Abstract
In the present study, we investigated the influence of diquat-induced oxidative stress on intestinal barrier, mitochondrial function, and the level of mitophagy in piglets. Twelve male Duroc × Landrace × Yorkshire 35-d-old pigs (weaned at 21 d of age), with an average body of 9.6 kg, were allotted to two treatments of six piglets each including the challenged group and the control group. The challenged pigs were injected with 100 mg/kg bodyweight diquat and control pigs injected with 0.9% (w/v) NaCl solution. The results showed that diquat injection decreased ADFI and ADG. Diquat decreased (P < 0.05) the activities of superoxide dismutase and glutathione peroxidase and increased (P < 0.05) the malondialdehyde concentrations. The lower (P < 0.05) transepithelial electrical resistance and higher (P < 0.05) paracellular permeability of fluorescein isothiocyanatedextran 4 kDa were found in diquat challenged piglets. Meanwhile, diquat decreased (P < 0.05) the protein abundance of claudin-1, occluding, and zonula occludens-1 in jejunum compared with the control group. Diquat-induced mitochondrial dysfunction, as demonstrated by increased (P < 0.05) reactive oxygen species production and decreased (P < 0.05) membrane potential of intestinal mitochondria. Diquat-injected pigs revealed a decrease (P < 0.05) of mRNA abundance of genes related to mitochondrial biogenesis and functions, PPARg coactivator-1α, mammalian-silencing information regulator-1, nuclear respiratory factor-1, mt transcription factor A, mt single-strand DNA-binding protein, mt polymerase r, glucokinase, citrate synthase, ATP synthase, and cytochrome coxidase subunit I and V in the jejunum. Diquat induced an increase (P < 0.05) in expression of mitophagy-related proteins, phosphatase and tensin homologue deleted on chromosome 10-induced putative kinase, and Parkin in the intestinal mitochondria, as well as an enhancement of the ratio of light chain 3-II (LC3-II) to LC3-I content in the jejunal mucosa. These results suggest that oxidative stress disrupted the intestinal barrier, caused mitochondrial dysfunction, and triggered mitophagy.
Keywords: intestinal barrier function, mitochondrial function, mitophagy, oxidative stress
INTRODUCTION
Weaning stress caused growth retardation, diarrhea, and intestinal barrier dysfunctional in piglets, as they are suddenly forced to accommodate to nutritional, immunological, and psychological disruptions (Wijtten et al. 2011; Hu et al. 2013). Moreover, weaning stress disrupted free-radical metabolism and antioxidative system, causing serious oxidative stress (Yin et al. 2014). Oxidative stress is considered to be an imbalance between the production of reactive oxygen species (ROS) and their elimination through antioxidative mechanisms (Bhat et al. 2015). Recently, studies showed that oxidative stress and disruption of cellular redox status impair intestinal function, intestinal turnover, and cell survival (Rahal et al. 2014; Rosero et al. 2015; Li et al. 2016). However, the connection between weaning oxidative stress and intestinal barrier function is still unclear.
Intestine has high demand for energy to support its integrity and function (Pi et al. 2014). Definitely, mitochondria are intracellular organelles that provide most of the energy consume (Marcu et al. 2017). However, mitochondria energy metabolism is an important source of ROS, but also as an important target for the damaging effects of ROS, which ultimately results in increased ROS overproduction by mitochondria and thus, leading to a serious oxidative stress (Jeong et al. 2016). ROS overproduction contributes to mitochondrial dysfunction, as well as causes disruption of ATP synthesis and activation of cell death pathways (Palikaras et al. 2016). Cells have developed a defense mechanism to selectively sequestrate and degrade of the dysfunctional mitochondria before it harms to the cells (Saita et al. 2013). This defense mechanism is called mitochondrial autophagy or mitophagy. However, no data are available regarding the effect of oxidative stress on intestinal mitochondrial function and the level of mitophagy. It would be of great interest to determine the effect of oxidative stress on intestinal mitochondrial function and the level of mitophagy. Furthermore, no data are available about the relation between intestinal mitochondrial function, level of mitophagy, and intestinal barrier function. In this study, we utilized a well-documented model for inducing oxidative stress by injecting diquat (Mao et al. 2014; Yin et al. 2015b). We aimed to determine whether the mitochondria dysfunction and mitophagy involved in the intestinal barrier alteration in the diquat-induced oxidative stress of piglets. We hypothesized that oxidative stress induced by diquat might induce the intestinal barrier disruption, mitochondria dysfunction, and trigger mitophagy.
MATERIALS AND METHODS
Animals, Housing, and Diet
All procedures were approved by the Zhejiang University Animal Care and Use Committee. Twelve male Duroc × Landrace × Yorkshire 35-d-old pigs (weaned at 21 d of age), with an average body of 9.6 kg, were allotted to two treatments of six piglets each including the challenged group and the control group. Diets were formulated to meet or exceed requirements suggested by the National Research Council (2012), and their compositions are shown in Table 1. At the beginning of the experiment, the challenged group received an intraperitoneal injection of diquat at 10 mg/kg of BW in one time. The dose of diquat was chosen to cause oxidative stress in piglets, in accordance with previous study (Lv et al. 2012). Diquat (dibromide monohydrate, Sigma-Aldrich, St. Louis, MO) was dissolved in 0.9% NaCl solution to a concentration of 10 mg/mL and filter-sterilized. Pigs were individually housed in pens (1.8~1.1 m2) in an environmentally controlled nursery barn. The piglets were ad libitum access to feed and water. The feeding trial lasted 1 wk. ADG, ADFI, and feed conversion efficiency were calculated.
Table 1.
Ingredient and chemical composition of the basal diet as fed basis
| Item, g/kg | |
|---|---|
| Maize | 500.8 |
| Extruded maize | 149 |
| Soybean meal | 143 |
| Extruded soybean | 112.5 |
| Fish meal | 46.5 |
| Soybean oil | 18 |
| Limestone | 6 |
| Dicalcium phosphate | 9 |
| Sodium chloride | 3 |
| L-Lysine hydrochloride | 1.5 |
| DL-Methionine | 0.7 |
| Vitamin-mineral premixa | 10 |
| Analysed composition, g/kg | |
| Digestible energyb, MJ/kg (calculated) | 14.57 |
| Crude protein | 214.87 |
| Lysine | 14.58 |
| Methionine | 4.58 |
| Calcium | 8.90 |
aProvided per kilogram of diet: vitamin A, 8,000 IU; vitamin D, 2,000 IU; vitamin E, 40 IU; vitamin K3, 1.5 mg; vitamin B1, 1.5 mg; vitamin B6, 1.6 mg; biotin, 0.10 mg; niacin, 30 mg; pantothenic acid, 25 mg; Zn, 100 mg; Fe, 110 mg; Cu, 15 mg; Mn, 16 mg; I, 0.3 mg; Se, 0.3 mg.
bDigestible energy was calculated from data provide by Feed Database in China (2012).
Sample Collection
After 7 d of experimental period, piglets were euthanasia with an intravenous injection of sodium pentobarbital (200 mg/kg BW) as described by Luo et al. (2015) and Zhang et al. (2016) and the gastrointestinal tract quickly removed. Segments of proximal jejunum were harvested immediately after killed, prepared for Ussing chamber studies and isolation of intestinal mitochondria. Intestinal segments measuring 10 cm in length were excised from the proximal jejunum, immediately placed in Ringer’s solution and mounted in Ussing chambers, as described by McLamb et al. (2013) and Hu et al. (2013). Jejunum mucosa was stripped from the seromuscular layer in oxygenated (95% O2–5% CO2) Ringer solution for Ussing chamber studies. About 5 cm adjacent portions of fresh jejunum was opened longitudinally and cleaned with ice-cold PBS and then clipped about 100 mg sample stored at 4 °C for isolation of intestinal mitochondria within an hour of killing pigs. About 10 cm adjacent portions of jejunum was opened longitudinally and cleaned with PBS. Mucosal scrapings from the adjacent jejunum were collected, rapidly frozen in liquid nitrogen, and stored at −80 °C.
Redox Status
Intestinal mucosa was used for the measurement of superoxide dismutase (SOD), glutathione peroxidase (GSH-Px), malondialdehyde (MDA) as previously described (Xu et al. 2014), using an ELISA kit specific for porcine following the manufacturer’s protocol (Nanjing Jiancheng Bioengineering Institute, Jiangsu, China).
Ex Vivo Ussing Chamber to Measure Intestinal Barrier Function
Tissues were mounted in EasyMount Ussing chamber system (model VCC MC6; Physiologic Instruments, San Diego, CA) as previously described (Hu et al. 2013; McLamb et al. 2013). Jejunum mucosa was stripped from the seromuscular layer in oxygenated (95% O2–5% CO2) Ringer solution. Tissues were then mounted in Ussing chambers. Tissues were bathed on the serosal and mucosal sides with 5mL of Ringer solution. The serosal bathing solution contained 10 mM glucose, which was osmotically balanced on the mucosal side with 10 mM mannitol. Bathing solutions were oxygenated (95% O2–5% CO2) and circulated in water-jacketed reservoirs maintained at 37 °C. The clamps were connected to acquire and analyze software (Physiologic Instruments, CA) for automatic data collection. Briefly, data were collected automatically using Acquire and Analyze software (Physiologic Instruments). Transepithelial electrical resistance (TER) was recorded at 15-min intervals over a 1-h period after a 15-min equilibration period. The flux of fluorescein isothiocyanate dextran 4 kDa (FD4) was used to evaluate the epithelial barrier function as previously described (Jiao et al. 2015a; Li et al. 2017). After a 15-min equilibration period on Ussing chambers, the FD4 (FD4-100MG; Sigma-Aldrich) (0.375 mg/ml) was added to the mucosal side of Ussing chamber-mounted tissues. The FD4 was allowed to equilibrate for 15 min after which 50 µL samples were taken from the serosal side of tissues at 15-min intervals over 60 min and transferred into a 96 well assay plate. The presence of FD4 fluorescence intensity of each sample was measured by Fluorescence Microplate Reader (FLx800, Bio-Tek Instruments Inc., Winooski, VT), and concentrations were determined from standard curves generated by serial dilution of FD4. FD4 flux was measured for 60 min and presented as the stable rate of FD4 flux (µg.cm−2.h−1).
Isolation of Mitochondria
All procedures for mitochondrial isolation were conducted at 4 °C. Mitochondria were isolated from jejunum mucosa by Tissue Mitochondria Isolation Kit (Beyotime Institute of Biotechnology, China) according to the manufacturer’s instructions. The purity of mitochondria was determined by the expression of voltage-dependent anion- selective channel protein 1 (VDAC) and β-actin using western blot (Larson-Casey et al. 2016). The β-actin is a marker protein of cytosol, and the VDAC is a marker protein of mitochondria. In our intestinal mitochondria samples, the expression of β-actin could not been found which means the mitochondria sample did not mix with other cytosol impurity protein. The yields of mitochondria were representing by total protein concentration of mitochondria samples determined by bicinchoninic acid protein assay as described in previous studies (Thummasorn et al. 2011; Pipatpiboon et al. 2012).
Intestinal Mitochondrial ROS Assay
Isolated intestinal mitochondria was treated with 2′,7′-dichlorohydro-fluorescein diacetate that can pass through the mitochondria membrane and is hydrolyzed by intracellular esterase. ROS oxidizes dichlorohydro-fluorescein and converts dichlorohydro-fluorescein to 2′,7′-dichlorodihydro-fluorescein diacetate, which is highly fluorescent at 485 nm, and the emission was detected at 528 nm. The isolated mitochondria (0.4 mg/ml total protein concentration of mitochondria samples) were treated with 2 µmol/L 2′,7′-dichlorohydro-fluorescein diacetate and incubated at room temperature for 20 min. The fluorescence intensity was detected using a fluorescence microplate reader as described (Pipatpiboon et al. 2012). The fluorescence intensity of all samples was expressed as fold changes, calculated relative to the control group according to Pipatpiboon et al. (2012).
Intestinal Mitochondrial Membrane Potential (∆Ψm) Assay
Changes in mitochondrial membrane potential (∆Ψm) were measured using mitochondrial membrane potential assay kit with JC-1 (Beyotime Institute of Biotechnology) according to the manufacturer’s instructions. Briefly, isolated mitochondria (0.1 mL of 0.4 mg/mL mitochondria total protein content) were suspended in 0.5 mL medium containing 5 mmol/L JC-1 and determination fluorescence immediately after blending. Samples were analyzed by automatic fluorescence microplate reader (FLx800, Bio-Tek Instruments, Inc.). The values of optical density (OD) at 590 nm and 530 nm were determined by a spectrofluorometry. As the ∆Ψm is proportional to the ratio of OD 590 nm to OD 530 nm, the ∆Ψm was expressed as OD590/OD530 (Li et al. 2011a, 2011b).
mRNA Expression Analysis by RT-PCR
The mRNA levels of peroxisomal proliferator-activated receptor-γ coactivator-1α (PGC-1α), mammalian-silencing information regulator-1 (SIRT-1), nuclear respiratory factor-1 (NRF-1), mitochondrial transcription factor A (TFAM), mitochondrial single-strand DNA-binding protein (mtSSB), mitochondrial polymeraser (mtpolr), glucokinase, citrate synthase (CS), ATP synthase (ATPS), cytochrome c oxidase I (CcOX I), cytochrome c oxidase IV (CcOX IV), cytochrome c oxidase V (CcOX V), Cytochrome c (Cyt c), and NADH dehydrogenase subunit 4 (ND4), were analyzed as described by Huang et al (2017). Sequence of primers used for the RT-PCR was shown in Table 2. Total RNA was extracted from jejunal mucosa using TRIzol reagent (Invitrogen, Carlsbad, CA), following the manufacturer’s guidelines. The concentration and purity of all RNA samples were measured using a Nano Drop spectrophotometer (ND-2000; NanoDrop Technologies, Wilmington, DE). Reverse transcription using the PrimeScripte RT reagent kit (TaKaRa Biotechnology, Dalian, China) was carried out following the manufacturer’s instructions. Quantitative analysis of PCR was carried out on a StepOne Plus real-time PCR system (Applied Biosystems, Foster City, CA) using SYBR Green Master mix (Promega, Madison, WI), according to the manufacturer’s instructions. Gene-specific amplification was determined by melting curve analysis and agarose gel electrophoresis. The 2−ΔΔCt method was used to analyze the relative expression (fold changes), calculated relative to the values from the control group. The 2−ΔΔCt method was used to analyze the relative changes in each target gene expression. The tight junction (TJ) proteins change (Δ) in Ct values in the each group was compared with the Ct value of β-actin (ΔCt). ΔΔCT was computed for each target gene from the treatment groups by subtracting the average ΔCT for the control group. The final fold differences were computed as 2−ΔΔCt for each target gene. All samples were run in triplicate. Our results showed that β-actin exhibited no difference among different groups.
Table 2.
Primer sequences used for real-time PCR
| Gene | 5′-Primer (F) | 3′-Primer (R) | Accession number | Length |
|---|---|---|---|---|
| PGC-1α | CCCGAAACAGTAGCAGAGACAAG | CTGGGGTCAGAGGAAGAGATAAAG | NM 213963 | 111 |
| NRF-1 | GCCAGTGAGATGAAGAGAAACG | CTACAGCAGGGACCAAAGTTCAC | AK237171.1 | 166 |
| TFAM | GGTCCATCACAGGTAAAGCTGAA | ATAAGATCGTTTCGCCCAACTTC | AY923074.1 | 167 |
| SIRT-1 | TGACTGTGAAGCTGTACGAGGAG | TGGCTCTATGAAACTGCTCTGG | EU030283.2 | 143 |
| mtSSB | CTTTGAGGTAGTGCTGTGTCG | CTCACCCCTGACGATGAAGAC | AK352341.1 | 143 |
| mtpolr | CTTTGAGGTTTTCCAGCAGCAG | GCTCCCAGTTTTGGTTGACAG | XM 001927064.1 | 119 |
| ND4 | TTATTGGTGCCGGAGGTACTG | CCCAGTTTATTCCAGGGTTCTG | NM 001097468 | 112 |
| Glucokinase | CTTTTCCCTCCCACACTGCTAT | GACTCCTCTTCCTGAGACCCTCT | AK233298.1 | 119 |
| CS | CCTTTCAGACCCCTACTTGTCCT | CACATCTTTGCCGACTTCCTTC | M21197.1 | 127 |
| CcOX I | ATTATCCTGACGCATACACAGCA | GCAGATACTTCTCGTTTTGATGC | AJ950517.1 | 127 |
| CcOX IV | CCAAGTGGGACTACGACAAGAAC | CCTGCTCGTTTATTAGCACTGG | AK233334.1 | 131 |
| CcOX V | ATCTGGAGGTGGTGTTCCTACTG | GTTGGTGATGGAGGGGACTAAA | AY786556.1 | 160 |
| Cyt c | TAGAAAAGGGAGGCAAACACAAG | GGATTCTCCAGGTACTCCATCAG | NM 001129970.1 | 154 |
| ATPS | TGTCCTCCTCCCTATCACACATT | TAGTGGTTATGACGTTGGCTTGA | AK230503 | 116 |
| β-actin | TCTGGCACCACACCTTCT | TGATCTGGGTCATCTTCTCAC | DQ178122 | 114 |
Protein Expression Analysis by Western Blot
Intestinal mucosal (100–150 mg) and isolated intestinal mitochondria (20–40 mg mitochondria total protein) were homogenized and lysed in chilled lysis buffer and mitochondrial lysis buffer (1 mL mitochondrial lysis buffer added 1 μL protease inhibitor, 5 μL phosphatase inhibition, and 1 μL DTT), respectively. The homogenates were centrifuged at 12,000 × g for 15 min at 4 °C and 15,000 × g for 10 min at 4 °C, respectively. The supernatant was recovered. Protein concentration was measured using the bicinchoninic acid protein assay kit (Applygen Technologies Co., Ltd., China). The western blot analysis was performed according to the procedures outlined by Hu et al. and Larson-Casey et al. (Hu et al. 2013; Larson-Casey et al. 2016). Briefly, after electrophoresis, the proteins were transferred to polyvinylidene difluoride membranes (Millipore, Bedford, MA). The membranes were incubated with primary Ab at 4 °C for 12 h and then with the secondary Ab for 2 h at room temperature. The primary Abs (occludin, claduin-1, zonula occludens-1 [ZO-1], light chain 3-I [LC3-I], LC3-II, Parkin, phosphatase and tensin homologue deleted on chromosome 10-induced putative kinase 1 [PINK1], VDAC, β-actin) were purchased from Santa Cruz Technology Inc. (Santa Cruz, CA). The secondary Ab was horseradish peroxidase-conjugated anti-rabbit Ab (Cell Signaling Technology, Danvers, MA). Western blot was detected with an enhanced chemiluminescence detection kit (Amersham, Arlington Heights, IL), photographed by a ChemiScope 3400 (Clinx Science Instruments, Shanghai, China), and analyzed using Quantity One software. β-Actin and VDAC were used as an internal control, which exhibited no difference among each group. The relative abundance of intestinal target proteins and mitochondria target proteins were expressed as target protein/β-actin, target protein/VDAC protein ratio, respectively. The protein expression of all samples was expressed as fold changes, calculated relative to the control group.
Statistical Analysis
One-way ANOVA was conducted using SPSS 20.0 statistical package (SPSS Inc., Chicago, IL). Differences among means were tested using Student’s t-test. Effects were considered significant at P < 0.05.
RESULTS
Growth Performance
The effect of diquat-induced oxidative stress on performance of piglets was shown in Table 3. Compared with the control group, diquat injection reduced (P < 0.05) ADG, ADFI. There was no difference in the feed:gain between the two groups.
Table 3.
Effect of oxidative stress on growth performance of pigs
| Items | Controla | Oxidative stressb | P value |
|---|---|---|---|
| ADG, g | 349.57 ± 21.04 | 242.81 ± 11.64 | <0.0001 |
| ADFI, g | 537.85 ± 21.75 | 400.71 ± 15.92 | <0.0001 |
| Feed:gain | 1.54 ± 0.13 | 1.46 ± 0.09 | 0.2320 |
aInjected with 0.9% (w/v) NaCl solution.
bInjected with diquat at 10 mg/kg BW.
Oxidative Stress Measurements
Table 4 showed the effect of oxidative stress on antioxidant enzyme activities and MDA concentrations in jejunal mucosa of weaned pigs. Compared with the control group, diquat injection decreased (P < 0.05) the activities of SOD and GSH-Px and increased (P < 0.05) the MDA concentrations of jejunal mucosa.
Table 4.
Effects of oxidative stress on activities of antioxidant enzymes and content of MDA in jejunum of piglets
| Items | Controla | Oxidative stressb | P value |
|---|---|---|---|
| SOD, U/mg protein | 100.32 ± 11.29 | 60.06 ± 8.01 | <0.0001 |
| GSH-Px, U/mg protein | 99.35 ± 8.31 | 57.88 ± 6.40 | <0.0001 |
| MDA, nmol/g protein | 0.54 ± 0.07 | 1.91 ± 0.10 | <0.0001 |
aInjected with 0.9% (w/v) NaCl solution.
bInjected with diquat at 10 mg/kg BW.
Intestinal Barrier Function and TJ Expression
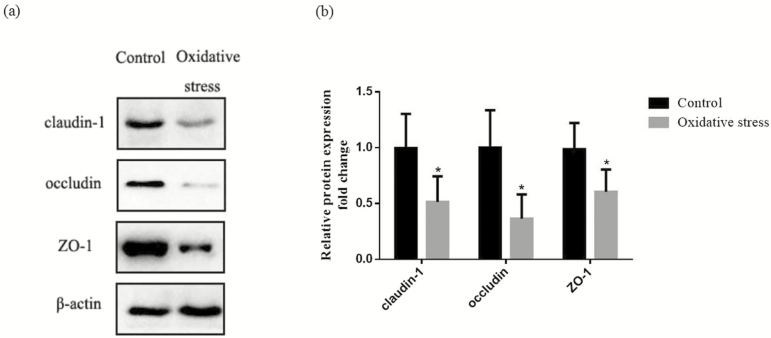
The effect of diquat-induced oxidative stress on intestinal barrier function of piglets was shown in Table 5. Compared with the control group, the pigs challenged with diquat-reduced (P < 0.05) TER in the jejunum, and also increased the mucosal-to-serosal flux of FD4. Figure 1 showed the protein expression of occludin, claudin-1, and ZO-1 in the jejunal mucosa. Compared with the control group, diquat challenge decreased (P < 0.05) protein levels of occludin, claudin-1, and ZO-1 in the jejunal mucosa.
Table 5.
Effect of oxidative stress on jejunal barrier function of piglets
| Items | Controla | Oxidative stressb | P value |
|---|---|---|---|
| Transepithelial electrical resistance, Ω·cm2 | 62.48 ± 7.30 | 45.22 ± 5.39 | 0.001 |
| FD4 flux, µg·cm−2·h−1 | 1.23 ± 0.61 | 2.51 ± 0.35 | 0.002 |
aInjected with 0.9% (w/v) NaCl solution.
bInjected with diquat at 10 mg/kg BW.
Figure 1.
Effect of oxidative stress on tight junction protein of piglets in jejunum of piglets. (a) Representative blots of claudin, occludin, ZO-1, and b-actin in the jejunal mucosa of piglets. (b) Summary of Western blot for n = 6 pigs per treatment in diquat-injected pigs and control pigs. Values are means and SD represented by vertical bars. Control group (black bars), injected with 0.9% (w/v) NaCl solution. *Means within a row with different letters differ significantly (P < 0.05).
Intestinal Mitochondrial ROS Production and Membrane Potentials (∆Ψm)
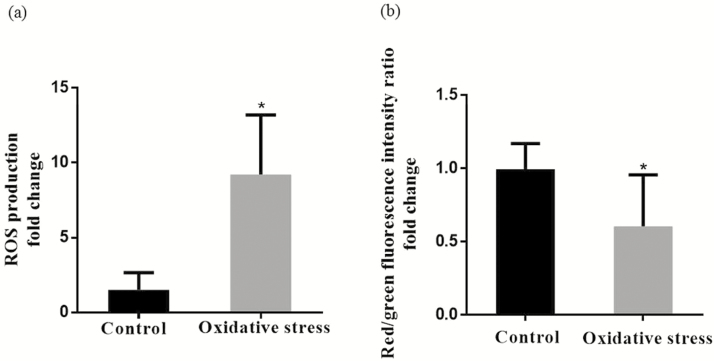
Mitochondrial ROS production and mitochondrial membrane potentials (∆Ψm) in the jejunum were presented in Figure 2a and b. Compared with the control group, diquat injection increased (P < 0.05) the mitochondrial ROS production and deceased (P < 0.05) mitochondrial membrane potentials (∆Ψm) in the jejunum.
Figure 2.
(a) Effect of diquat-induced oxidative stress on intestine mitochondrial ROS production of piglets. (b) Effect of diquat-induced oxidative stress on intestinal mitochondrial membrane potential (∆Ψm) change of piglets. Values are means and SD represented by vertical bars. Control group (black bars), injected with 0.9% (w/v) NaCl solution. *Means within a row with different letters differ significantly (P < 0.05).
mRNA Abundance of Genes Related to Mitochondrial Biogenesis
The mRNA abundance of genes related to mitochondrial biogenesis and function were shown in the Table 6. Compared with the control group, diquat injection resulted in decreased (P < 0.05) the mRNA abundance of PGC-1α, NRF-1, TFAM, SIRT-1, mtSSB, mtpolr, glucokinase, CS, ATPS, CcOX I, CcOX V in the jejunum. However, the mRNA abundance of CcOX IV, Cyt c, and ND4 were not influenced (P > 0.05) by diquat.
Table 6.
Effect of diquat-induced oxidative stress on mRNA abundance of genes related to mitochondrial biogenesis and functions in the jejunum of piglets
| Items | Controla | Oxidative stressb | P value |
|---|---|---|---|
| PGC-1α | 1.01 ± 0.35 | 0.44 ± 0.19 | 0.009 |
| NRF-1 | 1.00 ± 0.38 | 0.49 ± 0.17 | 0.019 |
| TFAM | 1.00 ± 0.31 | 0.47 ± 0.13 | 0.008 |
| SIRT-1 | 1.00 ± 0.40 | 0.43 ± 0.27 | 0.018 |
| mtSSB | 1.00 ± 0.39 | 0.45 ± 0.29 | 0.022 |
| mtpolr | 1.00 ± 0.34 | 0.40 ± 0.25 | 0.006 |
| Glucokinase | 1.00 ± 0.38 | 0.37 ± 0.19 | 0.007 |
| CS | 1.00 ± 0.39 | 0.43 ± 0.13 | 0.015 |
| ATPS | 1.00 ± 0.34 | 0.36 ± 0.16 | 0.004 |
| CcOX I | 1.00 ± 0.29 | 0.42 ± 0.23 | 0.003 |
| CcOX IV | 1.00 ± 0.33 | 0.90 ± 0.34 | 0.601 |
| CcOX V | 1.00 ± 0.31 | 0.39 ± 0.10 | 0.003 |
| Cyt C | 1.00 ± 0.30 | 0.93 ± 0.37 | 0.720 |
| ND4 | 1.00 ± 0.34 | 0.95 ± 0.41 | 0.821 |
aInjected with 0.9% (w/v) NaCl solution.
bInjected with diquat at 10 mg/kg BW.
Expression of Mitophagy-Related Proteins
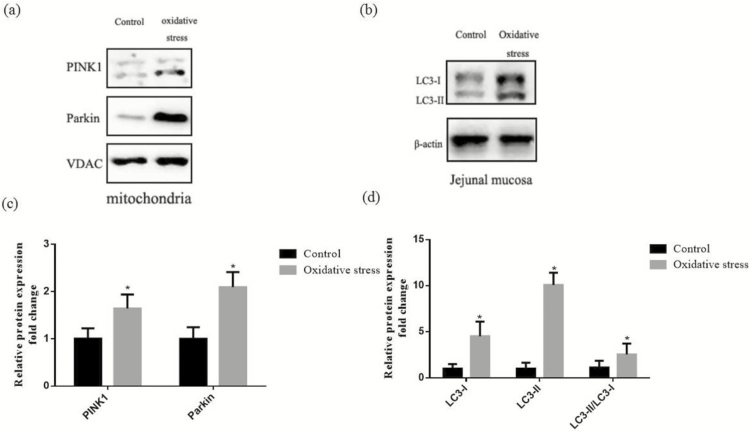
Figure 3 presented the expression of mitophagy-related proteins in the mitochondrial and jejunal mucosa of piglets. In comparison with the control group, diquat challenge increased (P < 0.05) expression of PINK1 and Parkin in the jejunal mitochondrial. Correspondingly, the results showed that administration of diquat enhanced (P < 0.05) both the abundances of LC3-I and LC3-II and the ratio of LC3-II to LC3-I content in the jejunal mucosa.
Figure 3.
Effect of diquat-induced oxidative stress on level of mitophagy-related protein in the jejunum of piglets. (a) Representative blots of PINK1, Parkin in the intestinal mitochondria. (b) Representative blots of LC3-I and LC3-II in the jejunum mucosa. (c) Shows relative PINK1 and Parkin expression. (d) Shows relative LC3-I and LC3-II expression. Summary of Western blot for n = 6 pigs per treatment in diquat-injected pigs and control pigs. Values are means and SD represented by vertical bars. *Means within a row with different letters differ significantly (P < 0.05).
DISCUSSION
Weaning disrupted oxidative balance between ROS generation and antioxidant system in piglets, and this imbalance can induce serious oxidative stress (Yin et al. 2014). Previous studies have confirmed that oxidative stress plays a principal role in disrupting intestinal function during weaning (Zhu et al. 2012; Yin et al. 2014). Therefore, in the present study, we utilized a well-established oxidative stress model by injecting diquat (Mao et al. 2014; Yin et al. 2015b). Diquat can produce H2O2 and O2 through the action of molecular oxygen, which can destroy the redox balance of the intestine, causing oxidative stress (Lv et al. 2012; Mao et al. 2014; Yuan et al. 2017). In the current study, we found that diquat injection decreased the activities of SOD and GSH-Px and increased MDA content in jejunal mucosa, which was consistent with previous reports (Zheng et al. 2010; Lv et al. 2012; Yin et al. 2015b). Liu et al. (2016) and Liu et al. (2018) had reported that antioxidants, vitamin E, and selenium protect intestinal barrier integrity, associated with a reduction in oxidative stress, as indicated by the increased level of GPX mRNA, GPX activity, and GSSG:GSH ratio. Additionally, we found oxidative stress induced by diquat decreased ADFI and ADG, which was consistent with Lv et al. (2012) and Zheng et al. (2010).
The intestinal epithelial barrier is the first line of defense against a hostile environment within the intestinal lumen (Wijtten et al. 2011). In vivo and in vitro studies indicated that diquat-induced oxidative stress can cause damage to intestinal epithelial barrier integrity, as demonstrated by disrupted TJs and decreased viability of epithelial cells, and then cause nutrients metabolize dysfunction (Jiao et al. 2015b; Liu et al. 2016; Zheng et al. 2017). In the present experiment, the Ussing chamber technique was used to monitor intestinal barrier function in terms of TER and flux of FD4. The TER is considered to reflect the opening of the TJs between epithelial cells and the paracellular permeability of the intestinal mucosa (Wijtten et al. 2011). The flux of intact FD4 across the intestinal epithelium occurs mainly through paracellular pathways (Jiao et al. 2015a). A decreased TER and increased flux of FD4 reflects an impaired intestinal barrier (Wijtten et al. 2011). We found that diquat injection disrupted the intestinal mucosal barrier function. Similarly, previous studies demonstrated that diquat-induced oxidative stress results in destruction of intestinal barrier as demonstrated by high level of serum lipopolysaccharide and activity of diamine oxidase of piglets (Wei et al. 2015; Yin et al. 2015b). The intestinal barrier is mainly formed by a layer of epithelial cells joined together by TJs which composed of a layer of columnar epithelium and interepithelial TJs (Pi et al. 2014). The result showed that protein abundance of occludin, claudin-1, ZO-1 of oxidative stress group was decreased compared with the control group. Therefore, diquat-induced oxidative stress increased the intestinal permeability in piglets and decreased the abundance of the TJ proteins in the jejunal mucosa.
Mitochondrion has a central role in energy metabolism homeostasis through mitochondrial respiratory chain. Meanwhile, the mitochondrial respiratory chain is the major source of intracellular ROS generation, but also as an important target for the damaging effects of ROS (Jeong et al. 2016). Mitochondrial insults, including oxidative damage itself, can cause an imbalance between ROS production and removal, resulting in ROS overproduction (Marcu et al. 2017). So far, the information regarding ROS production of intestinal mitochondrial in oxidative stress pigs is unavailable. In the present study, we demonstrated, for the first time, the ROS production of intestinal mitochondrial significantly increased in the diquat treatment of piglets. Thummasorn et al. (2011) had shown that the level of ROS production significantly increased under hydrogen peroxide–induced oxidative stress in isolated cardiac mitochondria. Zhang et al. (2016) had reported that oxidative stress known to be generated during ischemia is associated with a rapid increase in ROS production in cardiocytes. Grubbs et al. (2013) had reported that a lower production of ROS and amount of electron leakage from mitochondria isolated from muscle of pigs were found in pigs with a lower residual feed intake. The possible reason for the high level of ROS of mitochondrial may be that the antioxidative enzyme system had disrupted in intestinal porcine epithelial cell line 1 after administration with diquat (Jiao et al. 2015b), so the ROS degradation process is not sufficiently maintained, resulting in an excess of ROS. When ROS produced by the electron-transport chain is accumulated up to a threshold level, it triggers the opening of the inner membrane anion channel (IMAC). The opening of the IMAC releases the ROS from the mitochondrial matrix, resulting in mitochondrial membrane depolarization (Thummasorn et al. 2011). However, no data are available related to the influence of oxidative stress on polarized state of mitochondrial of piglets. In the current study, we demonstrated, for the first time, the diquat injection significantly decreased the ∆Ψm of intestinal mitochondrial in piglets. Liu et al. (2010) has discovered that oxidative stressors, hydrogen peroxide, 13-L-hydroperoxylinoleic acid, and xanthine + xanthine oxidase induced a significant increase ROS level simultaneously with the dramatic loss of ∆Ψm. We speculate that increased intestinal mitochondrial ROS production caused by diquat may cause the opening of mitochondrial permeability transition, leading to the depolarization of intestinal mitochondrial membrane.
We hypothesized that the alteration of ROS production and ∆Ψm of intestinal mitochondrial may be caused by abnormal mitochondrial biogenesis process, which was regulated by multiple transcriptional factors (Yoboue and Devin 2012). To better clarify the mitochondrial function status during oxidative stress condition, we determined, for the first time, whether injection with diquat influenced mRNA abundance of gene coding for mitochondrial biogenesis and function in piglets. PGC-1α and NRF-1, the primary regulator of mitochondrial biogenesis and proliferation, were found to be downregulated in oxidative stress group in the present study. Arany et al. (2008) found that the PGC-1α gene expression is declined in cultured skeletal myotubes with ischemia-like conditions, known as oxidative stress states. Gutsaeva et al. (2008) reported that the levels of NRF-1 were decreased in mouse cerebral subcortex under hypobaric hypoxia. The TFAM is a regulator of mitochondrial DNA (mtDNA) transcription and nucleoid formation that could be regulated by PGC-1α and NRF-1 in the maintenance of mtDNA (Cheng et al. 2012). SIRT1 influences the acetylation status of PGC-1α, so it is regard as a potential regulator to PGC-1α transcriptional activity and mitochondrial biogenesis (Tan et al. 2015). We found that the expressions of TFAM and SIRT1 were decreased in the jejunum during the oxidative stress condition. The mtSSB and the mtpolr play the role in stimulating the mtDNA helicase activity and promoting mtDNA synthesis (Oliveira and Kaguni 2011). In the current study, the expressions of mtSSB and mtpolr were decreased after treatment with diquat. Mitochondrial dysfunction could result in alterations in glycometabolism, oxidative phosphorylation, tricarboxylic acid (TCA) cycle, electron-transport respiratory chain, and ATP generation (Bhat et al. 2015). In the present study, Glucokinase, CS, ATPS, and CcOX I, V, the key components of glucose metabolism and TCA cycle, were decreased in the oxidative stress group. However, diquat injection had no effect on CcOX IV, Cyt c, and ND4.
In response to oxidative stress and disrupted mitochondria, cells have formed a self-protection mechanism to degrade the dysfunctional mitochondrion before it causes activation of cell death (Springer and Macleod 2016). This process is known as mitochondrial autophagy or mitophagy, which is triggered by ROS overproduction and depolarization of mitochondrial membrane (Springer and Macleod 2016). Nevertheless, no information is available regarding the mitophagy during the oxidative stress in piglets. To explore whether mitophagy involved in oxidative stress induced by diquat, we determined, for the first time, whether injection with diquat influenced expression level of mitophagy-related proteins in piglets. A slice of studies demonstrated that the PINK1 and parkin play the important role in mediating mitophagy (Eiyama and Okamoto 2015; Springer and Macleod 2016). The PINK1 expressed in healthy, polarized mitochondria, and then it would be rapidly degraded by proteolysis. Therefore, the expression of PINK1 was maintained at a very low level (Eiyama and Okamoto 2015). PINK1 accumulation on damage mitochondria is necessary for Parkin recruitment to induce mitophagy after mitochondrial depolarization stemming from overwhelming oxidative damage (Lazarou et al. 2015). So far, only a few studies have examined the role of PINK1 and Parkin during oxidative stress. Gautier et al. (2008) found that mice knocked down PINK1 exhibit increased sensitivity to oxidative stress and decreased mitochondrial function in the striatium. Narendra et al. (2010) demonstrated that PINK1 accumulates selectively on depolarized mitochondria to recruit Parkin to mitochondria in HeLa cells treated with carbonyl cyanide 3-chlorophenylhydrazone, known as oxidative stressor. In the present research, we revealed, for the first time, that oxidative stress induced by diquat increased protein abundance of PINK1 and Parkin in the mitochondrial. During initial phagophore formation in mitophagy process, LC3-I is modified and converted to LC3-II, which is translocated to the membrane of autophagosome and autolysosomes (Yin et al. 2015a). Our results showed that diquat promoted the conversion of LC3-II from LC3-I, indicating autophagosome contained dysfunctional mitochondrial formation in the jejunum. Mitophagy in this study may protect cells against cell death under oxidative stress induced by diquat in intestine of piglets due to the damaged mitochondrial to be taken up by autophagosomes, subsequently degraded by lysosomes, which contribute to the intestinal homeostasis and reduce further oxidative damage.
In the current study, diquat-induced oxidative stress in pigs disrupted intestinal barrier and caused mitochondrial dysfunction. Furthermore, levels of mitophagy-related proteins were upregulated in intestinal mitochondria in response to diquat injection. These results suggested that induction of mitophagy in intestine may play an important role in host response to intestinal barrier dysfunction induced by oxidative stress.
Footnotes
This research was supported by the National Key R & D Program (2016YFD0501210), National Natural Science Foundation of China (31472103) and Zhejiang Province Key R & D Project (2015C02022).
LITERATURE CITED
- Arany Z., Foo S. Y., Ma Y., Ruas J. L., Bommi-Reddy A., Girnun G., Cooper M., Laznik D., Chinsomboon J., Rangwala S. M.,. et al. 2008. Hif-independent regulation of vegf and angiogenesis by the transcriptional coactivator pgc-1alpha. Nature 451:1008–1012. doi:10.1038/nature06613 [DOI] [PubMed] [Google Scholar]
- Bhat A. H., Dar K. B., Anees S., Zargar M. A., Masood A., Sofi M. A., and Ganie S. A.. 2015. Oxidative stress, mitochondrial dysfunction and neurodegenerative diseases; a mechanistic insight. Biomed. Pharmacother. 74:101–110. doi:10.1016/j.biopha.2015.07.025 [DOI] [PubMed] [Google Scholar]
- Cheng A. W., Wan R. Q., Yang J. L., Kamimura N., Son T. G., Ouyang X., Luo Y. Q., Okun E., and Mattson M. P.. 2012. Involvement of PGC-1 alpha in the formation and maintenance of neuronal dendritic spines. Nat Commun 3:1250. doi:ARTN 1250 10.1038/ncomms2238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eiyama A., and Okamoto K.. 2015. PINK1/parkin-mediated mitophagy in mammalian cells. Curr. Opin. Cell Biol. 33:95–101. doi:10.1016/j.ceb.2015.01.002 [DOI] [PubMed] [Google Scholar]
- Gautier C. A., Kitada T., and Shen J.. 2008. Loss of pink1 causes mitochondrial functional defects and increased sensitivity to oxidative stress. Proc. Natl. Acad. Sci. U.S.A. 105:11364–11369. doi:10.1073/pnas.0802076105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grubbs J. K., Fritchen A. N., Huff-Lonergan E., Dekkers J. C., Gabler N. K., and Lonergan S. M.. 2013. Divergent genetic selection for residual feed intake impacts mitochondria reactive oxygen species production in pigs. J. Anim. Sci. 91:2133–2140. doi:10.2527/jas.2012-5894 [DOI] [PubMed] [Google Scholar]
- Gutsaeva D. R., Carraway M. S., Suliman H. B., Demchenko I. T., Shitara H., Yonekawa H., and Piantadosi C. A.. 2008. Transient hypoxia stimulates mitochondrial biogenesis in brain subcortex by a neuronal nitric oxide synthase-dependent mechanism. J. Neurosci. 28:2015–2024. doi:10.1523/JNEUROSCI.5654-07.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu C. H., Xiao K., Luan Z. S., and Song J.. 2013. Early weaning increases intestinal permeability, alters expression of cytokine and tight junction proteins, and activates mitogen-activated protein kinases in pigs. J. Anim. Sci. 91:1094–1101. doi:10.2527/jas.2012-5796 [DOI] [PubMed] [Google Scholar]
- Huang Q., Xu W., Bai K. W., He J. T., Ahmad H., Zhou L., Zhang L. L., and Wang T.. 2017. Protective effects of leucine on redox status and mitochondrial-related gene abundance in the jejunum of intrauterine growth-retarded piglets during early weaning period. Arch. Anim. Nutr. 71:93–107. doi:10.1080/1745039X.2017.1279712 [DOI] [PubMed] [Google Scholar]
- Jeong E. M., Chung J., Liu H., Go Y., Gladstein S., Farzaneh-Far A., Lewandowski E. D., and Dudley S. C.. 2016. Role of mitochondrial oxidative stress in glucose tolerance, insulin resistance, and cardiac diastolic dysfunction. J Am Heart Assoc 5:173. doi:ARTN e00304610.1161/JAHA.115.003046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao L. F., Ke Y. L., Xiao K., Song Z. H., Hu C. H., and Shi B.. 2015a. Effects of cello-oligosaccharide on intestinal microbiota and epithelial barrier function of weanling pigs. J. Anim. Sci. 93:1157–1164. doi:10.2527/jas.2014-8248 [DOI] [PubMed] [Google Scholar]
- Jiao N., Wu Z., Ji Y., Wang B., Dai Z., and Wu G.. 2015b. L-glutamate enhances barrier and antioxidative functions in intestinal porcine epithelial cells. J. Nutr. 145:2258–2264. doi:10.3945/jn.115.217661 [DOI] [PubMed] [Google Scholar]
- Larson-Casey J. L., Deshane J. S., Ryan A. J., Thannickal V. J., and Carter A. B.. 2016. Macrophage akt1 kinase-mediated mitophagy modulates apoptosis resistance and pulmonary fibrosis. Immunity 44:582–596. doi:10.1016/j.immuni.2016.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarou M., Sliter D. A., Kane L. A., Sarraf S. A., Wang C., Burman J. L., Sideris D. P., Fogel A. I., and Youle R. J.. 2015. The ubiquitin kinase PINK1 recruits autophagy receptors to induce mitophagy. Nature 524:309–314. doi:10.1038/nature14893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W. J., Chen Y., Nie S. P., Xie M. Y., He M., Zhang S. S., and Zhu K. X.. 2011a. Ganoderma atrum polysaccharide induces anti-tumor activity via the mitochondrial apoptotic pathway related to activation of host immune response. J. Cell. Biochem. 112:860–871. doi:10.1002/jcb.22993 [DOI] [PubMed] [Google Scholar]
- Li Y., Hansen S. L., Borst L. B., Spears J. W., and Moeser A. J.. 2016. Dietary iron deficiency and oversupplementation increase intestinal permeability, ion transport, and inflammation in pigs. J. Nutr. 146:1499–1505. doi:10.3945/jn.116.231621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W., Nie S., Chen Y., Wang Y., Li C., and Xie M.. 2011b. Enhancement of cyclophosphamide-induced antitumor effect by a novel polysaccharide from Ganoderma atrum in sarcoma 180-bearing mice. J. Agric. Food Chem. 59:3707–3716. doi:10.1021/jf1049497 [DOI] [PubMed] [Google Scholar]
- Li Y., Song Z., Kerr K. A., and Moeser A. J.. 2017. Chronic social stress in pigs impairs intestinal barrier and nutrient transporter function, and alters neuro-immune mediator and receptor expression. PloS one 12: e0171617.doi:10.1371/journal.pone.0171617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F., Celi P., Cottrell J. J., Chauhan S. S., Leury B. J., and Dunshea F. R.. 2018. Effects of a short-term supranutritional selenium supplementation on redox balance, physiology and insulin-related metabolism in heat-stressed pigs. J. Anim. Physiol. Anim. Nutr. (Berl). 102:276–285. doi:10.1111/jpn.12689 [DOI] [PubMed] [Google Scholar]
- Liu F., Cottrell J. J., Furness J. B., Rivera L. R., Kelly F. W., Wijesiriwardana U., Pustovit R. V., Fothergill L. J., Bravo D. M., Celi P.,. et al. 2016. Selenium and vitamin E together improve intestinal epithelial barrier function and alleviate oxidative stress in heat-stressed pigs. Exp. Physiol. 101:801–810. doi:10.1113/EP085746 [DOI] [PubMed] [Google Scholar]
- Liu W. B., Zhou J., Qu Y., Li X., Lu C. T., Xie K. L., Sun X. L., and Fei Z.. 2010. Neuroprotective effect of osthole on mpp+-induced cytotoxicity in pc12 cells via inhibition of mitochondrial dysfunction and ros production. Neurochemistry International 57:206–215. doi:10.1016/j.neuint.2010.05.011 [DOI] [PubMed] [Google Scholar]
- Luo Y. H., Yang C., Wright A. D., He J., and Chen D. W.. 2015. Responses in ileal and cecal bacteria to low and high amylose/amylopectin ratio diets in growing pigs. Appl. Microbiol. Biotechnol. 99:10627–10638. doi:10.1007/s00253-015-6917-2 [DOI] [PubMed] [Google Scholar]
- Lv M., Yu B., Mao X. B., Zheng P., He J., and Chen D. W.. 2012. Responses of growth performance and tryptophan metabolism to oxidative stress induced by diquat in weaned pigs. Animal 6:928–934. doi:10.1017/S1751731111002382 [DOI] [PubMed] [Google Scholar]
- Mao X. B., Lv M., Yu B., He J., Zheng P., Yu J., Wang Q. Y., and Chen D. W.. 2014. The effect of dietary tryptophan levels on oxidative stress of liver induced by diquat in weaned piglets. J. Anim. Sci. Biotechnol. 5:104–110. doi:Artn 4910.1186/2049-1891-5-49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcu R., Zheng Y., and Hawkins B. J.. 2017. Mitochondria and angiogenesis. Adv. Exp. Med. Biol. 982:371–406. doi:10.1007/978-3-319-55330-6_21 [DOI] [PubMed] [Google Scholar]
- McLamb B. L., Gibson A. J., Overman E. L., Stahl C., and Moeser A. J.. 2013. Early weaning stress in pigs impairs innate mucosal immune responses to enterotoxigenic E. coli challenge and exacerbates intestinal injury and clinical disease. Plos One 8:e59838. doi:10.1371/journal.pone.0059838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narendra D. P., Jin S. M., Tanaka A., Suen D. F., Gautier C. A., Shen J., Cookson M. R., and Youle R. J.. 2010. Pink1 is selectively stabilized on impaired mitochondria to activate parkin. Plos Biology 8. doi:ARTN e100029810.1371/journal.pbio.1000298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira M. T., and Kaguni L. S.. 2011. Reduced stimulation of recombinant DNA polymerase γ and mitochondrial DNA (mtDNA) helicase by variants of mitochondrial single-stranded DNA-binding protein (mtSSB) correlates with defects in mtDNA replication in animal cells. J. Biol. Chem. 286:40649–40658. doi:10.1074/jbc.M111.289983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palikaras K., Lionaki E., and Tavernarakis N.. 2016. Mitophagy: in sickness and in health. Mol. Cell. Oncol. 3:e1056332. doi:10.1080/23723556.2015.1056332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pi D. A., Liu Y. L., Shi H. F., Li S., Odle J., Lin X., Zhu H. L., Chen F., Hou Y. Q., and Leng W. B.. 2014. Dietary supplementation of aspartate enhances intestinal integrity and energy status in weanling piglets after lipopolysaccharide challenge. J. Nutr. Biochem. 25: 456–462. doi:10.1016/j.jnutbio.2013.12.006 [DOI] [PubMed] [Google Scholar]
- Pipatpiboon N., Pratchayasakul W., Chattipakorn N., and Chattipakorn S. C.. 2012. PPARγ agonist improves neuronal insulin receptor function in hippocampus and brain mitochondria function in rats with insulin resistance induced by long term high-fat diets. Endocrinology 153:329–338. doi:10.1210/en.2011-1502 [DOI] [PubMed] [Google Scholar]
- Rahal A., Kumar A., Singh V., Yadav B., Tiwari R., Chakraborty S., and Dhama K.. 2014. Oxidative stress, prooxidants, and antioxidants: the interplay. Biomed Res. Int. 2014:761264. doi:10.1155/2014/761264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosero D. S., Odle J., Moeser A. J., Boyd R. D., and van Heugten E.. 2015. Peroxidised dietary lipids impair intestinal function and morphology of the small intestine villi of nursery pigs in a dose-dependent manner. Br. J. Nutr. 114:1985–1992. doi:10.1017/S000711451500392X [DOI] [PubMed] [Google Scholar]
- Saita S., Shirane M., and Nakayama K. I.. 2013. Selective escape of proteins from the mitochondria during mitophagy. Nat. Commun. 4:1410. doi:10.1038/ncomms2400 [DOI] [PubMed] [Google Scholar]
- Springer M. Z., and Macleod K. F.. 2016. In brief: mitophagy: mechanisms and role in human disease. J. Pathol. 240:253–255. doi:10.1002/path.4774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan M., Tang C., Zhang Y., Cheng Y., Cai L., Chen X., Gao Y., Deng Y., and Pan M.. 2015. SIRT1/PGC-1α signaling protects hepatocytes against mitochondrial oxidative stress induced by bile acids. Free Radic. Res. 49:935–945. doi:10.3109/10715762.2015.1016020 [DOI] [PubMed] [Google Scholar]
- Thummasorn S., Kumfu S., Chattipakorn S., and Chattipakorn N.. 2011. Granulocyte-colony stimulating factor attenuates mitochondrial dysfunction induced by oxidative stress in cardiac mitochondria. Mitochondrion 11:457–466. doi:10.1016/j.mito.2011.01.008 [DOI] [PubMed] [Google Scholar]
- Wei H. K., Chen G., Wang R. J., and Peng J.. 2015. Oregano essential oil decreased susceptibility to oxidative stress-induced dysfunction of intestinal epithelial barrier in rats. J Funct Foods 18: 1191–1199.doi:10.1016/j.jff.2015.02.035 [Google Scholar]
- Wijtten P. J., van der Meulen J., and Verstegen M. W.. 2011. Intestinal barrier function and absorption in pigs after weaning: a review. Br. J. Nutr. 105:967–981. doi:10.1017/S0007114510005660 [DOI] [PubMed] [Google Scholar]
- Xu J., Xu C., Chen X., Cai X., Yang S., Sheng Y., and Wang T.. 2014. Regulation of an antioxidant blend on intestinal redox status and major microbiota in early weaned piglets. Nutrition 30:584–589. doi:10.1016/j.nut.2013.10.018 [DOI] [PubMed] [Google Scholar]
- Yin J., Duan J. L., Cui Z. J., Ren W. K., Li T. J., and Yin Y. L.. 2015a. Hydrogen peroxide-induced oxidative stress activates NF-kappa B and Nrf2/Keap1 signals and triggers autophagy in piglets. Rsc Adv 5: 15479–15486.doi:10.1039/c4ra13557a [Google Scholar]
- Yin J., Liu M. F., Ren W. K., Duan J. L., Yang G., Zhao Y. R., Fang R. J., Chen L. X., Li T. J., and Yin Y. L.. 2015b. Effects of dietary supplementation with glutamate and aspartate on diquat-induced oxidative stress in piglets. PloS one 10. doi:UNSP e012289310.1371/journal.pone.0122893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin J., Wu M. M., Xiao H., Ren W. K., Duan J. L., Yang G., Li T. J., and Yin Y. L.. 2014. Development of an antioxidant system after early weaning in piglets. J. Anim. Sci. 92:612–619. doi:10.2527/jas.2013-6986 [DOI] [PubMed] [Google Scholar]
- Yoboue E. D., and Devin A.. 2012. Reactive oxygen species-mediated control of mitochondrial biogenesis. Int. J. Cell Biol. 2012:403870. doi:10.1155/2012/403870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan D., Hussain T., Tan B., Liu Y., Ji P., and Yin Y.. 2017. The evaluation of antioxidant and anti-inflammatory effects of eucommia ulmoides flavones using diquat-challenged piglet models. Oxid. Med. Cell. Longev. 2017:8140962. doi:10.1155/2017/8140962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X. N., Yan H. J., Yuan Y., Gao J. Q., Shen Z., Cheng Y., Shen Y., Wang R. R., Wang X. F., Hu W. W.,. et al. 2013. Cerebral ischemia-reperfusion-induced autophagy protects against neuronal injury by mitochondrial clearance. Autophagy 9: 1321–1333. doi:10.4161/auto.25132 [DOI] [PubMed] [Google Scholar]
- Zhang Y., Zheng P., Yu B., He J., Yu J., Mao X. B., Wang J. X., Luo J. Q., Huang Z. Q., Cheng G. X.,. et al. 2016. Dietary spray-dried chicken plasma improves intestinal barrier function and modulates immune status in weaning piglets. J. Anim. Sci. 94:173–184. doi:10.2527/jas.2015–9530 [DOI] [PubMed] [Google Scholar]
- Zheng P., Yu B., He J., Yu J., Mao X., Luo Y., Luo J., Huang Z., Tian G., Zeng Q.,. et al. 2017. Arginine metabolism and its protective effects on intestinal health and functions in weaned piglets under oxidative stress induced by diquat. Br. J. Nutr. 117:1495–1502. doi:10.1017/S0007114517001519 [DOI] [PubMed] [Google Scholar]
- Zheng P., Yu B., Lv M., and Chen D. W.. 2010. Effects of oxidative stress induced by diquat on arginine metabolism of postweaning pigs. Asian Austral J Anim 23: 98–105. [Google Scholar]
- Zhu L. H., Zhao K. L., Chen X. L., and Xu J. X.. 2012. Impact of weaning and an antioxidant blend on intestinal barrier function and antioxidant status in pigs. J. Anim. Sci. 90:2581–2589. doi:10.2527/jas.2012-4444 [DOI] [PubMed] [Google Scholar]