Abstract
Bovine respiratory disease (BRD) is the most common cause of morbidity and mortality in North American beef cattle. Mannheimia haemolytica is the bacterial pathogen most often isolated from cattle with BRD, and the prevalence of antimicrobial resistance (AMR) in this organism has increased in recent years. Antimicrobials are commonly used to prevent BRD in cattle at high risk of developing BRD; however, recent work would suggest that this practice might be one factor contributing to the increased prevalence of AMR in M. haemolytica. We hypothesized that the administration of the short-acting fluoroquinolone, enrofloxacin, would be just as effective as the long-acting triamilide, tulathromycin, in preventing BRD but would be less likely to select for AMR M. haemolytica in stocker calves at high risk of developing BRD. Three hundred forty-one stocker calves were enrolled in the study with 172 calves in 4 pens being randomly assigned to treatment with enrofloxacin and 169 calves in 4 pens randomly assigned to treatment with tulathromycin. Calves within each treatment group were allocated to one of 4 replicate pens based on the week of enrollment. Of calves receiving enrofloxacin, 33.7% required treatment for BRD at least once within 45 d after arrival, compared with 18.3% of calves receiving tulathromycin (P = 0.040). The percentages of calves that required more than one treatment for BRD within 45 d after arrival did not differ statistically for those receiving enrofloxacin compared with those receiving tulathromycin (10.5% and 4.7%, respectively; P = 0.107) Likewise, the percentages of calves that died during the 45-d follow-up period did not differ for those receiving enrofloxacin compared with those receiving tulathromycin (12.2% and 10.1%, respectively; P = 0.592). Mannheimia haemolytica was cultured from 11% of calves sampled at arrival and from 50% of calves sampled at revaccination 14 to 17 d later. There was a significanst effect of sampling time on the proportion of calves carrying multidrug-resistant (MDR) isolates, with calves having a higher prevalence of MDR isolates at revaccination than arrival (100% vs. 13%; P < 0.001). Future research evaluating the impact of MDR on response to antimicrobial therapy is necessary.
Keywords: antimicrobial resistance, bovine respiratory disease, enrofloxacin, Mannheimia haemolytica, tulathromycin
INTRODUCTION
Bovine respiratory disease (BRD) is the most common cause of morbidity and mortality in North American beef cattle (Duff and Galyean, 2007). It has been estimated that BRD costs the beef industry in excess of $3 billion yearly with most economic losses coming from the costs of treatment, reductions in feed efficiency, and poorer carcass quality (Duff and Galyean, 2007; Tennant et al., 2014). Although both viral and bacterial agents have been associated with the development of BRD, Mannheimia haemolytica is most often associated with clinical disease (Griffin et al., 2010). As a result, many producers try to manage BRD risk by administering antimicrobials at arrival processing, a practice commonly referred to as metaphylaxis. Metaphylaxis has proven to be an effective means of reducing BRD risk (Nickell and White, 2010). However, recent studies have shown an increase in the proportion of M. haemolytica isolates resistant to multiple classes of antimicrobials isolated from high-risk stocker calves after arrival processing (Snyder et al., 2017). This work documenting a high prevalence of multidrug resistance (MDR) used long-acting macrolides. These drugs are popular because a single administration results in sustained concentrations in the lungs for several days (Nowakowski et al., 2004; Giguère et al., 2011; Menge et al., 2012). However, these drugs persist in the respiratory tract at subtherapeutic concentrations and sustained exposure of bacteria to low concentrations might select for resistant bacteria (Foster et al., 2016). A short-acting, concentration-dependent drug that does not persist at subtherapeutic concentration might be as effective as long-acting macrolides to control BRD while being less likely to select for antimicrobial resistance. We hypothesized that the administration of enrofloxacin to control BRD in high-risk calves would be less likely to select for MDR strains of M. haemolytica compared with calves administered tulathromycin. The objectives of this study were 2-fold: first, to determine the comparative efficacy of enrofloxacin and tulathromycin for the control of BRD in calves at high risk of developing respiratory tract disease, and second, to determine the prevalence of MDR M. haemolytica in cattle at arrival and revaccination 14 to 17 d after metaphylactic administration of enrofloxacin or tulathromycin at arrival processing.
MATERIALS AND METHODS
This study and all procedures were approved by the Clinical Research Committee of the University of Georgia College of Veterinary Medicine.
Animals, Housing, and Initial Processing
These studies were performed at a private stocker facility in central Georgia. Calves arriving at the operation between March 26 and April 29, 2017 were enrolled in the study and were followed for a period of 45 d to determine health outcomes. Client consent was obtained before beginning the trial and, as part of the consent agreement, the client was allowed to withdraw from participation in the trial at any time point. All calves were acquired from livestock auction markets in north and central Georgia. After arrival at the stocker facility, calves were provided with bedding, access to water and coastal bermudagrass hay, and allowed to rest overnight until processing the next morning. As part of the arrival processing protocol, calves received an autogenous multivalent modified live viral respiratory vaccine containing BHV-1, PI3, BVDV, and BRSV (Nold Animal Supply, Gettysburg, SD) and an autogenous inactivated bacterial respiratory vaccine containing M. haemolytica, Pasteurella multocida, Streptococcus durans, Histophilus somni, and Mycoplasma bovis (Nold Animal Supply, Gettysburg, SD). Calves were treated for external and internal parasites with topical moxidectin (Cydectin Pour-On, Boehringer-Ingelheim Vetmedica, St. Joseph, MO) and fenbendazole oral paste (Safe-Guard, Merck, Millsboro, DE). Calves also received an injectable multimineral supplement (Multimin 90, Multimin North America, Fort Collins, CO), they were implanted with Revalor G (Merck Animal Health, Madison, NJ), and they received an ear notch to determine BVD persistent infection status (PI, IDEXX Laboratories, Westbrook, ME). Bull calves were castrated at arrival using the California banding method (InoSol, El Centro, CA), and given tetanus antitoxin (Colorado Serum, Denver, CO). The arrival processing protocol was in place prior to beginning the study and was not changed for the purposes of data collection.
After arrival processing, calves were moved to holding pens specific to their assigned treatment group and given access to free-choice coastal bermudagrass hay (8% CP, 31.1% crude fiber (CF), and 55.6% TDN on a DM basis) and water. Each pen was filled with calves over the course of 1 wk, and calf groups were subsequently moved to larger coastal bermudagrass and fescue pastures at the end of the week. They were also provided a supplement consisting of 49.5% corn gluten feed, 49.5% soyhulls (18.7% CP, 15.1% CF, and 79.4% TDN on a DM basis), and added mineral. Calves were fed to an estimated intake of 4.5 kg/hd/d of the supplement for the duration of the study. The initial enrollment pen groupings were maintained throughout the follow-up period, with 4 replicate pens being enrolled for each treatment group.
Estimating the Cumulative Incidence of Bovine Respiratory Disease
This trial was designed as a randomized, double-blinded, clinical trial to evaluate the efficacy of enrofloxacin relative to tulathromycin. At the time of each day’s processing, calves were randomly assigned to receive either enrofloxacin (Baytril 100, Bayer Animal Health, Shawnee Mission, KS) at its label dose (7.5 mg/kg SQ once) or tulathromycin (Draxxin, Zoetis Animal Health, Florham Park, NJ) at its label dose (2.5 mg/kg SQ once) to control BRD using a random number generator in a commercially available spreadsheet program (Excel, Microsoft, Redmond, WA). Both study personnel and farm staff were blinded to treatments, and metaphylaxis was administered at the time of arrival processing for each group. After arrival processing and metaphylactic antimicrobial administration, cattle were sorted and housed in group pens according to the assigned treatment. Cattle in each treatment group were housed in separate pens to prevent transmission of contagious pathogens and were monitored for the development of signs consistent with BRD for 45 d after processing using visual observation of clinical signs. Cattle displaying signs consistent with BRD (DART [depression, lethargy, decreased gut fill, increased respiratory rate, ocular and nasal discharge]) were examined in a chute and had a rectal temperature taken (Wilson et al., 2015). Any animal with a rectal temperature > 104°F in conjunction with characteristic clinical signs was considered a clinical case and was treated per standard farm protocols with florfenicol (40 mg/kg SQ once, Nuflor Gold, Merck Animal Health, Madison, NJ). No postmetaphylactic interval was used in this study for calves assigned to either group to ensure study protocol compliance and maintain blinding. For an average pen size of 40 calves, it was determined that 6 replicate pens per group would be required to provide a power of 80% to detect a difference in BRD incidence of 25% in 1 group vs. 40% in the other group. This a priori sample size calculation assumed a type I error probability of 5%, a 2-sided alternative hypothesis, and an intra-pen correlation of 0.01 (PASS 15.0.1, NCSS LLC, Kaysville, UT). As noted below, the desired sample size of 6 replicate pens per group was not achieved because the owner of the stocker operation elected to terminate the study.
Estimating the Relative Effects of Enrofloxacin and Tulathromycin on the Prevalence of Antimicrobial Resistance in M. haemolytica
Assuming that M. haemolytica would be isolated from approximately 60% of cattle at resampling, it was determined that a sample size of 8 calves per pen would be required from each of 6 pens per treatment group to achieve a power of 80% for detecting a difference of 60% vs. 90% in the proportions of calves with MDR M. haemolytica isolates. Thus, each day that samples were acquired at processing, a subset of 8 calves was selected for nasal swab collection using a random number generator application (Random.org iOS app, version 1.1.2, Randomness and Integrity Services, Dublin, Ireland). Arrival processing samples were collected 2 to 3 d per week, depending on the operation’s receiving schedule.
Calves that were selected for sampling by deep nasopharyngeal swab (DNP) were restrained with a rope halter securing the head to the left side of the chute for safe and proper sample collection. A double guarded dry cotton swab (Continental Plastic, Delavan, WI) was inserted ventromedially into one nostril. On entrance into the nasopharynx, the swab was advanced through both sheaths, the cotton tip was swirled, and then retracted back into the sheaths prior to removal from the airway. The external sheath was discarded, the swab was sealed with provided caps, labeled with the calf’s unique identification number, and placed in a cooler on ice. Samples were immediately transported to the University of Georgia Athens Veterinary Diagnostic Laboratory after collection for aerobic culture and antimicrobial susceptibility testing. Calves that were swabbed were uniquely identified with a tag applied in the left ear. A second DNP was collected from each calf sampled at arrival processing at the time of revaccination 14 to 17 d later. If any calves that were selected for nasopharyngeal swabs were treated prior to revaccination, then the revaccination sample was collected prior to treatment.
Laboratory Procedures
The dry nasal swabs were transported to the diagnostic laboratory within 4 h after the beginning of sample collection. Each swab was soaked in sterile phosphate-buffered saline, and a drop of this saline was streaked onto blood and chocolate agar plates for aerobic culture. Matrix-assisted laser desorption ionization time of flight mass spectrometry (MALDI-TOF MS, bioMerieux Vitek, Durham, NC) was utilized to identify M. haemolytica isolates after 24 h of incubation. The manufacturer’s spectral database was used to compare the spectra of each isolate. Isolates that were identified as M. haemolytica with 99.9% or greater confidence were selected for inclusion in the study. Once the identity of the colonies had been confirmed for each individual bacterial isolate, 3 colonies were selected for Kirby–Bauer disk diffusion antimicrobial susceptibility testing. All isolates were grown to a 0.5 McFarland standard in Müeller–Hinton broth and plated on Müeller–Hinton agar supplemented with 5% sheep blood. The plates were incubated at 37°C in ambient air for 18 to 24 h prior to interpretation. The following antimicrobials were evaluated: ceftiofur, enrofloxacin, florfenicol, gamithromycin, tilmicosin, and tulathromycin. For each antimicrobial agent, isolates were characterized as susceptible, intermediate, or resistant according to the guidelines established by the Clinical Laboratory Standards Institute. Staphylococcus aureus ATCC 25923 and Escherichia coli ATCC 25922 were used for quality control, which was performed on a weekly basis. For the purposes of this study, MDR was defined as resistance to ≥2 antimicrobial classes (Snyder et al., 2017).
Statistical Analyses
A chi-square test of independence was used to assess the comparability of treatment groups with respect to calf sex and week of arrival at the stocker facility. A multilevel logistic regression model with random effects for pen and calf was used to evaluate the fixed effects of treatment group, sampling occasion, and the treatment by sampling occasion interaction, on the prevalence of M. haemolytica isolated from paired deep nasopharyngeal swabs collected from a randomly selected subset of calves at the time of arrival and at revaccination. Mixed logistic regression models with pen as a random effect were used to compare the treatment groups with respect to the proportions of calves that required at least one treatment for BRD, the proportions of calves that required more than 1 treatment for BRD, and the proportions of calves that died within 45 d of arrival. A Cox proportional hazards model with a shared pen frailty was used to compare treatment groups with respect to the time from arrival of calves until their first BRD treatment. The proportional hazards assumption was graphically assessed by plotting ln{−ln(survival)} curves and by using a test based on the scaled Shoenfeld residuals. Mixed logistic regression models with pen as a random effect were also used to compare treatment groups with respect to the proportions of calves having M. haemolytica isolates that were classified as susceptible to each antibiotic. All tests assumed a 2-sided alternative hypothesis, and P < 0.05 was considered statistically significant. Analyses were performed using commercially available software (Stata version 14.2, StataCorp LP, College Station, TX).
RESULTS
Population Characteristics
Due to the perception that the use of metaphylactic antimicrobial therapy increased the proportion of cattle with BRD requiring multiple treatments relative to before the beginning of the study, the producer elected to stop participation before enrollment of all animals was complete. Therefore, 341 stocker calves were enrolled between March 26 and April 29, 2017. Calves were grouped into 4 pens per treatment based on the week of enrollment. All of the enrolled calves were male; approximately 86% were bulls at the time of arrival, and 14% had already been castrated. The 2 treatment groups were similar with respect to the distributions of calf sex and week of arrival at the stocker facility (Table 1). Individual calf weights were not recorded, but mean calf weights for the truckloads of calves that were enrolled in the study ranged from 516 to 559 pounds, with an average of 535 pounds.
Table 1.
Distribution of 341 calves enrolled in a randomized trial of 2 metaphylactic antimicrobial treatments by sex and week of arrival at a Georgia stocker facility
| Variable | Treatment | |||
|---|---|---|---|---|
| Enrofloxacin | Tulathromycin | Total | P* | |
| Sex | ||||
| Bull | 151 (87.8) | 142 (84.0) | 293 (85.9) | 0.317 |
| Steer | 21 (12.2) | 27 (16.0) | 48 (14.1) | |
| Arrival week | ||||
| March 26–April 1 | 41 (23.8) | 38 (22.5) | 79 (23.2) | 0.698 |
| April 2–8 | 43 (25.0) | 35 (20.7) | 78 (22.9) | |
| April 9–15 | 54 (31.4) | 56 (33.1) | 110 (32.3) | |
| April 23–29 | 34 (19.8) | 40 (23.7) | 74 (21.7) | |
*Chi-square test of independence.
Cumulative Incidence of Bovine Respiratory Disease
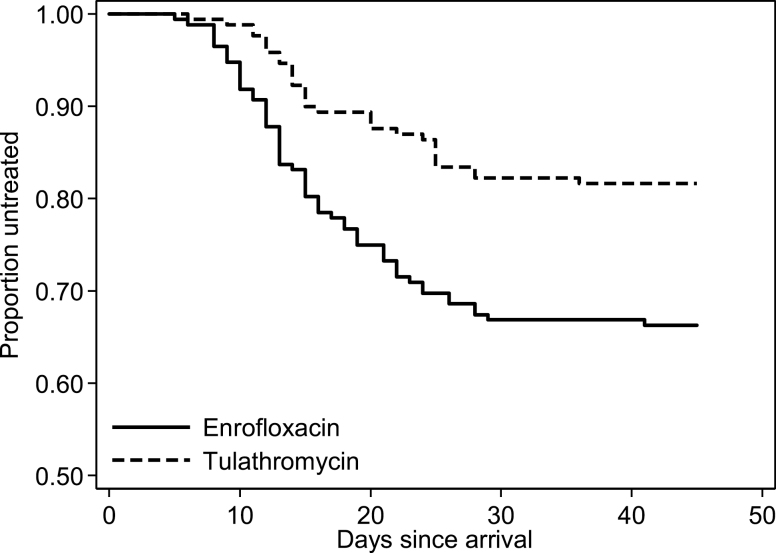
Fifty-eight (33.7%) of 172 calves receiving enrofloxacin required treatment for BRD at least once within 45 d after arrival, compared with 31 (18.3%) of 169 calves receiving tulathromycin. Percentages of calves in the 4 enrofloxacin-treated pens that required treatment for BRD by week of arrival were as follows: 24.4%, 25.6%, 29.6%, and 61.8%. Percentages of calves in the 4 tulathromycin-treated pens that required treatment for BRD by week of arrival were as follows: 13.2%, 14.3%, 14.3%, and 32.5%, respectively. When using a mixed logistic regression model to compare the cumulative BRD incidences of the 2 groups, the odds of BRD were approximately 58% lower for calves receiving tulathromycin compared with those receiving enrofloxacin (odds ratio = 0.42 [95% confidence interval {CI}: 0.18, 0.96]; P = 0.040). Likewise, when using a Cox frailty model to compare the times to first BRD treatment, the hazard rate was significantly lower for calves receiving tulathromycin compared with those receiving enrofloxacin (hazard ratio = 0.45 [95% CI: 0.21, 0.94]; P = 0.034). The times from arrival until first treatment for BRD are illustrated for both treatment groups in Figure 1.
Figure 1.
Kaplan–Meier survival curves depicting the time from arrival until treatment for BRD in 341 calves receiving 1 of 2 metaphylactic antimicrobial treatments at a Georgia stocker facility.
Eighteen (10.5%) of 172 calves receiving enrofloxacin required more than one treatment for BRD within 45 d after arrival, compared with 8 (4.7%) of 169 calves receiving tulathromycin. Percentages of calves in the 4 enrofloxacin-treated pens that required more than one treatment for BRD by week of arrival were: 12.2%, 4.7%, 5.6%, and 23.5%. Percentages of calves in the 4 tulathromycin-treated pens that required more than 1 treatment for BRD by week of arrival were as follows: 2.6%, 0.0%, 5.4%, and 10.0%. The odds of requiring a second BRD treatment were approximately 60% lower for calves receiving tulathromycin compared with those receiving enrofloxacin, but this was not statistically significant (odds ratio = 0.40 [95% CI: 0.13, 1.22]; P = 0.107).
Twenty-one (12.2%) calves receiving enrofloxacin died during the 45-d follow-up period, compared with 17 (10.1%) calves receiving tulathromycin. Percentages of calves in the 4 enrofloxacin-treated pens that died during the follow-up period by week of arrival were as follows: 7.3%, 11.6%, 7.4%, and 26.5%. Percentages of calves in the 4 tulathromycin-treated pens that died during the follow-up period by week of arrival were as follows: 5.3%, 8.6%, 7.1%, and 20.0%. The odds of death were approximately 22% lower for tulathromycin compared with enrofloxacin, but this was not statistically significant (odds ratio = 0.78 [95% CI: 0.32, 1.92]; P = 0.592).
Relative Effects of Enrofloxacin and Tulathromycin on the Prevalence of Antimicrobial Resistance in M. haemolytica
Deep nasopharyngeal swabs were collected from a random subset of 72 calves at the time of arrival, and repeat samples were collected from 70 of these same calves at the time of revaccination approximately 2 wk later. The mean numbers of days between the first and second sampling occasions were 15.6 d (SD = 1.8 d) and 15.8 d (SD = 0.8 d) for calves receiving enrofloxacin and tulathromycin, respectively. For calves receiving enrofloxacin, the time between sampling occasions ranged from 6 to 17 d, although only 1 calf was sampled for the second time at 6-d postarrival; all other calves were sampled for the second time 15- to 17-d postarrival. For calves receiving tulathromycin, the time between sampling occasions ranged from 14 to 17 d.
In calves receiving enrofloxacin, M. haemolytica was cultured from 4 (11.4%) of 35 calves sampled at arrival and from 17 (51.5%) of 33 calves sampled at revaccination. In calves receiving tulathromycin, M. haemolytica was cultured from 4 (10.8%) of 37 calves sampled at arrival and from 18 (48.7%) of 37 calves sampled at revaccination. In a multilevel logistic regression model with random effects for pen and calf, there was no significant effect of treatment (P = 0.842), and no significant interaction between treatment and sampling occasion (P = 0.953), but there was a significant effect of sampling occasion (P < 0.001), with calves from both groups having higher M. haemolytica prevalences at revaccination than at arrival. Mannheimia haemolytica isolates obtained from 7 of the 8 calves with a positive culture result at the time of arrival were susceptible to all 6 of the antimicrobials evaluated, whereas the remaining calf, which was assigned to treatment with enrofloxacin, had an isolate that was resistant to enrofloxacin, florfenicol, gamithromycin, tilmicosin, and tulathromycin. The antimicrobial susceptibility of M. haemolytica isolates at revaccination is summarized by treatment group in Table 2. All of the M. haemolytica isolates obtained from both treatment groups at revaccination were susceptible to ceftiofur, and all were classified as intermediate or resistant to enrofloxacin, gamithromycin, tilmicosin, and tulathromycin. Only the susceptibility to florfenicol varied, with 8 (47.1%) of 17 calves that received enrofloxacin that had M. haemolytica isolated at the time of revaccination having an isolate that was identified as resistant to florfenicol, compared with 16 (88.9%) of 18 calves that received tulathromycin. This apparent difference between treatments with respect to florfenicol susceptibility was not statistically significant, however, after adjusting for the clustering of calves within pens (P = 0.241). The intra-pen correlation for florfenicol resistance was 0.84, indicating that isolates obtained from calves within the same pen had a high level of similarity with respect to this resistance phenotype.
Table 2.
Summary of antimicrobial susceptibility testing results for Mannheimia haemolytica isolates collected from 35 stocker calves at the time of revaccination after metaphylactic treatment with enrofloxacin (ENR) or tulathromycin (TUL)
| Antimicrobial | Treatment | Number of calves (%) | P* | |
|---|---|---|---|---|
| Susceptible | Intermediate/resistant | |||
| Ceftiofur | ENR | 17 (100) | 0 (0.0) | 1.00 |
| TUL | 18 (100) | 0 (0.0) | ||
| Enrofloxacin | ENR | 0 (0.0) | 17 (100) | 1.00 |
| TUL | 0 (0.0) | 18 (100) | ||
| Florfenicol | ENR | 9 (52.9) | 8 (47.1) | 0.241 |
| TUL | 2 (11.1) | 16 (88.9) | ||
| Gamithromycin | ENR | 0 (0.0) | 17 (100) | 1.00 |
| TUL | 0 (0.0) | 18 (100) | ||
| Tilmicosin | ENR | 0 (0.0) | 17 (100) | 1.00 |
| TUL | 0 (0.0) | 18 (100) | ||
| Tulathromycin | ENR | 0 (0.0) | 17 (100) | 1.00 |
| TUL | 0 (0.0) | 18 (100) | ||
Calves were counted as having an intermediate or resistant isolate if any of the 1–3 colonies evaluated for each calf was classified as intermediate or resistant.
*Test of the treatment effect from a mixed logistic regression model with pen as a random effect.
There were too few calves with M. haemolytica isolated at arrival (n = 4 per group) to make separate comparisons of the change in antimicrobial susceptibility patterns between arrival and revaccination for each treatment group. If the calves from both treatment groups were combined, there was a significant increase in the percentage of calves having isolates that were resistant to enrofloxacin (P < 0.001), florfenicol (P = 0.048), gamithromycin (P < 0.001), tilmicosin (P < 0.001), and tulathromycin (P < 0.001), between the arrival and 14-d postarrival sampling occasions. None of the calves had an M. haemolytica isolate that was classified as resistant to ceftiofur on either sampling occasion (P = 1.00).
Antimicrobial susceptibility was evaluated for 1 to 3 (mean = 2.9) M. haemolytica colonies from each calf with a positive culture result. Only 2 calves had colonies with discrepant susceptibility testing results: one calf sampled at revaccination had 2 colonies that were classified as susceptible to florfenicol and 1 colony that was classified as resistant; another calf sampled at revaccination had 1 colony that was classified as intermediate for gamithromycin and 2 colonies that were classified as resistant. For all the remaining calves, multiple colonies obtained from the same calf on the same sampling occasion yielded identical susceptibility testing results. Only 1 colony was classified as having an intermediate susceptibility for any antimicrobial; all others were classified as either susceptible or resistant.
DISCUSSION
Bovine respiratory disease is the most common cause of morbidity and mortality in North American beef cattle. Stocker calves are classically considered to be at particularly high risk of developing BRD relative to calves in other segments of the beef industry due to the fact that they are often light-weight, unweaned, commingled, and of unknown health status. As a result, it is not unusual for more than 50% of calves in a stocker facility to be diagnosed with BRD at some point following arrival (Richeson et al., 2009). Stocker calves diagnosed with BRD have reduced average daily gains, poorer carcass quality, and an increased risk of mortality relative to cattle not diagnosed with BRD. Indeed, studies have shown that BRD can reduce per animal returns by 10% to 20% (Pinchak et al., 2004).
Because BRD is such a common occurrence in stocker calf populations, many producers and veterinarians administer antimicrobials at the time of arrival processing to reduce morbidity and mortality associated with the disease, a practice termed metaphylaxis (Nickell and White, 2010; Ives and Richeson, 2015). Metaphylaxis has been shown to be effective in reducing morbidity and mortality risk associated with BRD and, when used appropriately, can positively affect economic returns to the farm (Nickell and White, 2010; Tennant et al., 2014). Although multiple antimicrobials are labeled for metaphylaxis, tulathromycin is one of the most frequently used compounds currently available. A recent mixed treatment meta-analysis comparing efficacy of antimicrobials commonly used for metaphylaxis in cattle populations found that tulathromycin was superior to many of the other antimicrobials (ceftiofur, oxytetracycline, florfenicol, trimethoprim-sulfamethoxazole) evaluated for reducing BRD morbidity in the first 60 d on feed (Abell et al., 2017). Unfortunately, studies have shown a high prevalence of shedding of MDR M. haemolytica in stocker cattle following metaphylaxis with tulathromycin (Snyder et al., 2017).
The ultimate goal of the research reported here is to improve the health and welfare of high-risk stocker cattle through the development of evidence-based and judicious antimicrobial use practices that would decrease the rate of emergence of antimicrobial resistance in BRD pathogens while maintaining therapeutic efficacy. Many of the studies documenting MDR in M. haemolytica evaluate long-acting macrolide antimicrobials. These drugs are popular and convenient because a single administration results in sustained therapeutic concentrations at the site of infection for several days (Nowakowski et al., 2004; Giguère et al., 2011). However, this apparent pharmacokinetic advantage might contribute to development of resistance. As a result of their long elimination half-life, these drugs persist in the respiratory tract at potentially subtherapeutic concentrations (Foster et al., 2016). Recent work has shown that unbound concentrations of tulathromycin in pulmonary epithelial lining fluid of healthy cattle are below the minimum inhibitory concentration (MIC) of susceptible M. haemolytica at almost all time points after administration (Foster et al., 2016). In addition, there is a long tail of subtherapeutic drug exposure that persists for more than 12 d. The primary concern with this is that sustained exposure of bacteria to persistently low antimicrobial concentrations might select for resistant bacterial strains (Martinez et al., 2012).
Enrofloxacin is a fluoroquinolone antimicrobial labeled for the control and treatment of BRD in cattle. Recent meta-analysis data have shown enrofloxacin to have similar efficacy to tulathromycin for treating cattle with BRD (O’Connor et al., 2016). In addition, enrofloxacin has a much shorter elimination half-life in respiratory secretions (12 vs. 330 h) (Foster et al., 2016). Furthermore, enrofloxacin is a concentration-dependent antimicrobial (Drusano, 2004). This means that the duration of time that unbound drug concentrations are above the MIC of the infecting pathogen is not the primary determinant of drug efficacy. As a concentration-dependent drug, enrofloxacin’s efficacy is best determined by the ratio of area under the concentration over time curve (AUC)/MIC. As a general rule of thumb, achieving an AUC/MIC of >125 is necessary to optimize therapeutic response and suppress resistance when considering fluoroquinolones and Gram-negative pathogens (Drusano, 2004, 2007). Therefore, enrofloxacin was, theoretically, a more favorable antimicrobial from the standpoint of lower risk of selection pressure for the development of antimicrobial resistance. Unfortunately, the results of our study do not support this hypothesis. The prevalence of MDR M. haemolytica at revaccination in cattle receiving metaphylactic enrofloxacin was no different from the prevalence of MDR M. haemolytica in cattle that received metaphylactic tulathromycin. One of the reasons for this finding might be that the labeled metaphylactic dose of enrofloxacin (7.5 mg/kg SQ once) does not approach the optimal AUC/MIC ratio of 125. Studies have shown that, at this dose, the maximum AUC/MIC ratio that can be achieved in pulmonary epithelial lining fluid (PELF) is approximately 40 (Foster et al., 2016). At this magnitude of exposure, it is likely that MDR clones of M. haemolytica are being selected for and allowed to proliferate. Work from the same group has found that, when enrofloxacin is administered at a dose of 12.5 mg/kg SQ, the AUC/MIC ratio in PELF exceeds 400 (Foster et al., 2017). Although the significance of this difference in magnitude of exposure is not clear, work evaluating the impact of the higher dose of enrofloxacin on selection of MDR M. haemolytica is necessary. It is important to note, however, that the extra-label use of fluoroquinolones in food-producing species is prohibited by the US Food and Drug Administration and the use of enrofloxacin for control of BRD at a dose higher than 7.5 mg/kg is considered illegal and cannot be recommended.
In this study, the prevalence of shedding of M. haemolytica increased from arrival to revaccination in both treatment groups, with approximately 11% of calves shedding M. haemolytica at arrival and 50% of calves shedding M. haemolytica at revaccination. This finding is consistent with other work in similar populations of cattle (Snyder et al., 2017). Although the exact reasons for the increase in shedding of M. haemolytica between the 2 time points, despite the administration of antimicrobials, is not known, potential explanations include chronic stress and immune suppression. In addition to the increase in the prevalence of M. haemolytica shedding, the proportion of isolates classified as MDR increased between the time of arrival and revaccination, with more than 99% of M. haemolytica isolates being classified as MDR at the second sampling point. One of the primary limitations of the study reported here was the lack of an untreated control group. The lack of an untreated population of high-risk calves makes determining the reasons why the prevalence of resistance increases between arrival and revaccination impossible. Future studies using an untreated control group are needed to better assess the influence of metaphylaxis on MDR in M. haemolytica.
The high intra-pen correlation for florfenicol resistance suggests a high level of similarity of isolates in individual calves and contagious spread of M. haemolytica between calves within a given pen (Briggs et al., 1998). Although this phenomenon is not well understood, our findings are similar to those of Noyes et al. (2015). Traditionally, M. haemolytica has been thought to be a normal commensal of the upper respiratory tract of cattle. When cattle are stressed or infected with respiratory viruses, this commensal pathogen is allowed to colonize the lower airway and cause disease (Griffin et al., 2010). This work would suggest that MDR M. haemolytica can spread from calf to calf within a pen and is not restricted to a single animal (Briggs et al., 1998). Although the exact significance of this finding is not currently clear, spread of multiply resistant clones can disseminate antimicrobial resistance determinants and facilitate spread of MDR M. haemolytica to calves not even exposed to antimicrobials.
Numerous studies have been done comparing efficacy of metaphylactic administration of tulathromycin to other antimicrobials in the control of BRD in feedlot cattle; however, there is very limited data that pertains to stocker cattle (Godinho et al., 2005; Skogerboe et al., 2005; Booker et al., 2007; Tennant et al., 2014). The results of our study are consistent with the work of others showing that tulathromycin is superior to other antimicrobials for controlling BRD (Skogerboe et al., 2005; Booker et al., 2007; Van Donkersgoed et al., 2008; Tennant et al., 2014; Abell et al., 2017). Significantly, fewer calves that received tulathromycin required 1 treatment for BRD during the first 45 d after arrival.
The stocker operation’s decision to terminate the study early led to the enrollment of a smaller number of calves than originally planned. This was not only unfortunate but also unavoidable. Having a smaller sample size likely reduced the power of the study to identify differences between the groups, although the difference in BRD treatment incidences was still found to be statistically significant despite the reduced sample size. There was no difference between groups with respect to the percentages of MDR M. haemolytica isolates observed at the time of revaccination; 100% of the isolates from both groups were found to be MDR. Consequently, it seems unlikely that even if the original enrollment targets had been met that a significant difference would have been observed with respect to the prevalence of MDR M. haemolytica isolates.
In conclusion, the study reported here demonstrates that tulathromycin was superior to enrofloxacin for the control of BRD in high-risk stocker cattle under the management conditions used in this study. In addition, this study found an increase in the prevalence of shedding of M. haemolytica between arrival and resampling approximately 2 wk later in calves in both treatment groups. Also, and of most importance, we demonstrated an increase in the prevalence of MDR M. haemolytica isolates between the 2 sampling time points in calves of both treatment groups. An association between metaphylactic antimicrobial administration and MDR could not be demonstrated due to the study design and lack of an untreated control group, as having an untreated group was not feasible due to the fact that this was a commercial operation with privately owned cattle. Future studies with untreated groups should be performed to evaluate associations between mass antimicrobial administration and MDR M. haemolytica. In addition, studies evaluating nonantimicrobial methods of BRD control in high-risk cattle populations are warranted due to the apparent increase in prevalence of antimicrobial resistance being seen in M. haemolytica isolates from across the United States.
Footnotes
The research reported here was supported by Bayer Animal Health and USDA Animal Health Capacity Funds.
LITERATURE CITED
- Abell K. M., Theurer M. E., Larson R. L., White B. J., and Apley M.. 2017. A mixed treatment comparison meta-analysis of metaphylaxis treatments for bovine respiratory disease in beef cattle. J. Anim. Sci. 95:626–635. doi:10.2527/jas.2016.1062 [DOI] [PubMed] [Google Scholar]
- Booker C. W., Abutarbush S. M., Schunicht O. C., Jim G. K., Perrett T., Wildman B. K., Guichon P. T., Pittman T. J., Jones C., and Pollock C. M.. 2007. Evaluation of the efficacy of tulathromycin as a metaphylactic antimicrobial in feedlot calves. Vet. Ther. 8:183–200. [PubMed] [Google Scholar]
- Briggs R. E., Frank G. H., Purdy C. W., Zehr E. S., and Loan R. W.. 1998. Rapid spread of a unique strain of Pasteurella haemolytica serotype 1 among transported calves. Am. J. Vet. Res. 59:401–405. [PubMed] [Google Scholar]
- Drusano G. L. 2004. Antimicrobial pharmacodynamics: Critical interactions of ‘bug and drug’. Nat. Rev. Microbiol. 2:289–300. doi:10.1038/nrmicro862 [DOI] [PubMed] [Google Scholar]
- Drusano G. L. 2007. Pharmacokinetics and pharmacodynamics of antimicrobials. Clin. Infect. Dis. 45(Suppl 1):S89–S95. doi:10.1086/518137 [DOI] [PubMed] [Google Scholar]
- Duff G. C. and Galyean M. L.. 2007. Board-invited review: Recent advances in management of highly stressed, newly received feedlot cattle. J. Anim. Sci. 85:823–840. doi:10.2527/jas.2006-501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster D. M., Martin L. G., and Papich M. G.. 2016. Comparison of active drug concentrations in the pulmonary epithelial lining fluid and interstitial fluid of calves injected with enrofloxacin, florfenicol, ceftiofur, or tulathromycin. PLoS One 11:e0149100. doi:10.1371/journal.pone.0149100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster D. M., Sylvester H. J., and Papich M. G.. 2017. Comparison of direct sampling and brochoalveolar lavage for determining active drug concentrations in the pulmonary epithelial lining fluid of calves injected with enrofloxacin or tilmicosin. J. Vet. Pharmacol. Ther. 40:e45–e53. doi:10.1111/jvp.12412 [DOI] [PubMed] [Google Scholar]
- Giguère S., Huang R., Malinski T. J., Dorr P. M., Tessman R. K., and Somerville B. A.. 2011. Disposition of gamithromycin in plasma, pulmonary epithelial lining fluid, bronchoalveolar cells, and lung tissue in cattle. Am. J. Vet. Res. 72:326–330. doi:10.2460/ajvr.72.3.326 [DOI] [PubMed] [Google Scholar]
- Godinho K. S., Wolf R. M., Sherington J., Rowan T. G., Sunderland S. J., and Evans N. A.. 2005. Efficacy of tulathromycin in the treatment and prevention of natural outbreaks of bovine respiratory disease in European cattle. Vet. Ther. 6:122–135. [PubMed] [Google Scholar]
- Griffin D., Chengappa M. M., Kuszak J., and McVey D. S.. 2010. Bacterial pathogens of the bovine respiratory disease complex. Vet. Clin. North Am. Food Anim. Pract. 26:381–394. doi:10.1016/j.cvfa.2010.04.004 [DOI] [PubMed] [Google Scholar]
- Ives S. E. and Richeson J. T.. 2015. Use of antimicrobial metaphylaxis for the control of bovine respiratory disease in high-risk cattle. Vet. Clin. North Am. Food Anim. Pract. 31:341–50. doi:10.1016/j.cvfa.2015.05.008 [DOI] [PubMed] [Google Scholar]
- Martinez M. N., Papich M. G., and Drusano G. L.. 2012. Dosing regimen matters: The importance of early intervention and rapid attainment of the pharmacokinetic/pharmacodynamic target. Antimicrob. Agents Chemother. 56:2795–2805. doi:10.1128/AAC.05360-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menge M., Rose M., Bohland C., Zschiesche E., Kilp S., Metz W., Allan M., Röpke R., and Nürnberger M.. 2012. Pharmacokinetics of tildipirosin in bovine plasma, lung tissue, and bronchial fluid (from live, nonanesthetized cattle). J. Vet. Pharmacol. Ther. 35:550–559. doi:10.1111/j.1365-2885.2011.01349.x [DOI] [PubMed] [Google Scholar]
- Nickell J. S. and White B. J.. 2010. Metaphylactic antimicrobial therapy for bovine respiratory disease in stocker and feedlot cattle. Vet. Clin. North Am. Food Anim. Pract. 26:285–301. doi:10.1016/j.cvfa.2010.04.006 [DOI] [PubMed] [Google Scholar]
- Nowakowski M. A., Inskeep P. B., Risk J. E., Skogerboe T. L., Benchaoui H. A., Meinert T. R., Sherington J., and Sunderland S. J.. 2004. Pharmacokinetics and lung tissue concentrations of tulathromycin, a new triamilide antibiotic, in cattle. Vet. Ther. 5:60–74. [PubMed] [Google Scholar]
- Noyes N. R., Benedict K. M., Gow S. P., Booker C. W., Hannon S. J., McAllister T. A., and Morley P. S.. 2015. Mannheimia haemolytica in feedlot cattle: Prevalence of recovery and associations with antimicrobial use, resistance, and health outcomes. J. Vet. Intern. Med. 29:705–713. doi:10.1111/jvim.12547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor A. M., Yuan C., Cullen J. N., Coetzee J. F., da Silva N., and Wang C.. 2016. A mixed treatment meta-analysis of antibiotic treatment options for bovine respiratory disease – an update. Prev. Vet. Med. 132:130–139. doi:10.1016/j.prevetmed.2016.07.003 [DOI] [PubMed] [Google Scholar]
- Pinchak W. E., Tolleson D. R., McCloy M., Hunt L. J., Gill R. J., Ansley R. J., and Bevers S. J.. 2004. Morbidity effects on productivity and profitability of stocker cattle grazing in the southern plains. J. Anim. Sci. 82:2773–2779. doi:10.2527/2004.8292773x [DOI] [PubMed] [Google Scholar]
- Richeson J. T., Kegley E. B., Gadberry M. S., Beck P. A., Powell J. G., and Jones C. A.. 2009. Effects of on-arrival versus delayed clostridial or modified live respiratory vaccinations on health, performance, bovine viral diarrhea virus type I titers, and stress and immune measures of newly received beef calves. J. Anim. Sci. 87:2409–2418. doi:10.2527/jas.2008-1484 [DOI] [PubMed] [Google Scholar]
- Skogerboe T. L., Rooney K. A., Nutsch R. G., Weigel D. J., Gajewski K., and Kilgore W. R.. 2005. Comparative efficacy of tulathromycin versus florfenicol and tilmicosin against undifferentiated bovine respiratory disease in feedlot cattle. Vet. Ther. 6:180–196. [PubMed] [Google Scholar]
- Snyder E., Credille B., Berghaus R., and Giguère S.. 2017. Prevalence of multi drug antimicrobial resistance in isolated from high-risk stocker cattle at arrival and two weeks after processing. J. Anim. Sci. 95:1124–1131. doi:10.2527/jas.2016.1110 [DOI] [PubMed] [Google Scholar]
- Tennant T. C., Ives S. E., Harper L. B., Renter D. G., and Lawrence T. E.. 2014. Comparison of tulathromycin and tilmicosin on the prevalence and severity of bovine respiratory disease in feedlot cattle in association with feedlot performance, carcass characteristics, and economic factors. J. Anim. Sci. 92:5203–5213. doi:10.2527/jas.2014-7814 [DOI] [PubMed] [Google Scholar]
- Van Donkersgoed J., Merrill J., and Hendrick S.. 2008. Comparative efficacy of tilmicosin versus tulathromycin as a metaphylactic antimicrobial in feedlot calves at moderate risk for respiratory disease. Vet. Ther. 9:291–297. [PubMed] [Google Scholar]
- Wilson B. K., Step D. L., Maxwell C. L., Wagner J. J., Richards C. J., and Krehbiel C. R.. 2015. Evaluation of multiple ancillary therapies used in combination with an antimicrobial in newly received high-risk calves treated for bovine respiratory disease. J. Anim. Sci. 93:3661–3674. doi:10.2527/jas.2015-9023 [DOI] [PMC free article] [PubMed] [Google Scholar]