Abstract
Small noncoding RNA molecules (miRNA) regulate protein levels in a post-transcriptional manner by partial base pairing to the 3′-UTR of target genes thus mediating degradation or translational repression. Previous studies indicate that numerous miRNA regulate the biosynthesis of intraovarian hormones, and emerging evidence indicates that one of these, miRNA-221 (MIR221), may be a modulator of ovarian function. However, the hormonal control of ovarian MIR221 is not known. The objectives of this study were to investigate the developmental and hormonal regulation of MIR221 expression in granulosa (GC) and theca cell (TC) and its possible role in regulating follicular function. Bovine ovaries were collected from a local abattoir and GC and TC were obtained from small (<6 mm) and large (≥8 mm) follicles. In Exp. 1, GCs of small follicles had 9.7-fold greater (P < 0.001) levels of MIR221 than those of large follicles, and TCs of large follicles had 3.7-fold greater (P < 0.001) levels of MIR221 than those of small follicles. In large follicles, abundance of MIR221 was 66.6-fold greater (P < 0.001) in TCs than in GCs. In small follicles, MIR221 abundance did not differ (P = 0.14) between GC and TCs. In vitro Exp. 2, 3, and 4 revealed that treatment of bovine TCs with various steroids, phytoestrogens, IGF1, forskolin, and dibutyryl cyclic adenosine monophosphate had no effect (P > 0.35) on MIR221 expression, whereas treatment with fibroblast growth factor 9 (FGF9) and FGF2 increased (P < 0.001) TC MIR221 abundance 1.7- to 2.5-fold. In Exp. 5, FGF9 increased (P < 0.05) GC MIR221 abundance by 1.7- and 2.0-fold in small and large follicles, respectively. The role of MIR221 in GC steroidogenesis was investigated in Exp. 6 and it was found that transfection with a MIR221 mimic reduced (P < 0.01) GC estradiol and progesterone production induced by FSH and IGF1, whereas transfection with MIR221 inhibitor had little or no effect. We conclude that thecal MIR221 expression is increased by FGF9 and increased MIR221 may act to inhibit GC steroidogenesis in cattle.
Keywords: cattle, follicle growth, granulosa cell, microRNA 221 (MIR221), steroidogenesis, theca cell
INTRODUCTION
Recent research has demonstrated that microRNAs (miRNA) function to silence RNA and post-transcriptionally regulate gene expression in numerous tissues and biological functions in humans and animals, adding another dimension to gene regulation within the animal genome (Hossain et al., 2009; Li et al., 2011). These small noncoding RNA molecules regulate protein levels in a post-transcriptional manner by partial base pairing to the 3′-UTR of target genes thus mediating degradation or translational repression (He and Hannon, 2004; Ha and Kim, 2014). Studies have indicated that numerous miRNA regulate the biosynthesis of intraovarian hormones (Yao et al., 2010; Donadeu et al., 2012; Yin et al., 2012; Hu et al., 2013) and change within follicles. For example, MIR21, MIR26a, and MIR143 are elevated in ovaries of anestrous sheep (Di et al., 2014), whereas MIR21, MIR132, MIR212, and MIR224 are increased during follicular growth in mares (Schauer et al., 2013). Furthermore, MIR92a and MIR92b are lower in theca cells (TC) of women with polycystic ovarian syndrome (Lin et al., 2015).
Emerging evidence indicates that miRNA-221 (MIR221) may be a modulator of ovarian function. It was first reported that over expression of MIR221 was associated with human ovarian cancer (Dahiya et al., 2008). More recently, MIR221 abundance was found to be severalfold greater in granulosa cells (GC) of subordinate than of dominant follicles at d 3 of an estrous cycle in cattle but on d 7 MIR221 abundance in GC was severalfold less in subordinate than in dominant follicles (Salilew-Wondim et al., 2014). Blood plasma levels of MIR221 are greater in FSH-treated heifers and also increase between d 3 and 7 postestrus (Noferesti et al., 2015). However, no study has determined the hormonal regulation of ovarian cell MIR221 in cattle. Thus, the aims of the present work were to investigate the hormonal regulation of MIR221 expression in TC and GC, and its possible role in follicular steroidogenesis.
MATERIALS AND METHODS
Reagents and Hormones
Reagents and hormones used for cell preparation and culture were: gentamicin (catalog number G1397), glutamine (catalog number G6392), Ham’s F-12 (catalog number N4888), Dulbecco modified Eagle medium (DMEM) (catalog number D5921), sodium bicarbonate (catalog number S8761), trypan blue (catalog number T8154), protease (catalog number P5147), collagenase (catalog number C0130), hyaluronidase (catalog number H3506), deoxyribonuclease (DNase) (catalog number DN25), TRI reagent (catalog number T9424), and penicillin-streptomycin (catalog number P0781) from Sigma-Aldrich Chemical Company (St. Louis, MO); ovine FSH (175 x NIH-FSH-S1 U/mg) from the National Hormone and Peptide Program (Harbor-UCLA Medical Center, Torrance, CA); recombinant human IGF1 (catalog number 291-G1/CF), recombinant human fibroblast growth factor 9 (FGF9; catalog number 273-F9/CF), and recombinant bovine FGF2 (catalog number 2099-FB/CF) from R&D Systems (Minneapolis, MN); forskolin (catalog number F6886), dibutyryl cyclic adenosine monophosphate (dbcAMP) (catalog number D0260), estradiol (catalog number E8875), α-zearalenol (catalog number Z0166), and β-zearalenol (catalog number Z2000) from Sigma-Aldrich Chemical Company; genistein (catalog number EI-147) from Biomol Research Labs, Inc. (Plymouth Meeting, PA); androstenedione (catalog number A6030) and testosterone (catalog number A6950) from Steraloids (Wilton, NH); and fetal calf serum (FCS) (catalog number SR30-1572) from Equitech-Bio, Inc. (Kerrville, TX).
Cell Culture
Ovaries from beef heifers were collected from a local abattoir and transported to the lab on ice in saline with antibiotics (0.9% saline solution with 1% penicillin-streptomycin) as previously described (Langhout et al., 1991; Schreiber et al., 2012). Follicular fluid was aspirated from small (<6 mm) and large (≥8 mm) follicles (appeared healthy with good vascularity and moderately transparent follicular fluid) and GC and TC were collected as previously described (Stewart et al., 1995; Schreiber et al., 2012; Zhang et al., 2017). Isolated GC and TC were washed twice in serum-free medium (38.5 mM of sodium bicarbonate, 0.12 mM of gentamicin and 2.0 mM of glutamine within 1:1 DMEM and F12) and then re-suspended in serum-free medium containing collagenase (1.25 mg/mL) and DNase (0.5 mg/mL) to prevent cell clumping as previously described (Lagaly et al., 2008; Schreiber et al., 2012).
Viability of TC from large follicles and GC from small and large follicles was determined by trypan blue exclusion method (Adashi et al., 1987), and averaged 90.7 ± 2.2%, 81.8 ± 2.9% and 74.0 ± 6.1%, respectively. On average, 2.0 × 105 viable cells were plated on 24-well Falcon multiwell plates (No. 3047; Becton Dickinson, Lincoln Park, NJ) in 1 mL of medium and cultured in an environment of 38.5°C with 5% CO2 and 95% air in 10% FCS for the first 48 h to 144 h until cells reached 80% confluency with medium changes every 24 h. Cells were washed twice with 0.5 mL of serum-free medium, treatments were applied in serum-free medium for an additional 24 h or 48 h, and medium was either aspirated or collected from each well depending on the particular experiment. This culture system was developed to yield hormonally responsive nonluteinized GC and TC (Langhout et al., 1991; Stewart et al., 1995; Spicer and Chamberlain, 1998). First, progesterone production does not increase with time using this culture paradigm (Langhout et al., 1991; Stewart et al., 1995). Secondly, the morphology of the GC and TC retains a fibroblastic appearance (Chamberlain and Spicer, 2001). Third, aromatase activity of GC remains responsive to FSH, insulin and IGF-I and increases between d 3 and 4 of culture (Spicer et al., 1993; Spicer and Chamberlain, 1998), and the TC remain responsive to LH and IGF1 in terms of CYP17A1 mRNA and androstenedione production (Stewart et al., 1995; Spicer et al., 2008).
Experimental Design
Experiment 1 was designed to determine if the abundance of MIR221 differed between GC and TC and between small and large follicles. Therefore, GC and TC samples were collected from small and large follicles as described earlier. For large-follicle GC and TC, each of seven samples was collected from one large healthy follicle from one ovary. For small-follicle GC and TC, each sample had pooled cells from three to five small follicles from one ovary. Each cell type had seven samples collected from at least three animals. These fresh cells were lysed in TRI reagent and extracted for RNA as described below.
Because preliminary microarray data from our laboratory indicated FGF9 may induce MIR221 in TC, Exp. 2 was designed to test the effect of IGF1, FGF9, and FGF2 on MIR221 expression in large-follicle TC. Cells were cultured for 48 h in 10% FCS medium, washed twice with serum-free medium, and six treatments in a 2 × 3 factorial arrangement were applied for 24 h as follows: control, FGF9 (30 ng/mL), FGF2 (30 ng/mL), IGF1 (30 ng/mL), and IGF1 plus FGF9 or FGF2. Co-treatment with IGF1 was evaluated because many studies show a synergistic effect of IGF1 on hormone-induced cellular responses in ovarian cells (Spicer and Echternkamp, 1995; Spicer and Chamberlain, 1998). After 24 h of treatment, medium was aspirated and cellular RNA was collected as described below. Doses of FGF9 and FGF2 were selected based on previous studies showing that the doses used are effective in eliciting a functional response in cultured TC and GC (Vernon and Spicer, 1994; Schreiber et al., 2012; Totty et al., 2017).
To further explore possible hormonal regulation of MIR221 expression in TC and because a previous study reported an inhibitory effect of cAMP on MIR221 abundance (Castagnino et al., 2013), Exp. 3 was designed to test the effect of FGF9, dbcAMP, and forskolin-mediated cAMP on MIR221 expression in large-follicle TC. Forskolin is an inducer of adenyl cyclase activity increasing intracellular cAMP levels (Hedin and Rosberg, 1983; Adashi et al., 1989), whereas dbcAMP is a cAMP analog that mimics the effect of cAMP (Hedin and Rosberg, 1983; Schreiber et al., 2012). Cells were cultured for 72 h in 10% FCS medium, washed twice with serum-free medium, and six treatments were applied in a 2 × 3 factorial arrangement in serum-free medium as follows: control, dbcAMP (0.1 mg/mL; 0.2 mM), forskolin (4.1 μg/mL; 10 μM), and control plus FGF9 (30 ng/mL), dbcAMP plus FGF9 (30 ng/mL), forskolin plus FGF9 (30 ng/mL). After 24 h of treatment, medium was aspirated and cellular RNA was collected as described below. Doses of FGF9, dbcAMP, and forskolin were selected based on previous studies showing that the doses used are effective in eliciting a functional response in cultured TC and/or GC (Duleba et al., 1985; McArdle et al., 1991; Vernon and Spicer, 1994; Schreiber et al., 2012).
Because a previous study has shown that estrogens directly repress MIR221 in human breast cancer cell lines (Di Leva et al., 2010) and androgens repress MIR221 in human prostate cell lines (Ambs et al., 2008), Exp. 4 was designed to test the effect of steroids and phytoestrogens on MIR221 abundance in large-follicle TC. Cells were cultured for 144 h in 10% FCS medium, washed twice with serum-free medium, and six treatments were applied in serum-free medium as follows: control, genistein (300 ng/mL), estradiol (300 ng/mL), α-zearalenol (300 ng/mL), β-zearalenol (300 ng/mL), and androstenedione (300 ng/mL). After 24 h of treatment, medium was aspirated and cells were lysed in 0.5 mL of TRI reagent for RNA extraction as described below. Doses of the various steroids were selected based on previous studies showing that the doses used are effective in eliciting a functional response in cultured TC and/or GC (Spicer, 2005; Ranzenigo et al., 2008; Mlynarczuk et al., 2011; Aad et al., 2012; Zhang et al., 2017). Because previous studies have suggested that the phytoestrogens genistein, α-zearalenol, and β-zearalenol have estrogenic activity (Ranzenigo et al., 2008; Mlynarczuk et al., 2011; Pizzo et al., 2015; 2016), we decided to evaluate the possible effects of these phytoestrogens in addition to estradiol.
Experiment 5 was designed to test the effect of FGF9 on GC MIR221 expression in small and large follicles. Cells were cultured as in Exp. 2, washed twice with serum-free medium, and treated with either 0 or 30 ng/mL of FGF9. After 24 h of treatment, medium was aspirated and cellular RNA was collected as described below.
Because previous studies have indicated microRNAs can impact GC steroid production (Sirotkin et al., 2009; Xu et al., 2011; Dai et al., 2013), Exp. 6 was designed to determine the effects of MIR221 on steroidogenesis of small-follicle GC using transfection of MIR221 mimics and inhibitors. Cells were cultured as previously described for Exp. 3, washed twice with serum-free medium, and treated with transfection complexes (control, mimic, inhibitor) (see below) and incubated for 12 h, and then treatments of FGF9 (0 or 30 ng/mL) and IGF1 (0 or 30 ng/mL) were applied in a 3 × 2 × 2 factorial arrangement (see below). After 40 h of treatment, medium was collected for progesterone and estradiol determinations and cells were counted. For transfections, control, MIR221 mimic, or MIR221 inhibitor transfection medium was applied to GC for 12 h before treatment. Briefly, 1,300 μL Opti-MEM medium (catalog number 31985070; Invitrogen, Carlsbad, CA) and 39 μL Lipofectamine (catalog number 56531; Invitrogen) were combined, then 52 μL MIR221 mimic (5 pmol; AGCUACAUUGUCUGCUGGGUUU; catalog number 4464066) or inhibitor (5 pmol; UUUCCCUGCUGUCUUTGTUGCT; catalog number 4464084) purchased from (Life Technologies Corp., Carlsbad, CA) was added. Control transfection complex contained 1,300 μL Opti-MEM medium and 39 μL Lipofectamine. Attached GC were washed twice with 0.5 mL of serum-free medium, then 200 μL of serum-free medium and 50 μL of miRNA-transfection medium was applied to each well. GC were incubated with 250 μL of transfection medium for 12 h, after which an additional 250 μL of medium was added to each well containing control (no added hormones), IGF1, FGF9, or IGF1 plus FGF9 for an additional 40 h. Final concentrations of IGF1 and FGF9 were 30 ng/mL. A similar transfection procedure has been used and shown to be effective in this culture system (Spicer et al., 2006; 2008).
RNA Extraction and Real-Time PCR
For gene expression experiments, medium was aspirated and cells from two replicate wells were lysed in 0.5 mL of TRI reagent as previously described (Aad et al., 2012; Totty et al., 2017; Zhang et al., 2017). Briefly, 0.25 mL TRI reagent was added to all wells, cells were lysed by repeated pipetting and then combined with their respective replicates with each treatment containing four wells that generated two replicate samples of RNA. RNA from cell lysates were isolated using TRI reagent protocol and Phase Lock Gel Heavy tubes (5 Prime Inc., Germantown, MD) and quantitated as previously described (Voge et al., 2004; Aad et al., 2012; Schreiber et al., 2012; Zhang et al., 2017).
Quantification of MIR221 expression was determined using two-step RT-PCR using TaqMan Small RNA Assays (Life Technologies Corp.; catalog number 4427925) specific for MIR221. Complementary DNA (cDNA) was synthesized from total RNA samples using stem-loop primers, and TaqMan MicroRNA Reverse Transcription Kit (Life Technologies Corp.; catalog number 4366596). Reactions were performed on a CFX96 Real-Time System (Bio-Rad, Hercules, CA) in a 96-well plate with the following cycling conditions: 16 °C for 30 min, 42 °C for 30 min, and 85 °C for 5 min; reaction products were stored at −20 °C. Subsequent real-time quantitative PCR was performed using target-specific TaqMan Assays (Life Technologies Corp.; catalog number 4373318 with FAM dye) and the TaqMan Universal PCR Master Mix II, no UNG (Life Technologies Corp.; catalog number 4440040). Each 20 μL reaction volume contained the appropriate cDNA, specific TaqMan Assay, and TaqMan Universal PCR Master Mix II. All sample assays were performed in triplicate to determine an average threshold cycle (CT) value. PCR cycling conditions performed on CFX96 Real-Time System consisted of 95 °C for 10 min and 40 cycles of 95 °C for 15 s and 60 °C for 1 min in 96-well plates (Bio-Rad). The PCR intra-assay CV averaged 0.98%, and U6 snRNA (Life Technologies Corp.; catalog number 4395470) was used as an endogenous control to correct for discrepancies in RNA quantification and loading as previously described (Yang et al., 2012).
Radioimmunoassays
Progesterone concentrations in medium were determined using a double-antibody radioimmunoassay (RIA) as previously described (Langhout et al., 1991; Schreiber et al., 2012) and the intra-assay coefficient of variation averaged 10.6 ± 0.3%. Estradiol concentrations in medium were determined using a double-antibody RIA as previously described (Spicer et al., 1993; Schreiber and Spicer, 2012) and the intra-assay coefficient of variation averaged 9.0 ± 0.5%.
Determination of Cell Numbers
Cell numbers were determined using a Coulter Counter (Model Z2; Beckman Coulter, Hialeah, FL) as previously described (Langhout et al., 1991; Lagaly et al., 2008; Spicer et al., 2011).
Statistical Analysis
For Exp. 1, main effects in ANOVA were cell type, follicle size, pool, and their interactions. For Exp. 2 and 5, main effects in ANOVA were treatment, pool, and their interactions. For Exp. 3 and 4, main effects of FGF9 and treatment were analyzed in a 2 × 3 factorial ANOVA. For Exp. 6, main effects in a 3 × 2 × 2 factorial ANOVA with transfection complex (control, mimic, or inhibitor), FGF9 (+ or −), IGF1 (+ or −), pool, and their interactions. For the in vitro Exp. 2 to 5, each treatment was applied to three or four independent pools of large-follicle TC or GC collected from seven to eight follicles for each pool (biological replicate) and contained two replicates per treatment. For Exp. 5 and 6, each pool (biological replicate) of small-follicle GC was collected from 10 to 30 ovaries and contained two or three replicates per treatment. Treatment effects and interactions on dependent variables (e.g., MIR221 abundance and steroid production) were assessed using the GLM and ANOVA procedure of SAS (version 9.2, SAS Institute Inc., Cary, NY). To correct for heterogeneity of variance, data were analyzed after transformation to natural log (x + 1) when necessary. Mean differences were assessed using Fisher’s protected least significant differences test (Ott, 1977), if significant treatment effects in ANOVA were detected. Steroid production was expressed as ng/105 cells per 24 h and cell numbers at the end of the experiment were used for this calculation. Significance was declared at P < 0.05 and trends identified at P < 0.10. Data are presented as means ± pooled SEM of measurements from replicated experiments.
RESULTS
Changes in Abundance of MIR221 in GC and TC During Follicular Growth (Exp. 1)
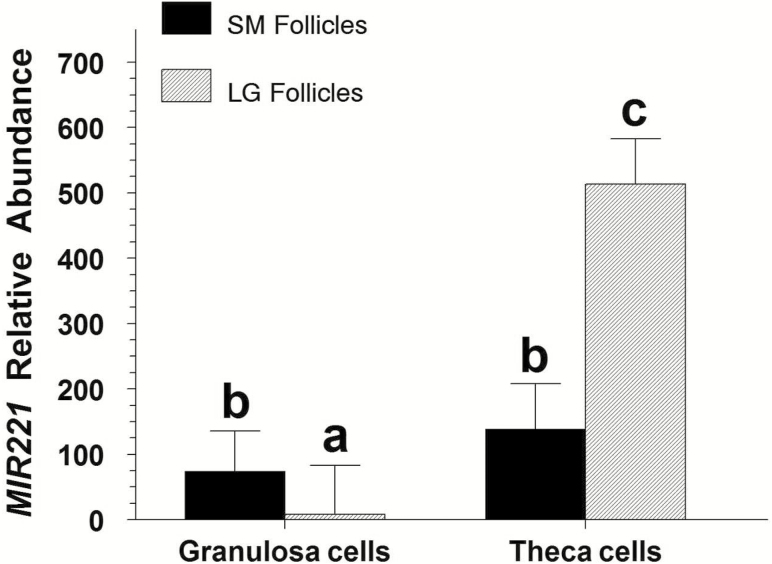
A size by cell-type interaction on MIR221 abundance was observed (P < 0.001). GCs of small follicles had 9.7-fold greater (P < 0.001) abundance of MIR221 than those of large follicles, and TC of large follicles had 3.7-fold greater (P < 0.001) abundance of MIR221 than those of small follicles (Figure 1). In large follicles, abundance of MIR221 was 66.6-fold greater (P < 0.001) in TC than in GC. In small follicles, MIR221 abundance did not differ (P = 0.14) between GC and TC (Figure 1).
Figure 1.
Abundance of MIR221 in freshly collected granulosa and theca cells from small (SM; <6 mm) and large (LG; ≥8 mm) bovine follicles (Exp. 1). Values are normalized to constitutively expressed U6 snRNA and expressed as fold of large-follicle granulosa cell values. a,b,cMeans (±pooled SEM) without a common letter differ (P < 0.001).
Effects of Growth Factors, cAMP Inducers, and Steroids on MIR221 Abundance in Large-Follicle TC
Exp. 2.
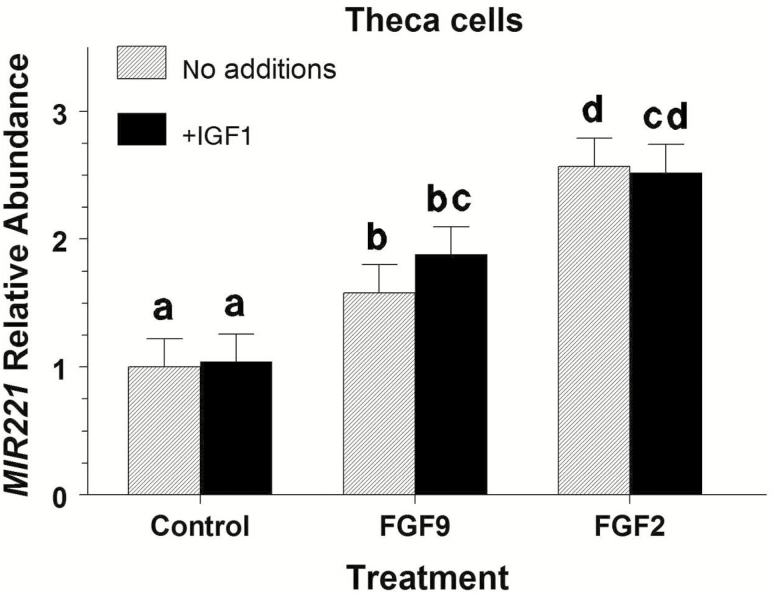
No significant interaction (P = 0.67) between FGF9 and IGF1 treatments on TC MIR221 abundance was observed. However, FGF9 and FGF2 treatments increased (P < 0.001) MIR221 expression by 1.7- and 2.5-fold (Figure 2), respectively. No significant effect of IGF1 on MIR221 abundance was observed (P =.36).
Figure 2.
Effect of FGF9 and FGF2 on MIR221 abundance in bovine large-follicle theca cells treated with or without IGF1 (Exp. 2). Theca cells were isolated and cultured for 48 h in 10% FCS after which cells were treated with 30 ng/mL of FGF9 or FGF2 and/or 30 ng/mL of IGF1 for 24 h. Values are normalized to constitutively expressed U6 snRNA and expressed as fold of control values with no additions. a,b,c,dMeans (±pooled SEM) without a common letter differ (P < 0.05).
Exp. 3.
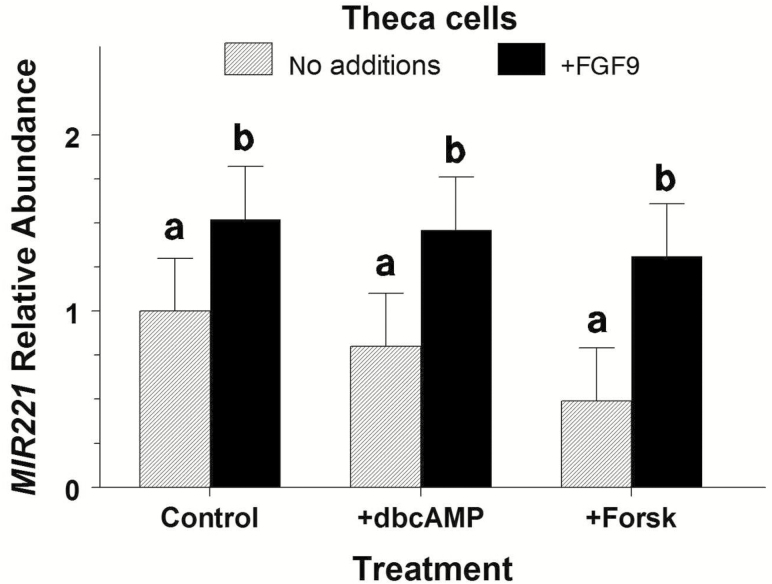
Main effect of FGF9 influenced (P = 0.03) TC MIR221 abundance but dbcAMP and forskolin treatments (P = 0.50) and their interaction with FGF9 (P = 0.52) did not affect MIR221 abundance (Figure 3). Averaged across treatments, FGF9 increased (P = 0.02) MIR221 abundance by 1.91-fold.
Figure 3.
Lack of effect of pharmacologic cAMP agents on MIR221 abundance in bovine large-follicle theca cells treated with or without FGF9 (Exp. 3). Theca cells were isolated and cultured for 72 h in 10% FCS and then treated with 0.1 mg/mL of dbcAMP, 4.1 μg/mL of forskolin (Forsk), and/or 30 mg/mL of FGF9 for 24 h. Values are normalized to constitutively expressed U6 snRNA and expressed as fold of control values with no additions. a,bMeans (±pooled SEM) without a common letter differ (P < 0.05).
Exp. 4.
Treatment of various steroids and phytoestrogens did not affect (P = 0.41) MIR221 abundance (data not shown). Relative abundance of MIR221 averaged 1.00, 1.34, 1.30, 0.95, 1.24, and 1.58 ± 0.22 for control, genistein, estradiol, α-zearalenol, β-zearalenol, and androstenedione, respectively.
Effect of FGF9 on MIR221 Abundance in Small- and Large-Follicle GC (Exp. 5)
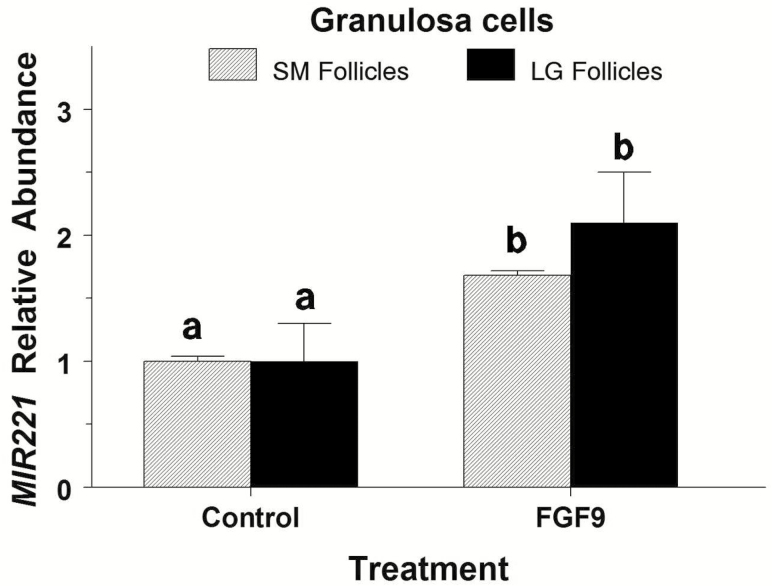
Data for small and large-follicle GC were analyzed separately. In GC from small and large follicles, FGF9 increased MIR221 abundance by 1.7-fold (P = 0.04) and 2.1-fold (P = 0.02), respectively (Figure 4).
Figure 4.
Effect of FGF9 on MIR221 abundance in bovine granulosa cells from small (SM) and large (LG) follicles (Exp. 5). Granulosa cells were isolated and cultured for 48 h in 10% FCS after which cells were treated with either 0 or 30 ng/mL of FGF9 for 24 h. Values are normalized to constitutively expressed U6 snRNA and expressed as fold of control values. a,bWithin follicle size group, means (±pooled SEM) without a common letter differ (P < 0.05).
Effect of Transfection of GC With MIR221 Mimic and Inhibitor on Steroidogenesis (Exp. 6)
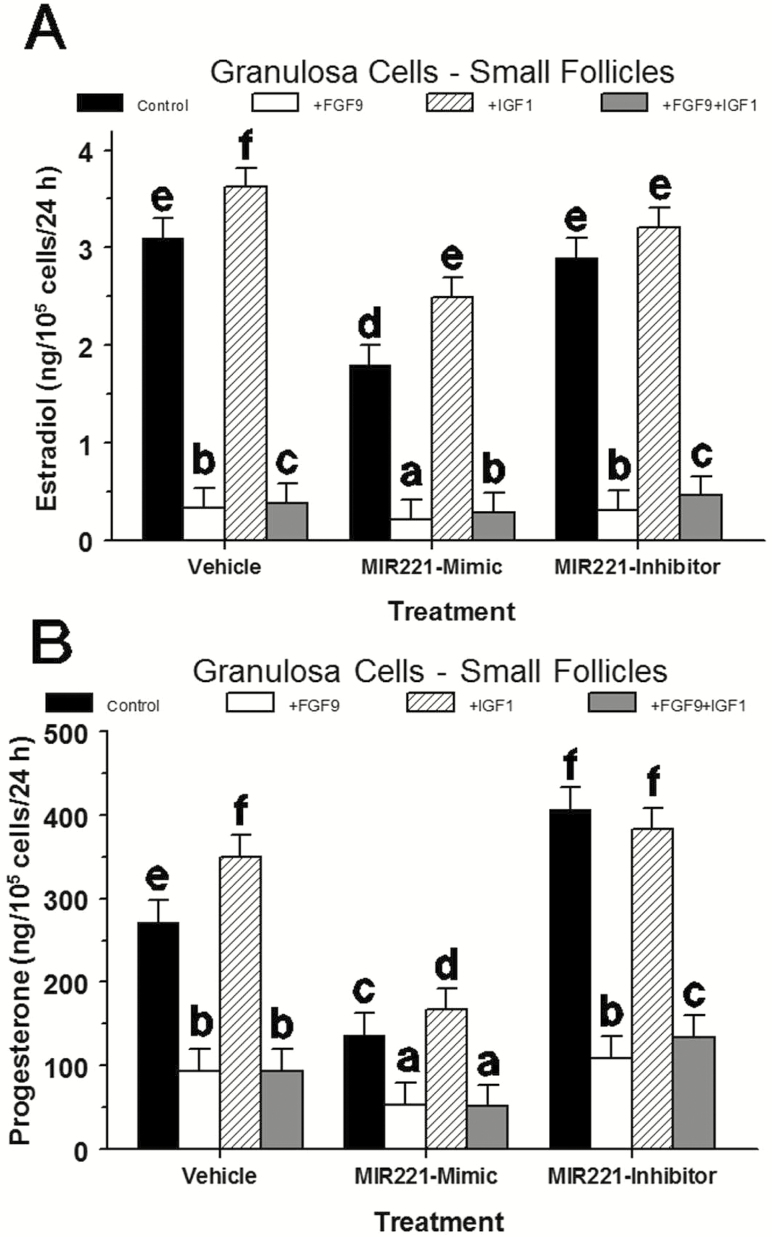
A significant treatment × IGF1 × FGF9 interaction existed (P < 0.01) such that FGF9 inhibited estradiol production by 86% to 89% regardless of the presence of IGF1 or MIR221 mimic or inhibitor (Figure 5A). Transfection of GC with MIR221 mimic inhibited (P < 0.001) estradiol production in control and IGF1-treated GC by 42% and 31%, respectively, and further reduced (P < 0.001) the FGF9 inhibition of estradiol production by 26% to 35%. Transfection of GC with MIR221 inhibitor only inhibited (P = 0.04) the IGF1-induced increase in estradiol production by 11% (Figure 5A).
Figure 5.
Effect of MIR221 mimic and MIR221 inhibitor on estradiol (A) and progesterone (B) production in bovine small-follicle granulosa cells treated with or without IGF1 and FGF9 (Exp. 6). Granulosa cells were isolated and cultured in 10% FCS and then transfected with either MIR221 mimic or MIR221 inhibitor for 12 h after which cells were treated with 0 or 30 ng/mL of FGF9, and 0 or 30 ng/mL of FSH + IGF1 for 40 h. a,b,c,d,e,fWithin a panel, means (±pooled SEM) without a common letter differ (P < 0.05).
No significant treatment × IGF1 × FGF9 (P = 0.19) or any two-way interaction existed (P = 0.19). Treatment (P < 0.001) and FGF9 affected (P < 0.001) progesterone production but IGF1 did not (P = 0.10). Specifically, FGF9 inhibited (P < 0.01) progesterone production 61% to 73% regardless of the presence of IGF1 or MIR221 mimic or inhibitor (Figure 5B). Transfection of GC with MIR221 mimic inhibited (P < 0.001) progesterone production in control and IGF1-treated GC by 50% and 52%, respectively, and further decreased (P < 0.001) progesterone production by 44% to 46%, respectively, in the presence of FGF9. Treatment with MIR221 inhibitor increased progesterone production in control cultures (by 50%; P < 0.01) and in those treated with IGF1 plus FGF9 (by 43%; P = 0.02) but did not affect progesterone production treated with IGF1 (P = 0.49) or FGF9 (P = 0.20) alone (Figure 5B).
DISCUSSION
MIR221 is expressed in a wide variety of tissues, including the breast (Di Leva et al., 2010), prostate (Sun et al., 2009), endometrium (Pan et al., 2007; Kuokkanen et al., 2010), and ovary (Dahiya et al., 2008; Salilew-Wondim et al., 2014). The present findings provide novel information on the dynamic regulation of MIR221 levels in bovine GC and TC. Specifically the present results indicated that: (1) MIR221 abundance in TC was severalfold greater than in GC of large follicles; (2) MIR221 increased with follicle size in TC but decreased with follicle size in GC; (3) IGF1, estrogenic compounds, dbcAMP, and forskolin had no effect on TC MIR221 abundance; (4) FGF9 increased both TC and GC MIR221 abundance; and (5) transfection of GC with MIR221 mimic reduced GC estradiol and progesterone production, whereas transfection with MIR221 inhibitor increased progesterone production. These findings suggest that TC may be the main source of MIR221 in large follicles and that FGF9 may modulate GC and TC function by influencing MIR221 expression in cattle.
Previous studies indicate that a single microRNA will target, on average, 200 transcripts (Krek et al., 2005) in part because as little as 6 bp that match with the target mRNA can be sufficient to suppress gene expression (Lewis et al., 2003; Brennecke et al., 2005). Also, it is thought that miRNA expression broadly contributes to tissue specificity of mRNA expression in many human tissues (Sood et al., 2006). A potential 5,760 gene targets for human MIR221 have been proposed (http://www.microrna.org/microrna/getTargets.do?matureName=hsa-miR-221&startIndex=0&organism=9606) and research with human SNU-398 heptocarcinoma cells reported 125 genes to be regulated by MIR221 (Lupini et al., 2013). Using TargetScan (release 7.1, Whitehead Institute for Biomedical Research; Agarwal et al., 2015) (see: http://www.targetscan.org/cgi-bin/targetscan/vert_71/targetscan.cgi?species=Cow&gid=&mir_sc=miR-221%2F222&mir_c=&mir_nc=&mir_vnc=&mirg=) 43 target genes of bovine MIR221 were identified. Therefore, further research will be required to identify and verify specific target genes of bovine MIR221 in TC and GC.
Determining the expression of MIR221 in GC and TC and the hormonal regulation of MIR221 expression is crucial to understanding its role in ovarian follicular function. In a recent study using cattle, MIR221 expression in GC was up regulated (by 1.8-fold) in subordinate vs. dominant follicles on d 3 postestrus only to be 3.2-fold less in GC of subordinate vs. dominant follicles on d 5 postestrus (Salilew-Wondim et al., 2014). Previously, dominant follicles on d 3 to 4 postovulation had significantly less FGF9 mRNA than subordinate follicles (Schütz et al., 2016). Thus, based on results of previous and present studies, changes in follicular cell MIR221 expression may involve changes in FGF9, which belongs to the 23-member FGF family (Chaves et al., 2012). To date, FGF1, FGF2, FGF7, FGF8, FGF9, FGF10, FGF17, and/or FGF18 have been described in ovaries of many species including rodents (Drummond et al., 2007) and domestic animals (Machado et al., 2009; Portela et al., 2010; Grado-Ahuir et al., 2011). Functions of FGF members in ovarian biological processes include regulation of steroidogenesis (Vernon and Spicer, 1994; Schreiber and Spicer, 2012; Evans et al., 2014), apoptosis and cell survival (Portela et al., 2010; Jiang and Price, 2012), and control of ovarian cell proliferation (Buratini et al., 2005; Schreiber and Spicer, 2012; Schreiber et al., 2012). It has been suggested that FGF9 is acting as an antidifferentiation factor stimulating GC and TC proliferation while inhibiting steroidogenesis in cattle (Schreiber and Spicer, 2012; Schreiber et al., 2012; Schütz et al., 2016). Based on results of the present study, both small and large follicles have greater MIR221 expression in TC than GC. Interestingly, MIR221 abundance increased in TC and decreased in GC as follicles enlarged, suggesting that control of MIR221 expression may differ in GC and TC. However, in the preovulatory dominant and subordinate follicles of heifers, TC and GC MIR221 abundance did not differ (Gebremedhn et al., 2015). In the present study, FGF9 and FGF2 increased MIR221 expression by 2- to 3-fold, whereas IGF1 had no effect. Whether changes in FGF2 and FGF9 (or other FGF) are regulating changes in GC or TC MIR221 abundance during follicular growth will require further elucidation.
In contrast to previous studies in nonovarian tissue, the present study indicates TC MIR221 expression is not regulated by steroids. Previously, estrogen receptor α stimulation directly repressed MIR221 expression in human breast cancer cell lines (Di Leva et al., 2010), whereas in human prostate cell lines, MIR221 expression is repressed by androgens (Ambs et al., 2008). Because our findings indicated that estrogens and androgens had no effect on MIR221 abundance in TC, it appears that as a follicle grows, changes in steroid hormones do not regulate the changes in MIR221 expression in bovine GC and TC. This latter suggestion is supported by the findings of Gebremedhn et al. (2015) who reported that abundance of MIR221 in TC and GC did not differ between dominant and subordinate preovulatory follicles in heifers. Together, the present and previous results suggest that steroid regulation of MIR221 expression may be tissue specific.
In the current study, enhancers of cAMP production/action had no effect on MIR221 abundance in TC, which is in contrast to findings of Castagnino et al. (2013) who found that 1 mM dbcAMP and forskolin inhibited MIR221 expression in cultured murine vascular smooth muscle cells. In cultured murine GC, treatment with 1 mM 8-bromo-cAMP had no effect on another microRNA, MIR21 (Carletti et al., 2010). Similarly, changes in MIR125b, MIR21, MIR145, and MIR34a in cultured bovine TC were independent of 10 µM forskolin treatment (McBride et al., 2012). In line with these findings, data from the present study indicate that the signal transduction of cAMP does not affect MIR221 abundance in vitro, as dbcAMP and forskolin did not alter MIR221 expression. We recognize that our study has limitations including the fact that only one dose of dbcAMP, forskolin, growth factors, and steroids were used, and thus, future studies should evaluate the effects of additional doses of these factors.
Small noncoding RNA molecules regulate protein levels in a post-transcriptional manner by partial base pairing to the 3′-UTR of target genes thus mediating degradation or translational repression (He and Hannon, 2004; Ha and Kim, 2014) and research indicates that miRNA can have both inhibiting and stimulating roles (Sirotkin et al., 2009; Xu et al., 2011; Dai et al., 2013) on ovarian steroid hormone production. For example, in human KGN (granulosa) cells, MIR133a stimulates estradiol production (Dai et al., 2013), whereas in porcine GC, MIR378 inhibits estradiol production (Xu et al., 2011). In the present study, MIR221 mimic reduced estradiol and progesterone production by bovine GC, suggesting that MIR221 may be inhibiting steroidogenesis in GC via translational repression of CYP19A1 and CYP11A1. Other studies have suggested direct targeting of gene(s) associated with steroid biosynthesis or indirect by affecting its precursors or processing and metabolism (Sirotkin et al., 2009; Yan et al., 2012; Dai et al., 2013). Further research will be required to ascertain whether MIR221 directly targets CYP19A1 (for estradiol production) or CYP11A1 (for progesterone production) in bovine GC. In androgen-independent human prostate cancer cell lines, the MIR221 cluster is known to interfere with androgen receptor transcriptional activity without affecting the androgen receptor itself (Sun et al., 2009). Transfection of GC with MIR221 inhibitor had little or no effect on steroid production in the present study, further implying that the main source of follicular fluid MIR221 may originate from TC.
Many miRNA have been suggested to be involved in regulating ovarian follicular function in cattle (Hossain et al., 2009; Salilew-Wondim et al., 2014; Gebremedhn et al., 2015), sheep (Baillet et al., 2008; Di et al., 2014), mares (Schauer et al., 2013), humans (Sirotkin et al., 2009; Lin et al., 2015), and rodents (Herrera et al., 2005; Yao et al., 2010). Examination of miRNA expression in follicles of sheep and cattle revealed some miRNA are expressed more abundantly in TC while others are expressed more abundantly in GC (McBride et al., 2012; Sohel et al., 2013; Gebremedhn et al., 2015). In addition, previous studies indicate that miRNA, secreted by exosomes (Sohel et al., 2013), are found circulating in serum and can be used as biomarkers for human disease (Friedman et al., 2012; Sochor et al., 2014; Langhe et al., 2015) including ovarian cancer (Hong et al., 2013) and polycystic ovarian syndrome (Ding et al., 2015; Jiang et al., 2016). Perhaps miRNA produced in TC are secreted via exosomes into the follicular fluid and act in a paracrine manner to regulate gene expression in GC (Camussi et al., 2010). In support of this suggestion, recent studies show that MIR221 can be detected in bovine follicular fluid (Gebremedhn et al., 2015). In addition, changes in blood levels of MIR221 have been observed during the estrous cycle in cattle (Noferesti et al., 2015) and may also contribute to changes in MIR221 in follicular fluid. Additional research will be required to verify these suggestions.
In summary, the results from the present study demonstrate that MIR221 abundance in GC decreases and in TC increases with follicular development in cattle, and that FGF9 and FGF2 increase TC MIR221 abundance while IGF1, steroids, and cAMP agonists have no effect on TC MIR221. We conclude that FGF9 increased GC and TC MIR221 expression, and that increased MIR221 may act to inhibit GC steroidogenesis in cattle.
Footnotes
The authors thank J. Ervin and E. Gove for laboratory assistance; the OSU Microarray Core Facility and OSU Recombinant DNA/Protein Resource Facility for use of equipment; Dr. A. F. Parlow, National Hormone and Peptide Program (Torrance, CA) for purified FSH; and Creekstone Farms (Arkansas City, KS) for their generous donation of bovine ovaries. Supported in part by The Endowment of Howard M. & Adene R. Harrington Chair in Animal Science (Project 21-58500 to L.J.S.), and the Oklahoma State University Agricultural Experiment Station (OKL02970 to L.J.S.). Approved for publication by the Director, Oklahoma Agric. Exp. Sta.
LITERATURE CITED
- Aad P.Y., Echternkamp S. E., Sypherd D. D., Schreiber N. B., and Spicer L. J.. 2012. The hedgehog system in ovarian follicles of cattle selected for twin ovulations and births: evidence of a link between the IGF and hedgehog systems. Biol. Reprod. 87:79. doi:10.1095/biolreprod.111.096735 [DOI] [PubMed] [Google Scholar]
- Adashi E. Y., Resnick C. E., Hernandez E. R., May J. V., Purchio A. F., and Twardzik D. R.. 1989. Ovarian transforming growth factor-beta (TGF beta): cellular site(s), and mechanism(s) of action. Mol. Cell. Endocrinol. 61:247–256. [DOI] [PubMed] [Google Scholar]
- Adashi E.Y., Resnick C. E., and Twardzik D. R.. 1987. Transforming growth factor-alpha attenuates the acquisition of aromatase activity by cultured rat granulosa cells. J. Cell. Biochem. 33:1–13. [DOI] [PubMed] [Google Scholar]
- Agarwal V., Bell G. W., Nam J. W., and Bartel D. P.. 2015. Predicting effective microRNA target sites in mammalian mRNAs. Elife. 4. doi:10.7554/eLife.05005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambs S., Prueitt R. L., Yi M., Hudson R. S., Howe T. M., Petrocca F., Wallace T. A., Liu C. G., Volinia S., Calin G. A.,. et al. 2008. Genomic profiling of microRNA and messenger RNA reveals deregulated microRNA expression in prostate cancer. Cancer Res. 68:6162–6170. doi:10.1158/0008-5472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baillet A., Mandon-Pepin B., Cabau C., Poumerol E., Pailhoux E., and Cotinot C.. 2008. Identification of transcripts involved in meiosis and follicle formation during ovine ovary development. BMC Genomics 9:436. doi:10.1186/1471-2164-9-436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennecke J., Stark A., Russell R. B., and Cohen S. M.. 2005. Principles of microRNA-target recognition. PLoS Biol. 3:e85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buratini J. Jr., Teixeira A. B., Costa I. B., Glapinski V. F., Pinto M. G., Giometti I. C., Barros C. M., Cao M., Nicola E. S., and Price C. A.. 2005. Expression of fibroblast growth factor-8 and regulation of cognate receptors, fibroblast growth factor receptor-3c and -4, in bovine antral follicles. Reproduction 130:343–350. [DOI] [PubMed] [Google Scholar]
- Camussi G., Deregibus M. C., Bruno S., Cantaluppi V., and Biancone L.. 2010. Exosomes/microvesicles as a mechanism of cell-to-cell communication. Kidney Interl. 78:838–848. doi:10.1038/ki.2010.278 [DOI] [PubMed] [Google Scholar]
- Carletti M.Z., Fiedler S. D. and Christenson L. K.. 2010. MicroRNA 21 blocks apoptosis in mouse periovulatory granulosa cells. Biol. Reprod. 83:286–295. doi:10.1095/biolreprod.109.081448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castagnino P., Kothapalli D., Hawthorne E. A., Liu S. L., Xu T., Rao S., Yung Y., and Assoian R. K.. 2013. miR-221/222 compensates for Skp2-mediated p27 degradation and is a primary target of cell cycle regulation by prostacyclin and cAMP. PLoS One 8:e56140. doi:10.1371/journal.pone.0056140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberlain C. S., and Spicer L. J.. 2001. Hormonal control of ovarian cell production of insulin-like growth factor binding proteins. Mol. Cell. Endocrinol. 182:69–81. [DOI] [PubMed] [Google Scholar]
- Chaves R. N., de Matos M. H., Buratini J. Jr., and de Figueiredo J. R.. 2012. The fibroblast growth factor family: involvement in the regulation of folliculogenesis. Reprod. Fertil. Develop. 24:905–915. doi:10.1071/RD11318 [DOI] [PubMed] [Google Scholar]
- Dahiya N., Sherman-Baust C. A., Wang T. L., Davidson B., Shih I. M., Zhang Y., Wood W. 3rd, Becker K. G., and Morin P. J.. 2008. MicroRNA expression and identification of putative miRNA targets in ovarian cancer. PLoS One 3:e2436. doi:10.1371/journal.pone.0002436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai A., Sun H., Fang T., Zhang Q., Wu S., Jiang Y., Ding L., Yan G., and Hu Y.. 2013. MicroRNA-133b stimulates ovarian estradiol synthesis by targeting Foxl2. FEBS Lett. 587:2474–2482. doi:10.1016/j.febslet.2013.06.023 [DOI] [PubMed] [Google Scholar]
- Di R., He J., Song S., Tain D., Liu Q., Liang X., Ma Q., Sun M., Wang J., Zhao W.,. et al. 2014. Characterization and comparative profiling of ovarian microRNAs during ovine anestrus and the breeding season. BMC Genomics 15:899. doi:10.1186/1471-2164-15-899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Leva G., Gasparini P., Piovan C., Ngankeu A., Garofalo M., Taccioli C., Iorio M. V., Li M., Volinia S., Alder H.,. et al. 2010. MicroRNA cluster 221–222 and estrogen receptor alpha interactions in breast cancer. J. Natl. Cancer Inst. 10:706–721. doi:10.1093/jnci/djq102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding C.F., Chen W. Q., Zhu Y. T., Bo Y. L., Hu H. M., and Zheng R. H.. 2015. Circulating microRNAs in patients with polycystic ovary syndrome. Hum. Fertil. (Camb). 18:22–29. doi:10.3109/14647273.2014.956811 [DOI] [PubMed] [Google Scholar]
- Donadeu F. X., Schauer S. N., and Sontakke S. D.. 2012. Involvement of miRNAs in ovarian follicular and luteal development. J. Endocrinol. 215:323–334. doi:10.1530/JOE-12-0252 [DOI] [PubMed] [Google Scholar]
- Drummond A. E., Tellbach M., Dyson M., and Findlay J. K.. 2007. Fibroblast growth factor-9, a local regulator of ovarian function. Endocrinology 148:3711–3721. [DOI] [PubMed] [Google Scholar]
- Duleba A. J., Kim K. S., Ho Yuen B., and Moon Y. S.. 1985. Inhibition of 20 alpha-hydroxysteroid dehydrogenase activity by follicle-stimulating hormone and androgens in cultured rat granulosa cells: a search for the mechanism of action. Biol. Reprod. 33:401–410. [DOI] [PubMed] [Google Scholar]
- Evans J. R., Schreiber N. B., Williams J. A., and Spicer L. J.. 2014. Effects of fibroblast growth factor 9 on steroidogenesis and control of FGFR2IIIc mRNA in porcine granulosa cells. J. Anim. Sci. 92:511–519. doi:10.2527/jas.2013-6989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman E. B., Shang S., de Miera E. V., Fog J. U., Teilum M. W., Ma M. W., Berman R. S., Shapiro R. L., Pavlick A. C., Hernando E.,. et al. 2012. Serum microRNAs as biomarkers for recurrence in melanoma. J. Transl. Med. 10:155. doi:10.1186/1479-5876-10-155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebremedhn S., Salilew-Wondim D., Ahmad I., Sahadevan S., Hossain M. M., Hoelker M., Rings F., Neuhoff C., Tholen E., Looft C.,. et al. 2015. MicroRNA expression profile in bovine granulosa cells of preovulatory dominant and subordinate follicles during the late follicular phase of the estrous cycle. PLoS One 10:e0125912. doi:10.1371/journal.pone.0125912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grado-Ahuir J. A., Aad P. Y., and Spicer L. J.. 2011. New insights into the pathogenesis of cystic follicles in cattle: microarray analysis of gene expression in granulosa cells. J. Anim. Sci. 89:1769–1786. doi:10.2527/jas.2010-3463 [DOI] [PubMed] [Google Scholar]
- Ha M., and Kim V. N.. 2014. Regulation of microRNA biogenesis. Nature Rev. Mol. Cell. Biol. 15:509–524. doi:10.1038/nrm3838 [DOI] [PubMed] [Google Scholar]
- He L., and Hannon G. J.. 2004. MicroRNAs: small RNAs with a big role in gene regulation. Nature Rev. Genet. 5:522–531. [DOI] [PubMed] [Google Scholar]
- Hedin L., and Rosberg S.. 1983. Forskolin effects on the cAMP system and steroidogenesis in the immature rat ovary. Mol. Cell. Endocrinol. 33:69–80. [DOI] [PubMed] [Google Scholar]
- Herrera L., Ottolenghi C., Garcia-Ortiz J. E., Pellegrini M., Manini F., Ko M. S., Nagaraja R., Forabosco A., and Schlessinger D.. 2005. Mouse ovary developmental RNA and protein markers from gene expression profiling. Develop. Biol. 279:271–290. [DOI] [PubMed] [Google Scholar]
- Hong F., Li Y., Xu Y., and Zhu L.. 2013. Prognostic significance of serum microRNA-221 expression in human epithelial ovarian cancer. J. Int. Med. Res. 41:64–71. doi:10.1177/0300060513475759 [DOI] [PubMed] [Google Scholar]
- Hossain M. M., Ghanem N., Hoelker M., Rings F., Phatsara C., Tholen E., Schellander K., and Tesfaye D.. 2009. Identification and characterization of miRNAs expressed in the bovine ovary. BMC Genomics 10:443. doi:10.1186/1471-2164-10-443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Z., Shen W. J., Cortez Y., Tang X., Liu L. F., Kraemer F. B., and Azhar S.. 2013. Hormonal regulation of microRNA expression in steroid producing cells of the ovary, testis and adrenal gland. PloS One 8:e78040. doi:10.1371/journal.pone.0078040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang L., Huang J., Chen Y., Yang Y., Li R., Li Y., Chen X., and Yang D.. 2016. Identification of several circulating microRNAs from a genome-wide circulating microRNA expression profile as potential biomarkers for impaired glucose metabolism in polycystic ovarian syndrome. Endocrine 53:280–290. doi:10.1007/s12020-016-0878-9 [DOI] [PubMed] [Google Scholar]
- Jiang Z., and Price C. A.. 2012. Differential actions of fibroblast growth factors on intracellular pathways and target gene expression in bovine ovarian granulosa cells. Reproduction 144:625–632. doi:10.1530/REP-12-0199 [DOI] [PubMed] [Google Scholar]
- Krek A., Grün D., Poy M. N., Wolf R., Rosenberg L., Epstein E. J., MacMenamin P., da Piedade I., Gunsalus K. C., Stoffel M.,. et al. 2005. Combinatorial microRNA target predictions. Nat. Genet. 37:495–500. [DOI] [PubMed] [Google Scholar]
- Kuokkanen S., Chen B., Ojalvo L., Benard L., Santoro N., and Pollard J. W.. 2010. Genomic profiling of microRNAs and messenger RNAs reveals hormonal regulation in microRNA expression in human endometrium. Biol. Reprod. 82:791–801. doi:10.1095/biolreprod.109.081059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagaly D. V., Aad P. Y., Grado-Ahuir J. A., Hulsey L. B., and Spicer L. J.. 2008. Role of adiponectin in regulating ovarian theca and granulosa cell function. Mol. Cell. Endocrinol. 284:38–45. doi:10.1016/j.mce.2008.01.007 [DOI] [PubMed] [Google Scholar]
- Langhe R., Norris L., Saadeh F. A., Blackshields G., Varley R., Harrison A., Gleeson N., Spillane C., Martin C., O’Donnell D. M.,. et al. 2015. A novel serum microRNA panel to discriminate benign from malignant ovarian disease. Cancer Lett. 356:628–636. doi:10.1016/j.canlet.2014.10.010 [DOI] [PubMed] [Google Scholar]
- Langhout D. J., Spicer L. J., and Geisert R. D.. 1991. Development of a culture system for bovine granulosa cells: effects of growth hormone, estradiol, and gonadotropins on cell proliferation, steroidogenesis, and protein synthesis. J. Anim. Sci. 69:3321–3334. [DOI] [PubMed] [Google Scholar]
- Lewis B. P., Shih I. H., Jones-Rhoades M. W., Bartel D. P., and Burge C. B.. 2003. Prediction of mammalian microRNA targets. Cell 115:787–798. [DOI] [PubMed] [Google Scholar]
- Li M., Liu Y., Wang T., Guan J., Luo Z., Chen H., Wang X., Chen L., Ma J., Mu Z.,. et al. 2011. Repertoire of porcine microRNAs in adult ovary and testis by deep sequencing. Interl. J. Biol. Sci. 7:1045–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin L., Du T., Huang J., Huang L. L., and Yang D. Z.. 2015. Identification of differentially expressed microRNAs in the ovary of polycystic ovary syndrome with hyperandrogenism and insulin resistance. Chinese Med. J. 128:169–174. doi:10.4103/0366-6999.149189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupini L., Bassi C., Ferracin M., Bartonicek N., D’Abundo L., Zagatti B., Callegari E., Musa G., Moshiri F., Gramantieri L.,. et al. 2013. miR-221 affects multiple cancer pathways by modulating the level of hundreds messenger RNAs. Front. Genet. 4:64. doi:10.3389/fgene.2013.00064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machado M. F., Portela V. M., Price C. A., Costa I. B., Ripamonte P., Amorim R. L., and Buratini J. Jr. 2009. Regulation and action of fibroblast growth factor 17 in bovine follicles. J. Endocrinol. 202:347–353. doi:10.1677/JOE-09-0145 [DOI] [PubMed] [Google Scholar]
- McArdle C. A., Kohl C., Rieger K., Groner I., and Wehrenberg U.. 1991. Effects of gonadotropins, insulin and insulin-like growth factor I on ovarian oxytocin and progesterone production. Mol. Cell. Endocrinol. 78:211–220. [DOI] [PubMed] [Google Scholar]
- McBride D., Carre W., Sontakke S. D., Hogg C. O., Law A., Donadeu F. X., and Clinton M.. 2012. Identification of miRNAs associated with the follicular-luteal transition in the ruminant ovary. Reproduction 144:221–233. doi:10.1530/REP-12-0025 [DOI] [PubMed] [Google Scholar]
- Mlynarczuk J., Wrobel M. H., and Kotwica J.. 2011. The adverse effect of phytoestrogens on the synthesis and secretion of ovarian oxytocin in cattle. Reprod Domest Anim. 46:21–28. doi:10.1111/j.1439-0531.2009.01529.x [DOI] [PubMed] [Google Scholar]
- Noferesti S. S., Sohel M. M., Hoelker M., Salilew-Wondim D., Tholen E., Looft C., Rings F., Neuhoff C., Schellander K., and Tesfaye D.. 2015. Controlled ovarian hyperstimulation induced changes in the expression of circulatory miRNA in bovine follicular fluid and blood plasma. J. Ovarian Res. 8:81. doi:10.1186/s13048-015-0208-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ott L. 1977. An introduction to statistical methods and data analysis. Scituate (MA): North Duxbury Press; p. 384–388. [Google Scholar]
- Pan Q., Luo X., Toloubeydokhti T., and Chegini N.. 2007. The expression profile of micro-RNA in endometrium and endometriosis and the influence of ovarian steroids on their expression. Mol. Hum. Reprod. 13:797–806. [DOI] [PubMed] [Google Scholar]
- Pizzo F., Caloni F., Schreiber N. B., Cortinovis C., and Spicer L. J.. 2016. In vitro effects of deoxynivalenol and zearalenone major metabolites alone and combined, on cell proliferation, steroid production and gene expression in bovine small-follicle granulosa cells. Toxicon 109:70–83. doi:10.1016/j.toxicon.2015.11.018 [DOI] [PubMed] [Google Scholar]
- Pizzo F., Caloni F., Schütz L. F., Totty M. L., and Spicer L. J.. 2015. Individual and combined effects of deoxynivalenol and α-zearalenol on cell proliferation and steroidogenesis of granulosa cells in cattle. Environ. Toxicol. Pharmacol. 40:722–728. doi:10.1016/j.etap.2015.08.025 [DOI] [PubMed] [Google Scholar]
- Portela V. M., Machado M., Buratini J. Jr., Zamberlam G., Amorim R. L., Goncalves P., and Price C. A.. 2010. Expression and function of fibroblast growth factor 18 in the ovarian follicle in cattle. Biol. Reprod. 83:339–346. doi:10.1095/biolreprod.110.084277 [DOI] [PubMed] [Google Scholar]
- Ranzenigo G., Caloni F., Cremonesi F., Aad P. Y., and Spicer L. J.. 2008. Effects of Fusarium mycotoxins on steroid production by porcine granulosa cells. Anim. Reprod. Sci. 107:115–130. [DOI] [PubMed] [Google Scholar]
- Salilew-Wondim D., Ahmad I., Gebremedhn S., Sahadevan S., Hossain M. D., Rings F., Hoelker M., Tholen E., Neuhoff C., Looft C.,. et al. 2014. The expression pattern of microRNAs in granulosa cells of subordinate and dominant follicles during the early luteal phase of the bovine estrous cycle. PloS One 9:e106795. doi:10.1371/journal.pone.0106795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber N. B., and Spicer L. J.. 2012. Effects of fibroblast growth factor 9 (FGF9) on steroidogenesis and gene expression and control of FGF9 mRNA in bovine granulosa cells. Endocrinology 153:4491–4501. doi:10.1210/en.2012-1003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber N. B., Totty M. L., and Spicer L. J.. 2012. Expression and effect of fibroblast growth factor 9 in bovine theca cells. J. Endocrinol. 215:167–175. doi:10.1530/JOE-12-0293 [DOI] [PubMed] [Google Scholar]
- Schauer S. N., Sontakke S. D., Watson E. D., Esteves C. L., and Danadeu F. X.. 2013. Involvement of miRNAs in equine follicle development. Reproduction 146:273–282. doi:10.1530/REP-13-0107 [DOI] [PubMed] [Google Scholar]
- Schütz L. F., Schreiber N. B., Gilliam J. N., Cortinovis C., Totty M. L., Caloni F., Evans J. R., and Spicer L. J.. 2016. Changes in fibroblast growth factor 9 mRNA in granulosa and theca cells during ovarian follicular growth in dairy cattle. J. Dairy Sci. 99:9143–9151. doi:10.3168/jds.2015-10667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirotkin A. V., Ovcharenko D., Grossmann R., Laukova M., Mlyncek M.. 2009. Identification of microRNAs controlling human ovarian cell steroidogenesis via a genome-scale screen. J. Cell. Physiol. 219:415–420. doi:10.1002/jcp.21689 [DOI] [PubMed] [Google Scholar]
- Sochor M., Basova P., Pesta M., Dusilkova N., Bartos J., Burda P., Pospisil V., and Stopka T.. 2014. Oncogenic microRNAs: miR-155, miR-19a, miR-181b, and miR-24 enable monitoring of early breast cancer in serum. BMC Cancer 14:448. doi:10.1186/1471-2407-14-448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohel M. M., Hoelker M., Noferesti S. S., Salilew-Wondim D., Tholen E., Looft C., Rings F., Uddin M. J., Spencer T. E., Schellander K.,. et al. 2013. Exosomal and non-exosomal transport of extra-cellular microRNAs in follicular fluid: implications for bovine oocyte developmental competence. PLoS One 8:e78505. doi:10.1371/journal.pone.0078505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sood P., Krek A., Zavolan M., Macino G., and Rajewsky N.. 2006. Cell-type-specific signatures of microRNAs on target mRNA expression. Proc. Natl. Acad. Sci. USA. 103:2746–2751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spicer L. J. 2005. Effects of estradiol on bovine thecal cell function in vitro: dependence on insulin and gonadotropins. J. Dairy Sci. 88:2412–2421. [DOI] [PubMed] [Google Scholar]
- Spicer L. J., Aad P. Y., Allen D., Mazerbourg S., and Hsueh A. J.. 2006. Growth differentiation factor-9 has divergent effects on proliferation and steroidogenesis of bovine granulosa cells. J Endocrinol. 189:329–339. [DOI] [PubMed] [Google Scholar]
- Spicer L. J., Aad P. Y., Allen D. T., Mazerbourg S., Payne A. H., and Hsueh A. J.. 2008. Growth differentiation factor 9 (GDF9) stimulates proliferation and inhibits steroidogenesis by bovine theca cells: influence of follicle size on responses to GDF9. Biol. Reprod. 78:243–253. [DOI] [PubMed] [Google Scholar]
- Spicer L. J., Alpizar E., and Echternkamp S. E.. 1993. Effects of insulin, insulin-like growth factor I, and gonadotropins on bovine granulosa cell proliferation, progesterone production, estradiol production, and(or) insulin-like growth factor I production in vitro. J. Anim. Sci. 71:1232–1241. [DOI] [PubMed] [Google Scholar]
- Spicer L. J., and Chamberlain C. S.. 1998. Influence of cortisol on insulin- and insulin-like growth factor 1 (IGF-1)-induced steroid production and on IGF-1 receptors in cultured bovine granulosa cells and thecal cells. Endocrine 9:153–161. [DOI] [PubMed] [Google Scholar]
- Spicer L. J., and Echternkamp S. E.. 1995. The ovarian insulin and insulin-like growth factor system with an emphasis on domestic animals. Domest. Anim. Endocrinol. 12:223–245. [DOI] [PubMed] [Google Scholar]
- Spicer L. J., Schreiber N. B., Lagaly D. V., Aad P. Y., Douthit L. B., and Grado-Ahuir J. A.. 2011. Effect of resistin on granulosa and theca cell function in cattle. Anim. Reprod. Sci. 124:19–27. doi:10.1016/j.anireprosci.2011.01.005 [DOI] [PubMed] [Google Scholar]
- Stewart R. E., Spicer L. J., Hamilton T. D., and Keefer B. E.. 1995. Effects of insulin-like growth factor I and insulin on proliferation and on basal and luteinizing hormone-induced steroidogenesis of bovine thecal cells: involvement of glucose and receptors for insulin-like growth factor I and luteinizing hormone. J. Anim. Sci. 73:3719–3731. [DOI] [PubMed] [Google Scholar]
- Sun T., Wang Q., Balk S., Brown M., Lee G. S., and Kantoff P.. 2009. The role of microRNA-221 and microRNA-222 in androgen-independent prostate cancer cell lines. Cancer Res. 69:3356–3363. doi:10.1158/0008-5472.CAN-08-4112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Totty M. L., Morrell B. C., and Spicer L. J.. 2017. Fibroblast growth factor 9 (FGF9) regulation of cyclin D1 and cyclin-dependent kinase-4 in ovarian granulosa and theca cells of cattle. Mol. Cell. Endocrinol. 440:25–33. doi:10.1016/j.mce.2016.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernon R. K., and Spicer L. J.. 1994. Effects of basic fibroblast growth factor and heparin on follicle-stimulating hormone-induced steroidogenesis by bovine granulosa cells. J. Anim. Sci. 72:2696–2702. [DOI] [PubMed] [Google Scholar]
- Voge J. L., Aad P. Y., Santiago C. A., Goad D. W., Malayer J. R., Allen D., and Spicer L. J.. 2004. Effect of insulin-like growth factors (IGF), FSH, and leptin on IGF-binding-protein mRNA expression in bovine granulosa and theca cells: quantitative detection by real-time PCR. Peptides 25:2195–2203. [DOI] [PubMed] [Google Scholar]
- Xu S., Linher-Melville K., Yang B. B., Wu D., and Li J.. 2011. Micro-RNA378 (miR-378) regulates ovarian estradiol production by targeting aromatase. Endocrinology 152:3941–3951. doi:10.1210/en.2011-1147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan G., Zhang L., Fang T., Zhang Q., Wu S., Jiang Y., Sun H., and Hu Y.. 2012. MicroRNA-145 suppresses mouse granulosa cell proliferation by targeting activin receptor IB. FEBS Lett. 586:3263–3270. doi:10.1016/j.febslet.2012.06.048 [DOI] [PubMed] [Google Scholar]
- Yang C. X., Du Z. Q., Wright E. C., Rothschild M. F., Prather R. S., and Ross J. W.. 2012. Small RNA profile of the cumulus-oocyte complex and early embryos in the pig. Biol. Reprod. 87:117. doi:10.1095/biolreprod.111.096669 [DOI] [PubMed] [Google Scholar]
- Yao N., Yang B. Q., Liu Y., Tan X. Y., Lu C. L., Yuan X. H., and Ma X.. 2010. Follicle-stimulating hormone regulation of microRNA expression on progesterone production in cultured rat granulosa cells. Endocrine 38:158–166. doi:10.1007/s12020-010-9345-1 [DOI] [PubMed] [Google Scholar]
- Yin M., Lu M., Yao G., Tian H., Lian J., Liu L., Liang M., Wang Y., and Sun F.. 2012. Transactivation of microRNA-383 by steroidogenic factor-1 promotes estradiol release from mouse ovarian granulosa cells by targeting RBMS1. Mol. Endocrinol. 26:1129–1143. doi:10.1210/me.2011-1341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L., Schütz L. F., Robinson C. L., Totty M. L., and Spicer L. J.. 2017. Evidence that gene expression of ovarian follicular tight junction proteins is regulated in vivo and in vitro in cattle. J. Anim. Sci. 95:1313–1324. doi:10.2527/jas.2016.0892 [DOI] [PubMed] [Google Scholar]