Abstract
This study estimated genetic parameters for ewe reproductive traits [number of lambs born (NLB) and weaned (NLW) per ewe lambing] and fecal egg counts (FEC) during the peri-parturient rise (PPR) for use in genetic evaluation of Katahdin sheep. Data included NLB and NLW for 23,060 lambings by 9,295 Katahdin ewes, 1,230 PPR at lambing (PPR0) for 750 ewes, 1,070 PPR at approximately 30 d postpartum (PPR30) for 611 ewes, BW at birth, weaning, and (or) post-weaning for 12,869 lambs, and FEC at weaning and (or) post-weaning for 4,676 lambs. Direct additive, permanent environmental, and residual (co)variances were estimated in univariate and bivariate animal models. Fixed effects included effects of ewe management group and ewe age for all traits, and, for PPR, a continuous effect of days between lambing and measurement. Effects of litter size on PPR0 and number of lambs suckled on PPR30 were included in univariate models but excluded from bivariate models for PPR and NLB or NLW. Heritability estimates in univariate models for NLB, NLW, PPR0, and PPR30 were 0.09 ± 0.01, 0.06 ± 0.01, 0.35 ± 0.06, and 0.24 ± 0.07, respectively. Estimates of permanent environmental variance as a proportion of total phenotypic variance were 0.02 ± 0.01 for NLB, 0.03 ± 0.01 for NLW, 0.05 ± 0.06 for PPR0, and 0.13 ± 0.07 for PPR30. Direct additive, phenotypic, permanent environmental, and residual correlations between NLB and NLW were 0.88 ± 0.03, 0.74 ± 0.004, 0.54 ± 0.15, 0.74 ± 0.003, respectively; corresponding correlations between PPR0 and PPR30 were 0.96 ± 0.07, 0.46 ± 0.03, 0.98 ± 0.50, 0.18 ± 0.05, respectively. The additive genetic correlation (rd) between ewe reproductive traits and PPR ranged from 0.12 to 0.18. Estimates of rd between lamb BW and subsequent ewe NLB and NLW ranged from 0.07 to 0.20, and those between PPR and lamb BW ranged from −0.03 to 0.29. The rd between ewe reproductive traits and lamb FEC ranged from 0.27 to 0.40, and those between PPR and lamb FEC ranged from 0.56 to 0.77. Correlations between maternal additive effects on BW and direct additive effects on PPR were low (−0.08 to 0.10), and those between maternal additive effects on BW and direct additive effects on ewe reproductive traits were variable (−0.36 to 0.11). We conclude that FEC in growing lambs and peri-parturient ewes are controlled by similar genes and that modest, but manageable, genetic antagonisms may exist between FEC and ewe productivity.
Keywords: ewe reproduction, fecal egg count, genetic parameters, Katahdin sheep, peri-parturient rise
INTRODUCTION
The Katahdin is a relatively prolific maternal composite breed of sheep developed in the late 1950s in Maine by crossing hair and wool breeds and is relatively resistant to gastrointestinal nematode parasites (Wildeus, 1997; Vanimisetti et al., 2004). The number of lambs born per ewe lambing (NLB) has an important effect on the efficiency of lamb production, but the number of lambs weaned (NLW) is more important, reflecting both the reproductive potential of the ewe and the survival of her lambs, and has the greatest financial impact on sheep production (Wang and Dickerson, 1991; Bradford, 2002; Borg et al., 2007). Genetic parameter estimates for ewe reproductive traits in different sheep breeds are readily available (Bradford, 2002; Safari et al., 2005; Hanford et al., 2006). However, the ewe is also a major contributor to pasture parasite load because of increased susceptibility of the ewe to parasite infection during the peri-parturient period of late pregnancy and early lactation (O’Sullivan and Donald, 1970; Notter et al., 2017). The peri-parturient rise is a suppression of the immune system during late pregnancy and early lactation that allows establishment of newly acquired larvae, and failure to suppress the adult worm production or expel the worm (Connan, 1968). The greatest contribution to pasture gastrointestinal nematodes is from peri-parturient ewes (Barger, 1996). Selection for increased reproductive performance in ewes may result in correlated responses in other traits, and attention to correlations among important traits is essential to the development of optimal selection strategies. The objective of this study, therefore, was to estimate genetic parameters for ewe reproductive traits and peri-parturient fecal egg counts (PPR), and assess their relationships with direct and maternal additive effects on lamb BW and fecal egg counts (FEC) in Katahdin sheep.
MATERIALS AND METHODS
Data
This study utilized records of NLB and NLW per ewe lambing from 23,060 lambings by 9,295 Katahdin ewes born between 2007 and 2013 in 100 Katahdin flocks that participated in the US National Sheep Improvement Program (NSIP; www.nsip.org). Ninety-four percent of these lambings occurred between January 1 and May 31. In addition, PPR were available near the time of lambing (PPR0) for 1,230 lambings by 750 ewes in 10 of these flocks and at approximately 30 d postpartum (PPR30) for 1,070 lambings by 611 ewes in 8 of these flocks. Peri-parturient FEC were available at both sampling times for 993 lambings, and averaged 36.6 d apart, consistent with the experimental design. Records for PPR0 were obtained between 7 d before and 35 d after lambing, and records for PPR30 were obtained between 8 and 53 d after lambing. Means for days postpartum at recording were 2.6 d for PPR0 and 34.0 d for PPR30. Details regarding recording of PPR can be found in Notter et al. (2017). Records of reproductive traits were restricted to ewes that were between 1 and 10 yr old at lambing; PPR records came from ewes that lambed at 1 to 7 yr of age. The study also used birth (BWT), weaning (WWT), and (or) post-weaning (PWWT) BW from 12,869 lambs from the 100 flocks (Ngere et al., 2017) and lamb FEC records at weaning (WFEC) and (or) post-weaning (PWFEC) for 4,676 lambs from 13 of these flocks (Ngere et al., 2018). Means ± SD for lamb ages at weaning and post-weaning were 66 ± 10 and 121 ± 21 d, respectively. Approximately 95% of the flocks that contributed data for this study were located in the eastern half of the United States (east of 97°W). Flocks that contributed FEC and PPR data were located between 31 and 41°N and between 74 and 94°W.
Statistical Analyses
The ASReml statistical package (Gilmour et al., 2015) was used for all analyses. Lamb FEC and ewe PPR were not normally distributed and were transformed as log10(FEC + 25) and log10(PPR + 25), respectively. A repeatability animal model, y = Xb + Zaa + Zcc + e, was used to analyze NLB, NLW, and PPR where y was a vector of observation for each trait; b was a vector of fixed effects; a was a vector of random animal additive effects; c, was a vector of random animal permanent environmental effects; e was a vector of random residual effects; X was an incidence matrix relating observations to fixed effects; and Za and Zc were incidence matrices relating observations to random direct additive and permanent environmental effects, respectively.
Univariate models for NLB, NLW, and PPR included fixed effects of management group and ewe age. Management group effects included effects of flock, year and season of lambing, and an optional owner-defined management group. Season of lambing was defined using rolling 35-d lambing date windows beginning on January 1 of each lambing year. Fixed effects for PPR0 also included an effect of litter size and a continuous effect of days between lambing and measurement, coded as a deviation from the mean of 2.6 d postpartum. Additional fixed effects for PPR30 were rearing type (defined as the number of lambs suckled at 14 d postpartum) and a continuous effect of days postpartum at measurement, coded as a deviation from the mean of 34.0 d. Distributions of NLB and NLW were presumably not normal, but with ranges of 1 to 4 lambs for NLB and 1 to 3 lambs for NLW, we assumed that the assumption of normality would not meaningfully affect resulting parameter estimates.
Models for lamb BW (Ngere et al., 2017) included fixed effects of management group, dam age in years, and either litter size (for BWT) or (for WWT and PWWT) a joint effect of the number of lamb born and reared (i.e., present at 14 d postpartum), a continuous linear effect of age at weighing for WWT and PWWT, a random additive animal effects, and random additive maternal, permanent environmental, and temporary environmental (i.e., litter) effects of the dam. Models for lamb FEC (Ngere et al., 2018) included the same fixed effects fitted for lamb BW, a continuous linear effect of age at measurement, and random additive animal and litter effects. Additive maternal and dam permanent environmental effects on lamb FEC were tested in preliminary models but were not significant and were removed from the final models.
Direct additive, animal permanent environmental, and residual effects were assumed to be normally distributed, with means of 0 and variances A , Ia, and Ie, respectively, where A was the additive numerator relationship matrix, Ia and Ie were identity matrices with dimensions equal to the numbers of individuals and observations, respectively, and , , and were direct additive, permanent environmental, and residual variance components, respectively. For each trait, the phenotypic variance () was the sum of all variance components included in the model. Heritabilities (h2) and proportions of attributable to permanent environmental effects (c2) were estimated as / and /, respectively. Bivariate models were used to estimate covariances among ewe traits and between ewe and lamb traits, and included fixed effects used in corresponding univariate analyses. However, effects of numbers of lambs born or suckled were excluded from models for PPR in bivariate analyses of PPR and NLB or NLW. For all models, convergence was assumed when log likelihoods in successive iterations changed less than 0.002 × the current iteration number.
RESULTS
Fixed Effects on Ewe Reproductive Traits and PPR
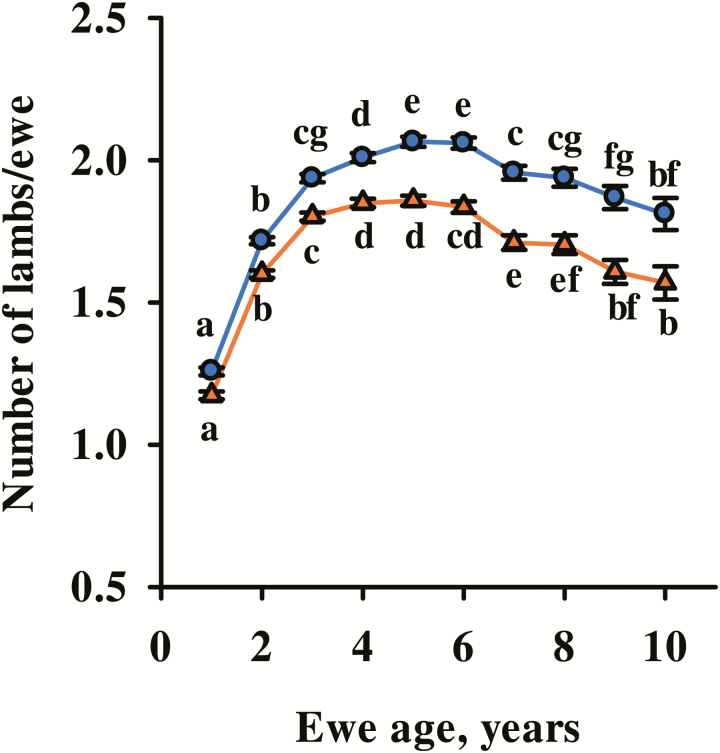
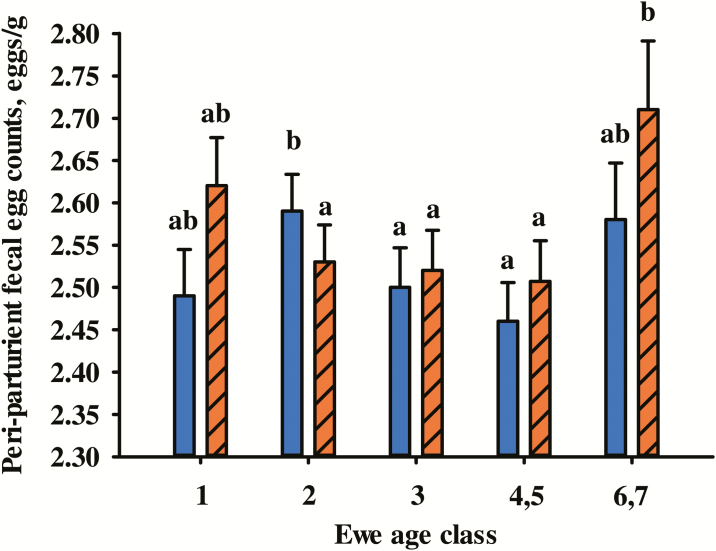
Data for NLB, NLW, and PPR were summarized in Table 1. Fixed effects of ewe management group (P < 0.001) and ewe age (P < 0.05) were significant for all traits. Both NLB and NLW increased as ewe age increased from 1 to 6 yr and then declined for older ewes (Fig. 1). Two-year-old ewes had higher PPR0 than 3-, 4-, and 5-yr-old ewes but did not differ from 1-yr-old or 6- and 7-yr-old ewes (Fig. 2). At 30 d postpartum, 6- and 7-yr-old ewes had higher PPR than 2- through 5-yr-old ewes (P < 0.05) but did not differ (P = 0.30) from yearling ewes.
Table 1.
Descriptive statistics for number of lambs born (NLB), number of lambs weaned (NLW), and ewe peri-parturient rise fecal egg counts (PPR; eggs per g of feces) at lambing (PPR0) and 30 d postpartum (PPR30) for Katahdin ewes
| Trait | Numbers of: | Minimum | Maximum | Mean | SD | CV, % | |||
|---|---|---|---|---|---|---|---|---|---|
| Ewes | Sires | Dams | Records | ||||||
| NLB | 9,295 | 975 | 3,969 | 23,060 | 1 | 4 | 1.82 | 0.68 | 37 |
| NLW | 9,295 | 975 | 3,969 | 23,060 | 0 | 4 | 1.66 | 0.68 | 41 |
| PPR0a | 750 | 136 | 442 | 1,230 | 0 | 16,400 | 933 | 1,665 | 178 |
| PPR30a | 611 | 115 | 358 | 1,070 | 0 | 19,600 | 1,145 | 2,002 | 175 |
aAfter transformation of PPR as log10(PPR + 25), means, SD, and CV were 2.49, 0.74, and 30%, respectively, for PPR0 and 2.60, 0.70, and 27%, respectively, for PPR30.
Figure 1.
Effect of ewe age class on least squares means and SE of number of lambs born (blue circles) and weaned (orange triangles) per ewe exposed. Means within a trait with different letters differ (P < 0.05).
Figure 2.
Effect of ewe age class on least squares means and SE of transformed peri-parturient rise (PPR) fecal egg count (FEC) at lambing (PPR0; solid blue) and at 30-d post-lambing (PPR30; diagonal orange). Fecal egg counts at lambing (PPR) were transformed as [log10(PPR FEC + 25)]. Means within a trait with different letters differ (P < 0.05).
The PPR was affected by both litter size and rearing type (P < 0.001). Ewes with twin births had greater PPR0 than ewes with single or triplet births (Table 2). However, at 30 d postpartum, ewes that nursed 2 or 3 lambs had greater PPR30 than ewes that nursed single lambs. Regression coefficients describing associations between transformed values of PPR0 and PPR30 with days from lambing were negative but small (−0.01 ± 0.01 and −0.002 ± 0.003 per day, respectively; P ≥ 0.32). Notter et al. (2017) discussed fixed effects on PPR in detail.
Table 2.
Effect of birth type and rearing type on peri-parturient rise fecal egg counts (PPR) at lambing (PPR0) and 30 d postpartum (PPR30)a
| Birth type | PPR0 | Rearing type | PPR30 |
|---|---|---|---|
| Single | 2.46a | Single | 2.44a |
| Twin | 2.60b | Twin | 2.62b |
| Triplet | 2.46a | Triplet | 2.61b |
aThe PPR were transformed as log10(PPR + 25) before analysis.
a,bMeans with different superscripts are significantly different (P < 0.05).
Variance Component Estimates in Univariate Models
Numbers of lambs born and weaned.
Heritability estimates were low and similar for NLB and NLW (Table 3). Estimates of c2 were also relatively small. Resulting estimates of the repeatability of NLB and NLW across repeated lambings were 0.11 and 0.09, respectively.
Table 3.
Genetic parameters and SE for number of lambs born (NLB) and weaned (NLW) per ewe lambing and peri-parturient rise fecal egg counts of the ewe at lambing (PPR0) and 30 d postpartum (PPR30)a
| Trait | c 2 | h 2 | ||||
|---|---|---|---|---|---|---|
| NLB | 0.03 ± 0.003 | 0.01 ± 0.003 | 0.31 ± 0.003 | 0.34 ± 0.003 | 0.02 ± 0.01 | 0.09 ± 0.01 |
| NLW | 0.02 ± 0.003 | 0.01 ± 0.003 | 0.33 ± 0.003 | 0.37 ± 0.003 | 0.03 ± 0.01 | 0.06 ± 0.01 |
| PPR0 | 0.13 ± 0.03 | 0.02 ± 0.02 | 0.23 ± 0.01 | 0.38 ± 0.02 | 0.05 ± 0.06 | 0.35 ± 0.06 |
| PPR30 | 0.09 ± 0.03 | 0.05 ± 0.02 | 0.23 ± 0.02 | 0.37 ± 0.02 | 0.13 ± 0.07 | 0.24 ± 0.07 |
a = direct additive variance; = permanent environmental variance; = residual variance; = phenotypic variance = + + ; c2 = /; h2 = heritability = /.
Peri-parturient fecal egg counts.
Heritability estimates for PPR were moderate and somewhat lower for PPR30 than for PPR0 (Table 3). Estimates of c2 were higher for PPR30 than for PPR0, leading to similar estimates for the repeatability across repeated lambings of PPR0 (0.40) and PPR30 (0.37).
Correlations Among Traits
Correlations among traits measured on the ewes.
Direct additive, permanent environmental, and residual covariances were estimated between NLB and NLW and between PPR0 and PPR30. Resulting correlations (Table 4) were all positive and significant. The direct additive correlation between NLB and NLW was large, and permanent environmental and phenotypic correlations between NLB and NLW were substantial. Direct additive and permanent environmental correlations between PPR0 and PPR30 approached 1.0. Residual correlations between NLB and NLW and between PPR0 and PPR30 were much smaller than corresponding direct additive correlations.
Table 4.
Estimates of correlations and SE between number of lambs born (NLB) and weaned (NLW) per ewe lambing and peri-parturient rise fecal egg counts of the ewe at lambing (PPR0) and 30 d postpartum (PPR30)a
| Trait 1 | Trait 2 | r d | r c | r e | r p |
|---|---|---|---|---|---|
| NLB | NLW | 0.88 ± 0.03 | 0.54 ± 0.15 | 0.74 ± 0.004 | 0.74 ± 0.004 |
| PPR0 | PPR30 | 0.96 ± 0.07 | 0.98 ± 0.50 | 0.18 ± 0.05 | 0.46 ± 0.03 |
| NLB | PPR0 | 0.17 ± 0.16 | −0.10 ± 0.89 | 0.15 ± 0.04 | 0.14 ± 0.03 |
| NLB | PPR30 | 0.12 ± 0.21 | 0.24 ± 0.53 | 0.15 ± 0.04 | 0.14 ± 0.03 |
| NLW | PPR0 | 0.18 ± 0.18 | 0.12 ± 0.70 | 0.13 ± 0.04 | 0.12 ± 0.03 |
| NLW | PPR30 | 0.18 ± 0.22 | 0.12 ± 0.46 | 0.19 ± 0.04 | 0.17 ± 0.03 |
a r d = direct additive correlation; rc = correlation between permanent environmental effects; re = residual correlation; rp = phenotypic correlation (see text for additional details).
In bivariate models that excluded effects of NLB or NLW on PPR, direct additive, residual, and phenotypic correlations were similar in magnitude and ranged from 0.12 to 0.19 (Table 4). Only 342 or 770 ewes had PPR records in >1 yr. Permanent environmental correlations between PPR and NLB or NLW had correspondingly large SE but were consistent with observed direct additive and residual correlations. Inclusion of effects of numbers of lambs born or suckled in the model for PPR precluded estimation of permanent environmental or residual correlations, but direct additive correlations, based on resemblances among relatives such as sire progenies, were similar to those in Table 4 and ranged from 0.11 to 0.14 (not shown). Effects of numbers of lambs born or suckled on PPR were significant, but removal of these effects from models for PPR reduced by < 2.5% and reduced heritability estimates for PPR by only 0.01 compared to values in Table 3. These results suggest small, but manageable, antagonisms between ewe reproductive performance and PPR.
Correlations between NLB and NLW and lamb BW
Bivariate models were used to estimate covariances between direct and maternal additive effects on lamb BW and subsequent direct additive effects on NLB and NLW for lambs that were retained as replacement females. Data for these pairs of traits commonly included a single BW record and a series of records for NLB and NLW, which precluded straightforward estimation of covariances involving permanent environmental and residual effects on NLB and NLW and residual effects on BW. Covariances between these pairs of effects were assumed to be 0, thereby assuming that residual effects on lamb BW were independent of future permanent environmental and residual effects on NLB and NLW in retained replacement females. Resulting direct additive correlations between NLB and NLW and lamb BW were consistently positive, but small (Table 5). These correlations slightly exceeded their SE for NLB but were less than their SE for NLW. By contrast, correlations between direct additive effects on NLB and maternal additive effects on lamb BW were consistently negative, but significant only for NLB and BW at birth (−0.36 ± 0.09). Correlations between direct additive effects on NLW and maternal additive effects on lamb BW did not show a consistent pattern and were smaller than their SE.
Table 5.
Correlation estimates and SE for numbers of lambs born (NLB) and weaned (NLW) per ewe lambing and lamb birth (BWT), weaning (WWT), and post-weaning (PWWT) BWa,b
| Trait 1 | Trait 2 | r d | r am | r p |
|---|---|---|---|---|
| NLB | BWT | 0.16 ± 0.12 (0.10 ± 0.14) | −0.36 ± 0.09 (−0.34 ± 0.10) | −0.04 ± 0.03 (−0.04 ± 0.03) |
| NLB | WWT | 0.18 ± 0.14 (0.09 ± 0.16) | −0.07 ± 0.12 (−0.02 ± 0.13) | 0.02 ± 0.12 (0.02 ± 0.11) |
| NLB | PWWT | 0.20 ± 0.16 (0.10 ± 0.19) | −0.02 ± 0.13 (0.04 ± 0.15) | −0.06 ± 0.19 (−0.07 ± 0.19) |
| NLW | BWT | 0.12 ± 0.14 (0.06 ± 0.15) | −0.03 ± 0.11 (−0.001 ± 0.12) | 0.001 ± 0.03 (−0.001 ± 0.03) |
| NLW | WWT | 0.07 ± 0.16 (0.03 ± 0.17) | 0.08 ± 0.13 (0.11 ± 0.14) | 0.02 ± 0.11 (0.02 ± 0.11) |
| NLW | PWWT | 0.11 ± 0.18 (0.07 ± 0.20) | 0.11 ± 0.15 (0.14 ± 0.16) | −0.06 ± 0.19 (−0.06 ± 0.18) |
a r d = direct additive correlation; ram = correlation between direct additive effects on NLB or NLW and maternal additive effects on BW; rp = phenotypic correlation.
bValues in parentheses were obtained after removing records from yearling ewes from the data.
Environmental correlations between lamb BW and future ewe NLB and NLW were not considered in results shown in Table 5. Such an association, if present, would potentially inflate estimates of the direct additive covariances and be most important in young ewes. These bivariate analyses were therefore repeated after excluding records of NLB and NLW for yearling ewes (Table 5). Excluding records of yearling ewes reduced estimates of direct additive correlations between lamb BW and ewe NLB and NLW by approximately 50%, suggesting that there may have been a small positive phenotypic carryover effect of lamb BW on reproductive performance in yearling ewes. Estimates of covariances between maternal additive effects on lamb BW and ewe NLB and NLW were, however, essentially unchanged.
Correlations between ewe PPR and lamb BW.
Bivariate models for lamb BW and ewe PPR included covariances between direct and maternal additive effects on lamb BW and direct additive effects on ewe PPR. Covariances between residual effects on lamb BW and future permanent environmental and residual effect on ewe PPR were assumed to be 0. Resulting correlations between PPR0 and lamb BW were small, generally inconsistent in sign, and consistently less than their SE (Table 6). However, corresponding phenotypic correlations were consistently negative. For PPR30, direct additive correlations with lamb BW were generally positive but only the estimate of the correlation between PPR30 and PWWT exceeded its SE (0.29 ± 0.22).
Table 6.
Correlation estimates and SE between peri-parturient rise fecal egg count at lambing (PPR0) and 30 d postpartum (PPR30) and lamb birth (BWT), weaning (WWT), and post-weaning (PWWT) BWa
| Trait 1 | Trait 2 | r d | r am | r p |
|---|---|---|---|---|
| PPR0 | BWT | −0.10 ± 0.15 | 0.06 ± 0.13 | −0.03 ± 0.04 |
| PPR0 | WWT | 0.08 ± 0.17 | −0.08 ± 0.15 | −0.02 ± 0.11 |
| PPR0 | PWWT | −0.03 ± 0.20 | −0.08 ± 0.15 | −0.13 ± 0.19 |
| PPR30 | BWT | 0.01 ± 0.19 | 0.06 ± 0.16 | 0.001 ± 0.05 |
| PPR30 | WWT | 0.04 ± 0.22 | 0.09 ± 0.18 | 0.001 ± 0.12 |
| PPR30 | PWWT | 0.29 ± 0.22 | 0.10 ± 0.19 | −0.06 ± 0.19 |
a r d = direct additive correlation; ram = correlation between direct additive effects on PPR0 or PPR30 and maternal additive effects on BW; rp = phenotypic correlation.
Correlations between NLB and NLW and lamb FEC.
Bivariate models for lamb FEC and ewe NLB and NLW included only direct additive covariances between the pairs of traits. Univariate models for lamb FEC (Ngere et al., 2018) revealed no significant maternal additive or dam permanent environmental effects on lamb FEC, and these effects therefore did not appear in bivariate models involving lamb FEC. Covariances between residual effects on lamb FEC and future permanent environmental and residual effects on ewe NLB and NLW were also assumed equal to 0. Both NLB and NLW had similar direct additive and phenotypic associations with lamb FEC (Table 7). Estimates of direct additive correlations were moderate and positive between lamb WFEC and subsequent NLB and NLW in retained replacement females. Estimates of direct additive correlations between lamb PWFEC and subsequent NLB and NLW were larger than those obtained for WFEC and consistently exceeded 2 times their SE. However, because of high CV and low to modest heritabilities for these traits, phenotypic correlations were generally low, and positive phenotypic associations among these traits would have been difficult to observe in small flocks under typical production conditions. In contrast to results obtained for lamb BW, removing records of NLB and NLW for yearling ewes from the data had essentially no effect on estimates of the direct additive correlations between lamb FEC and subsequent ewe reproductive performance (results not shown). These results thus do not provide evidence for residual carryover effects of lamb FEC on future reproductive performance.
Table 7.
Correlation estimates and SE between numbers of lambs born (NLB) and weaned (NLW) per ewe lambing and ewe peri-parturient rise fecal egg counts at lambing (PPR0) and 30 d postpartum (PPR30) and lamb weaning (WFEC) and post-weaning (PWFEC) fecal egg countsa
| Trait 1 | Trait 2 | r d | r p |
|---|---|---|---|
| NLB | WFEC | 0.27 ± 0.16 | 0.04 ± 0.02 |
| NLB | PWFEC | 0.38 ± 0.13 | 0.06 ± 0.02 |
| NLW | WFEC | 0.32 ± 0.18 | 0.04 ± 0.02 |
| NLW | PWFEC | 0.40 ± 0.15 | 0.05 ± 0.02 |
| PPR0 | WFEC | 0.56 ± 0.02 | 0.15 ± 0.04 |
| PPR0 | PWFEC | 0.56 ± 0.13 | 0.16 ± 0.04 |
| PPR30 | WFEC | 0.77 ± 0.16 | 0.19 ± 0.04 |
| PPR30 | PWFEC | 0.73 ± 0.13 | 0.19 ± 0.04 |
a r d = direct additive correlation; rp = phenotypic correlation.
Correlations between ewe PPR and lamb FEC.
Bivariate models for ewe PPR and lamb FEC also included only direct additive covariances between the pairs of traits. Direct additive correlations of PPR0 and PPR30 with WFEC and PWFEC were relatively large, positive, and exceeded 4 times their SE (Table 7), suggesting that substantial numbers of genes had consistent impacts on parasite resistance in both lambs and peri-parturient ewes. Estimates of phenotypic correlations were also positive and significant but lower than direct additive correlations.
DISCUSSION
Estimates of h2 and c2 for NLB of 0.09 ± 0.01 and 0.02 ± 0.01, respectively, were somewhat smaller than averages of 0.13 and 0.05, respectively, reported by Safari et al. (2005) but consistent with estimates obtained for other U.S. breeds (Rao and Notter, 2000). Estimates of h2 and c2 for NLW of 0.06 ± 0.01 and 0.03 ± 0.01, respectively, however, were close to averages reported by Safari et al. (2005). Direct additive and phenotypic correlations between NLB and NLW (0.88 ± 0.03 and 0.74 ± 0.004, respectively) were higher than the average value of 0.70 reported by Safari et al. (2005) and estimates of 0.70 to 0.75 reported by Vanimisetti et al. (2007) in an earlier analyses of NSIP Katahdin data. A higher value for the correlation between NLB and NLW in the current study compared with earlier studies in this, and other, breeds may have been associated with a trend toward reductions in voluntary lamb removal (i.e., fostering of lambs to other ewes or removal for artificial rearing) in NSIP Katahdin flocks in order to more accurately assess ewe genetic potentials for NLW.
Generally positive, but small, direct additive associations between lamb BW and subsequent ewe NLB were consistent with averages presented by Safari et al. (2005). However, both results of the current study and averages presented by Safari et al. (2005) indicated that additive effects on BW were essentially independent of additive effects on subsequent NLW. In other NSIP breeds, Rao and Notter (2000) observed that direct additive correlations between weaning weights and NLB were positive and often significant in Targhee and Suffolk sheep but did not differ from zero in Polypay sheep. Estimates of correlations between maternal additive effects on weaning weight and subsequent direct additive effects on NLB in that study were positive for Suffolk sheep but did not differ from zero for Targhee or Polypay sheep. Pre-adjustment of lamb BW for effects of litter size and rearing type accounted for antagonistic phenotypic effects of these variables on BW. These phenotypic effects were considered to be mainly environmental in origin and are commonly accounted for in estimation of genetic parameters involving lamb growth and ewe reproduction, but adjustment of lamb BW for effects of litter size and rearing type in bivariate models potentially removed transmitted additive effects of genes from the dam with additive effects on both BW and NLB or NLW. Genetic correlations in Table 5 therefore do not reflect anticipated antagonistic associations between genes for NLB or NLW in the dam and the phenotypic expression of BW in their lambs. They instead estimate additive genetic associations between ewe NLB and NLW and lamb growth in ewes with the same distributions of NLB or NLW. Antagonisms between NLB and NLW and lamb BW are, however, important at the phenotypic level and must be considered in developing breeding objectives and economic weightings (e.g., Borg et al., 2007).
Heritability estimates for FEC in peri-parturient ewes were 0.35 ± 0.06 at lambing and 0.24 ± 0.07 at 30 d postpartum and somewhat higher than heritability estimates for FEC of 0.19 to 0.24 in growing Katahdin lambs (Ngere et al., 2018). These results suggest that genetic improvement in parasite resistance in Katahdin sheep is possible for both growing lambs and peri-parturient ewes. Similarly, Gray (1991) noted a positive phenotypic correlation between FEC in lambs and in the same animals during the peri-parturient rise. Results from the current study differed from results reported by Goldberg et al. (2012) in Merino sheep in Uruguay indicating that the heritability of FEC in growing lambs (0.25 ± 0.03) was higher than that observed in peri-parturient ewes (0.08 ± 0.03). However, heritability estimates for FEC in peri-parturient ewes from the current study were similar to the estimate of 0.37 ± 0.06 obtained by Morris et al. (1998) for FEC in Romney ewes in New Zealand sampled at 1 to 2 mo after lambing and representing lines selected for high or low FEC in naturally infected 4- to 7-mo-old lambs that grazed pastures contaminated with a mixed population of gastrointestinal nematodes.
Direct additive correlations between FEC in growing lambs and peri-parturient ewes were 0.56 for ewes evaluated around the time of lambing and approximately 0.75 at 30 d postpartum. These estimates were consistent with the estimate of 0.81 for the direct additive correlation between FEC in growing lambs and peri-parturient ewes reported by Goldberg et al. (2012). In that study, FEC were determined in naturally infected post-weaning lambs at 9 to 12 mo of age and in peri-parturient ewes evaluated up to 3 times from 50 d before to 68 d after lambing. Experimental confirmation of a positive association between parasite resistance in growing lambs and peri-parturient ewe also can be found in the significant positive correlated responses in FEC in peri-parturient ewes obtained in lines of artificially infected Australian Merino (Woolaston, 1992) and naturally infected New Zealand Romney (Morris et al., 1998) sheep selected for high or low FEC in growing lambs. Morris et al. (1998) reported estimates of the genetic correlation between FEC in growing lambs and peri-parturient ewes of 0.70 based on animal-model analysis of covariance and of 0.58 based on realized selection responses to divergent selection for FEC in growing lambs. The literature thus suggests that similar sets of genes are involved in expression of parasite resistance in growing lambs and peri-parturient ewes. The current study further indicated that this positive association can be extended to Katahdin lambs evaluated at relatively young ages (i.e., 42 to 90 d of age) and with limited prior exposure to gastrointestinal nematodes.
Estimates of additive and phenotypic correlations between PPR and NLB or NLW were all positive, but estimates of additive genetic correlations were similar in magnitude to their SE. These results thus indicate that there may be a small genetic antagonism between ewe prolificacy and parasite susceptibility in the first 30 d of lactation. However, associations between PPR, NLB, and NLW are potentially complex combinations of genetic, environmental, and causal phenotypic effects and must be carefully interpreted and properly implemented in terms of their impact on net economic merit. Litter size and NLW are indicators of genetic merit for prolificacy but also had significant causal phenotypic effect on PPR (Table 2). Ewes that gestated and, particularly, suckled larger litters presumably had greater nutrient demands during gestation and early lactation and may have been less able to respond to the peri-parturient rise. Results in Table 2 were, at first sight, somewhat surprising. As might have been expected, ewes that suckled twins and triplets had higher PPR30 than ewes that suckled singles. However, ewes that produced triplet litters had lower PPR0 than ewes that produced twins and did not differ from ewes that produced singles. This result may have been due to sampling errors associated with relatively small numbers of triplet litters. However, it is also possible that ewes that were able to produce triplet litters were better-conditioned and more fit at lambing than ewes that produced twins but could not retain this initial advantage while suckling 3 lambs. Adjustment of PPR for phenotypic effects of NLB or NLW is appropriate if associations between PPR and NLB or NLW were predominantly environmental and may, in fact, be causal and, therefore, unidirectional. Adjustment of PPR for effects of NLB or NLW would be expected to improve accuracy of EBV for PPR, but there was little effect on heritability estimates in the current study.
Measurement of PPR in peri-parturient ewes requires that the measurement protocol be integrated into normal flock management procedures in a way that allows expression of genetic differences in PPR but does not unduly compromise the health and welfare of gestating and lactating ewes and their offspring. Changes in PPR in Katahdin ewes in late gestation and early lactation were relatively dynamic, involving nonlinear increases in PPR in late gestation and subsequent reductions in PPR after about 28 d postpartum as ewes begin to control the peri-parturient infection (Notter et al., 2017). Notter et al. (2017) concluded that measurements of PPR for use in genetic evaluation should be taken between 7 d before and 35 d after lambing. This recommendation is supported by the estimated direct additive correlation of 0.96 ± 0.07 between PPR at lambing and 30 d postpartum.
In warm, subhumid regions of the United States where Katahdin sheep are popular, ewes often lamb outdoors in mid- to late spring. Sampling protocols to assess FEC in peri-parturient ewes must be designed to accommodate (and, when possible, take advantage of) typical management activities. For ewes that are housed, or otherwise closely monitored at lambing, collection of peri-parturient FEC is probably best done when groups of ewes in late pregnancy are brought into the lambing area on a daily basis as ewes lamb, or when groups of postpartum ewes leave the lambing pens. Regardless of the level of confinement around the time of lambing, tagging of lambs at or near the time of birth is an essential activity in pedigreed flocks, and collection of fecal samples from the ewes can potentially accompany tagging of the lambs.
Previous recommendations to control parasitic disease associated with the PPR in ewes considered deworming all ewes at or before lambing. However, because refugia (proportion of gastrointestinal nematodes unexposed to anthelmintic; Van Wyk, 2001) are low at this time, faster development of anthelmintic resistance occurred (Leathwick et al., 2006). Anthelmintic resistance is now highly prevalent (Howell et al., 2008), and current recommendations include selective treatment of ewes showing signs of parasitism (Van Wyk and Bath, 2002; Bath and Van Wyk, 2009; Kenyon et al., 2009). Recording of PPR for use in genetic evaluation, however, may require that treatment of peri-parturient ewes be deferred to allow expression of genetic differences in parasite resistance, increasing their vulnerability to negative effects of parasitism. Careful monitoring of ewes is therefore necessary. For flocks that practice strategic deworming based on FAMACHA scores or other indicators of parasitism, ewes can be assessed when fecal samples are collected for determination of PPR and treated strategically as necessary. However, treatment of the whole flock prior to the start of lambing would likely preclude meaningful assessment of PPR.
Estimates of heritabilities and additive and phenotypic correlations for lamb BW and FEC and ewe NLB, NLW, and PPR from the current study and from Ngere et al. (2017, 2018) are summarized in Supplementary Tables S1 and S2. The overall additive covariance matrix for these traits was positive definite, although the determinant was small, indicating that the covariance matrix was internally consistent. Absolute values for estimates of additive correlations between FEC in either lambs or ewes and other recorded variables were generally below 0.4 and only rarely exceeded 2 times their SE. Attention to breeding value predictions for the full range of economically important traits should thus permit simultaneous genetic improvement in all traits. However, occasional significant or near-significant genetic antagonisms were observed between measures of maternal productivity and parasite resistance. Examples include the positive correlation between direct additive effects on post-weaning FEC in lambs and NLB (0.38), NLW (0.40), and maternal effects on lamb post-weaning weight (0.29; Ngere et al., 2018). Similar positive, but smaller, correlations were also observed between these maternal traits and lamb FEC at weaning and between NLB and NLW and measures of PPR in ewes. Brown and Fogarty (2017) also reported only small correlations between FEC and growth, carcass quality, and reproduction traits in the Australian Merino sheep.
Previous studies demonstrated that genetic improvement in number of lambs weaned dominated breeding objectives for meat sheep (Borg et al., 2007; Vanimisetti et al., 2007). Estimates of genetic associations between NLW and NLB and other variables are therefore critical to predict correlated responses to selection to improve ewe productivity. Parameterization of multivariate models involving NLB and NLW is likewise critical, as are inferences regarding impacts of resulting EBV on breeding objectives. In the current context, BV predictions for health, reproductive, and fitness traits in Katahdin sheep involved several traits with causal phenotypic associations. Indeed, NLW itself has a strong causal phenotypic association with NLB and may be viewed as a proxy variable that combines effects of NLB of the dam and direct and maternal effects on lamb survival. An analogous situation existed for lamb BW and FEC (Ngere et al., 2018), which are co-expressed in time and may also have simultaneous recursive phenotypic effects on one another. Smaller lambs are more vulnerable to parasitism and more highly parasitized lambs grow less rapidly, potentially leading to antagonistic synergy between BW and FEC. Somewhat surprisingly, Ngere et al. (2018) did not find significant effects of ewe litter size on lamb FEC in these data and reported only small phenotypic correlations between lamb BW and FEC (−0.07 to 0.01). These results allowed use of relatively simple models for lamb FEC and avoided problems of inference analogous to those experienced for ewe PPR and NLB or NLW. However, modeling of NLB, NLW, and PPR and lamb BW and FEC as structurally interrelated traits would have potential to better explain the genetic architecture of these traits. Apparent differences between yearling and older ewes in the association between lamb BW and ewe NLB (Table 5) also suggested a possible structural association between these variables in yearling ewes. Models for structurally related traits were described by Wright (1960), discussed in the context of antagonisms between productive and reproductive traits by Notter (1986), rigorously modeled by Gianola and Sorensen (2004), and applied to productive and fitness traits in dairy cattle by de los Campos et al. (2006). Understanding interrelationships among production, reproduction, and fitness traits is particularly important for development of breeding objectives. In the presence of causal phenotypic associations, a unit change in BV for a given trait may affect phenotypically correlated traits without producing changes in BV for those traits. Thus, in the current study, a unit change in BV for NLW would be expected to have a negative effect on lamb BW and ewe PPR30, but would not necessarily be associated with changes in BV for these traits.
In conclusion, FEC in peri-parturient Katahdin ewes were heritable and should both respond to direct selection and exhibit a positive correlated response to selection for low FEC in growing lambs. Similar heritability estimates for lamb FEC and ewe PPR indicated that incorporation of measurements of FEC in peri-parturient ewes into NSIP genetic evaluations should increase the accuracy of evaluation of parasite resistance in both lambs and ewes. No evidence was found for serious genetic antagonisms between FEC in ewes or lambs and other economically important traits. However, the data suggested that modest genetic antagonisms may exist between expressions of ewe maternal capacity (NLB, NLW, maternal effects on lamb BW) and parasite resistance. The definition of comprehensive breeding objectives that include consideration of the anticipated level of parasite challenge and the measurement of the full spectrum of economically important traits is thus recommended to optimize selection responses.
SUPPLEMENTARY DATA
Supplementary data are available at Journal of Animal Science online.
ACKNOWLEDGMENTS
Supported by the USDA National Institute of Food and Agriculture Organic Research and Education Initiative (project number 2016-51300-25723). This manuscript is dedicated to Dr Charles Parker by the authors and the cooperating Katahdin producers for his advocacy for more than 40 yr to the importance of selecting sheep resistant to gastrointestinal nematodes and use of quantitative genetics. Without his tireless promulgation, the Katahdin flocks would have failed to be recruited to meet his challenge. Mention of trade names or commercial products in this manuscript is solely for providing specific information and does not imply recommendation or endorsement by the U.S. Department of Agriculture (USDA). The USDA prohibits discrimination in all its programs and activities on the basis of race, color, national origin, age, disability, and where applicable, sex, marital status, familial status, parental status, religion, sexual orientation, genetic information, political beliefs, reprisal, or because all or part of an individual’s income is derived from any public assistance program. (Not all prohibited bases apply to all programs.) Persons with disabilities who require alternative means for communication of program information (Braille, large print, audiotape, etc.) should contact USDA’s TARGET Center at (202) 720-2600 (voice and TDD). To file a complaint of discrimination, write to USDA, Director, Office of Civil Rights, 1400 Independence Avenue, S.W., Washington, DC 20250-9410, or call (800) 795-3272 (voice) or (202) 720-6382 (TDD). USDA is an equal opportunity provider and employer.
LITERATURE CITED
- Barger I. A. 1996. The impact of adult Sheep on nematode parasitism – an Australian perspective. In: Proc. 2nd Pan Pacific Vet. Conf.: Sheep Sess , Christchurch, NZ p. 66–72. [Google Scholar]
- Bath G. F., and Van Wyk J. A.. 2009. The five point check© for targeted selective treatment of internal parasites in small ruminants. Sm. Rumin. Res. 86:6–13. doi:10.1016/j.smallrumres.2009.09.009 [Google Scholar]
- Borg R. C., Notter D. R., Kuehn L. A., and Kott R. W.. 2007. Breeding objectives for Targhee sheep. J. Anim. Sci. 85:2815–2829. doi:10.2527/jas.2006-064 [DOI] [PubMed] [Google Scholar]
- Bradford G. E. 2002. Selection for reproductive efficiency. Sheep Goat Res. J. 17:6–10. [Google Scholar]
- Brown D. J., and Fogarty N. M.. 2017. Genetic relationships between internal parasite resistance and production traits in Merino sheep. Anim. Prod. Sci. 57:209–215. doi:10.1017/AN15469 [Google Scholar]
- Connan R. M. 1968. Studies on the worm populations in the alimentary tract of breeding ewes. J. Helminthol. 42:9–28. doi:10.1017/S0022149X00027176 [DOI] [PubMed] [Google Scholar]
- de los Campos G., Gianola D., and Heringstad B.. 2006. A structural equation model for describing relationships between somatic cell score and milk yield in first-lactation dairy cows. J. Dairy Sci. 89:4445–4455. doi:10.3168/jds.S0022-0302(06)72493-6 [DOI] [PubMed] [Google Scholar]
- Gianola D. and Sorensen D.. 2004. Quantitative genetic models for describing simultaneous and recursive relationships between phenotypes. Genetics 167:1407–1424. doi:10.1534/genetics.103.025734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmour A. R., Gogel B. J., Cullis B. R., Welham S. J., and Thompson R.. 2015. ASReml user guide release 4.1. structural specification. VSN International Ltd, Hemel Hempstead, UK. [Google Scholar]
- Goldberg V., Ciappesoni G., and Aguilar I.. 2012. Genetic parameters for nematode resistance in periparturient ewes and post-weaning lambs in Uruguayan Merino sheep. Livest. Sci. 147:181–187. doi:10.1016/j.livsci.2012.05.003 [Google Scholar]
- Gray G. D. 1991. Breeding for resistance to trichostrongyle nematodes in sheep. In: R. F. E. Axford and J. B. Owen, editors, Breeding for disease resistance in farm animals. Comm. Agric. Bureau Intern, Wallingford, UK: p. 139–161. [Google Scholar]
- Hanford K. J., Van Vleck L. D., and Snowder G. D.. 2006. Estimates of genetic parameters and genetic trend for reproduction, weight, and wool characteristics of Polypay sheep. Livest. Sci. 102:72–82. doi:10.1016/j.livsci.2005.11.002 [Google Scholar]
- Howell S. B., J. M. Burke J. E. Miller T. H. Terrill E. Valencia M. J. Williams L. H. Williamson A. M. Zajac, and Kaplan R. M.. 2008. Prevalence of anthelmintic resistance on sheep and goat farms in the southeastern United States. J. Am. Vet. Med. Assoc. 233:1913–1919. doi:10.2460/javma.233.12.1913 [DOI] [PubMed] [Google Scholar]
- Kenyon F., A. W. Greer G. C. Coles G. Cringoli E. Papadopoulos J. Cabaret B. Berrag M. Varady J. A. Van Wyk E. Thomas, et al. 2009. The role of targeted selective treatments in the development of refugia-based approaches to the control of gastrointestinal nematodes of small ruminants. Vet. Parasitol. 164:3–11. doi:10.1016/j.vetpar.2009.04.015 [DOI] [PubMed] [Google Scholar]
- Leathwick D. M., C. M. Miller D. S. Atkinson N. A. Haack R. A. Alexander A. M. Oliver T. S. Waghorn J. F. Potter, and Sutherland I. A.. 2006. Drenching adult ewes:implications of anthelmintic treatments pre- and post-lambing on the development of anthelmintic resistance. N. Z. Vet. J. 54:297–304. doi:10.1080/00480169.2006.36714 [DOI] [PubMed] [Google Scholar]
- Morris C. A., Bisset S. A., Vlassoff A., West C. J., and Wheeler M.. 1998. Faecal nematode egg counts in lactating ewes from Romney flocks selectively bred for divergence in lamb faecal egg count. Anim. Sci. 67: 283–288. doi:10.1017/S1357729800010043 [Google Scholar]
- Ngere L., Burke J. M., Morgan J. L. M., Miller J. E., and Notter D. R.. 2018. Genetic parameters for fecal egg counts and their relationship with body weights in Katahdin lambs. J. Anim. Sci. (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngere L., J. M. Burke D. R. Notter, and Morgan J. L. M.. 2017. Variance components for direct and maternal effects on body weights of Katahdin lambs. J. Anim. Sci. 95:3396–3405. doi:10.2527/jas.2017.1596 [DOI] [PubMed] [Google Scholar]
- Notter D. R. 1986. Feed intake specifications in defining breeding objectives. Proc. 3rd World Congr. Genet. Appl. Livestock Prod. 9:232–243. [Google Scholar]
- Notter D. R., J. M. Burke J. E. Miller, and Morgan J. L.. 2017. Factors affecting fecal egg counts in periparturient Katahdin ewes and their lambs. J. Anim. Sci. 95:103–112. doi:10.2527/jas.2016.0955 [DOI] [PubMed] [Google Scholar]
- O’Sullivan B. M., and Donald A. D.. 1970. A field study of nematode parasite populations in the lactating ewe. Parasitology 61:301–315. doi:10.1017/S0031182000041135 [DOI] [PubMed] [Google Scholar]
- Rao S. and Notter D. R.. 2000. Genetic analysis of litter size in Targhee, Suffolk, and Polypay sheep. J. Anim. Sci. 78:2113–2120. doi:10.2527/2000.7882113x [DOI] [PubMed] [Google Scholar]
- Safari E., Fogarty N. M., and Gilmour A. R.. 2005. A review of genetic parameter estimates for wool, growth, meat and reproduction traits in sheep. Lives. Prod. Sci. 92:271–289. doi:10.1016/j.livprodsci.2004.09.003 [Google Scholar]
- Vanimisetti H. B., S. P. Greiner A. M. Zajac, and Notter D. R.. 2004. Performance of hair sheep composite breeds:resistance of lambs to Haemonchus contortus. J. Anim. Sci. 82:595–604. doi:10.2527/2004.822595x [DOI] [PubMed] [Google Scholar]
- Vanimisetti H. B., D. R. Notter, and Kuehn L. A.. 2007. Genetic (co)variance components for ewe productivity traits in Katahdin sheep. J. Anim. Sci. 85:60–68. doi:10.2527/jas.2006-248 [DOI] [PubMed] [Google Scholar]
- Van Wyk J. A. 2001. Refugia–overlooked as perhaps the most potent factor concerning the development of anthelmintic resistance. Onderstepoort J. Vet. Res. 68:55–67. [PubMed] [Google Scholar]
- Van Wyk J. A. and Bath G. F.. 2002. The FAMACHA system for managing haemonchosis in sheep and goats by clinically identifying individual animals for treatment. Vet. Res. 33:509–529. doi:10.1051/vetres:2002036 [DOI] [PubMed] [Google Scholar]
- Wang C. T. and Dickerson G. E.. 1991. Simulation of life-cycle efficiency of lamb and wool production for genetic levels of component traits and alternative management options. J. Anim. Sci. 69:4324–4337. doi:10.2527/1991.69114312x [DOI] [PubMed] [Google Scholar]
- Wildeus S. 1997. Hair sheep genetic resources and their contribution to diversified small ruminant production in the United States. J. Anim. Sci. 75:630–640. doi:10.2527/1997.753630x [DOI] [PubMed] [Google Scholar]
- Woolaston R. R. 1992. Selection of merino sheep for increased and decreased resistance to Haemonchus contortus: peri-parturient effects on faecal egg counts. Int. J. Parasitol. 22:947–953. doi:10.1016/0020-7519(92)90052-M [DOI] [PubMed] [Google Scholar]
- Wright S. 1960. The treatment of reciprocal interaction, with or without lag, in path analysis. Biometrics 16:423–445. doi:10.2307/2527693 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.