sampler (CAI, Inc., Orange, CA) that directed the flow of gas from each fermentor vat. Each cycle (sampling and line flush) required 140 cm3 of the approximately 1.50 L of headspace gas available. Total daily CH4 production was calculated by determining the difference in CH4 volume (measured CH4 concentration multiplied by the headspace volume) between each of the 10-min samples and then summing these values for every 24-h period during the collection days. Total daily CH4 production = Σ [CH4 volumea − CH4 volumeb], where CH4 volumea was CH4 volume was determined by multiplying the headspace volume by the measured CH4 concentration and volumea was taken 10 min after volumeb over each 24-h period during collection days.
Nutrient Analyses
Samples of orchardgrass, annual ryegrass, canola, rapeseed, and turnip were analyzed by wet chemistry (Dairy One Laboratories, Ithaca, NY) according to the following procedures: DM (method 930.15; AOAC, 2006), CP (method 990.03; AOAC, 2006), RDP (Cornell Streptomyces griseus enzymatic digestion; Coblentz et al., 1999), fat (method 2003.05; AOAC, 2006), and NDF [Ankom model A200; Mertens (2002) with heat stable α-amylase and sodium sulfite used in the NDF procedures (inclusive of ash)]. Water-soluble carbohydrates were determined using a Thermo Scientific Genesys 10S Vis Spectrophotometer after incubation in 40°C water both for 1 h, and acid hydrolysis with H2SO4 (Smith, 1969). Potassium ferricyanide was used for the colorimetric reaction rather than iodide-potassium oxalate, as potassium ferricyanide proves a more stable reaction for detecting reducing sugars (Miller-Webster et al., 2002). Ethanol-soluble carbohydrates (Hall et al., 1999), starch (Application Note #319; YSI, Inc. Life Sciences, Yellow Springs, OH), minerals (Ca, P, Mg, K, Na, S; Thermo IRIS Advantage HX; CEM Application Note for Acid Digestion, CEM Corp., Matthews, NC), and ether extract (method 2003.045; AOAC, 2006) were also determined. Individual and total GLS concentrations were determined by extracting ground, freeze–dried forage in 70% methanol at 60°C for 15 min. The extracted liquid was filtered through a 0.45 µm syringe filter and analyzed using an ICS-5000+ chromatography system (Thermo-Fisher Scientific, Sunnyvale, CA) interfaced to a Q-Exactive orbitrap mass spectrometer (Thermo-Fisher Scientific, Bremen, Germany) equipped with an ultra aqueous polar end-capped analytic column (Restek Corp., Bellefonte, PA).
Samples from effluent and microbial pellets were analyzed for DM (method 930.15; AOAC, 2006), OM (method 942.05, AOAC, 2006), and CP (micro-Kjeldahl digestion using 75-mL calibrated tubes with CuSO4/K2SO4 catalyst, method 976.06; AOAC, 2006). Neutral detergent fiber of effluent was determined in the same manner as forage. Concentrations of total purines (Zinn and Owens, 1986) in bacterial isolates and effluent were used to partition effluent N flow into bacterial and nonbacterial fractions and to calculate true digestibilities and flows. Apparent (DM, OM, NDF, and ADF) and true (DM, OM, and CP) nutrient digestibilities were calculated using the following equations (DM as an example):
DM apparently digested(% of total DM) = [(g of DM fed − g of effluent flow DM) ÷ g of DM fed] × 100, with effluent corrected for g of buffer DM.
DM truly digested(% of total DM) = {[g of DM fed − (g of effluent flow DM − g of microbial DM)] ÷ g of DM fed} × 100, with effluent corrected for g of buffer DM.
Nitrogenous fraction flows were calculated as follows:
Bacterial efficiency was calculated as follows:
Statistical Analysis
Data were analyzed as a 4 × 4 Latin square design using the GLIMMIX procedure of SAS (SAS Institute, Inc., Cary, NC) using fermentor as the experimental unit and fitted to the following model:
where Yijk = the observations for dependent variables, µ = population mean, Pi = mean effect of ith period, Fj = mean effect of jth fermentor, Tk = mean effect of kth diet, and eijk = residual error. Fermentor, period, and error were considered random effects and diet was considered a fixed effect.
Temporal analysis of CH4 concentrations was analyzed using the following model:
where Yijk = the observations for dependent variables, µ = population mean, Pi = mean effect of ith period, Fj = mean effect of jth fermentor, Tk = mean effect of kth diet, E1ijk = whole-plot error, Hl = mean effect of lth hour of day analyzed as repeated measures, HTlk = interaction between lth hour of day and kth diet, and E2ijkl = subplot residual error. Diet was considered a fixed effect and all other parameters were considered to be random. All reported values are least squares means and were compared by least squared minimum difference. Pearson correlation coefficients between dependent variables and forage characteristics were conducted using PROC CORR of SAS. Stepwise linear regression was conducted using PROC REG of SAS to detect predictive statistical associations between forage characteristics and dependent variables. Statistical significant was declared at P ≤0.05, and tendencies at 0.05 < P ≤ 0.10 for all analyses. There were no period or period × diet interactions; therefore, only main effects are reported.
RESULTS AND DISCUSSION
Diet Composition and Nutrient Digestibility
The chemical composition of dietary ingredients and diets are reported in Table 1 and GLS concentration in Table 2. The use of composite sampling for nutrient and GLS analyses precluded the ability for statistical comparison among diets. The orchardgrass used in the current study was of higher quality (greater CP and lower NDF and ADF) than that reported in previous studies (Hafla et al., 2016; Dillard et al., 2017) and is of great nutritive value based on the description of Cherney and Allen (1995; 18–24% DM, 18–25% CP, 40–50% NDF, and 1.53–1.67 Mcal/kg NEL). The greater quality of the orchardgrass compared with previous studies was likely result of an early harvest date (late April) and differences in fertilization management of the forage used in the current experiment compared with previous studies. Among the brassicas, all quality parameters were similar, while ARG had numerically greater CP and fiber fractions than the brassica forage. All forage used in the current study had numerically higher CP (>23%) and lower NDF and ADF (16.1–29.7% and 10.8–21.2%, respectively) compared to typical cool-season perennial forages (National Academies of Sciences, Engineering, and Medicine, 2016), and contained 1.61 to 1.98 Mcal/kg NEL. Furthermore, unlike the majority of forage grasses and legumes, the nutritive quality of brassicas does not decrease significantly as the plants mature (Smith and Collins, 2003). The resulting diets were all of extremely high nutritive value (>28% CP, <36% NDF, and <22% ADF) and of higher quality than the 3-yr average of 14 pasture-based dairy farms throughout the northeastern United States (19.5, 51.0, and 31.4% CP, NDF, and ADF, respectively; Hafla et al. 2016). All diets used in the current study were of sufficient quality to meet the CP and energy requirements of a mid-lactation dairy cow (680 kg BW, 45 kg milk/d; NRC, 2001) or a yearling stocker (300 kg BW, 1.35 kg ADG; National Academies of Sciences, Engineering, and Medicine, 2016). However, due to their low NDF concentrations, these diets may not have sufficient fiber to optimize nutrient utilization due to rapid passage rate (van Soest, 1994), regardless of sufficient CP and energy.
Table 2.
Total and individual glucosinolate concentrations (mg/g DM) of individual forages and diets fed during continuous culture fermentation
| Glucosinolate† | Ingredient | Diet* | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Annual Ryegrass | Canola | Rapeseed | Turnip | Orchardgrass | ARG | CAN | RAP | TUR | |
| Glucobrassicanapin | 0.00 | 5.72 | 5.13 | 17.29 | 0.00 | 0.00 | 2.86 | 2.57 | 8.65 |
| Progoitrin | 0.00 | 3.04 | 9.66 | 15.26 | 0.00 | 0.00 | 1.52 | 4.83 | 7.63 |
| Gluconapin | 0.00 | 1.00 | 1.42 | 4.15 | 0.00 | 0.00 | 0.50 | 0.71 | 2.08 |
| Glucobrassicin | 0.00 | 0.95 | 1.25 | 1.96 | 0.00 | 0.00 | 0.48 | 0.63 | 0.98 |
| Gluconasturtiin | 0.00 | 0.68 | 1.16 | 3.95 | 0.00 | 0.00 | 0.34 | 0.58 | 1.98 |
| Glucoraphanin | 0.00 | 0.16 | 0.63 | 0.42 | 0.00 | 0.00 | 0.08 | 0.32 | 0.21 |
| Glucoerucin | 0.00 | 0.01 | 0.05 | 0.41 | 0.00 | 0.00 | 0.01 | 0.03 | 0.21 |
| Sinigrin | 0.00 | 0.08 | 0.23 | 0.31 | 0.00 | 0.00 | 0.04 | 0.12 | 0.16 |
| Glucoraphenin | 0.00 | 0.04 | 0.04 | 0.05 | 0.00 | 0.00 | 0.02 | 0.02 | 0.03 |
| Total | 0.00 | 11.68 | 19.51 | 43.80 | 0.00 | 0.00 | 5.84 | 9.76 | 21.90 |
*Diets calculated using actual nutrient composition and proportion of individual forages (DM basis); ARG = 50% orchardgrass + 50% annual ryegrass; CAN = 50% orchardgrass + 50% canola; RAP = 50% orchardgrass + 50% rapeseed; TUR = 50% orchardgrass + 50% turnip.
†No detectable levels of gluoiberin, glucoiberverin, gluochlearin, or sinalbin were found in any of the forages or diets tested.
As expected, no GLS were observed in either orchardgrass or annual ryegrass (Table 2). The turnip diet had, numerically, the greatest concentration of GLS of all diets; both CAN and RAP also contained GLS, but in lesser quantities. Among all diets containing GLS, glucobrassicanapin, gluconapin, gluconasturtiin, and progoitrin represented the largest fraction of individual GLS. Sinigrin, glucerucin, glucoraphanin, and glucoraphenin were also observed in diets, but in lesser amounts. However, sinalbin, glucohlearin, glucoiberin, and glucoiberverin were not observed in any of the forage samples. While GLS concentrations vary considerably with species, variety, and growing conditions (Gustine and Jung, 1985), both total and individual GLS present in all brassicas tested were within the range of those reported in the literature (Font et al., 2005; Cartea and Velasco, 2008; Velasco et al., 2008).
There were no differences (P > 0.28) in apparent DM, OM, and NDF digestibilities or true DM and OM digestibilities among diets (Table 3). Apparent ADF digestibility was greatest (P ≤ 0.04) in CAN, while no differences (P > 0.27) were observed among the other diets. Both Cassida et al. (1994) and Lambert et al. (1987) reported greater apparent DM, OM, and NDF digestibilities for diets containing 40 to 52% brassica and 60 to 48% mixed-grass hay. The apparent ADF digestibilities of ARG, RAP, and TUR were similar to that reported by Cassida et al. (1994) and Lambert et al. (1987), but the CAN diet used in the current study was greater than that reported in either study.
Table 3.
Nutrient digestibility of annual ryegrass, canola, rapeseed, and turnip fed with orchardgrass during continuous culture fermentation
| Item | Diet* | SEM | |||
|---|---|---|---|---|---|
| ARG | CAN | RAP | TUR | ||
| Apparent digestibility | |||||
| DM, % | 44.5 | 44.7 | 45.3 | 46.0 | 2.77 |
| OM, % | 61.6 | 62.5 | 63.6 | 65.0 | 2.84 |
| NDF, % | 38.1 | 52.8 | 40.9 | 44.5 | 5.57 |
| ADF, % | 52.1a | 64.0b | 48.8a | 53.9a | 3.15 |
| True digestibility | |||||
| DM, % | 70.0 | 66.4 | 69.5 | 62.4 | 2.86 |
| OM, % | 89.7 | 86.4 | 90.1 | 82.6 | 3.13 |
*ARG = 50% orchardgrass + 50% annual ryegrass; CAN = 50% orchardgrass + 50% canola; RAP = 50% orchardgrass + 50% rapeseed; TUR = 50% orchardgrass + 50% turnip.
a–bWithin a row, means without a common superscript differ (P ≤ 0.05).
Fermentor pH, VFA, and CH4 Production
There were no differences (P > 0.11) in mean, minimum, and maximum pH among diets (Table 4). Lower fiber diets tend to have higher rates of digestion and acid production, thereby decreasing ruminal pH (van Soest, 1994); however, lack of differences in pH suggest that fiber differences between the diets were not great enough to exceed the buffering capacity of the system. Keogh et al. (2009) reported that pregnant, nonlactating dairy cows fed 60% forage kale had a mean ruminal pH of 6.32, similar to the mean pH of the brassica diets in the current study. However, ruminal pH of lambs fed 100% forage rapeseed was lower than that of lambs fed 100% perennial ryegrass (6.02 and 6.71, respectively; Sun et al., 2015), likely due to less effective fiber in the 100% rapeseed diet.
Table 4.
Fermentor pH, VFA concentration and molar proportion, and CH4 output of annual ryegrass, canola, rapeseed, and turnip fed with orchardgrass during continuous culture fermentation
| Item | Diet* | SEM | |||
|---|---|---|---|---|---|
| ARG | CAN | RAP | TUR | ||
| pH | |||||
| Mean | 6.61 | 6.29 | 6.41 | 6.57 | 0.142 |
| Minimum | 6.40a | 6.08b | 6.14a,b | 6.16a,b | 0.078 |
| Maximum | 6.95 | 6.73 | 7.04 | 6.69 | 0.201 |
| Total VFA, mM | 90.2 | 84.8 | 88.9 | 84.9 | 4.68 |
| Individual VFA, mol/100 mol | |||||
| Acetate (A) | 48.10 | 42.30 | 45.14 | 42.72 | 2.353 |
| Propionate (P) | 25.82a | 22.28b | 21.80b | 23.66a,b | 1.092 |
| Butyrate (B) | 11.44a | 15.53a,b | 16.80b | 14.21a,b | 1.478 |
| Isobutyrate | 0.84a | 0.39b | 0.42b | 0.43b | 0.044 |
| Valerate (V) | 3.22a | 3.98a,b | 4.37b | 3.55a,b | 0.256 |
| Isovalerate | 0.74a | 0.30b | 0.33b | 0.32b | 0.047 |
| A:P | 1.86a | 1.90a | 2.08b | 1.80a | 0.049 |
| A + B:P + V | 2.05a | 2.20a,b | 2.36b | 2.09a | 0.057 |
| CH4 | |||||
| mg CH4/d | 68.9a | 13.1b | 7.4b | 13.1b | 5.77 |
| mg of CH4/g of OM fed | 0.94a | 0.18b | 0.18b | 0.10b | 0.079 |
| mg of CH4/g of NDF fed | 2.37a | 0.56b | 0.31b | 0.55b | 0.204 |
| mg of CH4/g of digestible OM fed | 2.22a | 0.30b | 0.22b | 0.37b | 0.139 |
| mg of CH4/g of digestible NDF fed | 1.37a | 0.25b | 0.14b | 0.24b | 0.119 |
*ARG = 50% orchardgrass + 50% annual ryegrass; CAN = 50% orchardgrass + 50% canola; RAP = 50% orchardgrass + 50% rapeseed; TUR = 50% orchardgrass + 50% turnip.
a–cWithin a row, means without a common superscript differ (P ≤ 0.05).
Neither total VFA nor acetate (A) concentrations were different (P > 0.34) among diets (Table 4). Propionate (P) was greater (P ≤ 0.05) in ARG than CAN or RAP, and butyrate (B) was greater (P = 0.03) in RAP than ARG. Valerate (V) was greater (P = 0.011) in RAP than ARG. Isobutyrate and isovalerate concentrations were greater (P < 0.01) in ARG than the brassica diets. All VFA ratios (A:P and A+B:P+V) were greater (P < 0.01) in RAP than ARG, with CAN and TUR having median values. The lower A:P ratio observed in ARG was the result of a greater proportion of propionate in ARG at the expense of butyrate. Sun et al. (2012) reported that total VFA concentrations after feeding were greater in rapeseed and turnip than perennial ryegrass (97.0 and 74.2 mol/100 mol, respectively). Keogh et al. (2009) found that the A:P ratio of pregnant, nonlactating dairy cows consuming 60% kale and 40% perennial ryegrass (Lolium perenne L.) silage was not different than cows consuming 100% perennial ryegrass silage. However, total VFA concentrations were greater in the 60% kale diet than the 100% perennial ryegrass diet.
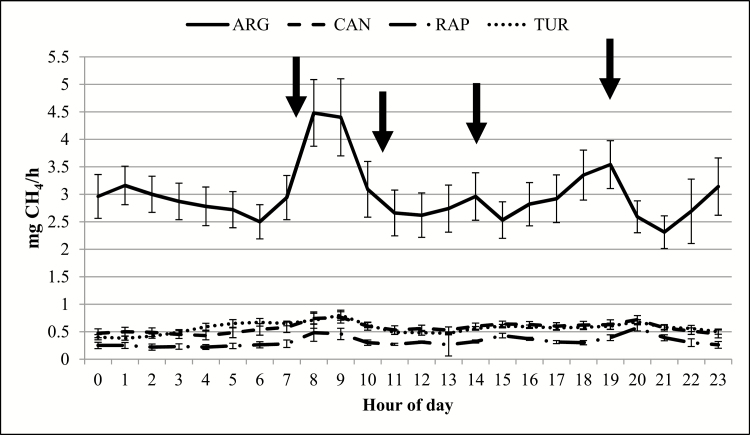
Methane production (mg/d) was 84% greater (P < 0.01) in ARG than the brassica diets, while no difference (P > 0.28) was observed among brassicas (Table 4). Furthermore, CH4 per gram of OM fed, per gram of NDF fed, per gram of digestible OM fed, and per gram of digestible NDF fed also followed a similar pattern with no differences (P > 0.18) observed among brassicas, but all brassicas being lower (P < 0.01) than ARG. A significant (P < 0.01) diet × hour of day interaction was observed, such that a diurnal pattern in CH4 production was observed in ARG (Figure 1). However, there was no difference (P > 0.05) in hourly CH4 production throughout the day. This response was due to CH4 production of each of the brassica diets being at least 6 times lower than the ARG diet resulting in a SEM (0.51 mg CH4/h) that was similar to the hourly mean of the brassica diets. Sun et al. (2012) reported that gram of CH4 per kilogram of DMI was 25% lower in lambs fed forage rapeseed than perennial ryegrass. However, the authors reported no differences in gram of CH4 per kilogram of DMI between perennial ryegrass and turnip, disagreeing with the current study. These differences are due to the reduced DMI observed in the lambs fed turnip compared to those fed perennial ryegrass, while DMI was similar for lambs consuming rapeseed and perennial ryegrass (Sun et al., 2012). In a separate study, Sun et al. (2015) reported a 22% reduction in CH4 production in lambs fed forage rapeseed compared with perennial ryegrass for 15 weeks. Brask et al. (2013b) reported that lactating dairy cattle consuming a total mixed ration (TMR) with and without different rapeseed by-products (i.e., rapeseed cake, whole cracked rapeseed, and rapeseed oil) included had similar daily milk production (28.3 kg/d), milk fat content (39.7 g/kg), and milk protein concentration (33.0 g/kg), while CH4 emissions were lower in the rapeseed supplemented cattle (17.5 vs. 20.4 L/kg of energy corrected milk and 5.44 vs. 6.32% of gross energy intake, respectively). Storlien et al. (2017) reported that inclusion of a rapeseed concentrate in TMR-supplemented, pasture-based, lactating dairy cattle increased milk production (31.7 vs. 29.6 kg/d, with and without rapeseed, respectively), but no difference was observed in energy-corrected milk (26.6 kg/d). Furthermore, milk fat and protein were lower in cattle consuming the rapeseed supplement (30.4 vs. 34.8 g/kg and 29.8 vs. 30.3 g/kg, respectively). Enteric CH4 measured was lower in rapeseed supplemented cattle, regardless of measurement method (221 vs. 251 g/day, 8.1 vs. 9.0 g/kg of energy-corrected milk, and 27.4 vs. 33.6 g/kg of concentrate fed, respectively). Methane reduction in the current study was greater than that reported in previous studies; however, this could be attributed to inherent differences between in vivo and in vitro studies (Hristov et al., 2012).
Figure 1.
Temporal CH4 production of orchardgrass fed with annual ryegrass (ARG), canola (CAN), rapeseed (RAP), and turnip (TUR) during continuous culture fermentation. Error bars indicate standard errors. Vertical arrows indicate times of feeding.
In the current study, differences in CH4 production did not follow typical patterns for VFA ratios. This was also observed by Sun et al. (2012) who reported no correlation between CH4 yield as g CH4/kg DMI and total and individual VFA. This suggests that GLS directly affect methanogens or protozoa populations, but do not alter gram-positive bacteria populations. Ohene-Adjei et al. (2008) indicated that phylogenetic analysis of ruminal fluid from sheep fed a barley-based diet and supplemented with garlic oil (contains organosulphur compounds similar to GLS) inhibited Methanobrevibacter ruminantium when compared to nonsupplemented sheep. Furthermore, Patra et al. (2010) reported no significant changes in protozoal populations in sheep supplemented with garlic oil compared to nonsupplemented sheep. The authors then concluded that organosulphur compounds inhibited methanogenic arachaea without affecting other microorganisms found in the rumen. Busquet et al. (2005) proposed that reduction in methanogens was due to inhibition of 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase, thus affecting the unique membrane lipids that contain ether-linked glycerols and long-chain isoprenoid alcohols, which are not present in other microorganisms found in the rumen (De Rosa et al., 1986).
Correlation analyses between daily CH4 production and the individual GLS that were present in the diets revealed a significant (P < 0.045) negative relationship between daily CH4 production and glucobrassicanapin, sinigrin, glucobrassicin, gluconapin, glucoraphanin, glucoraphenin, progoitrin, and total GLS concentration (Table 5). Gluconasturtiin tended (P = 0.076) to be negatively correlated; however, glucoerucin was not significantly correlated (P = 0.207) with daily CH4 production. Stepwise multiple regression analysis between CH4 production and nutritive quality parameters, total GLS, and individual GLS concentration showed that NDF explained 75% of the variation in daily CH4 production and CH4 production per OM fed, 73% of the variation in CH4 per digestible OM fed, and 85% of the variation in CH4 per digestible NDF fed (Table 6). Furthermore, when methane was expressed per gram of NDF fed, NFC was the only independent variable to enter the model and explained 73% of the variation in CH4 production. Therefore, even though a significant negative correlation was observed between CH4 production and total GLS concentration, NDF still plays the most important role in determining CH4 production from forage-based diets. Previous studies (Brask et al., 2013b; Storlien et al., 2017) showed that addition of rapeseed cake to dairy cows on pasture or consuming a TMR also significantly reduced CH4 per kg of concentrate and CH4 as % of GE intake, respectively. These studies were able to more closely control NDF and fat concentrations of the diets, allowing the researchers to remove these confounding factors. In order to better determine the direct effect of GLS on CH4 production when grazing forage brassicas on pasture, diets will need to be balanced for fiber and NFC components to prevent the effects of NDF masking any possible effects of GLS on CH4 production.
Table 5.
Correlation coefficients (r) between individual and total glucosinolate concentrations (mg/g DM) and daily CH4 production (mg/d) of fermentors fed annual ryegrass, canola, rapeseed, or turnip with orchardgrass
| Glucosinolate* | r | P value |
|---|---|---|
| Glucobrassicanapin | −0.523 | 0.038 |
| Sinigrin | −0.643 | 0.007 |
| Glucobrassicin | −0.732 | 0.001 |
| Glucoerucin | −0.333 | 0.207 |
| Gluconapin | −0.509 | 0.044 |
| Gluconasturtiin | −0.456 | 0.076 |
| Glucoraphanin | −0.670 | 0.005 |
| Glucoraphenin | −0.787 | <0.001 |
| Progoitrin | −0.593 | 0.015 |
| Total | −0.567 | 0.022 |
*No correlation was tested between daily CH4 production and sinalbin, glucohlearin, glucoiberin, or glucoibeverin due to lack of measurable concentrations in any forage analyzed.
Table 6.
Multiple regression of nutritive quality parameters and glucosinolate concentrations on daily CH4 production, CH4 production per OM fed, CH4 production per NDF fed, CH4 production per digestible OM fed, and CH4 production per digestible NDF fed of fermentors fed annual ryegrass, canola, rapeseed, and turnip with orchardgrass
| Independent variable | Partial r2 | P value | |
|---|---|---|---|
| Daily CH4 production, mg/d | NDF | 0.75 | <0.0001 |
| CH4 production per OM fed, mg/g | NDF | 0.75 | <0.0001 |
| CH4 production per NDF fed, mg/g | NFC | 0.73 | <0.0001 |
| CH4 production per digestible OM fed, mg/g | NDF | 0.73 | <0.0001 |
| CH4 production per digestible NDF fed, mg/g | NDF | 0.85 | <0.0001 |
Nitrogen Metabolism
While DM intake was kept constant, due to differences in CP concentration of diets, dietary N was greatest (P < 0.01) in CAN and TUR and least (P < 0.01) in RAP (Table 7). Luo et al. (2015) found that DMI of lambs consuming fresh forage rapeseed tended to be greater than lambs fed perennial (0.895 vs. 0.826 kg/d, respectively). Furthermore, N concentration of rapeseed was 8% greater than the perennial ryegrass; this led to a 23% higher daily N intake in lambs fed rapeseed compared to perennial ryegrass. Effluent NH3-N concentration was 26% greater in ARG than the brassica diets and the same pattern was observed in NH3-N flows. Kaur et al. (2010) found that daily ruminal NH3-N concentrations were not different in lambs consuming 10, 25, or 40% forage rapeseed with a corn silage-based TMR (22.6 ± 1.4 mg/dL). Furthermore, the authors reported no differences in N intake among diets. In the current study, true CP digestibility was 25% greater (P < 0.01) in ARG and CAN than RAP and TUR. Sun et al. (2012) reported that the apparent CP digestibility of forage rapeseed and turnip in lambs was greater than perennial ryegrass (30 and 17%, respectively). Furthermore, Cassida et al. (1994) reported that apparent CP digestibility increased linearly with increasing amounts of tyfon (B. campestrics var rapa L. × B. pekinensis [Lour.] Rupr.) in lambs fed a diet of mixed-grass hay and tyfon. Total N flow was greater (P = 0.04) in ARG than CAN in the current study, while RAP and TUR had intermediate values. However, no difference (P > 0.43) was observed in non-ammonia-N flows among all diets.
Table 7.
Nitrogen metabolism of annual ryegrass, canola, rapeseed, and turnip fed with orchardgrass during continuous culture fermentation
| Item | Diet* | SEM | |||
|---|---|---|---|---|---|
| ARG | CAN | RAP | TUR | ||
| N intake, g/d† | 4.33b | 4.83a | 4.27c | 4.83a | 0.004 |
| NH3-N, mg/dL | 28.4a | 21.1b | 22.2b | 20.1b | 0.91 |
| True CP digestibility, % | 94.3a | 91.0a | 69.6b | 70.4b | 2.84 |
| N flows, g/d | |||||
| Total N | 2.95a | 2.67b | 2.70ab | 2.70ab | 0.100 |
| NH3-N | 1.14a | 0.85b | 0.90b | 0.80b | 0.038 |
| NAN | 1.80 | 1.83 | 1.80 | 1.90 | 0.092 |
| Bacterial N | 1.60a | 1.46a | 0.73b | 0.89b | 0.159 |
| Dietary N | 0.20a | 0.37a | 1.07b | 1.21b | 0.115 |
| Bacterial efficiency | |||||
| g N/kg DM truly digested | 29.9a | 27.5a | 16.6b | 15.4b | 2.63 |
| g N/kg OM truly digested | 27.5a | 24.7a | 14.4b | 13.2b | 2.40 |
*ARG = 50% orchardgrass + 50% annual ryegrass; CAN = 50% orchardgrass + 50% canola; RAP = 50% orchardgrass + 50% rapeseed; TUR = 50% orchardgrass + 50% turnip.
†N intake (g/d) = dietary N (g/d) + urea-N from buffer (g/d).
a,bWithin a row, means without a common superscript differ (P ≤ 0.05).
Bacterial N flows were 47% greater (P ≤ 0.01) in ARG and CAN than RAP and TUR, while dietary N flows were 75% greater (P < 0.01) in RAP and TUR than ARG and CAN (Table 7). Furthermore, bacterial efficiency, measured as g N/kg of truly digestible DM and OM, was greater (P ≤ 0.01) in ARG and CAN than RAP and TUR. Busquet et al. (2005) found no differences in dietary N flow, bacterial N flow, or bacterial efficiency per OM truly digested in a continuous culture system fed a 50:50 forage:concentrate diet and supplemented with or without garlic oil. In the current study, the greater bacterial efficiency observed in CAN compared to RAP and TUR could be a result of the greater apparent ADF digestibility of CAN.
CONCLUSIONS
When mixed with orchardgrass, forage brassicas can provide a suitable alternative to cool-season annual grass pastures such as ARG during times when productivity of cool-season grasses is low (i.e., late fall and summer forage slump). In addition to being high in nutritive quality, all three brassica diets (CAN, RAP, and TUR) also significantly lowered CH4 production compared to ARG. Furthermore, CAN provided greater ruminal bacterial efficiency than the other brassicas, making it the superior variety in this study. While other studies (Brask et al., 2013b; Storlien et al., 2017) have established a link between organosulphur secondary plant metabolites and CH4 reduction, in the current study, we were unable to establish a causal relationship between individual or total GLS and CH4 production even though a significant CH4 reduction occurred. Use of brassicas in a ruminant grazing system could extend the fall grazing season and reduce winter feed costs while increasing animal efficiency and decreasing greenhouse gas emissions. However, more research is needed on the long-term effects of grazing brassicas to determine if these effects can be observed in pasture-based ruminants.
LITERATURE CITED
- AOAC 2006. Official methods of analysis. 18th ed Assoc. Off. Anal. Chem., Gaithersburg, MD. [Google Scholar]
- Bargo F., Varga G. A., Muller L. D., and Kolver E. S.. 2003. Pasture intake and substitution rate effects on nutrient digestion and nitrogen metabolism during continuous culture fermentation. J. Dairy Sci. 86, 1330–1340. doi:10.3168/jds.S0022- 0302(03)73718-7 [DOI] [PubMed] [Google Scholar]
- Brask M., Lund P., Hellwing A. L. F., Poulsen M., and Weisbjerg M. R.. 2013a. Enteric methane production, digestibility and rumen fermentation in dairy cows fed different forages with and without rapeseed fat supplementation. Anim. Feed Sci. Tech. 184:67–79. doi:10.1016/j.anifeedsci.2013.06.006 [Google Scholar]
- Brask M., Lund P., Weisbjerg M. R., Hellwing A. L. F., Poulsen M., Larsen M. K., and Hvelplund T.. 2013b. Methane production and digestion of different physical forms of rapeseed as fat supplements in dairy cows. J. Dairy Sci. 96:2356–2365. doi:10.3168/jds.2011-5239 [DOI] [PubMed] [Google Scholar]
- Busquet M., Calsamiglia S., Ferret A., Carro M. D., and Kamel C.. 2005. Effect of garlic oil and four of its compounds on rumen microbial fermentation. J. Dairy Sci. 88:4393–4404. doi:10.3168/jds.S0022-0302(05)73126-X [DOI] [PubMed] [Google Scholar]
- Cartea M. E., and Velasco P.. 2008. Glucosinolates in Brassica foods: bioavailability in food and significance for human health. Phytochem. Rev. 7:213–229. doi:10.1007/s11101- 007-9072-2 [Google Scholar]
- Cassida K. A., Barton B. A., Hough R. L., Wiedenhoeft M. H., and Guillard K.. 1994. Feed intake and apparent digestibility of hay-supplemented brassica diets for lambs. J. Anim. Sci. 72:1623–1629. doi:10.2527/1994.7261623x [DOI] [PubMed] [Google Scholar]
- Cherney J. H., and Allen V. G.. 1995. Forages in a livestock system. In: R. F. Barnes D. A. Miller, and C. J. Nelson, editors, Forages vol. 1: an introduction to grassland agriculture. 5th ed Ames, IA: Iowa State Uni. Press, p. 182. [Google Scholar]
- Coblentz W. K., Abdelgadir I. E., Cochran R. C., Fritz J. O., Fick W. H., Olson K. C., and Turner J. E.. 1999. Degradability of forage proteins by in situ and in vitro enzymatic methods. J. Dairy Sci. 82:343–354. doi:10.3168/jds.S0022-0302(99)75241-0 [DOI] [PubMed] [Google Scholar]
- Darby H., Blair K., Cummings E., Monahan S., Post J., and Ziegler S.. 2015. 2014 forage brassica planting date trial. Tech. Bull. Univ. of Vermont Extension. University of Vermont, p. 1–7. [accessed March 5, 2018]. http://www.uvm.edu/extension/cropsoil/wp-content/uploads/2014-Forage-Brassica-Planting-Date.pdf. [Google Scholar]
- Darby H., Harwood H., Cummings E., Madden R., and Monahan S.. 2013. 2012 forage brassica variety trial. Tech. Bull. Univ. of Vermont Extension. University of Vermont, p. 1–8. [accessed March 5, 2018]. http://www.uvm.edu/extension/cropsoil/wp-content/uploads/2012_ForageBrassicaVarietyTrialfinal.pdf. [Google Scholar]
- De Rosa M., Gambacorta A., and Gliozzi A.. 1986. Structure, biosynthesis, and physicochemical properties of archaebacterial lipids. Microbiol. Rev. 50:70–80. [accessed March 5, 2018]. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC373054/pdf/microrev00052-0078.pdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillard S. L., Hafla A. N., Roca-Fernández A. I., Brito A. F., Rubano M. D., and Soder K. J.. 2017. Effect of feeding warm-season annuals with orchardgrass on ruminal fermentation and methane output in continuous culture. J. Dairy Sci. 100:1179–1188. doi:10.3168/jds.2016-11510 [DOI] [PubMed] [Google Scholar]
- Erwin E. S., Marco G. J., and Emery E. M.. 1961. Volatile fatty acid analysis of blood and rumen fluid by gas chromatography. J. Dairy Sci. 44:1768–1771. doi:10.3168/jds.S0022- 0302(61)89956-6 [Google Scholar]
- Font R., del Río-Celestino M., Rosa E., Aires A., and de Haro-Bailón A.. 2005. Glucosinolate assessment in Brassica oleracea leaves by near-infrared spectroscopy. J. Agr. Sci. 143:65–73. doi:10.1017/S0021859605004806 [Google Scholar]
- Griswold K. E., Hoover W. H., Miller T. K., and Thayne W. V.. 1996. Effect of form of nitrogen on growth of ruminal microbes in continuous culture. J. Anim. Sci. 74:483–491. doi:10.2527/1996.742483x [DOI] [PubMed] [Google Scholar]
- Gustine D. L., and Jung G. A.. 1985. Influence of some management parameters on glucosinolate levels in Brassica forage. Agron. J. 77:593–597. doi:10.2134/agronj1985.00021962007700040020x [Google Scholar]
- Hafla A. N., Soder K. J., Brito A. F., Kersbergen R., Benson A. F., Darby H., Rubano M. D., and Reis S. F.. 2016. Case study: feeding strategy and pasture quality relative to nutrient requirements of dairy cows in the northeastern United States. Prof. Anim. Sci. 32:523–530. doi:10.15232/pas.2015-01500 [Google Scholar]
- Hall M. B., Hoover W. H., Kennings J. P., and Miller-Webster T. K.. 1999. A method for partitioning neutral detergent-soluble carbohydrates. J. Sci. Food Agr. 79:2079–2086. doi:10.1002/(SICI)1097-0010(199912)79:15<2079::AID-JSFA502>3.0.CO;2-Z [Google Scholar]
- Hall M. H., and Jung G. A.. 2008. Use of brassica crops to extend the grazing season. Tech. Bull. No. 33. University Park: Penn State University. [accessed March 5, 2018]. https://extension.psu.edu/use-of-brassica-crops-to-extend-the-grazing-season. [Google Scholar]
- Hoover W. H., Crooker B. A., and Sniffen C. J.. 1976. Effects of differential solid-liquid removal rates on protozoa numbers in continuous cultures of rumen contents. J. Anim. Sci. 43, 528–534. doi:10.2527/jas1976.432528x [Google Scholar]
- Hristov A. N., Lee C., Hristova R., Huhtanen P., and Firkins J. L.. 2012. A meta-analysis of variability in continuous-culture ruminal fermentation and digestibility data. J. Dairy Sci. 95:5299–5307. doi:10.3168/jds.2012-5533 [DOI] [PubMed] [Google Scholar]
- Jones D. I. H., and Bailey R. W.. 1972. The hydrolysis of cell wall polysaccharides from freeze- dried and oven-dried herbage by rumen and mould carbohydrases. J. Sci. Food Agr. 23:609–614. doi:10.1002/jsfa.2740230509 [DOI] [PubMed] [Google Scholar]
- Kaur R., Garcia S. C., Fulkerson W. J., and Barchia I.. 2010. Utilisation of forage rape (Brassica napus) and Persian clover (Trifolium resupinatum) diets by sheep: effects on whole tract digestibility and rumen parameters. Anim. Prod. Sci. 50:59–67. doi:10.1071/EA08309 [Google Scholar]
- Keogh B., French P., Murphy J. J., Mee J. F., McGrath T., Storey T., Grant J., and Mulligan F. J.. 2009. A note on the effect of dietary proportions of kale (Brassica oleracea) and grass silage on rumen pH and volatile fatty acid concentrations in dry dairy cows. Livest. Sci. 126:302–305. doi:10.1016/j.livsci.2009.06.010 [Google Scholar]
- Lambert M. G., Abrams S. M., Harpster H. W., and Jung G. A.. 1987. Effect of hay substitution on intake and digestibility of forage rape (Brassica napus) fed to lambs. J. Anim. Sci. 65:1639–1646. doi:10.2527/jas1987.6561639x [DOI] [PubMed] [Google Scholar]
- Luo J., Sun X. Z., Pacheco D., Ledgard S. F., Lindsey S. B., Hoogendoorn C. J., Wise B., and Watkins N. L.. 2015. Nitrous oxide emission factors for urine and dung from sheep fed either fresh forage rape (Brassica napus L.) Or fresh perennial ryegrass (Lolium perenne L.). Animal 9:534–543. doi:10.1017/S1751731114002742 [DOI] [PubMed] [Google Scholar]
- Mertens D. R. 2002. Gravimetric determination of amylase-treated neutral detergent fiber in feeds with refluxing in beakers or crucibles: collaborative study. J. Aoac Int. 85:1217–1240. [PubMed] [Google Scholar]
- Miller-Webster T., Hoover W. H., Holt M., and Nocek J. E.. 2002. Influence of yeast culture on ruminal microbial metabolism in continuous culture. J. Dairy Sci. 85:2009–2014. doi:10.3168/jds.S0022-0302(02)74277-X [DOI] [PubMed] [Google Scholar]
- National Academies of Sciences, Engineering, and Medicine 2016. Nutrient requirements of beef cattle. 8th rev ed. Washington (DC): National Academic Press. [Google Scholar]
- NRC 2001. Nutrient requirements of dairy cattle. 7th rev. ed Washington (DC): National Academic Press. [Google Scholar]
- Ohene-Adjei S. A. V. Chaves T. A. McAllister C. Benchaar R. M. Teather, and Forster R. J.. 2008. Evidence of increased diversity of methanogenic arachaea with plant extract supplementation. Microb. Ecol. 56:234–242. doi:10.1007/s00248-007-9340-0 [DOI] [PubMed] [Google Scholar]
- Patra A. K., Kamra D. N., and Agarwal N.. 2010. Effects of extracts of spices on rumen methanogenesis, enzyme activities and fermentation of feeds in vitro. J. Sci. Food Agric. 90:511–520. doi:10.1002/jsfa.3849 [DOI] [PubMed] [Google Scholar]
- Rico D. E., Chung Y. H., Martinez C. M., Cassidy T. W., Heyler K. W., Varga G. A, 2012. Effects of partially replacing dietary starch with dry glycerol in a lactating cow diet on ruminal fermentation during continuous culture. J. Dairy Sci. 95, 3310–3317. doi:10.3168/jds.2011–5059 [DOI] [PubMed] [Google Scholar]
- Simon K., Jones S., Jennings J., Rhein R., and Philipp D.. 2014. Forage brassica variety trial in Northwest Arkansas. Tech. Bull. No. 2014. Fayetteville: Univ. of Arkansas, p. 50–52. [Google Scholar]
- Smith D. 1969. Removing and analyzing total nonstructural carbohydrates from plant tissue. Tech. Bull No. 41. Madison: University of Wisconsin, p. 1. [Google Scholar]
- Smith D. H., and Collins M.. 2003. Forbs. In: Barnes, R. F., C. J. Nelson, M. Collins, K. J. Moore, editors, Forages: an introduction to grassland agriculture. Ames (IA): Iowa State Press, p. 215–236. [Google Scholar]
- Soder K. J., Brito A. F., Hafla A. N., and Rubano M. D.. 2016. Effect of starchy or fibrous carbohydrate supplementation of orchardgrass on ruminal fermentation and methane output in continuous culture. J. Dairy Sci. 99:4464–4475. doi:10.3168/jds.2015-10471 [DOI] [PubMed] [Google Scholar]
- van Soest P. J. 1994. Nutritional ecology of the ruminant. 2nd ed Ithaca (NY): Cornell University Press; p. 363. [Google Scholar]
- Storlien T. M., Prestløkken E., Beauchemin K. A., McAllister T. A., Iwaasa A., and Harstad O. M.. 2017. Supplementation with crushed rapeseed causes reduction of methane emissions from lactating dairy cows on pasture. Anim. Prod. Sci. 57:8–89. doi:10.1071/AN15287 [Google Scholar]
- Sun X., Henderson G., Cox F., Molano G., Harrison S. J., Luo D., Janssen P. H., and Pacheco D.. 2015. Lambs fed fresh winter forage rape (Brassica napus L.) emit less methane than those fed perennial ryegrass (Lolium perenne L.) and possible mechanisms behind the difference. PLoS ONE. 10:e0119697. doi:10.1371/journal.pone.0119697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X. Z, Waghorn G. C., Hoskin S. O., Harrison S. J., Muetzel S., and Pacheco D.. 2012. Methane emissions from sheep fed fresh brassicas (Brassica spp.) compared to perennial ryegrass (Lolium perenne). Anim. Feed Sci. Tech. 176:107–116. doi:10.1016/ j.anifeedsci.2012.07.013 [Google Scholar]
- Tripathi M. K., and Mishra A. S.. 2007. Glucosinolates in animal nutrition: a review. Anim. Feed Sci. Tech. 132:1–27. doi:10.1016/j.anifeddsci.2006.03.003 [Google Scholar]
- Velasco P., Soengas P., Vilar M., and Cartea M. E.. 2008. Comparison of glucosinolate profiles in leaf and seed tissues of difference Brassica napus crops. J. Am. Soc. Hortic. Sci. 133:551–558. doi:10.1093/jxb/err373 [Google Scholar]
- Villalobos L. A., and Brummer J. E.. 2015. Forage brassicas stockpiled for fall grazing: yield and nutritive value. Crop Forage Turfgrass Manag. 1:1–6. doi:10.2134/ cftm2015.0165 [Google Scholar]
- Weller R. A., and Pilgrim A. F.. 1974. Passage of protozoa and volatile fatty acids from the rumen of the sheep and from a continuous in vitro fermentation system. Br. J. Nutr. 32:341–351. [DOI] [PubMed] [Google Scholar]
- Williams S. R. O., Moate P. J., Deighton M. H., Hannah M. C., Wales W. J., and Jacobs J. L.. 2016. Milk production and composition, and methane emissions from dairy cows fed lucerne hay with forage brassica or chicory. Anim. Prod. Sci. 56:304–311. doi:10.1071/AN15528 [Google Scholar]
- Yang C. M. J., and Varga G. A.. 1989. Effect of three concentrate feeding frequencies on rumen protozoa, rumen digesta kinetics, and milk yields in dairy cows. J. Dairy Sci. 72:950–957. doi:10.3168/jds.S0022-0302(89)79188-8 [DOI] [PubMed] [Google Scholar]
- Zinn R. A., and Owens F. N.. 1986. A rapid procedure for purine measurement and its use for estimating net ruminal protein synthesis. Can. J. Anim. Sci. 66:157–166. doi:10.4141/cjas86-017 [Google Scholar]