Abstract
Cortisol levels reflect hypothalamic-pituitary-adrenocortical (HPA) axis activity. While most plasma cortisol is supposed to be bound to the soluble corticosteroid-binding globulin (CBG), only free cortisol (FC) actively regulates metabolic and immunological processes. We aimed to establish a multispecies suitable method to assess FC in cows and horses which in combination with total cortisol (TC) allows interpreting proportional changes of cortisol in saliva as well as in blood in response to a standardized HPA axis activation via ACTH. We further investigated if the ratios of cortisol fractions as obtained at basal levels in healthy horses (herbivorous and monogastric) and dairy cows (herbivorous and ruminant) change during HPA axis activation, and to which extent saliva cortisol (SC) is representative for alterations in plasma FC and adrenal cortex reactivity. However, it was not the objective of the present study to directly compare the two species. Dosages of ACTH applied in cows and horses were based on published data. Synthetic ACTH was intravenously administered to eight dairy cows (0.16 µg/kg BW) and five horses (1 µg/kg BW). Blood and saliva were collected every 30 min for 3 h from a jugular vein catheter, and analyzed for TC and SC, the ratio of free cortisol (rFC), and the concentration of FC (cFC) in plasma. During the entire sampling period of the ACTH test, plasma TC was paralleled by blood cFC, rFC, and SC in both cows and horses. All cortisol fractions increased within 30 min of ACTH administration compared to basal values (0 min, P < 0.05). Peak TC concentration reached 63.2 ± 9.6 ng/mL and 73.2 ± 11.8 ng/mL in bovine and equine plasma, respectively. Peak values of rFC averaged 17.9 ± 4.5% in cows and 19.2 ± 7.8% in horses. The ratio of SC to cFC in horses remained similar during the ACTH challenge suggesting that SC is recruited from plasma FC. However, SC increased less compared to plasma TC and FC during HPA axis activation in cows. In conclusion, the short-term activation of the HPA axis caused not only an elevation of TC, but also a similar increase of rFC in both species. SC closely reflected changes of FC in horses, but less accurately in cows. The concomitant evaluation of changes among cortisol fractions might give further indications on adaptation mechanisms in glucocorticoid regulation as well as differentiate cortisol-related health disorders.
Keywords: ACTH challenge, corticosteroid-binding globulin, cortisol, free cortisol, hypothalamic-pituitary-adrenocortical axis
INTRODUCTION
Glucocorticoids affect metabolism, growth, reproduction, and resource allocation (Breuner et al., 2013). Cortisol is also an established marker to assess hypothalamic-pituitary-adrenocortical (HPA) axis activation (Otovic and Hutchinson, 2015). Although specific proteins binding cortisol affecting the circulating pool of bioactive free cortisol (FC) in plasma have been described (as reviewed in Breuner and Orchinik, 2002), analytic methods are inconsistent and data on a more profound analysis of cortisol fractions are scarce.
While basal cortisol concentrations are low in cows, horses show high basal levels of cortisol (Gayrard et al., 1996). These species differences in cortisol concentrations may be due to the volatile fatty acid-based intermediary metabolism in cows and the carbohydrate-based metabolism in horses. Moreover, the HPA axis reactivity toward an ACTH challenge revealed a higher response of total cortisol (TC) in cows than in horses (review by van der Kolk et al., 2016), although direct species comparisons are limited by the use of different dosages for the ACTH tests that have been established for horses and cows. It must be emphasized that the objective of the present study is not a direct species comparison of dairy cows and horses. However, up to now no study used a comprehensive approach to investigate changes in FC and further cortisol fractions during HPA axis activation within each of these two species. Furthermore, the contribution of altered blood FC to saliva cortisol (SC), as well as the relation to overall plasma TC concentration under HPA axis stimulation was not studied yet. Therefore, the aim of the present study was to establish a multispecies suitable method for assessing plasma FC in horses and dairy cows that allows interpretation of proportional changes in cortisol fractions in saliva and blood following HPA axis activation.
MATERIALS AND METHODS
The experimental procedures followed the Swiss Law on animal protection and welfare, and were approved by the responsible cantonal committees (Fribourg and Bern, approval no. 2012-12-FR and BE2616). The analytical procedures described in the following part were based on two sampling sets. First, the method for determination of plasma FC using an ultrafiltration (UF) method was adjusted to fit for horses and cows. All preliminary tests were carried out in pooled plasma samples of cows and horses. Together with the established analysis of SC and plasma TC, a comprehensive and validated analytical repertoire could be provided for the evaluation of cortisol fractions in different matrices.
Second, we measured these different cortisol fractions in dairy cows and horses during an ACTH challenge test. These measurements were performed to evaluate proportional changes of cortisol fractions measured in parallel in saliva and blood following HPA axis activation and at clarifying the question, which substrate, time point, and cortisol fraction best characterizes changes in adrenocortical reactivity within the respective species.
Preliminary Tests and Establishment of Cortisol Fraction Measurements
Blood collection and plasma harvest for pool samples.
The bovine plasma pool was obtained by collecting plasma samples of 10 healthy dairy cows randomly selected from the experimental herd of the Agroscope Research Station (Posieux, Switzerland). The equine plasma pool was obtained by collecting blood samples from three healthy geldings (two warmbloods, one Freiberger) housed at the Swiss Institute of Equine Medicine ISME (Bern, Switzerland). Blood was collected from the jugular vein into tubes containing EDTA, immediately put on wet ice, centrifuged (3,000 × g, 20 min, 4 °C), and the pooled plasma was stored in 1.5 mL aliquots at −20 °C until analysis.
Analysis and calculations of cortisol and cortisol fractions in plasma pool samples.
All measurements in the preliminary tests were performed in duplicate. TC in blood was measured using a commercially available RIA (REF IM1841, Beckman Coulter GmbH, Sinsheim, Germany). The manufacturer’s procedure was modified and validated according to Fureix et al. (2013) as horse plasma contains more interfering proteins. The analytical range of the test was between 3.625 and 725 ng/mL. Plasma samples of both species spiked with the calibrators showed full parallelism with the standard curve.
The ratio of free cortisol (rFC) in plasma was measured using a modified UF method as previously described for humans by Lewis et al. (2003) and validated for horses by Hart et al. (2011). In a first step 10 µL of 1,2-3H radiolabeled cortisol in ethanol equaling 0.1 µCi was placed into 2 mL polystyrene tubes and allowed to evaporate to dryness. Afterwards 400 µL of plasma were added to each tube, vortexed, and incubated at 37 °C for 30 min to ensure equilibration of bound and unbound radiolabeled cortisol. UF devices (UFC501008 Amicon Ultra-0.5 mL Centrifugal Filter Concentrator, Millipore, Tullagreen, Carrigtwohill, Co. Cork, Ireland) were prepared according to the manufacturer’s protocol. Equilibrated plasma samples were vortexed and diluted with 400 µL of PBS (0.14 M sodium chloride and 0.01 M sodium phosphate, pH 7.0) containing 0.1% gelatin. After short mixing, 400 µL of the diluted sample were centrifuged at 14,000 × g for 60 min at 25 °C.
These finally used plasma dilution, centrifugation time, and temperature were determined based on preliminary tests. For this, dilutions of bovine and equine plasma (1:2, 1:4, and 1:20) with PBS-G as well as undiluted plasma were measured in duplicate (after centrifugation at 14,000 × g, 60 min, 25 °C) for filtration efficiency. The filtration devices, the amount of plasma, and the filtrate were weighed, and the recovery rate determined by calculating the weight ratio of filtrate to original plasma. Furthermore, the optimal centrifugation time was established by centrifugation of duplicates of 1:2 pool plasma dilutions at 14,000 × g and 25 °C for 10, 30, 60, and 120 min. To determine the optimal centrifugation temperature, 1:2 diluted pool plasma samples were measured in duplicate after centrifugation at 14,000 × g for 60 min at temperatures of 0, 25, 37, and 40 °C as compiled from literature. Samples diluted 1:2 and processed at 25 °C for 60 min revealed a recovery rate of 90.2% in equine plasma and 89.2% in bovine plasma with rFC values of 10.4% and 11.2%, respectively. For the data presented in this manuscript, the final dilution of plasma samples of 1:2 with centrifugation for 60 min at 25 °C was chosen.
Total radioactivity of the equilibrated plasma sample (representing all labeled cortisol, both unbound and bound to protein (i.e., corticosteroid-binding globulin [CBG] and albumin) and of the ultrafiltrate (representing the unbound labeled cortisol fraction) were measured after adding 50 µL of sample to 2 mL of scintillation fluid (Lumasafe, PerkinElmar Inc., Waltham, MA, United States) in a liquid scintillation counter (Tri-Carb 2910 TR, PerkinElmar Inc., Waltham, MA, United States). The intra-assay CV for equine and bovine plasma was 5.0% and 7.0%, the inter-assay CV 11% and 2.0%, respectively. The rFC was finally calculated by dividing the measured radioactivity in the ultrafiltrate by the total radioactivity in the unfiltrated sample. The concentration of free cortisol (cFC, in ng/mL) was the product of total plasma cortisol concentration (TC) and rFC.
ACTH Challenge Tests in Dairy Cows and Horses
ACTH injection and sampling of blood and saliva.
For the ACTH challenge eight multiparous lactating Holstein dairy cows from the experimental herd were weighed and fitted with a sterile indwelling jugular catheter (16 gauge × 32 cm; Cavafix Certo Splittocan, B. Braun Melsungen AG, Melsungen, Germany). ACTH (ACTH Fragment 1-24, catalogue no. A0298, Sigma-Aldrich Chemie GmbH, Buchs, Switzerland) was administered intravenously (0.16 µg/kg BW), and blood was sampled before and at 30-min intervals for 180 min after the injection of ACTH.
At the Swiss Institute of Equine Medicine ISME, five healthy horses were exposed to an ACTH challenge. Horses were weighed and fitted with a sterile indwelling jugular catheter (14 gauge × 13 cm; MILACATH, catalogue no. 1411, MILA International Inc., KY, United States) the evening before the test. A dose of 1 µg/kg BW of the synthetic ACTH1-24 (Synacthen tetracosactidum, 0.25 mg/mL equivalent to 25 IU/mL, Novartis, Vilvoorde, Belgium) was administered intravenously, and blood was sampled before and at 30-min intervals for the following 180 min after the ACTH injection.
In horses and cows, blood was collected from the jugular vein catheter into tubes containing EDTA, cooled down on wet ice, centrifuged (3,000 × g, 20 min, 4 °C), and the harvested plasma was stored at −20 °C until analysis. Saliva samples of cows and horses were collected in parallel to blood samples during the ACTH challenge tests by using saliva collection tubes (Salivette, Sarstedt, Nuembrecht, Germany). Each saliva sample was taken within 1 min after the respective blood sample. The sponge of the collection device was manually inserted in the oral cavity for approximately 1 min, and after being soaked put back into the plastic container. Saliva samples of horses and cows were immediately cooled down and stored at −20 °C after centrifugation.
Analysis and calculation of cortisol fractions in blood and saliva.
TC and cortisol fractions in bovine and equine plasma samples during the ACTH challenge were measured as described above. Furthermore, pUC and cFC were determined as stated above. SC in cows and horses was measured with an ELISA (Salimetrics Cortisol Enzyme Immunoassay Kit, Salimetrics, State College, PA, United States) according to the manufacturer’s protocol and previously described in more detail by Schwinn et al. (2016) for cows and Scheidegger et al. (2016) for horses. The sensitivity of the assay was 0.07 ng/mL, the intra-assay was CV 8.5%, and the inter-assay CV was 8.7%. Regarding the question, to which extent SC itself reflects solely FC of blood, the ratios of SC (saliva) to TC (plasma) and of SC to cFC (plasma) were calculated.
Statistical Analysis
Data presented in the text, tables, and figures are mean values ± SD. All statistical analyses were carried out with the statistical software package SAS (Version 9.4, SAS Institute, Cary, NC, United States). Pearson’s correlation coefficients between SC, plasma cortisol, and cortisol fractions, as well as the ratios of SC to TC and SC to cFC at the respective time points during the 3-h recording period of the ACTH challenge were evaluated with the CORR procedure of SAS to assess the congruence in the dynamic pattern of cortisol fractions. In addition, a Bland–Altman analysis was conducted that revealed the agreement between SC and cFC, i.e., to which extent SC represents FC as observed in plasma. Differences in cortisol, cortisol fractions, and ratios of advanced sampling time points (30 to 180 min after ACTH application) in relation to initial values and between time points within horses and cows were evaluated by the MIXED procedure of SAS with species and time point as fixed effects. The individual animal was considered as repeated subject. Significant effects were assumed at P < 0.05.
RESULTS
Basal Concentrations of Cortisol and Cortisol Fractions in Horses and Cows Prior to the ACTH Challenge
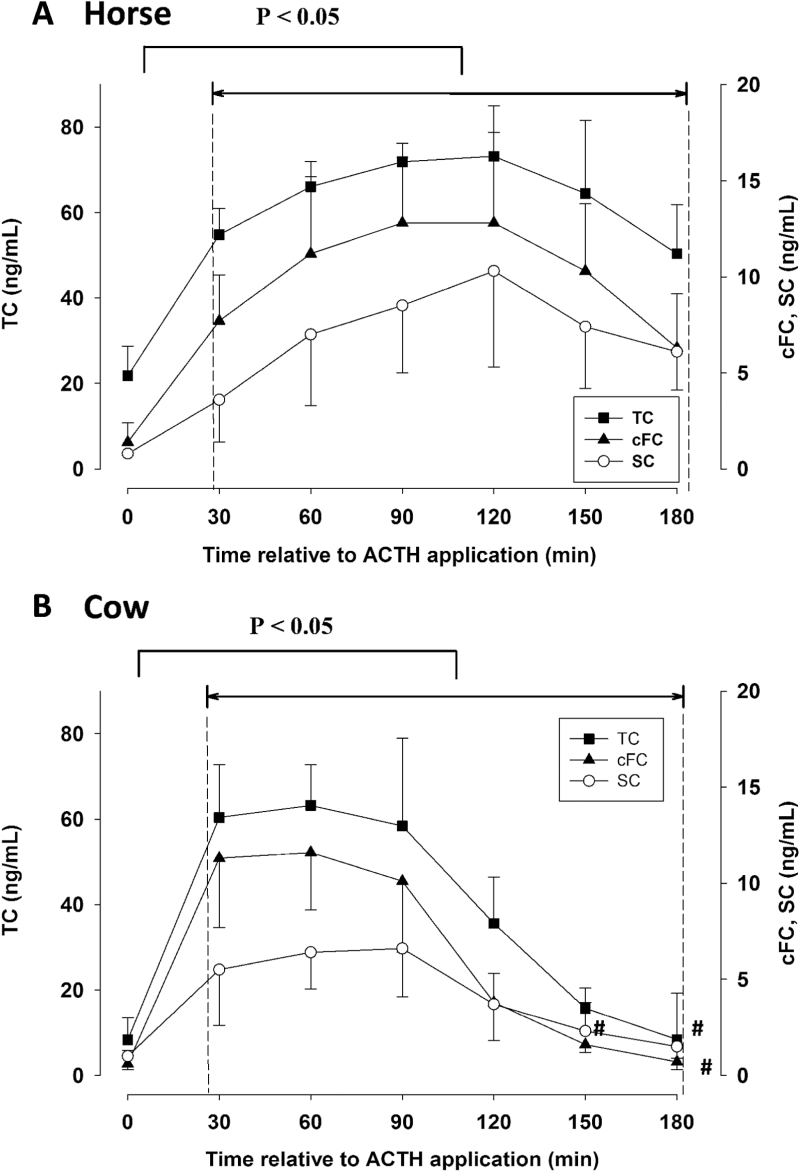
Prior to the ACTH application, horses had basal plasma TC concentrations of 21.8 ± 7.0 ng/mL, while initial TC concentrations in cows were considerably lower (8.3 ± 5.2 ng/mL; Figure 1A and B). Basal cFC plasma were 1.4 ± 1.0 ng/mL in horses, and 0.6 ± 0.7 ng/mL in cows (Figure 1A and B). With 6% and 9%, respectively, horses and cows had a similar range of rFC before the ACTH challenge (Table 1). Initial SC concentrations prior to the ACTH challenge were 0.8 ± 0.1 ng/mL and 1.01 ± 0.7 ng/mL in horses and cows, respectively (Figure 1). Prior to the ACTH test, SC represented 4% and 14% relative to plasma TC of horses and cows, respectively (Table 1). The initial ratio of SC to cFC averaged 0.85 ± 0.61 in horses and 1.62 ± 0.94 in cows (Table 1).
Figure 1.
Concentrations of TC, cFC, and SC during an ACTH challenge test in horses (A; n = 5; 1 µg/kg BW) and cows (B; n = 8; 0.16 µg/kg BW). ACTH was injected directly after the first blood sample at t = 0 min. Data are presented as mean values ± SD. Values between 30 and 180 min were compared to the respective basal concentrations. Time points that are not different (P > 0.05) from basal concentrations (min = 0) are marked with #.
Table 1.
Changes of the rFC in plasma, and the ratios of SC to TC in plasma (SC/TC), and SC to the cFC (SC/cFC) during an ACTH challenge test in horses (n = 5) and cows (n = 8)
| Time relative to ACTH application (min) | rFC | SC/TC | SC/cFC | |||
|---|---|---|---|---|---|---|
| Horse | Cow | Horse | Cow | Horse | Cow | |
| 0 | 0.06 ± 0.03b | 0.09 ± 0.01b | 0.04 ± 0.01c | 0.14 ± 0.08ab | 0.85 ± 0.61ab | 1.62 ± 0.94ab |
| 30 | 0.14 ± 0.05a | 0.19 ± 0.08a | 0.07 ± 0.04bc | 0.10 ± 0.05b | 0.45 ± 0.17b | 0.53 ± 0.37b |
| 60 | 0.17 ± 0.06a | 0.18 ± 0.04a | 0.11 ± 0.06ab | 0.11 ± 0.04b | 0.62 ± 0.26ab | 0.57 ± 0.17b |
| 90 | 0.18 ± 0.05a | 0.17 ± 0.05a | 0.12 ± 0.05ab | 0.12 ± 0.06b | 0.64 ± 0.1ab | 0.74 ± 0.38b |
| 120 | 0.17 ± 0.05a | 0.11 ± 0.01b | 0.14 ± 0.06a | 0.12 ± 0.07b | 0.78 ± 0.17ab | 1.17 ± 0.71b |
| 150 | 0.16 ± 0.05a | 0.10 ± 0.01b | 0.12 ± 0.05ab | 0.16 ± 0.10ab | 0.72 ± 0.25ab | 1.47 ± 0.86ab |
| 180 | 0.13 ± 0.05a | 0.09 ± 0.01b | 0.12 ± 0.04ab | 0.15 ± 0.06a | 0.96 ± 0.41a | 2.91 ± 3.61a |
Different superscripts within a parameter indicate significant differences (P < 0.05). Data are shown as mean values ± SD.
Dynamic Changes of Cortisol and Cortisol Fractions During the ACTH Challenge
Doses of ACTH we used base on previous reports for cattle (Verkerk et al., 1994; Gross et al., 2018) and horses (Bousquet-Mélou et al., 2006; Peeters et al., 2011; Scheidegger et al., 2016). Therefore, absolute values are not directly comparable between species. However, in both species, concentrations of TC, cFC, and SC were increased compared to basal values at 30 min after ACTH injection (P < 0.05, Figure 1A and B). In horses, concentrations of TC, cFC, and SC remained elevated (P < 0.05) until the end of sampling (Figure 1A). In cows, TC and SC remained elevated compared to basal concentrations until 120 min and cFC until 150 min (P < 0.05, Figure 1B).
In horses, highest TC concentrations in plasma reached 73.2 ± 11.8 ng/mL, while in cows up to 63.2 ± 9.6 ng/mL were observed (Figure 1A and B). Concentrations of cFC increased up to 12.8 ± 4.7 ng/mL in horses and 11.6 ± 3.0 ng/mL in cows (Figure 1A and B). Cortisol concentrations in saliva increased up to 10.3 ± 5.0 ng/mL in horses and 6.6 ± 2.5 ng/mL in cows after ACTH application (Figure 1A and B).
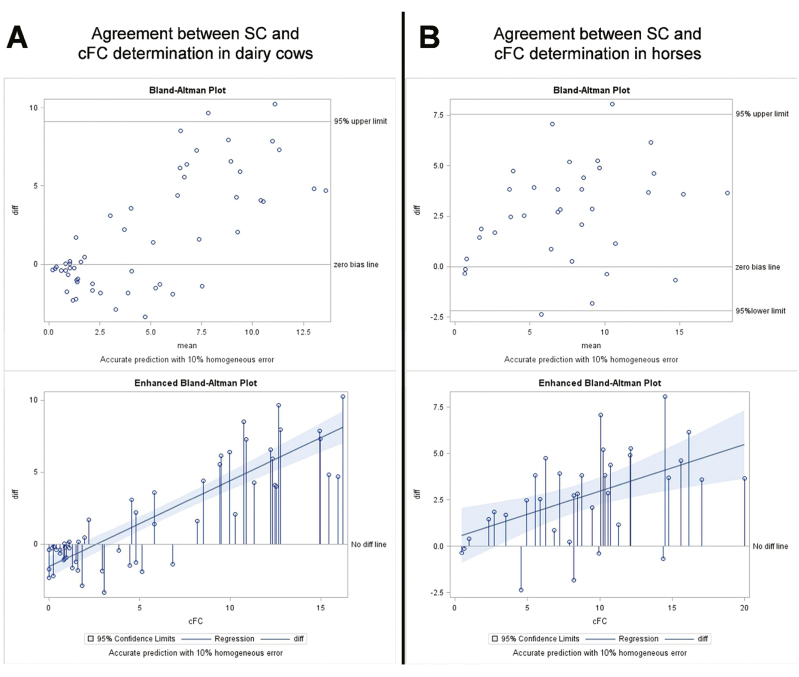
Across all sampling time points of the ACTH test, highly positive correlation coefficients were observed between the different cortisol fractions in both species, indicating the parallel pattern of concentration changes of the respective cortisol fractions following HPA axis activation (P < 0.01; e.g., cFC–TC: r = 0.92 in cows and r = 0.80 in horses; SC–TC: r = 0.66 in cows and r = 0.72 in horses). The Bland–Altman plots in Figure 2 show that SC reflected cFC as measured in plasma to a higher extent in horses than in cows, although Pearson’s correlation coefficients revealed parallel changes of SC and cFC during the ACTH sampling period in both species (r = 0.76 in cows, r = 0.87 in horses, P < 0.01).
Figure 2.
Bland–Altman analysis of the agreement between concentrations of cFC and SC in dairy cows (A; n = 8) and horses (B, n = 5).
Alterations of Cortisol Binding Pattern in Plasma, and of the Saliva to Blood Cortisol Ratio During the ACTH Challenge
Compared to initial values, rFC increased (P < 0.05) after 30 min in both species and remained elevated until 180 min in horses (P < 0.05) and 90 min in cows (P < 0.05; Table 1). In both species, rFC reached almost 20% during the ACTH challenge (Table 1).
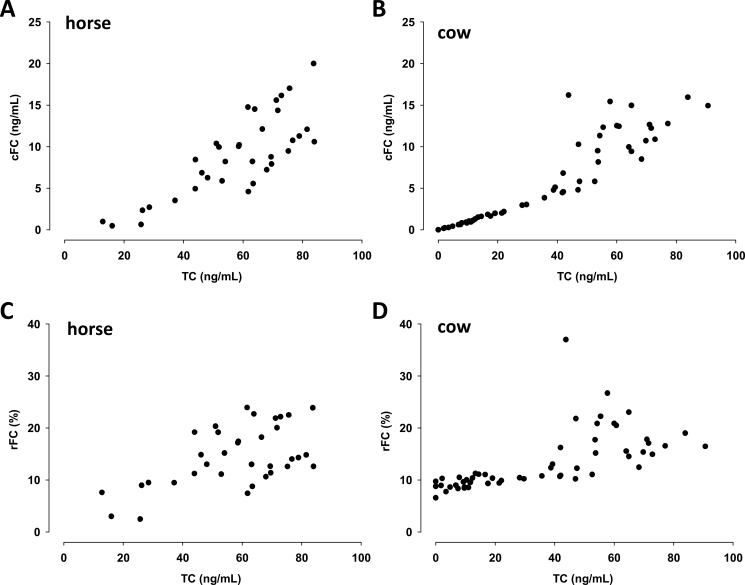
The ratio of SC to plasma TC concentration decreased slightly in cows and increased again at the end of the sampling period, whereas in horses the SC to TC ratio gradually increased during the ACTH challenge (Table 1). In contrast, the SC to cFC ratio was more constant during the ACTH challenge in horses than in cows, where it tended to decrease compared to the initial values, and to increase again toward the end of the sampling period (Table 1). In both species, rFC and cFC increased almost linearly with increasing plasma TC concentrations up to 40 ng/mL (Figure 3). Beyond this threshold of plasma TC, the relationship toward rFC and cFC was less well defined (Figure 3).
Figure 3.
Concentrations of cFC and rFC vs. TC concentrations in horses (A, C; n = 5) and cows (3B, D; n = 8) exposed to an ACTH challenge (1 µg/kg BW in horses; 0.16 µg/kg BW in cows). Data represent all analyzed concentrations of cFC and rFC in relation to the respective TC concentration measured sorted in ascending order.
DISCUSSION
Complexity in the Analysis of Cortisol and Cortisol Fractions in Blood and Saliva Between Different Species
Although equilibrium dialysis is accepted to be the gold standard for directly measuring FC, it is rather costly in terms of labor and complexity, while UF was reported to provide just as reliable results (Levine et al., 2007). The combination of UF with the addition of labeled cortisol that binds to the inherent CBG offers a method to estimate FC besides the cortisol binding capacity of an individual animal’s sample. Although basal TC concentrations were twice as high in horses than in cows, the UF as described in the present manuscript accurately measured rFC in both species within a wide range of cortisol concentrations. In our study, rFC values in both species were relatively unaffected with increasing concentrations of TC up to approximately 40 ng/mL. Additionally, we observed a linear increase of FC up to TC concentrations of 40 ng/mL. Beyond the supra-physiological threshold of 40 ng TC/mL, variation in the relationship between rFC and cFC increased. This finding might indicate a maximal binding capacity of CBG in horse and cow plasma at approximately 40 ng cortisol/mL.
Estimating FC by measuring of SC represents a noninvasive approach, thereby assuming SC to reflect plasma FC that diffuses unhindered into saliva (Brossaud et al., 2015). SC is used in horses and dairy cows to estimate cortisol concentrations in blood (Scheidegger et al., 2016; Schwinn et al., 2016). Vining and McGinley (1987) confirmed that the equilibrium between concentrations of FC in plasma and cortisol in saliva establishes due to passive diffusion of plasma FC across the acinar cells of the salivary gland. SC concentrations, at least in cows, were further shown to be independent of the animal’s feeding actions, e.g., drinking, eating, and ruminating (Schwinn et al., 2016). At basal cortisol concentrations, we observed only weak correlations between SC, FC, and TC, indicating that individual animals exhibit a considerable biological variation. However, during manifest changes of circulating cortisol concentrations (e.g., by activation of the HPA axis), changes in SC paralleled nicely the pattern of cortisol and cortisol fractions in plasma resulting in overall high correlation coefficients. SC and cFC increased similarly in horses. In cows, SC increased more slowly, which manifests in decreasing SC to cFC, and SC to TC ratios during the ACTH challenge. Even though SC represents plasma FC quite well in horses, SC does not closely reflect the changes of plasma FC concentrations manner during HPA axis activation in cows. In contrast to our findings, Vining et al. (1983) reported that SC represents FC very well but not necessarily TC. Several factors can be speculated to contribute to the observed discrepancy of SC reflecting cortisol in blood, such as conversion of cortisol to corticosterone in saliva (Levine et al., 2007). Additionally, saliva constituents, such as proteins, were reported to change considerably under stress (Kerémi et al., 2017). In accordance with that Brossaud et al. (2015) demonstrated that different methods to measure FC are closely but not synchronously correlated to TC. Certainly, SC as noninvasive biomarker reflecting FC in blood might be advantageous. On the other hand, the UF method provides more accurate FC values and an estimate about the binding pattern of cortisol in blood.
Changes of Cortisol Fractions, the Blood to Saliva Ratio, and Their Significance During HPA Axis Activation
Injection of ACTH results in an immediate and broad ranged supra-physiological elevation of cortisol above its basal concentration, which provides a suitable model to evaluate the relationships of cortisol fractions in blood and saliva. Presently no study has focused on a comprehensive description of the extent of changes among different cortisol fractions during an ACTH challenge.
Our findings regarding the pattern of TC and FC increasing after ACTH injection in horses are confirmed by earlier reports of Bousquet-Mélou et al. (2006) and Peeters et al. (2011). For dairy cows, we observed a similar increase of TC concentrations during the ACTH challenge as described earlier (Yoshida and Nakao, 2005; Schwinn et al., 2016; Gross et al., 2018). The rising rFC demonstrates a greater increase of FC compared to TC, thereby confirming previous results of Hart et al. (2011). Approximately 80% of cortisol is assumed to be bound to CBG, 10% to albumin, and 6% to 14% to remain free in different species (Gayrard et al., 1996). However, as we observed during the ACTH challenge, the proportion of FC may change. In particular, Hammond et al. (1990) showed that cortisol is released from CBG at local sites of inflammation. Furthermore, acute inflammation decreases CBG concentration rapidly (Bartalena et al., 1993). Alexander and Irvine (1998) showed a decrease in cortisol binding capacity after 3 to 4 d in horses exposed to social stress, whereas simultaneously TC values remained unaffected. In previous studies of stress associated diseases, such as the equine glandular gastric disease, gastric ulcers were associated with higher concentrations of SC (Scheidegger et al., 2017). These findings further emphasize the potential of the UF method and the concomitant investigation of cortisol binding instead of merely evaluating changes in plasma TC concentrations.
Whereas the overall correlation between SC and TC was high during the ACTH challenge, we could additionally identify changes in the ratios of SC to TC in both dairy cows and horses. With increasing TC concentrations after ACTH application, the SC to TC ratio increased in horses, whereas it tended to decrease in cows. This could potentially indicate species differences in the transfer rate of cortisol from blood into saliva during HPA axis activation. One might assume that observations can be attributed to species differences in composition and flow rate of saliva (60 to 160 L/d in cows, 5 to 10 L/d in horses). Cortisol passively diffuses trough the lipophilic membranes of blood vessels and acinus cells into saliva and this transit is thus independent of flow rate (Vining and McGinley, 1987). However, exocytosis of protein storage granules, aquaporines and electrolyte secretion are stimulated by the autonomic nerve system and also affect saliva flow rate, composition, and osmolarity (Hosoi, 2016; Proctor, 2016; Kerémi et al., 2017), and also affect SC concentrations. The ratio of SC to cFC during the ACTH challenge remained similar in horses implying that SC reliably represents FC in blood in this species. In cows, however, SC increased slower compared with FC and TC. Thus, SC only incompletely reflects circulating FC in cows.
In conclusion, the measurement of either FC or TC from saliva or blood gives reasonable estimates of response to ACTH in either cattle or horses. The UF and SC are suitable for assessing FC in horses, whereas in cows, changes in SC represented FC in circulation only to a limited extent. Especially when cortisol binding is impaired, e.g., under acute inflammation, TC might not accurately represent effects of the active FC. The concomitant evaluation of changes among cortisol fractions might give further insight into adaptive mechanisms in glucocorticoid regulation.
ACKNOWLEDGMENTS
The expert laboratory assistance by C. Philipona is gratefully acknowledged. This work was supported by the H. Wilhelm Schaumann-Stiftung (Hamburg, Germany) by providing a scholarship to A.-C.S. We thank the Swiss Federal Food Safety and Veterinary Office (ARAMIS-Nr. 2.17.02) and ISMEquine Research for funding this study.
LITERATURE CITED
- Alexander S. L., and Irvine C. H.. 1998. The effect of social stress on adrenal axis activity in horses: the importance of monitoring corticosteroid-binding globulin capacity. J. Endocrinol. 157:425–432. [DOI] [PubMed] [Google Scholar]
- Bartalena L., Hammond G. L., Farsetti A., Flink I. L., and Robbins J.. 1993. Interleukin-6 inhibits corticosteroid-binding globulin synthesis by human hepatoblastoma-derived (Hep G2) cells. Endocrinol. 133:291–296. [DOI] [PubMed] [Google Scholar]
- Bousquet-Mélou A., Formentini E., Picard-Hagen N., Delage L., Laroute V., and Toutain P. L.. 2006. The adrenocorticotropin stimulation test: contribution of a physiologically based model developed in horse for its interpretation in different pathophysiological situations encountered in man. Endocrinol. 147:4281–4291. doi:10.1210/en.2005-1161 [DOI] [PubMed] [Google Scholar]
- Breuner C. W., Delehanty B., and Boonstra R.. 2013. Evaluating stress in natural populations of vertebrates: total CORT is not good enough. Funct. Ecol. 27: 24–36. doi:10.1111/1365-2435.12016 [Google Scholar]
- Breuner C. W., and Orchinik M.. 2002. Plasma binding proteins as mediators of corticosteroid action in vertebrates. J. Endocrinol. 175:99–112. [DOI] [PubMed] [Google Scholar]
- Brossaud J., Gatta B., Tabarin A., and Corcuff J. B.. 2015. Different methods to estimate serum free cortisol: a comparison during cortisol tetracosactide testing. Clin. Chem. Lab. Med. 53:1367–1373. doi:10.1515/cclm-2014-0912 [DOI] [PubMed] [Google Scholar]
- Fureix C., Benhajali H., Henry S., Bruchet A., Prunier A., Ezzaouia M., Pamle R., and Jego P.. 2013. Plasma cortisol and faecal cortisol metabolites concentrations in stereotypic and non-stereotypic horses: do stereotypic horses cope better with poor environmental conditions?BMC Vet. Res. 9:3. doi:10.1186/1746-6148-9-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gayrard V., Alvinerie M., and Toutain P. L.. 1996. Interspecies variations of corticosteroid-binding globulin parameters. Domest. Anim. Endocrinol. 13:35–45. [DOI] [PubMed] [Google Scholar]
- Gross J. J., Zbinden R. S., Dohme‐Meier F., and Bruckmaier R. M.. 2018. Adrenal cortex reactivity in dairy cows differs between lactational stages and between different feeding levels. J. Anim. Physiol. Anim. Nutr. doi:10.1111/jpn.12746 [DOI] [PubMed] [Google Scholar]
- Hammond G. L., Smith C. L., Paterson N. A., and Sibbald W. J.. 1990. A role for corticosteroid-binding globulin in delivery of cortisol to activated neutrophils. J. Clin. Endocrinol. Metab. 71:34–39. [DOI] [PubMed] [Google Scholar]
- Hart K. A., Barton M. H., Ferguson D. C., Berghaus R., Slovis N. M., Heusner G. L., and Hurley D. J.. 2011. Serum free cortisol fraction in healthy and septic neonatal foals. J. Vet. Intern. Med. 25:345–355. doi:10.1111/j.1939-1676.2010.0667.x [DOI] [PubMed] [Google Scholar]
- Hosoi K. 2016. Physiological role of aquaporin 5 in salivary glands. Pflugers Arch. 468:519–539. doi:10.1007/s00424-015-1749-6 [DOI] [PubMed] [Google Scholar]
- Kerémi B., Beck A., Fábián T. K., Fábián G., Szabó G., Nagy Á., and Varga G.. 2017. Stress and Salivary Glands. Curr. Pharm. Des. doi:10.2174/1381612823666170215110648. [DOI] [PubMed] [Google Scholar]
- Levine A., Zagoory-Sharon O., Feldman R., Lewis J. G., and Weller A.. 2007. Measuring cortisol in human psychobiological studies. Physiol. Behav. 90:43–53. doi:10.1016/j.physbeh.2006.08.025 [DOI] [PubMed] [Google Scholar]
- Lewis J. G., Lewis M. G., and Elder P. A.. 2003. An enzyme-linked immunosorbent assay for corticosteroid-binding globulin using monoclonal and polyclonal antibodies: decline in CBG following synthetic ACTH. Clin. Chim. Acta. 328:121–128. doi:10.1016/S0009-8981(02)00417-5 [DOI] [PubMed] [Google Scholar]
- Otovic P., and Hutchinson E.. 2015. Limits to using HPA axis activity as an indication of animal welfare. ALTEX 32:41–50. doi:10.14573/altex.1406161 [DOI] [PubMed] [Google Scholar]
- Peeters M., Sulon J., Beckers J. F., Ledoux D., and Vandenheede M.. 2011. Comparison between blood serum and salivary cortisol concentrations in horses using an adrenocorticotropic hormone challenge. Equine Vet. J. 43:487–493. doi:10.1111/j.2042-3306.2010.00294.x [DOI] [PubMed] [Google Scholar]
- Proctor G. B. 2016. The physiology of salivary secretion. Periodontol. 2000. 70:11–25. doi:10.1111/prd.12116 [DOI] [PubMed] [Google Scholar]
- Scheidegger M. D., Gerber V., Bruckmaier R. M., van der Kolk J. H., Burger D., and Ramseyer A.. 2017. Increased adrenocortical response to adrenocorticotropic hormone (ACTH) in sport horses with equine glandular gastric disease (EGGD). Vet. J. 228:7–12. doi:10.1016/j.tvjl.2017.09.002 [DOI] [PubMed] [Google Scholar]
- Scheidegger M. D., Gerber V., Ramseyer A., Schüpbach-Regula G., Bruckmaier R. M., and van der Kolk J. H.. 2016. Repeatability of the ACTH stimulation test as reflected by salivary cortisol response in healthy horses. Domest. Anim. Endocrinol. 57:43–47. doi:10.1016/j.domaniend.2016.04.002 [DOI] [PubMed] [Google Scholar]
- Schwinn A. C., Knight C. H., Bruckmaier R. M., and Gross J. J.. 2016. Suitability of saliva cortisol as a biomarker for hypothalamic–pituitary–adrenal axis activation assessment, effects of feeding actions, and immunostimulatory challenges in dairy cows. J. Anim. Sci. 94:2357–2365. doi:10.2527/jas.2015-0260 [DOI] [PubMed] [Google Scholar]
- Van der Kolk J. H., Fouché N., Gross J. J., Gerber V., and Bruckmaier R. M.. 2016. A comparison between the equine and bovine hypothalamus-pituitary-adrenocortical axis. Domest. Anim. Endocrinol. 56:S101–S111. doi:10.1016/j.domaniend.2016.02.008 [DOI] [PubMed] [Google Scholar]
- Verkerk G. A., Macmillan K. L., and McLeay L. M.. 1994. Adrenal cortex response to adrenocorticotropic hormone in dairy cattle. Domest. Anim. Endocrinol. 11:115–123. [DOI] [PubMed] [Google Scholar]
- Vining R. F., and McGinley R. A.. 1987. The measurement of hormones in saliva: possibilities and pitfalls. J. Steroid Biochem. 27:81–94. [DOI] [PubMed] [Google Scholar]
- Vining R. F., McGinley R. A., Maksvytis J. J., and Ho K. Y.. 1983. Salivary cortisol: a better measure of adrenal cortical function than serum cortisol. Ann. Clin. Biochem. 20:329–335 [DOI] [PubMed] [Google Scholar]
- Yoshida C., and Nakao T.. 2005. Response of plasma cortisol and progesterone after ACTH challenge in ovariectomized lactating dairy cows. J. Reprod. Dev. 51:99–107. [DOI] [PubMed] [Google Scholar]