Abstract
The aim of this study was to examine whether the combination of dietary soluble fiber and cellobiose exerts a synergistic effect on growth performance, health status, fermentation traits, and immune response in rabbits. Six treatments in a 3 × 2 factorial arrangement were used: 3 cellobiose concentrations in drinking water (0.0, 7.5, and 15.0 g/L) × 2 dietary levels of soluble fiber (84.0 and 130 g/kg DM, for the low soluble fiber [LSF] and high soluble fiber [HSF] diets, respectively). A total of 318 young rabbits (53/treatment) were weaned at 34 d of age and had ad libitum access to feed and water. At 46 d of age, 9 rabbits/treatment were slaughtered and ileal and cecal digesta were collected to analyze VFA profile and the immune response in the cecal appendix mucosa. At 48 d of age, the cellobiose supplementation was withdrawn and the experimental diets were replaced by a standard commercial diet until 61 d of age. From 34 to 48 d of age, there was a linear increase of mortality with the level of cellobiose in the HSF group (0% vs. 17.1%; P = 0.017). In contrast, a quadratic effect of cellobiose level on mortality was observed in the LSF group, the rabbits offered 7.5-cellobiose showing the lowest mortality (5.7% vs. 21.4%; P = 0.030). Cellobiose level had a quadratic effect on ADFI, ADG, and G:F in this period (P ≤ 0.047), with the 7.5-cellobiose groups having the best growth performance. In contrast, only minor changes on these traits were observed from 48 d of age onwards. Cellobiose level influenced quadratically the ileal VFA concentrations (P = 0.014), showing the maximal value in the 7.5-cellobiose groups. In rabbits fed 7.5-cellobiose-LSF, a change of acetate to propionate, butyrate, and valerate was observed in the ileum. Increasing cellobiose levels reduced linearly cecal VFA concentrations in HSF fed rabbits, but no effect was detected in LSF groups (P = 0.046). The level of soluble fiber increased VFA concentrations in both the ileum (by 22%; P < 0.001), and the cecum (by 11%; P = 0.005). The relative gene expression of IL-6, IL-10, TNF-α, iNOS, MUC-1, and toll-like receptors (TLR-2 and TLR-4) in the cecal appendix increased linear and quadratically with increasing levels of cellobiose (P ≤ 0.063). In conclusion, in rabbits fed LSF diets, a dose of 7.5 g cellobiose/L drinking water would be recommended, whereas these levels of cellobiose supplementation should be avoided in rabbits fed HSF diets.
Keywords: cellobiose, immune response, rabbit, soluble dietary fiber, volatile fatty acids
INTRODUCTION
Epizootic rabbit enteropathy (ERE) is a frequent digestive disease in rabbits after weaning (Licois et al., 2006; Bäuerl et al., 2014; Badiola et al., 2016). The incorporation of moderate levels of dietary soluble fiber as sugar beet pulp in rabbit diets improved the functionality of the intestinal mucosa (Gómez-Conde et al., 2007; El Abed et al., 2011), modified the intestinal microbiota (Gómez-Conde et al., 2009; El Abed et al., 2013), and reduced the mortality in the post-weaning period in farms affected by ERE (Martínez-Vallespín et al., 2011; Trocino et al., 2013). These effects might be related to the amount of fermentable fiber both in the small intestine and in the cecum (Abad-Guamán et al., 2015). However, the use of moderate levels of soluble fiber alone does not reduce mortality below an acceptable threshold (Trocino et al., 2013). Therefore, it might be useful to find other nutrients with synergistic effects with soluble/fermentable fiber to reinforce rabbit health status after weaning. Part of the beneficial effects of fermentable fiber might be mediated through the low-molecular-weight sugars (mono-, di-, and oligosaccharides) produced by microbial cell wall degradation in the small intestine as shown in pigs (Pedersen et al., 2015). Sugars can be included directly in the diet, and cello-oligosaccharides supplementation (0.15% to 0.3% diet) have proven to positively affect the intestinal mucosa and microbiota in pigs and poultry (Song et al., 2013; Jiao et al., 2014). Nevertheless, in rats widely variable results were reported using higher doses (10% to 15%; Moinuddin and Lee, 1958; Umeki et al., 2004).
Our hypothesis was that cellobiose might improve the health status of weaned rabbits, but it might depend on the dietary soluble fiber content. The aim of this work was to study the effect of increasing levels of cellobiose in drinking water and its potential additive effect with the dietary level of soluble fiber on growth and fermentation traits in growing rabbits.
MATERIALS AND METHODS
All procedures involving animals were carried out in accordance with the Spanish guidelines for experimental animal protection (Spanish Royal Decree 53/2013; BOE, 2013) after being approved by the Animal Ethics Committee of the Universidad Politécnica de Madrid. A total of 264 crossbred mixed-sex rabbits (New Zealand White × Californian, V × R from UPV, Valencia, Spain) weaned at 34 d of age were used in this study.
Treatments
Six treatments in a 3 × 2 factorial arrangement (3 cellobiose concentrations × 2 dietary levels of soluble fiber) were used. Three concentrations of cellobiose were used in the drinking water: 0.0, 7.5, and 15.0 g/L (d-cellobiose, NPC Cello-Oligo, Nippon Paper Industries Co., Tokyo, Japan. According to the manufacturer contained 96.6% cellobiose β1–4, 1.9% cello-oligosaccharide, 1.5% glucose, and no nitrogen). These concentrations were selected to obtain a wider range of cellobiose supplementation than those used in previous studies conducted with poultry and pigs (0.3% to 0.6% in the diet; Song et al., 2013; Jiao et al., 2014). Cellobiose was supplemented in drinking water because in sick rabbits the water intake is less affected than feed intake that is clearly reduced compared with healthy rabbits (Delgado et al., 2015). Two experimental diets were formulated to differ in their dietary soluble fiber concentration (84.0 and 130 g/kg DM, for the low soluble fiber [LSF] and high soluble fiber [HSF] diets, respectively), the latter met the recommendations of Trocino et al. (2013), starch (226 and 182 g/kg DM for LSF and HSF, respectively), and acid detergent fiber (165 and 185 g/kg DM for LSF and HSF, respectively), whereas NDF remained constant and similar to the value proposed by Gutiérrez et al. (2002) (310 g/kg DM on average, corrected for ash and protein, or 333 g/kg DM only corrected for ash). The increase of soluble/fermentable fiber was obtained by replacing wheat straw and bran in the LSF diet by sugar beet pulp, that provides not only of soluble fiber but also of insoluble fermentable fiber. Dietary CP was minimized in order to limit ERE incidence but fixed to meet rabbit requirements (Carabaño et al., 2009; Xiccato et al., 2011). Ingredients and chemical composition of diets are shown in Table 1.
Table 1.
Ingredient and chemical composition of the experimental diets
| LSF | HSF | |
|---|---|---|
| Ingredient, g/kg as-fed | ||
| Wheat bran | 280 | 130 |
| Wheat straw | 100 | 50.0 |
| Beet pulp | 0.00 | 180 |
| Sunflower meal | 99.7 | 129.7 |
| Dehydrated alfalfa | 150 | 150 |
| Soybean meal | 80.0 | 80.0 |
| Wheat | 227 | 217 |
| High oleic sunflower oil | 8.50 | 8.50 |
| Sunflower oil | 21.5 | 21.5 |
| l-Lysine HCl | 4.40 | 4.40 |
| dl-Methionine | 0.80 | 0.60 |
| l-Threonine | 3.10 | 3.20 |
| Calcium carbonate | 12.0 | 7.00 |
| Sodium chloride | 3.00 | 3.10 |
| Calcium phosphate | 5.00 | 10.0 |
| Vitamin/mineral premix1 | 5.00 | 5.00 |
| Analyzed chemical composition, g/kg DM | ||
| DM | 908 | 908 |
| Ash | 70.8 | 67.5 |
| CP | 167 | 165 |
| CP-NDF | 19.7 | 27.5 |
| TDF | 391 | 442 |
| NDF2 | 307 | 312 |
| ADF3 | 165 | 185 |
| ADL3 | 31.0 | 33.0 |
| Soluble fiber (TDF–NDF) | 84.0 | 130 |
| Starch | 226 | 182 |
| Ether extract | 53.8 | 48.7 |
| Sugars | 79.9 | 81.7 |
1Provided by Trouw Nutrition (Madrid, Spain). Mineral and vitamin composition (per kg of complete diet): 20 mg of Mn as MnO; 59.2 mg of Zn as ZnO; 10 mg of Cu as CuSO4 5H2O; 1.25 mg of I as KI; 0.495 mg of Co as CoCO3 H2O H2O; 76 mg of Fe as FeCO3; 8,375 UI of vitamin A; 750 UI of vitamin D3, 20 UI of vitamin E as dl-α-tocopherol acetate, 1.0 mg of vitamin K; 1.0 mg of vitamin B1; 2 mg of vitamin B2; 1 mg of vitamin B6; 20 mg of Niacin; 54.1 mg of betaine; 137,5 mg of choline chloride; 66 mg of robenidine; 50 mg of ethoxyquin.
2Values were corrected for ash and crude protein.
3Values were corrected for ash.
Growth Trial
Two hundred and ten rabbits (779 ± 7.3 g BW) were blocked by litter, randomly assigned to each of the six experimental treatments (35 rabbits/treatment) and housed individually with ad libitum access to feed and water. The ADFI, ADG, and mortality were recorded individually. Water intake could not be individually measured and was estimated according to the water/feed intake ratio previously reported in rabbits fed the same experimental diets by Delgado et al. (2015). Before weaning rabbits had access to their mothers’ feed (180 CP, 329 NDF, and 93.4 soluble fiber, all in g/kg DM). At 48 d of age, the supplementation of cellobiose in the drinking water was withdrawn and the experimental diets were replaced by a standard commercial diet (164 CP, 341 NDF, and 58.7 soluble fiber, all in g/kg DM) for all rabbits. The experiment finished at 61 d of age.
Ileal and Cecal Fermentation, Sucrose Activity, and Immune Response Trial
Another 54 rabbits (620 ± 14.7 g BW) were also blocked by litter, and randomly assigned to one of the six treatments (9 rabbits/treatment). They were housed individually and had ad libitum access to feed and water. After 12 d adaptation period, they were slaughtered by head concussion between 1900 and 2100 h. After slaughter, the whole gastrointestinal tract was removed and weighed. The cecum was removed and its full weight was recorded. The cecal content was then extracted, weighed, and homogenized, and the pH was immediately measured with a Crison Basic 20 pH meter (Crison Instruments, Barcelona, Spain). About 2 g of cecal content were weighed, mixed with 2 mL of 0.5 N HCl, and immediately frozen (−20°C) until analysis of VFA concentrations by gas chromatography as described by Carro et al. (1992). The pH of the ileal content was measured and a sample (1 g) was taken for VFA analysis. The remaining cecal and ileal content was used to determine DM content. In addition, 6 cm samples were excised from the middle part of the jejunum, flushed with saline solution, frozen in dry ice, and immediately stored at −20°C to determine sucrose activity as described by Góméz-Conde et al. (2007). Finally, 2-cm segments of the distal part of the cecal appendix were collected to characterize the immune response in 8 rabbits/treatment. Samples were cleaned with saline solution (ClNa, 0.9%), cut longitudinally and scraped to obtain approximately 50 mg of mucosa. Samples were placed in vials containing 1 mL of RNA preserving solution (RNA Later, Applied Biosystems, Foster City, CA) and frozen at −80°C. Tissue disruption for RNA isolation from cecal appendix was performed using Trizol reagent (Sigma-Aldrich, St Louis, MO, USA) and a mixer mill MM-200 (Restch, Stuttgart, Germany). Total RNA was isolated using the GenElute Mammalian Total RNA Miniprep kit (Sigma-Aldrich, St Louis, MO, USA) according to manufacturer’s instructions. A DNAse treatment step using RNase-Free DNase Set (Qiagen, Hilden, Germany) was added to prevent genomic DNA contamination. The RNA concentration was measured by spectrophotometry (Epoch, BioTek, Winooski, VT) combined with the Take3 Micro-Volume Plate (BioTek, Santa Barbara, CA). The extracted A260/A280 ratio was used to calculate the quantity of diluted RNA for the following reverse transcription.
First strand cDNA was synthesized using the High-Capacity cDNA Archive Kit (Applied Biosystems, Foster City, CA) according to the manufacturer’s instructions. The relative gene expression of selected cytokines was determined using real-time, quantitative PCR. The specific primers for rabbit glyceraldehyde-3 phosphate (GADPH) and hypoxanthine–guanine phosphoryltransferase (HPRT; housekeepings), IL-10 and tumor necrosis factor-alpha (TNF-α) were taken from Godornes et al. (2007) and Chamorro et al. (2010). Primers for tool-like receptors (TLR-2 and TLR-4), transmembrane glycoprotein Mucin 1 and 13 (MUC-1 and MUC-13) were taken from Bäuerl et al. (2014) and those for IL-6 and the inducible nitric oxide synthase (iNOS) were designed by using Primer Express v.2 (Applied Biosystems, Foster City, CA). The specificity of the amplified product was confirmed through melting curves analysis and further confirmed by gel electrophoresis. The quantitative PCR was performed in an ABI Prism 7300 Sequence Detector System (Applied Biosystems, Foster City, CA). Each reaction mix consisted of around 100 ng of first strand cDNA as a template, specific primers, ultrapure water and SYBR Green Master Mix (Applied Biosystems Foster City, CA) as fluorescent DNA intercalating agent. All samples were run in triplicate and quantified by normalizing the target gene signal to that of the GADPH and HPRT geometric mean.
Analytical Methods
Procedures of the AOAC (2000) were used to determine the concentrations of DM (934.01), ash (967.05), CP (968.06), ether extract (920.39), starch (amyloglucosidase-α-amylase method, 996.11), and total dietary fiber (985.29, TDF). Dietary NDF was determined using the filter bag system (Ankom Technology, New York) according to Mertens et al. (2002), and a thermostable amylase without any sodium sulfite added. Values were corrected for ash and protein. Dietary ADF and ADL were analyzed according to AOAC (2000) (method 973.187) and Goering and Van Soest (1970), respectively. The soluble fiber content was calculated by difference as TDF–NDF, and did not contain the low-molecular-weight fibrous carbohydrates. Sugars were analyzed according to Yemm and Willis (1954).
Statistical Analysis
The results of the growth trial were analyzed by using a mixed model (PROC MIXED, SAS Inst. Inc., Cary, NC) that included as fixed factors the level of soluble fiber and cellobiose and their interaction, and the litter as a random effect. Weaning weight was included as a linear covariate. Mortality was analyzed using a logistic model (GENMOD procedure of SAS considering a binomial distribution) including the level of soluble fiber and cellobiose, and their interaction in the model, and the results were transformed from the logit scale. Non-orthogonal contrast was used to compare the LSF and HSF groups with no cellobiose supplementation. Analysis of gene expression was determined using a mixed model in which the levels of soluble fiber and cellobiose were included as fixed factors and the sample as a random effect (Steibel et al., 2009). For genes displaying efficiencies different from 2 (E ≠ 2), Ct values were adjusted according to the model described by Steibel et al. (2009). SE was used to recalculate the lower and upper 95% confidence intervals for each fold change. In all cases, linear and quadratic polynomial contrasts were used according to Kaps and Lamberson (2004) to test the linear and quadratic effects of the level of cellobiose and their interactions with the level of soluble fiber as follows: linear effect of cellobiose (+1 0 −1 + 1 0 −1, for treatments LSF_0, LSF_7.5, LSF_15, HSF_0, HSF_7.5, and HSF_15, respectively), quadratic effect of cellobiose (+1 −2 + 1 +1 −2 + 1), interaction of soluble fiber with the linear effect of cellobiose (+1 0 −1 −1 0 + 1), and the interaction of soluble fiber with the quadratic effect of cellobiose (+1 −2 + 1 −1 + 2 −1). When any interaction between cellobiose and dietary fiber was significant, specific linear and quadratic contrasts to study the effect of cellobiose were done within each level of soluble fiber.
RESULTS
No interaction (P > 0.05) between the level of soluble fiber and cellobiose was observed for growth traits. The ADFI from 34 to 48 d of age decreased by 9% in the HSF compared with the LSF-fed group (102 vs. 109 g/d; P < 0.001; Table 2), but no differences were observed either in ADG or G:F (P ≥ 0.12). The level of cellobiose had a quadratic effect on ADFI and ADG in this period, showing the 7.5-cellobiose groups the highest values (108 vs. 102 g/d, and 52.7 vs. 47.9 g/d, respectively; P ≤ 0.031). This led to a quadratic effect of cellobiose on G:F (P = 0.047), with the 7.5-cellobiose group having the highest values (0.487 vs. 0.460, averaged value for 0 and 15.0 cellobiose; P = 0.047).
Table 2.
Effect of the level of cellobiose in drinking water and dietary soluble fiber on the growth performance of rabbits
| Soluble fiber | Low | High | SEM | P | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cellobiose, g/L | 0.00 | 7.50 | 15.0 | 0.00 | 7.50 | 15.0 | Cov2 | Soluble fiber | Cellobiose | Cellobiose × soluble fiber | |||
| n 1 | 26 | 32 | 29 | 33 | 30 | 27 | Lineal | Quadratic | Lineal | Quadratic | |||
| 34–48 d3 | |||||||||||||
| BW at 34 d, g | 768 | 794 | 795 | 791 | 771 | 770 | 21.0 | – | 0.55 | 0.86 | 0.92 | 0.10 | 0.43 |
| ADFI, g/d | 111 | 112 | 104 | 98.9 | 104 | 95.9 | 3.20 | <0.001 | <0.001 | 0.13 | 0.031 | 0.48 | 0.72 |
| ADG, g/d | 49.9 | 54.5 | 48.3 | 47.6 | 51.0 | 45.8 | 2.18 | 0.31 | 0.12 | 0.45 | 0.010 | 0.84 | 0.77 |
| G:F, g/g | 0.449 | 0.487 | 0.452 | 0.471 | 0.487 | 0.470 | 0.016 | <0.001 | 0.28 | 0.95 | 0.047 | 0.61 | 0.43 |
| Water intake, g/d4 | 166 | 168 | 156 | 173 | 183 | 168 | 5.21 | – | – | – | – | – | – |
| Cellobiose intake, g/d | 0.00 | 1.26 | 2.34 | 0.00 | 1.37 | 2.51 | 0.053 | – | – | – | – | – | – |
| Mortality, % | 25.7 | 5.71 | 17.1 | 0 | 5.71 | 17.1 | – | – | 0.020 | 0.029 | 0.84 | 0.002 | 0.087 |
| 48–61 d5 | |||||||||||||
| BW at 48 d, g | 1,481 | 1,546 | 1,460 | 1,449 | 1,497 | 1,423 | 30.6 | <0.001 | 0.12 | 0.45 | 0.010 | 0.84 | 0.77 |
| ADFI, g/d | 175 | 171 | 175 | 166 | 164 | 175 | 5.40 | 0.071 | 0.23 | 0.40 | 0.27 | 0.58 | 0.79 |
| ADG, g/d | 48.6 | 44.6 | 47.5 | 45.9 | 46.2 | 43.9 | 2.13 | 0.73 | 0.37 | 0.47 | 0.56 | 0.66 | 0.19 |
| G:F, g/g | 0.279 | 0.247 | 0.276 | 0.276 | 0.284 | 0.221 | 0.020 | 0.17 | 0.67 | 0.16 | 0.89 | 0.85 | 0.063 |
| Mortality, % | 0.00 | 2.86 | 0.00 | 5.71 | 8.57 | 5.71 | – | – | 0.010 | 1.00 | 0.12 | 0.88 | 0.23 |
| 34–61 d | |||||||||||||
| BW at 61 d, g | 2,113 | 2,126 | 2,077 | 2,046 | 2,097 | 1,993 | 39.6 | <0.001 | 0.066 | 0.28 | 0.11 | 0.88 | 0.49 |
| ADFI, g/d | 141 | 140 | 138 | 131 | 133 | 134 | 3.56 | <0.001 | 0.014 | 0.90 | 0.81 | 0.46 | 0.99 |
| ADG, g/d | 49.3 | 49.7 | 47.9 | 46.8 | 48.7 | 44.8 | 1.47 | 0.30 | 0.066 | 0.28 | 0.11 | 0.88 | 0.49 |
| G:F, g/g | 0.350 | 0.354 | 0.349 | 0.356 | 0.367 | 0.328 | 0.0066 | <0.001 | 0.97 | 0.034 | 0.011 | 0.36 | 0.083 |
| Mortality, % | 25.7 | 8.57 | 17.1 | 5.71 | 14.3 | 22.9 | – | – | 0.53 | 0.28 | 0.36 | 0.012 | 0.16 |
1 n = number of rabbits at the end of the fattening period and used to calculate growth traits. For mortality values, the initial number of rabbits was 35/treatment.
2Live weight at weaning was used as covariate.
3Period during which rabbits were supplemented cellobiose in drinking water and fed the two experimental diets.
4Estimated according to Delgado et al. (2015) that obtained a ratio water to feed intake of 1.50 and 1.75 for LSF and HSF diets, respectively.
5Period in which both cellulose and experimental diets were withdrawn and rabbits received a standard feed and no additive in water.
Estimations of water and cellobiose intake are shown in Table 2. The supplementation with 7.5 and 15 g cellobiose/L drinking water was estimated to be equivalent to a dietary level of inclusion of cellobiose of 1.1% and 2.2% (as fed) for LSF group, and to 1.3% and 2.6% for HSF group, respectively. Once the cellobiose was withdrawn (at 48 d of age) and all rabbits received a standard diet, less differences among groups were observed. A trend (P = 0.063) for an interaction between the quadratic effect of cellobiose and the level of soluble fiber affected G:F ratio.
The effects of the experimental factors in the whole fattening period were similar than those observed from 34 to 48 d of age. The high level of soluble fiber impaired ADFI by 5% (140 vs. 133 g/d; P = 0.014), and tended to reduce ADG (P = 0.066) with no influence on G:F. Cellobiose supplementation had no influence on G:F in the LSF-fed group but exerted a quadratic effect in the HSF group (P = 0.003), and a trend for an interaction between the quadratic effect of cellobiose supplementation and the level of soluble fiber (P = 0.083) was detected.
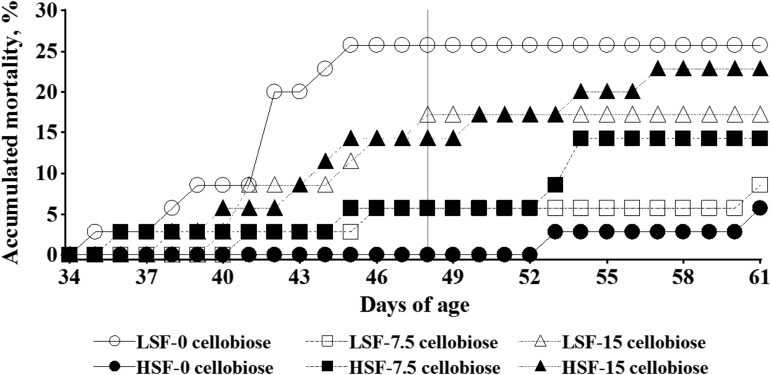
Mortality rate was influenced both by cellobiose, and soluble fiber levels, and there were linear and quadratic interactions between the two factors. From 34 to 48 d of age, there was a linear increase of mortality with increasing cellobiose levels in the HSF group (from 0% to 17.1%; P = 0.017), and this effect tended to remain when the whole fattening period was considered (P = 0.065). In contrast, a quadratic effect of the level of cellobiose on mortality was observed in the LSF group, showing the rabbits offered 7.5-cellobiose the lowest mortality from 34 to 48 d of age (5.7% vs. 21.4%, averaged value for 0 and 15.0 cellobiose; P = 0.030), and this effect tended to be observed when the whole fattening period was considered (P = 0.091). The mortality of rabbits that received no cellobiose was lower with the HSF than with the LSF diet both from 34 to 48 d (0 vs. 25.7; P = 0.030), and in the whole fattening period (5.7% vs. 25.7%; P = 0.017). However, when rabbits were fed a standard diet (from 48 to 61 d), the mortality was higher in the HSF compared with the LSF group, indicating that mortality occurred later (but in a lower proportion) in HSF than in LSF rabbits. The accumulated mortality revealed a different evolution of mortality among rabbits from different treatments (Figure 1). Once cellobiose and the experimental diets were withdrawn, mortality increased in rabbits previously fed the HSF diet, with no changes in those fed the LSF diet. The observed symptoms were the emission of small quantities of watery droppings, cecal impaction, liquid stomach, and intestinal distension with gas and mucus.
Figure 1.
Effect of the level of cellobiose in drinking water (0, 7.5, and 15.0 g/L) and dietary soluble fiber content (low: LSF; high: HSF) on the accumulated mortality of rabbits from weaning to 61 d of age. The vertical line at 48 d of age indicates the end of experimental treatments (cellobiose and diets) and the change to a common commercial diet to all rabbits.
A cellobiose × soluble fiber interaction (P = 0.023) was observed for the sucrose activity in the jejunal mucosa, as the 7.5 cellobiose group showed a maximal value with the LSF diet (232 vs. 191 µmol glucose/g protein) but a minimal value with the HSF diet (187 vs. 229 µmol glucose/g protein) compared with the 0 or 15.0 cellobiose groups (Table 3). The relative weight of the digestive tract was not influenced by the level of soluble fiber (P = 0.51), but tended to increase quadratically with increasing cellobiose levels (P = 0.071; Table 3). There was no effect of treatments on the relative weight of the empty cecum or cecal digesta (P ≥ 0.18). The experimental treatments had no influence on ileal pH (P > 0.26), but an interaction between the level of cellobiose and that of soluble fiber was observed for cecal pH (P = 0.045; Table 4). The 7.5 cellobiose group had the highest cecal pH values when the LSF diet was fed (5.50 vs. 5.36, averaged value for 0 and 15.0 g/L cellobiose), but the lowest values with the diet HSF (5.26 vs. 5.36). The pH was higher in the ileum than in the cecum (P < 0.001) for all treatments, and cecal digesta had about two times more DM content than the ileal one (P < 0.001). The DM content of both ileal and cecal digesta was not influenced by cellobiose level, but cecal DM content decreased with the level of soluble fiber (20.9% vs. 19.4%; P = 0.031).
Table 3.
Effect of the level of cellobiose in drinking water and dietary soluble fiber on digestive traits in 46-d-old rabbits
| Soluble fiber |
Low | High | SEM | P | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cellobiose, g/L |
0 | 0.75 | 1.5 | 0 | 0.75 | 1.5 | Soluble fiber | Cellobiose | Soluble fiber × Cellobiose | |||
| n 1 | 7 | 9 | 8 | 9 | 8 | 9 | Lineal | Quadratic | Lineal | Quadratic | ||
| BW, g | 1,231 | 1,316 | 1,311 | 1,308 | 1,353 | 1,296 | 60.70 | 0.51 | 0.58 | 0.36 | 0.54 | 0.95 |
| Sucrose activity in jejunal mucosa, µmol glucose/g protein | 200 | 232 | 182 | 212 | 187 | 227 | 17.1 | 0.79 | 0.94 | 0.76 | 0.70 | 0.023 |
| Weight of the digestive tract, % BW | 28.3 | 27.8 | 28.9 | 28.8 | 26.9 | 29.6 | 0.97 | 0.91 | 0.48 | 0.071 | 0.74 | 0.41 |
| Cecum | ||||||||||||
| Empty weight, % BW | 2.52 | 2.54 | 2.71 | 2.51 | 2.44 | 2.74 | 0.15 | 0.84 | 0.18 | 0.32 | 0.95 | 0.69 |
| Digesta, % BW | 8.57 | 7.65 | 8.35 | 7.82 | 8.34 | 8.75 | 0.56 | 0.81 | 0.53 | 0.44 | 0.19 | 0.38 |
1 n = number of rabbits.
Table 4.
Effect of the level of cellobiose in drinking water and dietary soluble fiber on the total and molar VFA at ileal and cecal concentration in 46-d-old rabbits
| Soluble fiber | Low | High | SEM | P | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cellobiose, g/L | 0.00 | 7.50 | 15.0 | 0.00 | 7.50 | 15.0 | Soluble fiber | Cellobiose | Soluble fiber × Cellobiose | |||
| n 1 | 7 | 8 | 6 | 8 | 8 | 8 | Lineal | Quadratic | Lineal | Quadratic | ||
| n 2 | 7 | 8 | 8 | 9 | 8 | 9 | ||||||
| Ileum | ||||||||||||
| DM, % | 10.5 | 12.9 | 11.4 | 10.5 | 10.7 | 10.7 | 0.97 | 0.23 | 0.57 | 0.21 | 0.40 | 0.25 |
| pH | 6.91 | 6.84 | 6.99 | 6.90 | 6.97 | 6.97 | 0.065 | 0.59 | 0.27 | 0.49 | 0.63 | 0.21 |
| Total VFA, mmol/g fresh digesta | 19.8 | 25.1 | 20.9 | 25.9 | 27.6 | 26.5 | 1.41 | <0.001 | 0.56 | 0.014 | 0.41 | 0.18 |
| Molar proportion, mol/100 mol | ||||||||||||
| Acetate | 93.9 | 91.2 | 95.4 | 93.6 | 94.0 | 95.0 | 0.91 | 0.34 | 0.14 | 0.019 | 0.34 | 0.043 |
| Propionate | 4.37 | 5.65 | 2.67 | 3.70 | 4.38 | 3.46 | 0.58 | 0.42 | 0.11 | 0.005 | 0.68 | 0.19 |
| Butyrate | 1.49 | 2.44 | 1.45 | 1.89 | 1.34 | 1.41 | 0.42 | 0.48 | 0.56 | 0.36 | 0.19 | 0.086 |
| Isobutyrate | 0.00 | 0.096 | 0.015 | 0.040 | 0.00 | 0.008 | 0.046 | 0.58 | 0.85 | 0.41 | 0.26 | 0.16 |
| Valerate | 0.024 | 0.096 | 0.027 | 0.18 | 0.004 | 0.049 | 0.058 | 0.56 | 0.29 | 0.69 | 0.067 | 0.075 |
| Isovalerate | 0.091 | 0.068 | 0.22 | 0.18 | 0.080 | 0.085 | 0.050 | 0.79 | 0.75 | 0.11 | 0.099 | 0.68 |
| Caproate | 0.11 | 0.50 | 0.23 | 0.40 | 0.17 | 0.040 | 0.14 | 0.50 | 0.41 | 0.25 | 0.032 | 0.13 |
| Cecum | ||||||||||||
| DM, % | 19.5 | 20.9 | 22.4 | 19.2 | 19.9 | 19.0 | 0.86 | 0.031 | 0.13 | 0.64 | 0.22 | 0.57 |
| pH | 5.37 | 5.50 | 5.35 | 5.39 | 5.26 | 5.33 | 0.067 | 0.17 | 0.57 | 0.72 | 0.23 | 0.045 |
| Total VFA, mmol/g fresh digesta | 81.0 | 86.7 | 82.9 | 99.5 | 94.5 | 84.5 | 3.81 | 0.005 | 0.092 | 0.28 | 0.046 | 0.74 |
| Molar proportion, mol/100 mol | ||||||||||||
| Acetate | 83.5 | 83.4 | 82.5 | 84.2 | 87.1 | 84.8 | 1.27 | 0.036 | 0.83 | 0.18 | 0.30 | 0.31 |
| Propionate | 2.85 | 3.29 | 2.30 | 2.94 | 2.58 | 3.02 | 0.40 | 0.92 | 0.56 | 0.66 | 0.91 | 0.12 |
| Butyrate | 11.8 | 11.6 | 13.5 | 11.9 | 9.43 | 11.2 | 0.97 | 0.076 | 0.60 | 0.066 | 0.18 | 0.52 |
| Isobutyrate | 0.017 | 0.094 | 0.011 | 0.027 | 0.00 | 0.00 | 0.017 | 0.024 | 0.33 | 0.027 | 0.039 | 0.002 |
| Valerate | 0.26 | 0.24 | 0.20 | 0.20 | 0.12 | 0.15 | 0.035 | 0.011 | 0.13 | 0.52 | 0.73 | 0.26 |
| Isovalerate | 0.15 | 0.20 | 0.18 | 0.10 | 0.060 | 0.15 | 0.022 | <0.001 | 0.095 | 0.43 | 0.35 | 0.010 |
| Caproate | 1.38 | 1.22 | 1.37 | 0.60 | 0.65 | 0.70 | 0.20 | <0.001 | 0.84 | 0.66 | 0.64 | 0.64 |
1Number of ileal VFA samples.
2Number of cecal VFA samples.
Both ileal and cecal total VFA concentrations were higher (22% and 11%, respectively; P ≤ 0.005) in HSF-fed rabbits than in those fed the LSF diet (Table 4). The level of cellobiose influenced quadratically the ileal total VFA concentrations, with the 7.5-cellobiose group showing the maximal value (26.3 vs. 23.3 mmol/g fresh digesta; P = 0.014). In the cecum, total VFA concentrations decreased linearly with the level of cellobiose in HSF-fed rabbits (P = 0.031), whereas no effect was observed in LSF groups, resulting in an interaction between the level of cellobiose and the level of soluble fiber (P = 0.046). Total VFA concentrations were higher in cecal than in ileal digesta (87.5 vs. 24.3 mmol/g fresh digesta; values averaged across experimental treatments; P < 0.001; Table 4). Cecal VFA concentrations were negatively correlated to mortality from 34 to 48 d of age (r = −0.89; P = 0.018; n = 6), and a trend was observed for the mortality in the whole fattening period (r = −0.77; P = 0.076; n = 6).
The VFA profile in the ileal and cecal digesta was influenced by both cellobiose and soluble fiber levels. In the ileal digesta, acetate and butyrate proportions were affected quadratically by the cellobiose level in LSF-fed rabbits, with no effect in HSF-fed rabbits, which resulted in a trend to an interaction cellobiose × soluble fiber (P ≤ 0.086; Table 4). A similar result was observed for propionate proportions, but no cellobiose × soluble fiber interaction was observed (P = 0.19). The rabbits fed the LSF diet and supplemented with 7.5 cellobiose had lower acetate, and greater propionate, and butyrate proportions in the ileum compared with those on 0 and 15.0 cellobiose treatments. In contrast, no effects of cellobiose supplementation on the main VFA proportions were observed in rabbits fed HSF diet, but caproate proportion decreased linearly with cellobiose inclusion (P = 0.032), and valerate and isovalerate proportions tended to increase and decrease, respectively (P ≤ 0.099). A negative correlation between ileal caproate concentration and mortality rate in the whole fattening period was observed (r = −0.86; P = 0.019; n = 6).
Acetate proportions were higher, and those of butyrate, isobutyrate, valerate, isovalerate, and caproate were lower in the cecal digesta of rabbits fed the HSF diet compared with LSF-fed rabbits (P ≤ 0.076). Cellobiose supplementation had less influence on VFA proportions in the cecum than in the ileum. Cellobiose influenced quadratically (P ≤ 0.066) the proportions of butyrate in both LSF and HSF rabbits (minimal values for 7.5 cellobiose groups), isobutyrate in LSF-fed rabbits (maximal values for 7.5 cellobiose group), and isovalerate in HSF rabbits (minimal values for 7.5 cellobiose group). The VFA profile differed between the ileal and cecal digesta (P < 0.001), with the exception of isobutyrate and isovalerate proportions. Ileal digesta had higher (P < 0.001) proportions of acetate (93.8% vs. 84.4%; values averaged across treatments), and propionate (4.04 vs. 2.82) than cecal digesta, but lower (P < 0.001) proportions of butyrate (1.67% vs. 11.5%), valerate (0.06% vs. 0.19%), and caproate (0.24% vs. 0.98%).
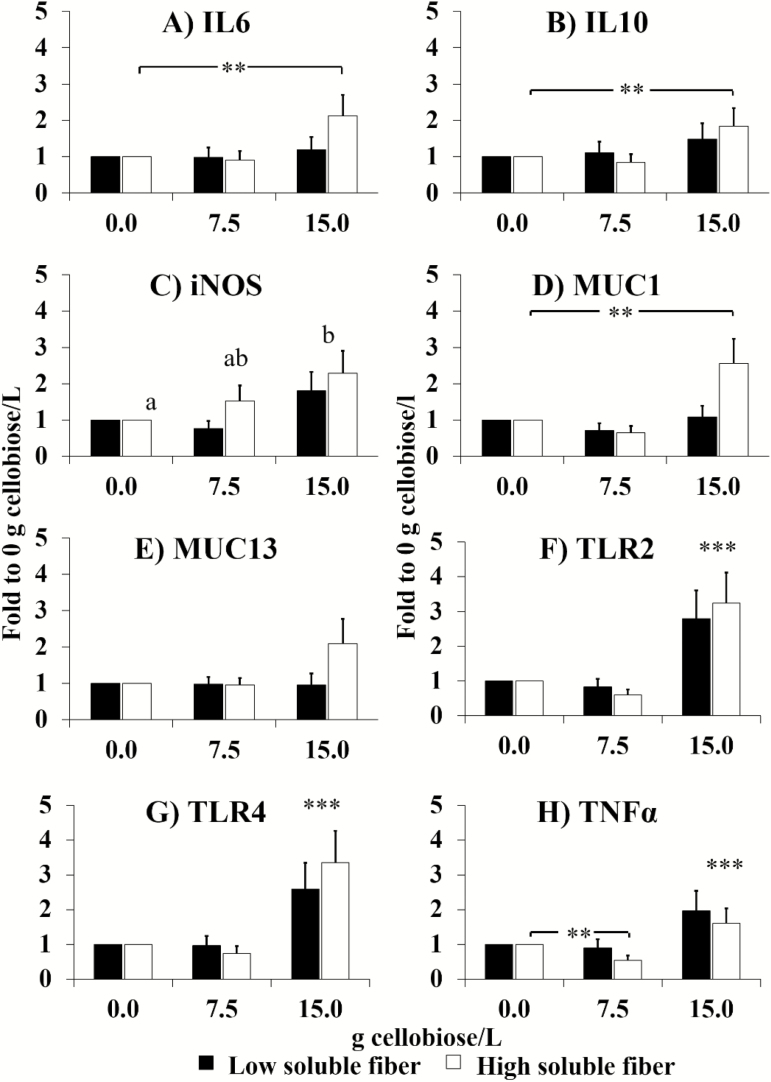
The relative gene expression of all studied cytokines (IL-6, IL-10, and TNF-α), iNOS, MUC-1, and toll-like receptors (TLR-2 and TLR-4) in the cecal appendix increased linear and quadratically with the level of cellobiose (P ≤ 0.063; Figure 2), resulting in higher gene expression for HSF than for LSF-fed rabbits when combined with 15.0 cellobiose (P ≤ 0.058), except for iNOS. The relative gene expression of MUC-13 was not influenced by the experimental treatments (P > 0.10).
Figure 2.
Effect of the level of cellobiose in drinking water (0, 7.5, and 15 g/L) and dietary soluble fiber on mRNA levels interleukin 6 and 10 (IL-6, IL-10), inducible nitric oxide synthase (iNOS), MUC-1, TLR-2, TLR-4, and TNF-α. Relative gene expression values are fold change of 7.5- and 15-cellobiose groups relative to the 0-cellobiose (control), for each level of fiber. Bars indicate the 95% confidence interval (fold change up–fold change low). n = 8/treatment (**P < 0.01; ***P < 0.001). Lower case letters indicate differences of expression among cellobiose levels.
DISCUSSION
Moderate levels of dietary soluble fiber can reduce the mortality of rabbits affected by ERE (Trocino et al., 2013). The results obtained in this study are in agreement, as mortality was reduced in rabbits fed the HSF compared with those fed the LSF diet when rabbits received no cellobiose supplementation. In these diets, the minimal insoluble fiber requirements were met, and we attributed most of this positive effect to the soluble fiber fraction, although a positive influence of dietary ADF cannot be discarded as it increased in parallel with soluble fiber. This positive effect might be related to beneficial changes in the intestinal microbiota promoted by soluble fiber (Gómez-Conde et al., 2007, 2009). However, it was noticeable the interaction between cellobiose and soluble fiber detected for rabbit mortality, which was reduced by cellobiose supplementation in LSF-fed rabbits (only for 7.5 g cellobiose/L) but linearly increased in rabbits fed the HSF diet. These results were confirmed in two subsequent experiments (Ocasio-Vega, 2018). They might indicate a negative effect of cellobiose on the intestinal microbiota of HSF-fed rabbits, as the increase in mortality was accompanied with a reduction of cecal total VFA concentrations. Cellobiose can be hydrolyzed in the small intestine by the enzyme lactase that has β-glucosidase and β-galactosidase activities (Dahlqvist, 1962; Nakamura, 2005; Morita et al., 2008). However, intestinal lactase activity in weaned rabbits is extremely low (Marounek et al., 1995; Gutiérrez et al., 2002), and cellobiose hydrolysis might be also very slow (Fischer and Sutton, 1957). Therefore, it would be expected that most of the cellobiose was undigested and could be completely fermented by the intestinal microbiota. Whether cellobiose was fermented before or in the cecum is not clear due to the lack of differences in the ileal and cecal pH, although the observed differences in VFA concentrations, and profile in the ileum, and cecum might suggest that cellobiose would be fermented in both segments.
The two groups with the lowest mortality (HSF-0 cellobiose and LSF-7.5 cellobiose) also had the highest butyrate proportions in the ileum, which might be related to a better integrity of the intestinal mucosa in these animals (Guilloteau et al, 2010), and/or changes in their microbiota metabolism, and profile (Gantois et al., 2006; Song et al., 2013; Jiao et al., 2015). Intestinal VFA concentrations measured at a single time point are the balance between the production rate, and absorption, and cannot be considered as a good indicator of VFA production, and/or absorption (Van der Klis and Jansman, 2002). Butyrate has showed higher absorption rates compared with acetate and propionate (Vernay, 1987; Von Engelhardt et al., 1989), and despite of this butyrate proportion in the ileum was higher in the two groups with the lowest mortality (HSF-0 cellobiose and LSF-7.5 cellobiose) than in the others. It might indicate that the effect on the butyrate absorption could be even stronger than that observed on the ileal butyrate concentration. In this way, several in vitro studies have reported a greater production of butyrate when cellobiose was fermented with cecal content or soft feces from rabbits (Yang et al., 2010; Ocasio-Vega et al., 2018a, b), and feces from pigs (Tran et al., 2016), or humans (Van Zanten et al., 2012) compared with other carbohydrates. Nevertheless, the reduction of mortality might be also related to other factors. Rabbits from the groups with the lowest mortality (HSF-0 cellobiose and LSF-7.5 cellobiose) also showed higher caproate proportions in the ileum, and caproate has shown antimicrobial activity by reducing in vitro the concentration of viable cells of an enteropathogenic E. coli strain (Skrivanová and Marounek, 2007).
The negative influence of cellobiose supplementation on the health of rabbits fed the HSF diet might be related to changes in the intestinal microbiota, as indicated by the reduction observed in butyrate, and caproate proportions in the ileum, and total VFA concentrations in the cecum. The latter might suggest a relevant negative effect of cellobiose on cecal fermentation of HSF-fed rabbits, especially at the highest dose, and a potential dysbiosis. In fact, dietary cellobiose supplementation at higher doses (15% of diet) produced a general diarrhea in rats (Moinuddin and Lee, 1958), although this effect was not always observed at 10% dietary cellobiose (Umeki et al., 2004; Nishimura et al., 2010), suggesting an interaction of cellobiose with the host microbiota. The dietary supplementation with lactose, which is hydrolyzed by the same enzymes that cellobiose, up to 14% during the post-weaning period also showed a negative effect on the mortality of rabbits affected by ERE (Gutiérrez et al., 2002). All these results are in agreement with the highly variable response of rabbits to the supplementation of different types of oligosaccharides as reviewed by Falcão-e-Cunha et al. (2007), which may be also related to the dose used in the different studies and variations in the host microbiota.
Cellobiose has been previously reported to exert a positive effect by reducing inflammatory markers in mice with induced colitis, which was attributed to a prebiotic effect related to increases in butyrate production (Nishimura et al., 2010). However, in this study, a pro-inflammatory response was observed in the cecal appendix of rabbits fed with the highest dose of cellobiose supplementation (15.0 g/L), especially in those fed the HSF diet. These effects are in agreement with those obtained by supplementing a higher dose of cellobiose (15%) to rats (Moinuddin and Lee, 1958), suggesting that the positive or negative interaction of cellobiose with the host microbiota and hence its prebiotic effects might be dose dependent, and independent of the existence of an ERE outbreak. Moreover, at the time of sampling for the immune response analysis (46 d of age), the mortality in the groups supplemented with 15.0 cellobiose was still increasing, which might account for the strong immune response observed, even when all slaughtered rabbits were apparently healthy. The upregulation of the toll-like receptors TLR-4 and TLR-2 in the cecal appendix might indicate an activation of the innate immune response usually triggered by a pathogen-associated molecular pattern (Williams, 2012). This would lead to the upregulation of the pro-inflammatory mediators such as TNF-α, iNOS, and IL-6 (only in the HSF group), which partially agrees with the cytokine profile previously reported in the cecal appendix and cecal mucosa of rabbits affected by ERE (Bäuerl et al., 2014). The upregulation of MUC-1 in HSF fed rabbits might suggest a higher mucin secretion, although it also might play an anti-inflammatory role together with IL-10 to counterbalance the pro-inflammatory response (Opal and DePalo, 2000; Sheng et al., 2013). Therefore, the present results indicated the immune response in a specific moment (46 d of age), and remarks the importance of sampling time when characterizing the immune response in ERE-affected rabbits in relation with the development of the disease and the interest to develop procedures that allow to assess the evolution of immune response along time.
Part of the mortality observed in rabbits fed the HSF diet, and supplemented with 0, 7.5, and 15.0 cellobiose was produced once the experimental diets, and cellobiose supplementation were withdrawn. The commercial diet supplied in this period was low in soluble fiber, as LSF diet (58.7 and 84.0 g/kg DM, respectively), but contained more insoluble fiber (341 and 307 g NDF/kg DM). This might have altered the intestinal microbiota in HSF-fed rabbits and increased the mortality at the end of the experimental period. This effect was almost not observed in the HSF-fed rabbits receiving no cellobiose, but was more evident in the HSF-fed rabbits receiving 7.5 cellobiose, indicating that cellobiose withdrawn in this group might have also influenced this increase in mortality.
In conclusion, our results indicate that in rabbits fed LSF diets a dose of 7.5 g cellobiose/L drinking water could be recommended, whereas no cellobiose supplementation seems to be adequate for rabbits fed HSF diets. Further research is required to determine the optimal dose of cellobiose supplementation for diets differing in fiber content and composition.
ACKNOWLEDGMENTS
We are grateful to the Ministerio de Ciencia e Innovación (Spain. Project AGL2011-23885), and the Comunidad Autónoma de Madrid (Project MEDGAN ABI-2913) for the financial support, to Nippon Paper Industries (Tokyo, Japan) for providing the cellobiose, and to Dr. Enrique Blas and his team (Universidad Politécnica de Valencia, Spain) for the manufacturing of experimental diets.
LITERATURE CITED
- Abad-Guamán R., Carabaño R., Gómez-Conde M. S., and García J.. 2015. Effect of type of fiber, site of fermentation, and method of analysis on digestibility of soluble and insoluble fiber in rabbits. J. Anim. Sci. 93:2860–2871. doi:10.2527/jas.2014-8767 [DOI] [PubMed] [Google Scholar]
- AOAC 2000. Official methods of analysis. 17th ed Arlington, VA: AOAC Int. [Google Scholar]
- Badiola I., Pérez de Rozas A., González J., Aloy N., García J., and Carabaño R.. 2016. Recent advances in ERE in growing rabbits. In: Qin Y., F. Li T. Gidenne, editors. Proceedings of the 11th World Rabbit Congress June 15 to 18; Qingdao, China. [Google Scholar]
- Bäuerl C., Collado M. C., Zúñiga M., Blas E., and Pérez Martínez G.. 2014. Changes in cecal microbiota and mucosal gene expression revealed new aspects of epizootic rabbit enteropathy. Plos One. 9:e105707. doi:10.1371/journal.pone.0105707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boletín Oficial del Estado 2013. Real Decreto 53/2013. Normas básicas aplicables para la protección de los animales utilizados en experimentación y otros fines científicos, incluyendo la docencia. B.O.E. 34:11370–11421. [Google Scholar]
- Carabaño R., Villamide M. J., García J., Nicodemus N., Llorente A., Chamorro S., Menoyo D., García-Rebollar P., García-Ruiz A. I., and De Blas C.. 2009. New concepts and objectives for protein-amino acid nutrition in rabbits: a review. World Rabbit Sci. 17:1–14. [Google Scholar]
- Carro M. D., Lebzien P., and Rohr K.. 1992. Influence of yeast culture on the in vitro fermentation (Rusitec) of diets containing variable portions of concentrates. Anim. Feed Sci. Technol. 37:209–220. doi:10.1016/0377-8401(92)90005-Q [Google Scholar]
- Chamorro S., C. de Blas G. Grant I. Badiola D. Menoyo, and Carabaño R.. 2010. Effect of dietary supplementation with glutamine and a combination of glutamine-arginine on intestinal health in twenty-five-day-old weaned rabbits. J. Anim. Sci. 88:170–180. doi:10.2527/jas.2008-1698 [DOI] [PubMed] [Google Scholar]
- Dahlqvist A. 1962. Specificity of the human intestinal disaccharidases and implications for hereditary disaccharide intolerance. J. Clin. Invest. 41:463–470. doi:10.1172/JCI104499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado R., Abad-Guamán R., Nicodemus N., Sastre J., Menoyo D., Carabaño R., and García J.. 2015. Effect of level of soluble fibre and omega-6/omega-3 ratio on water intake in growing rabbits. World Rabbit Sci. 23:133. (abst). doi:10.4995/wrs.2015.3901 [Google Scholar]
- El Abed N., Badiola I., de Rozas A. P., González J., Menoyo D., Carabaño R., and García J.. 2013. Effect of soluble and insoluble fractions of sugar beet pulp on ileal and caecal microbiota of rabbits after weaning. World Rabbit Sci. 21:207–208. (abst). [Google Scholar]
- El Abed N., Delgado R., Abad R., Romero C., Villamide M. J., Menoyo D., Carabaño R., and García J.. 2011. Soluble and insoluble fibre from sugar beet pulp enhance intestinal mucosa morphology in young rabbits. In: Proc. 62nd Annual meeting of the European Federation (Stavanger, Norway) of Animal Science, Book of abstracts Wageningen, The Netherlands: Wageningen Academic Publishers; p. 159 (abst.). [Google Scholar]
- Falcão-e-Cunha L., Castro-Solla L., Maertens L., Marounek M., Pinheiro V., Freire J., and Mourão J.. 2007. Alternatives to antibiotic growth promoters in rabbit feeding: a review. World Rabbit Sci. 15:127–140. doi:10.4995/wrs.2007.597 [Google Scholar]
- Fischer J. E., and Sutton T. S.. 1957. The hydrolysis of sucrose, lactose, and cellobiose by small intestinal mucosa in vitro: relationship to laxation in the rat produced by these disaccharides in vivo. Ohio J. Sci. 57:75–80. [Google Scholar]
- Gantois I., R. Ducatelle F. Pasmans F. Haesebrouck I. Hautefort A. Thompson J. C. Hinton, and Van Immerseel F.. 2006. Butyrate specifically down-regulates salmonella pathogenicity island 1 gene expression. Appl. Environ. Microbiol. 72:946–949. doi:10.1128/AEM.72.1.946-949.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godornes C., B. T. Leader B. J. Molini A. Centurion-Lara, and Lukehart S. A.. 2007. Quantitation of rabbit cytokine mRNA by real-time rt-pcr. Cytokine. 38:1–7. doi:10.1016/j.cyto.2007.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goehring H. K., and Van Soest P. J.. 1970. Forage fibre analyses (apparatus, reagents, procedures and some applications). Agric. Handbook no. 379. Washington, DC: ARS, USDA. [Google Scholar]
- Gómez-Conde M. S., de Rozas A. P., Badiola I., Pérez-Alba L., de Blas C., Carabaño R., and García J.. 2009. Effect of neutral detergent soluble fibre on digestion, intestinal microbiota and performance in twenty five day old weaned rabbits. Livest. Sci. 125:192–198. doi:10.1016/j.livsci.2009.04.010 [Google Scholar]
- Gómez-Conde M. S., J. García S. Chamorro P. Eiras P. G. Rebollar A. Pérez de Rozas I. Badiola C. de Blas, and Carabaño R.. 2007. Neutral detergent-soluble fiber improves gut barrier function in twenty-five-day-old weaned rabbits. J. Anim. Sci. 85:3313–3321. doi:10.2527/jas.2006-777 [DOI] [PubMed] [Google Scholar]
- Guilloteau P., Martin L., Eeckhaut V., Ducatelle R., Zabielski R., and Van Immerseel F.. 2010. From the gut to the peripheral tissues: the multiple effects of butyrate. Nutr. Res. Rev. 23:366–384. doi:10.1017/S0954422410000247 [DOI] [PubMed] [Google Scholar]
- Gutiérrez I., Espinosa A., García J., Carabaño R., and de Blas J. C.. 2002. Effect of levels of starch, fiber, and lactose on digestion and growth performance of early-weaned rabbits. J. Anim. Sci. 80:1029. doi:10.2527/2002.8041029x [DOI] [PubMed] [Google Scholar]
- Jiao L. F., Ke Y. L., Xiao K., Song Z. H., Hu C. H., and Shi B.. 2015. Effects of cello-oligosaccharide on intestinal microbiota and epithelial barrier function of weanling pigs. J. Anim. Sci. 93:1157–1164. doi:10.2527/jas.2014-8248 [DOI] [PubMed] [Google Scholar]
- Jiao L. F., Song Z. H., Ke Y. L., Xiao K., Hu C. H., and Shi B.. 2014. Cello-oligosaccharide influences intestinal microflora, mucosal architecture and nutrient transport in weaned pigs. Anim. Feed Sci. Technol. 195:85–91. doi:10.1016/j.anifeedsci.2014.05.014 [Google Scholar]
- Kaps M., and Lamberson W.. 2004. Analysis of numerical treatment level. In: Biostatistics for animal science. Cambridge (MA): CABI Publishing; p. 384–393. [Google Scholar]
- Licois D., Coudert P., and Marlier D.. 2006. Epizootic rabbit enteropathy. In: Maertens L. and P. Coudert, editors. Recent advances in rabbit science. Melle, Belgium: Institute for Agricultural and Fisheries Research (ILVO); pp. 163–170. [Google Scholar]
- Marounek M., Vovk S. J., and Skrivanová V.. 1995. Distribution of activity of hydrolytic enzymes in the digestive tract of rabbits. Br. J. Nutr. 73:463–469. doi:10.1079/BJN19950048 [DOI] [PubMed] [Google Scholar]
- Martínez-Vallespín B., Martínez-Paredes E., Ródenas L., Cervera C., Pascual J. J., and Blas E.. 2011. Combined feeding of rabbit female and young: partial replacement of starch with acid detergent fibre or/and neutral detergent soluble fibre at two protein levels. Livest. Sci. 141:155–165. [Google Scholar]
- Mertens D. R. 2002. Gravimetric determination of amylase-treated neutral detergent fiber in feeds with refluxing in beakers or crucibles: collaborative study. J. aoac Int. 85:1217–1240. [PubMed] [Google Scholar]
- Moinuddin J. F. and Lee H. W.. 1958. Effects of feeding diets containing sucrose, cellobiose or glucose on the dry weights of cleaned gastrointestinal organs in the rat. Am. J. Physiol. 192:417–420. doi:10.1152/ajplegacy.1958.192.2.417 [DOI] [PubMed] [Google Scholar]
- Morita T., M. Ozawa H. Ito S. Kimio, and Kiriyama S.. 2008. Cellobiose is extensively digested in the small intestine by beta-galactosidase in rats. Nutrition. 24:1199–1204. doi:10.1016/j.nut.2008.06.029 [DOI] [PubMed] [Google Scholar]
- Nakamura S. 2005. Bioavailability of cellobiose and other non-digestible and/or non-absorbable sugar substitutes and related topics. Nutrition. 21:1158–1159. doi:10.1016/j.nut.2005.08.006 [DOI] [PubMed] [Google Scholar]
- Nishimura T., Andoh A., Hashimoto T., Kobori A., Tsujikawa T., and Fujiyama Y.. 2010. Cellobiose prevents the development of dextran sulfate sodium (dss)-induced experimental colitis. J. Clin. Biochem. Nutr. 46:105–110. doi:10.3164/jcbn.09-72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ocasio-Vega C. 2018. Effect of cellobiose and dietary soluble fibre on fermentation, growth performance and health in rabbits [PhD thesis]. Madrid: Universidad Politécnica de Madrid. [Google Scholar]
- Ocasio-Vega C., Abad-Guaman R., Delgado R., Carabano R., Carro M. D., and Garcia J.. 2018a. In vitro caecal fermentation of carbohydrate-rich feedstuffs in rabbits as affected by substrate pre-digestion and donors’ diet. World Rabbit Sci. 26:15–25. doi:10.4995/wrs.2018.7854 [Google Scholar]
- Ocasio-Vega C., Abad-Guamán R., Delgado R., Carabaño R., Carro M. D., and García J.. 2018b. Effect of cellobiose supplementation and dietary soluble fibre on in vitro caecal fermentation of carbohydrate-rich substrates in rabbits. Arch. Anim. Nutr. doi:10.1080/1745039X.2018.1458459 [DOI] [PubMed] [Google Scholar]
- Opal S. M. and DePalo V. A.. 2000. Anti-inflammatory cytokines. Chest. 117:1162–1172. doi:10.1378/chest.117.4.1162 [DOI] [PubMed] [Google Scholar]
- Pedersen M. B., Yu S., Arent S., Dalsgaard S., Bach Knudsen K. E., and Lærke H. N.. 2015. Xylanase increased the ileal digestibility of nonstarch polysaccharides and concentration of low molecular weight nondigestible carbohydrates in pigs fed high levels of wheat distillers dried grains with solubles. J. Anim. Sci. 93:2885–2893. doi:10.2527/jas.2014-8829 [DOI] [PubMed] [Google Scholar]
- Sheng Y. H., S. Triyana R. Wang I. Das K. Gerloff T. H. Florin P. Sutton, and McGuckin M. A.. 2013. muc1 and muc13 differentially regulate epithelial inflammation in response to inflammatory and infectious stimuli. Mucosal Immunol. 6:557–568. doi:10.1038/mi.2012.98 [DOI] [PubMed] [Google Scholar]
- Skrivanová E. and Marounek M.. 2007. Influence of pH on antimicrobial activity of organic acids against rabbit enteropathogenic strain of Escherichia coli. Folia Microbiol. (Praha). 52:70–72. doi:10.1007/BF02932141. [DOI] [PubMed] [Google Scholar]
- Song J., Jiao L. F., Xiao K., Luan Z. S., Hu C. H., Shi B., and Zhan X. A.. 2013. Cello-oligosaccharide ameliorates heat stress-induced impairment of intestinal microflora, morphology and barrier integrity in broilers. Anim. Feed Sci. Technol. 185:175–181. doi:10.1016/j.anifeedsci.2013.08.001 [Google Scholar]
- Steibel J. P., Poletto R., Coussens P. M., and Rosa G. J.. 2009. a powerful and flexible linear mixed model framework for the analysis of relative quantification rt-pcr data. Genomics. 94:146–152. doi:10.1016/j.ygeno.2009.04.008 [DOI] [PubMed] [Google Scholar]
- Tran T. H. T., Boudry C., Everaert N., and Bindelle J.. 2016. Prebiotic potential of novel carbohydrates in an in vitro co-inoculation fermentation model of the bacteria isolated from pig intestine and Salmonella. J. Anim. Sci. 94:58–61. doi:10.2527/jas.2015-976026812312 [Google Scholar]
- Trocino A., García J., Carabaño R., and Xiccato G.. 2013. A meta-analysis on the role of soluble fibre in diets for growing rabbits. World Rabbit Sci. 21:1–15. doi:10.4995/wrs.2013.1285 [Google Scholar]
- Umeki M., K. Oue S. Mochizuki Y. Shirai, and Sakai K.. 2004. Effect of lactobacillus rhamnosus ky-3 and cellobiose as synbiotics on lipid metabolism in rats. J. Nutr. Sci. Vitaminol. (Tokyo). 50:330–334. doi:10.3177/jnsv.50.330 [DOI] [PubMed] [Google Scholar]
- Van der Klis J. D., and Jansman A. J.. 2002. Optimising nutrient digestion, absorption and gut barrier function in monogastrics: reality or illusion. In: Blok M. C., H. A. Vahl L. de Lange A. E. van de Braak G. Hemke, and M. Hessing, editors. Nutrition and health of the gastrointestinal tract. Wageningen, The Netherlands: Wageningen Academic Publishers; p. 15–36. [Google Scholar]
- Vernay M. 1987. Origin and utilization of volatile fatty acids and lactate in the rabbit: influence of the faecal excretion pattern. Br. J. Nutr. 57:371–381. doi:10.1079/BJN19870045 [DOI] [PubMed] [Google Scholar]
- Von Engelhardt W., Rönnau K., Rechkemmer G., and Sakata T.. 1989. Absorption of short-chain fatty acids and their role in the hindgut of monogastric animals. Anim. Feed Sci. Technol. 23:43–53. doi:10.1016/0377-8401(89)90088-6 [Google Scholar]
- Williams A. E. 2012. Immunology. Mucosal and body surface defenses. Oxford, UK: Wiley-Blackwell. [Google Scholar]
- Xiccato G., A. Trocino D. Majolini M. Fragkiadakis, and Tazzoli M.. 2011. Effect of decreasing dietary protein level and replacing starch with soluble fibre on digestive physiology and performance of growing rabbits. Animal. 5:1179–1187. doi:10.1017/S1751731111000243 [DOI] [PubMed] [Google Scholar]
- Yang H. J., Cao Y. C., and Zhang D. F.. 2010. Caecal fermentation patterns in vitro of glucose, cellobiose, microcrystalline cellulose and NDF separated from alfalfa hay in the adult rabbit. Anim. Feed Sci. Technol. 162:149–154. doi:10.1016/j.anifeedsci.2010.09.008 [Google Scholar]
- Yemm E. W., and Willis A. J.. 1954. The estimation of carbohydrates in plant extracts by anthrone. Biochem. J. 57:508–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Zanten G. C., Knudsen A., Röytiö H., Forssten S., Lawther M., Blennow A., Lahtinen S. J., Jakobsen M., Svensson B., and Jespersen L.. 2012. The effect of selected synbiotics on microbial composition and short-chain fatty acid production in a model system of the human colon. Plos One. 7:e47212. doi:10.1371/journal.pone.0047212 [DOI] [PMC free article] [PubMed] [Google Scholar]