Abstract
Objectives were to document effects of the Texel myostatin mutation (MSTN) on growth and carcass traits and also test whether or not interactions with the callipyge mutation (CLPG) could be detected. Twelve rams heterozygous at both loci on the two different chromosomes were mated to 215 terminal-sire type composite crossbred ewes genotyped as non-carriers for both loci. A total of 365 lambs were born, 362 of those were genotyped and 236 lambs contributed carcass data to estimate effects and interactions among the four genotype combinations produced. The four genotype combinations were defined as follows: ++/++ for wild-type at both loci; ++/C+ for wild-type at MSTN and heterozygous at CLPG; M+/++ for heterozygous at MSTN and wild-type at CLPG; and M+/C+ for heterozygous at both loci. The two independently segregating sire-derived alleles represent different breed-of-origin contrasts at each locus (Texel vs. composite origin for MSTN and Dorset vs. Texel origin for CLPG). Birth weight was recorded on all lambs, and subsequent body weights were adjusted to 56 (weaning), 70, and 140 d of age. Within sire-sex-genotype subgroups, naturally reared lambs were assigned to one of eight slaughter groups accounting for variation in birth date. Lambs were serially slaughtered at weekly intervals, 30 lambs per group, from roughly 26 to 33 wk of age. In addition to standard carcass traits, subjective leg scores were assigned and widths of carcasses were measured at the widest points of the shoulder and rump. Differences in birth weight were detected (P < 0.01) for the combination of the two loci and birth type, with single-born differences among genotypes exceeding differences among twin born progeny. Those interaction differences among genotypes were not as important at weaning (P = 0.36). Impact on growth rate differences among the genotypes during the post-weaning period were variable and dependent on sex of the lamb (P < 0.01). A synergistic interaction between MSTN and CLPG was observed for leg muscling scores (P < 0.05) but no other measures of carcass shape were affected. One copy of MSTN had a more modest impact on fat deposition and muscle conformation than did CLPG and did not interact (all values P > 0.20). Although some non-additive interactions that vary by trait and sex were detected, in general the data are consistent with the two mutations acting on muscle growth through independent pathways.
Keywords: carcass, callipyge, growth, lamb, myostatin
INTRODUCTION
The callipyge mutation (CLPG) on ovine chromosome 18 (Freking et al., 2002; Smit et al., 2003) and myostatin (MSTN) on ovine chromosome 2 (Clop et al., 2006) are two known functional mutations that affect muscle development in sheep via two distinct signaling pathways. CLPG acts by altering the expression of delta-like homolog 1 (DLK1), a member of the EGF-like family of homeotic proteins involved with cell–cell communication. DLK1 has known roles in adipocyte differentiation and muscle development, interacting with the notch signaling pathway, and the influence of CLPG leads to hypertrophic growth of specific fiber types, with inheritance following a pattern of paternal over-dominance (White et al, 2008; Bidwell et al., 2014). In contrast, MSTN acts additively by reducing translation of myostatin protein (Clop et al., 2006), a member of the transforming growth factor-β super family of growth and differentiation factors acting through the SMAD signaling pathway. Myostatin functions as a negative regulator of muscle growth, and reduced protein levels lead to increased muscle growth primarily reflected in increased fiber number, in addition to fiber hypertrophy.
CLPG beneficial impacts on carcass composition have not been exploited by the sheep industry due to severe adverse effects on tenderness of lamb (Koohmaraie et al., 1995; Freking et al., 1999). MSTN effects on tenderness of lamb has been studied to a lesser degree (Johnson et al., 2005; Hope et al., 2013) with some muscles showing improved tenderness, whereas myostatin mutations generally improve beef tenderness (Arthur, 1995; Wheeler et al., 1996; Wheeler et al., 2001). It is not known if CLPG and MSTN genotypes interact with each other, generating epistatic effects for either muscle development or meat quality. We report the first results of an experiment designed to estimate MSTN effects on growth, carcass, and meat quality traits and to test for additivity or interactions of MSTN and CLPG in sheep.
MATERIALS AND METHODS
General Experimental Design
This study was conducted using standard production and experimental practices that were in accordance with the Guide for the Care and Use of Agricultural Animals in Agricultural Research and Teaching (FASS, 2010) and were approved by the U.S. Meat Animal Research Center (USMARC) Animal Care and Use committee. The design is a simple factorial arrangement of genotype combinations from two loci, with each lamb having 0 or 1 copies of MSTN and 0 or 1 copies of CLPG. Genotypes were defined as follows: ++/++ for wild-type at both loci; ++/C+ for wild-type at MSTN and heterozygous at CLPG; M+/++ for heterozygous at MSTN and wild-type at CLPG; and M+/C+ for heterozygous at both loci. The two independently segregating sire-derived alleles represent different breed-of-origin contrasts at each locus (Texel vs. composite origin for MSTN and Dorset vs. Texel origin for CLPG).
To be able to produce large contemporary groups of progeny to fulfill this design, crossbred sires were first produced that were heterozygous at both loci. Three Texel rams at USMARC were available for breeding in October of 2006. Two rams were homozygous for the MSTN mutation and the third ram was a heterozygote. These rams were mated to ewes from the CLPG line of composite sheep created at USMARC. This composite was created by multiple generations of introgression of the Dorset callipyge allele into a terminal sire composite population (Leymaster, 1991) that was composed of 1/2 Columbia, 1/4 Suffolk, and 1/4 Hampshire breeds. These composite ewes were all genotyped ++/CC for the two loci involved. Resulting ram lambs born in 2007 were bled via jugular venipuncture and genotyped at both MSTN and CLPG loci. Twelve ram lambs (4 from each Texel sire) heterozygous at both loci were then used in a single sire mating season of 35 d starting on October 29, 2007 with a group of non-carrier ewes (n = 215). These non-carrier ewes (++/++ genotypes) were born in the fall of 2006 and the breed composition was 1/2 Composite (Leymaster, 1991), 1/4 Romanov with the remaining 1/4 consisting of one of four breeds (Dorper, Dorset, Katahdin, or Rambouillet). Lambs for this experiment (n = 365; 186 female, 179 male) segregating at both loci in approximately equal frequencies from the matings of the carrier rams and the non-carrier ewes were born in March to April of 2008.
Management of Lambs
Ewes were limited to rearing 1 or 2 lambs (no grafting) to address uniformity issues of maternal environment. Additional lambs were artificially reared in the nursery (n = 23) without regard to sex of lamb, but carcass and meat quality data were not collected on artificially reared lambs. Ram lambs were castrated and tails were docked on all lambs when processed within 2 d of birth. Wether and ewe lambs were penned together by contemporary groups according to birth dates. Lambs were provided ad libitum access to a total mixed diet (18% crude protein) from creep to about 23 kg body weight (BW) and then fed ad libitum a total mixed diet (2.96 Mcal of metabolizable energy/kg of dry matter with 14.5% crude protein) during the finishing period. Lamb BW was recorded at 0 (birth), 8 (weaning), 10, and 20 wk of age. Lambs were bled after 10 wk of age to determine MSTN and CLPG genotypes. Each lamb was manually restrained and a whole blood sample (3 mL) was taken via jugular venipuncture using a 16 or 18 gauge needle with 4% ethylenediaminetetraacetate. Ear tissue was collected from lambs that died prior to the sample time for blood collection.
Collection of Carcass Data
Within sire-sex-genotype subgroups, naturally reared lambs were assigned to eight slaughter groups accounting for variation in birth date. Lambs (n = 236) were serially slaughtered at weekly intervals, ~30 lambs per group, ranging from roughly 26 (week of 29 September 2008) to 33 (week of 17 November 2008) weeks of age. Live BW was recorded in the sheep area on the day prior to slaughter. Kidney-pelvic fat and hot carcass weights were recorded. Following a 24-h chill, subjective leg scores were assigned and widths of carcasses measured at the widest points of the shoulder and rump were obtained by calipers. Subjective leg scores that appeared to exceed the prime plus category (score = 15) were developed to represent data recorded. Carcasses were ordered on the rail according to subjective leg score appearance and categories were added from 16 to 20 to reflect visually noticeable differences in conformation that were observed. At 48 h postmortem, chilled carcass weight was recorded, carcasses were split along the midline, and the right-side weights recorded. Carcass length was measured from the anterior edge of the first rib to the anterior edge of the aitch bone. The right side was split between the 12th and 13th ribs to measure longissimus area and 12th rib fat-depth.
DNA Isolation and Genotyping
Genomic DNA was extracted from blood samples using Gentra Generation Capture kits (Gentra Systems Inc., Minneapolis, MN). Alternatively, when a blood sample was not available, genomic DNA was extracted from ear tissue samples using the Wizard SV Genomic DNA Purification kit (Promega, Madison, WI). Genotypes were generated using the Sequenom MASSARRAY® system (Sequenom, San Diego, CA) using a duplex design created from massarray® Assay Design software. Amplification primers, amplicon lengths, and extension products are listed in Table 1. Approximately 75 ng of DNA was subjected to duplex PCR reactions and hME chemistry as suggested by the manufacturer (Sequenom, San Diego, CA).
Table 1.
Oligo primers and analytes for the duplex mass spec genotyping assay with A/C terminator mix
| Locus | Oligo primer (5ʹ–3ʹ) | Function | Mass | Allele represented |
|---|---|---|---|---|
| CLPGa | ACGTTGGATGGTGTCCTGGTCTATTTTCGG | 5ʹ Capture Primer | ||
| ACGTTGGATGAGCTGGGGAAAGGATCTGAC | 3ʹ Capture primer | |||
| AAGGATCTGACAGGTGG | Extend primer | 5299.5 Da | ||
| AAGGATCTGACAGGTGGC | Analyte G | 5572.6 Da | CLPG | |
| AAGGATCTGACAGGTGGTC | Analyte A | 5876.8 Da | + | |
| MSTNb | ACGTTGGATGGTTAAATCATTTTGGTTTGC | 5ʹ Capture primer | ||
| ACGTTGGATGCGTGATGGCTGTATAATGTG | 3ʹ Capture primer | |||
| GTCATTGTATTCAAATCTCAAC | Extend primer | 6668.4 Da | ||
| GTCATTGTATTCAAATCTCAACA | Analyte A | 6965.6 Da | + | |
| GTCATTGTATTCAAATCTCAACGTTC | Analyte G | 7879.2 Da | MSTN |
aCLPG PCR amplicon is 116 bp in length.
bMSTN PCR amplicon is 137 bp in length.
Statistical Analysis
Data were analyzed with the mixed-model analysis of variance procedure of SAS (SAS Inst., Inc., Cary, NC). Preliminary models tested for the effects of variation in genetic background of the non-carrier ewes with 1/4 of the breed composition of those ewes varying among Dorper, Dorset, Katahdin, or Rambouillet which contributed to 1/8 of the variation in the lambs used in this study. No significant effects of this ewe breed variability were detected for any trait and were thus not considered in any further analyses. A general model in common for all traits included fixed effects of sex (ewes, wethers), copies of CLPG alleles (0, 1), copies of MSTN alleles (0, 1) and all possible two-way interactions. Sire (n = 12 sires) of the lamb was included as a random effect. The Kenward–Roger option was used to approximate denominator degrees of freedom associated with the random effect of sire. Compared to this general model the following additional effects were added specific to the trait(s) analyzed. The model for birth weight also fitted a fixed effect for birth type (single, twin) and all possible two- and three-way interactions. Similarly, the model for weaning weight and post-weaning growth traits included fitting a fixed effect for type of rearing (single, twin) and all possible two- and three-way interactions. Carcass traits did not include fixed effects for birth or rearing type but did include a linear covariate of chilled carcass weight fitted within each sex and genotype subclass and the additional random effect of slaughter date. Chilled carcass weight was analyzed using slaughter age as the linear covariate. Linear contrasts were made among the main effect means when the F-tests for the interactions were not significant and the main effect was significant at the P < 0.05 level.
RESULTS AND DISCUSSION
General Results
Number of observations represented in each genotypic combination are presented in Table 2. There were 365 lambs born but 362 lambs with DNA samples to be genotyped. Triplet birth types (n = 11) were deemed too infrequent to estimate with any precision within genotypes and were excluded from all analyses due to interaction of birth type with other fixed effects. Animals successfully reared in the nursery were also excluded from the analysis for weaning and carcass traits. A total of 236 lambs contributed carcass data to estimate effects and interactions among the four genotype combinations produced.
Table 2.
Observations present by genotype combination at different endpoints
| Genotype combinations for MSTN/CLPG | |||||
|---|---|---|---|---|---|
| Endpoint | Age | ++/++ | M+/++ | ++/C+ | M+/C+ |
| Birth | d 0 | 92 | 90 | 101 | 79 |
| Weaning | d 56 | 78 | 76 | 86 | 64 |
| Carcass | wk 26 to 33 | 66 | 61 | 62 | 47 |
Results are presented for different traits by sources of variation in Tables 3 to 5. Levels of significance and least squares means are reported for effects of the three-way interaction of genotype combinations with birth type (Table 3), with sex of lamb (Table 4), or in the case of carcass traits adjusted to a constant chilled carcass weight, just the two-way locus interaction (Table 5). As indicated previously, the two independently segregating sire-derived alleles represent different breed-of-origin contrasts at each locus (Texel vs. composite origin for MSTN and Dorset vs. Texel origin for CLPG).
Table 3.
Least squares means and average standard errors of growth traits for the interaction effect of CLPG by MSTN genotypes by type of birth or rearing
| Trait | Least squares means for genotypes | Average SEM | Level of significance | |||
|---|---|---|---|---|---|---|
| ++/++ | M+/++ | ++/C+ | M+/C+ | |||
| Birth wt., kg | 0.01 | |||||
| Single | 4.37a | 5.98c | 5.59b | 5.23b | .31 | |
| Twin | 4.32 | 4.43 | 4.39 | 4.40 | .11 | |
| Adjusted 56 d wt, kg | 0.36 | |||||
| Single | 18.3 | 20.3 | 19.5 | 22.1 | .89 | |
| Twin | 14.6 | 15.2 | 15.3 | 15.3 | .45 | |
a,b,cValues with different superscripts within a row are significantly different (P < 0.05).
Table 5.
Least squares means and average standard errors of carcass traits adjusted to a constant chilled carcass weight for the interaction effect of CLPG by MSTN genotypes
| Trait | Least squares means for genotypes | Average SEM | Level of significance | |||
|---|---|---|---|---|---|---|
| ++/++ | M+/++ | ++/C+ | M+/C+ | |||
| Live wt at slaughter, kg | 58.0 | 57.9 | 54.6 | 54.2 | .46 | 0.63 |
| Hot carcass wt, kg | 30.3 | 30.3 | 30.3 | 30.3 | .05 | 0.90 |
| Kidney-Pelvic fat, kg | 1.22 | 1.06 | .79 | .71 | .05 | 0.25 |
| 4th sacral vertebra fat, mm | 18.8 | 17.8 | 14.0 | 12.5 | .88 | 0.64 |
| 12th rib fat, mm | 6.7 | 5.8 | 3.9 | 3.1 | .38 | 0.79 |
| Shoulder width, cm | 21.6 | 21.9 | 22.4 | 22.6 | .15 | 0.85 |
| Rump width, cm | 23.5 | 23.6 | 24.8 | 25.2 | .11 | 0.31 |
| Leg scorea | 12.3b | 12.5b | 16.3c | 17.1d | .22 | 0.04 |
| Longissimus muscle area, cm2 | 16.5 | 16.9 | 22.3 | 23.5 | .47 | 0.20 |
| Carcass length, cm | 64.7 | 64.0 | 62.1 | 61.4 | .28 | 0.91 |
aAverage choice = 11, average prime = 14.
b,c,dValues with different superscripts within a row are significantly different (P < 0.05).
Table 4.
Least squares means and average standard errors of growth traits for the interaction effect of CLPG by MSTN genotypes by sex of lamb
| Trait | Least squares means for genotypes | Average SEM | Level of significance | |||
|---|---|---|---|---|---|---|
| ++/++ | M+/++ | ++/C+ | M+/C+ | |||
| Adjusted 56 d wt, kg | 0.04 | |||||
| Ewe | 16.7a | 16.9a | 17.4a | 19.4b | 0.66 | |
| Wether | 16.4a | 18.7a,b | 17.6a,b | 18.6b | 0.72 | |
| Adjusted 70 d wt, kg | 0.25 | |||||
| Ewe | 21.2 | 21.7 | 21.7 | 23.9 | 0.87 | |
| Wether | 20.9 | 23.4 | 22.2 | 23.9 | 0.94 | |
| Adjusted 140 d wt, kg | 0.01 | |||||
| Ewe | 43.4a,b | 42.6a,b | 40.7a | 45.0b | 1.48 | |
| Wether | 43.5 | 44.9 | 46.0 | 43.6 | 1.59 | |
| Post-weaning ADG, g/d | 0.03 | |||||
| Ewe | 304b | 283a | 273a | 277a | 11 | |
| Wether | 307a,b | 317b | 316b | 290a | 13 | |
| Chilled Carcass wt, kg | < 0.01 | |||||
| Ewe | 28.4a | 28.0a | 28.2a | 30.9b | 0.83 | |
| Wether | 29.3a | 29.8a | 32.1b | 28.7a | 0.87 | |
a,bValues with different superscripts within a row are significantly different (P < 0.05).
Body Weight and Growth Traits
Genotype combinations influenced weight of lambs at birth while interacting with birth type (P = 0.01; Table 3). Twin-born lambs were similar across all four genotype combinations, while single-born lambs differed. Differences between single- and twin-born progeny were larger in the M+/++ genotype, intermediate in the ++/C+ and M+/C+ genotype classes and much smaller in the ++/++ genotype class. This is a somewhat surprising result given previous data comparing Texel, Suffolk, and the terminal composite breed used in this study did not detect differences in birth weight associated with Texel genetics (Leymaster and Jenkins, 1993; Freking et al., 2000; Leeds et al., 2012). Differences between CLPG genotypes also were not previously detected at birth (Freking et al., 1998). There were far fewer single-born lambs (n = 34) than twin lambs (n = 298) in the current experiment, so a few relatively large single lambs perhaps influenced this interaction to a greater extent. This three-way interaction was not apparent at adjusted 56-d BW taken at weaning (P = 0.36; Table 3).
Genotype combinations interacted with sex of lamb for most measures of growth from weaning and throughout the post-weaning period to final weights when sacrificed for slaughter (Table 4). At weaning, changes in both rank and magnitude of differences contributed to the significant three-way interaction effect for d-56 BW (P = 0.04). The largest difference between sexes was for the M+/++ class while the largest numeric values were for the M+/C+ class that also switched ranks with ewes weighing heavier than wethers for this combination. Following weaning, this three-way interaction was not detected for d-70 BW (P = 0.25). Growth rate during the period from 10 wk to 20 wk of age again showed a significant effect for the three-way interaction (P = 0.03). Effects of this interaction were due to differences in magnitude of growth rate differences between the sexes rather than any changes in rank across the four genotypes. All ewes grew more slowly than did their wether counterparts. The largest difference between the sexes occurred with the M+/++ and ++/C+ genotype classes compared to the other two classes. Similar reasons were responsible for the interaction observed (P = 0.01) at BW to d-140. The final growth measurement to exhibit this three-way interaction (P < 0.01) was for chilled carcass weight, which was adjusted to a constant age at slaughter. Interaction observed in this case was primarily due to the change in rank of the M+/C+ genotype class relative to the other three combinations. For this genotype class the ewes produced heavier carcasses than wethers at the same average age-constant endpoint compared to the other three combinations. Previous experiments with CLPG did not detect differences between genotypes or interaction with sexes in growth rates to any slaughter endpoint (Freking et al. 1998). Likewise, most studies consistently show Texel genetics to grow at a slower rate but with enhanced muscling and less fat (reviewed by Tellam et al., 2012). Kijas et al. (2007) found that under Australian conditions, MSTN had significant effects on slaughter measurements of muscling and fatness, but only minor impact on live weight and growth.
Carcass Traits on a Constant Chilled Carcass Weight Basis
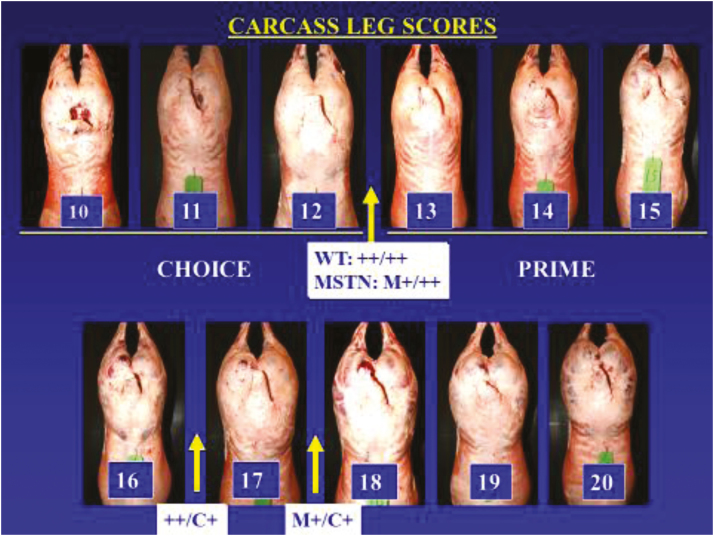
While traits adjusted to an age-constant basis typically showed interactions with sex of lamb, traits evaluated at a constant carcass weight did not. Least squares means are presented in Table 5 for the effect of the two-way interaction between CLPG and MSTN genotype combinations. The only trait that we detected a significant two-way interaction was for subjective leg conformation score (Table 5; Figure 1). Subjective score of 11 is equivalent to average choice and 14 is equivalent to average prime. The initial standard scale had a maximum of 15 = prime plus. Over the course of experiment it was apparent that there were examples of carcasses that exceeded that scale and those are represented by the pictures in Figure 1. Genotype combinations ++/++ and M+/++ both averaged between 12 and 13; which is between choice plus and low prime. The impact of a single copy of MSTN had less impact on leg conformation than did the CLPG mutation. Genotype combination ++/C+ averaged between 16 and 17, while genotype M+/C+ averaged over 17. The extreme carcasses observed in the M+/C+ are quite similar in appearance to those reported by Boman et al. (2010) where they evaluated the combined effects of two different mutations within MSTN. The Texel-derived g+6723G>A is the same mutation evaluated in our study.
Figure 1.
Synergistic interaction effect of CLPG and MSTN genotypes for subjective leg score. Subjective score of 11 is equivalent to average choice and 14 is equivalent to average prime. The initial standard scale had a maximum of 15 = prime plus. There were examples of carcasses that exceeded that scale and are represented by the pictures. Genotype combinations ++/++ and M+/++ both averaged between 12 and 13; which is between choice plus and low prime. Genotype combination ++/C+ averaged between 16 and 17, while genotype M+/C+ averaged over 17.
Additional quantitative measurements associated with carcass shape (shoulder width, rump width, longissimus muscle area, and carcass length) did not detect significant differences for the two locus interaction. In each of these cases, numerical values for the M+/C+ genotype combination were the most extreme values but did not differ statistically from the ++/C+ group.
Relative to the ++/++ genotype group, the overall net effect of a single copy of MSTN on carcass shape was 0.2 leg score units, 0.4 cm2 longissimus area, 0.3 cm in shoulder width, 0.3 cm in rump width, and −0.7 cm carcass length. Corresponding values for a single copy of CLPG were 4.0, 5.8, 0.8, 0.7, and −2.6, respectively.
Measures of carcass fatness followed a similar pattern to carcass shape measurements. While there were no significant two-way interactions detected, the most extreme genotype combination was always the M+/C+ group for fat depot measurements but did not differ (P > 0.05) statistically from the ++/C+ group. Relative to the ++/++ genotype group, the overall net effect of a single copy of MSTN on carcass fatness was −0.16 kg kidney-pelvic fat, −0.9 mm fat at the 12th rib, and −1.0 mm fat at the 4th sacral vertebrae. Corresponding values for a single copy of CLPG were −0.43, −2.8, and −4.8 respectively.
Others have sufficiently reviewed the independent impacts of CLPG or MSTN genotypes on growth and carcass composition (Tellam et al., 2012). We report here for the first time the combined effects of a synergistic interaction between the two loci that influence at least some subjective measures of carcass shape although the magnitude of that interaction is variable depending on the trait considered. Improved estimates for the two loci were obtained in this experiment because we only used sires that were heterozygous for both mutations and produced them in one large contemporary group. Impact of a single copy of MSTN was relatively modest for carcass shape and fatness relative to CLPG. It is important to take these results in context; it is not intended to promote use of CLPG by the U.S. sheep industry to improve carcass composition without consideration of meat quality effects.
Conflict of interest statement. None declared.
ACKNOWLEDGMENTS
Mention of trade names or commercial products in this publication is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the U.S. Department of Agriculture. The authors acknowledge Kreg A. Leymaster (retired) who provided the primary leadership for conceiving, designing, and conducting this experiment. The authors also acknowledge Stephanie Schmidt for assistance with manuscript preparation, Troy Gramke for technical assistance, the USMARC sheep operations for animal husbandry. The U.S. Department of Agriculture (USDA) prohibits discrimination in all its programs and activities on the basis of race, color, national origin, age, disability, and where applicable, sex, marital status, familial status, parental status, religion, sexual orientation, genetic information, political beliefs, reprisal, or because all or part of an individual’s income is derived from any public assistance program. (Not all prohibited bases apply to all programs.) Persons with disabilities who require alternative means for communication of program information (Braille, large print, audiotape, etc.) should contact USDA’s TARGET Center at (202) 720-2600 (voice and TDD). To file a complaint of discrimination, write to USDA, Director, Office of Civil Rights, 1400 Independence Avenue, S.W., Washington, D.C. 20250-9410, or call (800) 795-3272 (voice) or (202) 720-6382 (TDD). USDA is an equal opportunity provider and employer.
REFERENCES
- Arthur P.F. 1995. Double muscling in cattle: a review. Aust. J. Agric. Res. 46:1493–1515. doi:10.1071/AR9951493 [Google Scholar]
- Bidwell C.A., Waddell J.N., Taxis T.M., Yu H., Tellam R.L., Neary M.K., and Cockett N.E.. 2014. New insights into polar overdominance in callipyge sheep. Anim. Genet. 45(Suppl 1). 51–61. doi:10.1111/age.12132 [DOI] [PubMed] [Google Scholar]
- Boman I.A., Klemetsdal G., Nafstad O., Blichfeldt T., and Våge D.I.. 2010. Impact of two myostatin (MSTN) mutations on weight gain and lamb carcass classification in Norwegian White Sheep (Ovis aries). Genet. Sel. Evol. 42:4. doi:10.1186/1297-9686-42-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clop A., Marcq F., Takeda H., Pirottin D., Tordoir X., Bibe B., Bouix J., Caiment F., Elsen J.M., Eychenne F.,. et al. 2006. A mutation creating a potential illegitimate microRNA target site in the myostatin gene affects muscularity in sheep. Nat. Genet. 38:813–818. doi:10.1038/ng1810 [DOI] [PubMed] [Google Scholar]
- FASS 2010. Guide for the Care and Use of Agricultural Animals in Research and Teaching, Third Edition. Champaign, IL: Federation of Animal Science Societies; [accessed September 28, 2015] Available from http://www.fass.org/docs/agguide3rd/Ag_Guide_3rd_ed.pdf. [Google Scholar]
- Freking B.A., Keele J.W., Nielsen M.K., and Leymaster K.A.. 1998. Evaluation of the ovine callipyge locus: II. Genotypic effects on growth, slaughter, and carcass traits. J. Anim. Sci. 76:2549–2559. doi:10.2527/1998.76102549x [DOI] [PubMed] [Google Scholar]
- Freking B.A., Keele J.W., Shackelford S.D., Wheeler T.L., Koohmaraie M., Nielsen M.K., and Leymaster K.A.. 1999. Evaluation of the ovine callipyge locus: III. Genotypic effects on meat quality. J. Anim. Sci. 77:2336–2344. doi:10.2527/1999.7792336x [DOI] [PubMed] [Google Scholar]
- Freking B.A., Leymaster K.A., and Young L.D.. 2000. Evaluation of dorset, finnsheep, romanov, texel, and montadale breeds of sheep: I. Effects of ram breed on productivity of ewes of two crossbred populations. J. Anim. Sci. 78:1422–1429. doi:10.2527/2000.7861422x [DOI] [PubMed] [Google Scholar]
- Freking B.A., Murphy S., Wylie A., Jirtle R.L., Rhodes S., Keele J.W., Leymaster K.A., and Smith T.P. L.. 2002. Identification of the single base change causing the callipyge muscle hypertrophy phenotype, the only known example of polar overdominance in mammals. Genome Res. 12:1496–1506. doi:10.1101/gr.571002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hope M.F. Haynes H. Oddy M. Koohmaraie A. Al-Owaimer, and Geesink G.. 2013. The effects of the myostatin g+6723G>A mutation on carcass and meat quality of lamb. Meat Sci. 95:118–122. doi:10.1016/j.meatsci.2013.03.029 [DOI] [PubMed] [Google Scholar]
- Johnson P.L., McEwan J.C., Dodds K.G., Purchas R.W., and Blair H.T.. 2005. Meat quality traits were unaffected by a quantitative trait locus affecting leg composition traits in Texel sheep. J. Anim. Sci. 83:2729–2735. doi:10.2527/2005.83122729x [DOI] [PubMed] [Google Scholar]
- Kijas J.W., McCulloch R., Edwards J.E.H., Oddy V.H., Lee S.H., and vander Werf J.. 2007. Evidence for multiple alleles effecting muscling and fatness at the Ovine GDF8 locus. BMC Genetics. 8:80. doi:10.1186/1471-2156-8-80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koohmaraie M., Shackelford S.D., Wheeler T.L., Lonergan S.M., and Doumit M.E.. 1995. A muscle hypertrophy condition in lamb (callipyge): Characterization of effects on muscle growth and meat quality traits. J. Anim. Sci. 73:3596–3607. doi:10.2527/1995.73123596x [DOI] [PubMed] [Google Scholar]
- Leeds T.D., Notter D.R., Leymaster K.A., Mousel M.R., and Lewis G.S.. 2012. Evaluation of Columbia, USMARC-composite, suffolk, and texel rams as terminal sires in an extensive rangeland production system: I. Ewe productivity and crossbred lamb survival and preweaning growth. J. Anim. Sci. 90:2931–2940. doi:10.2527/jas.2011-4640 [DOI] [PubMed] [Google Scholar]
- Leymaster K.A. 1991. Straightbred comparison of a composite population and the Suffolk breed for performance traits of sheep. J. Anim. Sci. 69: 993–999. doi:10.2527/1991.693993x [DOI] [PubMed] [Google Scholar]
- Leymaster K.A. and Jenkins T.G.. 1993. Comparison of Texel- and Suffolk-sired crossbred lambs for survival, growth, and compositional traits. J. Anim. Sci. 71:859–869. doi:10.2527/1993.714859x [DOI] [PubMed] [Google Scholar]
- Smit M.A., Segers K., Carrascosa L.G., Shay T.L., Baraldi F., Gyapay G., Snowder G., Georges M., Cockett N., and Charlier C.. 2003. Mosaicism of solid gold supports the causality of a noncoding A-to-G transition in the determinism of the callipyge phenotype. Genetics 163:453–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tellam R.L., Cockett N.E., Vuocolo T., and Bidwell C.A.. 2012. Genes contributing to genetic variation of muscling in sheep. Front Genet. 3:164. doi:10.3389/fgene.2012.00164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler T.L., Cundiff L.V., Koch R.M., and Crouse J.D.. 1996. Characterization of biological types of cattle (Cycle IV): carcass traits and longissimus palatability. J. Anim. Sci. 74:1023–1035. doi:10.2527/1996.7451023x [DOI] [PubMed] [Google Scholar]
- Wheeler T.L., Shackelford S.D., Casas E., Cundiff L.V., and Koohmaraie M.. 2001. The effects of Piedmontese inheritance and myostatin genotype on the palatability of longissimus thoracis, gluteus medius, semimembranosus, and biceps femoris. J. Anim. Sci. 79:3069–3074. doi:10.2527/2001.79123069x [DOI] [PubMed] [Google Scholar]
- White J.D., Vuocolo T., McDonagh M., Grounds M.D., Harper G.S., Cockett N.E., and Tellam R.. 2008. Analysis of the callipyge phenotype through skeletal muscle development; association of Dlk1 with muscle precursor cells. Differentiation 76:283–298. doi:10.1111/j.1432-0436.2007.00208.x [DOI] [PubMed] [Google Scholar]