Abstract
Prior work using a contractility bioassay determined that the serotonin (5-HT) receptor subtype 5-HT2A is present in bovine lateral saphenous veins and plays a role in ergot alkaloid (EA)-induced vascular contraction in steers grazing endophyte-infected (Epichloë coenophiala) tall fescue (Lolium arundinaceum). Ergot alkaloids have also been shown to be vasoactive in bovine gut vasculature. To determine what 5-HT receptors are involved in vasoconstriction of gut vasculature, contractility of ruminal and mesenteric arteries and veins collected from cattle was evaluated in the presence of agonists selective for 5-HT1B (CP 93129), 5-HT1D (L-694, 247), 5-HT2A (TCB-2), 5-HT2B (BW 723C86), 5-HT4 (BIMU-8), and 5-HT7 (LP 44) receptors. Segments of ruminal and mesenteric veins and arteries were collected and suspended in a multimyograph containing continuously oxygenated Krebs–Henseleit buffer. Blood vessels were exposed to increasing concentrations of 5-HT agonists every 15 min and contractile response data were normalized as a percentage of the maximum contractile response induced by 120 mM KCl. Analysis of variance was evaluated using mixed models procedure of SAS for effects of agonist concentration for each vessel type. Receptor agonists for 5-HT2B, 5-HT1D, and 5-HT7 did not induce a contractile response for ruminal or mesenteric vasculature (P > 0.05). However, when exposed to agonists for 5-HT2B or 5-HT1D, mesenteric veins relaxed below zero (P < 0.05). Exposure of all 4 blood vessel types to 5-HT2A agonist induced contractile responses (P < 0.05). The findings of this study indicate that 5-HT1D and 5-HT2B are present in mesenteric veins and may play a role in vasorelaxation. Further, 5-HT2A is present in ruminal and mesenteric vasculature, plays a role in vasoconstriction of these vessels, and could be influenced by EA exposure as has been demonstrated in peripheral blood vessels.
Keywords: bovine, mesenteric artery and vein, ruminal artery and vein, serotonin, vasoconstriction
INTRODUCTION
Epichlöe coenophiala is an endophyte symbiotically related with tall fescue grass (Lolium arundinaceum). Epichlöe coenophiala produces numerous ergot alkaloids (EA) which have been identified as causative agents of vasoconstriction associated with fescue toxicosis. Various studies have reported that EA induce vasoconstriction in bovine uterine and umbilical arteries (Dyer, 1993), right ruminal artery and vein (Foote et al., 2011), mesenteric artery and vein (Egert et al., 2014), and lateral saphenous vein (Klotz et al., 2008, 2010). Ergot alkaloids have structural similarities with biogenic amines, such as serotonin (5-hydroxytryptamine, 5-HT), and cause vasoconstriction by binding to receptors (Berde, 1980; Dyer, 1993), possibly causing decreased blood flow observed in gastrointestinal vasculature (Rhodes et al., 1991; Foote et al., 2011).
Foote et al. (2011) found that EA could play a role in vasoconstriction of the bovine right ruminal artery and vein, possibly reducing blood flow to or from the rumen and resulting in decreased absorption rates of nutrients and fermentation products. Ergot alkaloid-induced vasoconstriction was not only seen in ruminal vasculature but also in mesenteric vasculature. Egert et al. (2014) demonstrated that various EA were vasoactive in bovine mesenteric vasculature. Further, steers with prior exposure to endophyte-infected tall fescue had a diminished response to many EA and 5-HT in the mesenteric vasculature. Because there are a number of different serotonin receptor subtypes (Hoyer et al., 1994), it is necessary to assess the receptor populations available for possible EA interaction. Therefore, the objective of this study was to pharmacologically determine which 5-HT receptors are involved in contractility of bovine gut vasculature using agonists selective for 5-HT1D, 5-HT1B, 5-HT2A, 5-HT2B, 5-HT4, and 5-HT7 receptors.
MATERIALS AND METHODS
No live animals were involved in this study, thus approval from the University of Kentucky Animal Care and Use Committee was not required.
Animals and Tissue Collection
Blood vessels were collected from the carcasses of 24 mixed breed and gender cattle between 10 and 30 mo of age (542 ± 23 kg) that were slaughtered at the University of Kentucky Meats Lab or Hi-View Meats (Sadieville, KY). As previously described by Klotz and Barnes (2014), the gastrointestinal tract was removed and the ileocecal fold and ileal flange were identified as landmarks in order to remove multiple branches of common mesenteric artery (MA) and vein (MV) segments. The ventral coronary and caudal grooves were identified as landmarks to remove segments of the right ruminal artery (RA) and vein (RV) as previously described by Klotz et al. (2011). Vessel segments of 2–3 cm were immersed and transported in oxygenated Krebs–Henseleit buffer (95% O2/5% CO2; pH = 7.4; 11.1 mM D-glucose; 1.2 mM MgSO4; 1.2 mM KH2PO4; 4.7 mM KCl; 118.1 mM NaCl; 3.4 mM CaCl2; 24.9 mM NaHCO3; Sigma Chemical Co., St. Louis, MO). The MA, MV, RA, and RV were stored on ice until vessels were manually separated from surrounding fascia and adipose tissues. Vessels were sliced into approximately 2-mm segments and viewed under a dissection microscope (Stemi 2000-C, Carl Zeiss Inc., Oberkochen, Germany) at 12.5× magnification to check for vessel abnormalities, such as damage incurred during dissection and branches, and to assess vascular dimensions. Cross sections with structural damage were replaced with undamaged segments. Because MV and RV are pliant, vascular dimensions were only recorded for MA and RA cross sections using Axiovision (version 20, Carl Zeiss Inc.).
Standard Preparations
All receptor agonists were obtained from Tocris Bioscience (Minneapolis, MN). Stock solutions of the agonists CP 93129 dihydrochloride (CP 93129; 5-HT1B; Cat. No. 1032), L-694, 247 (L-694, 247; 5-HT1D; Cat. No. 0781), TCB-2 (TCB-2; 5-HT2A; Cat. No. 2592), BW 723C86 hydrochloride (BW 723C86; 5-HT2B; Cat. No. 1059), BIMU-8 (BIMU-8; 5-HT4; Cat. No. 4374), and LP 44 (LP 44; 5-HT7; Cat. No. 2534) (Table 1) were diluted to corresponding concentrations for final working concentrations in tissue wells of 1 × 10−11 to 1 × 10−4 M for LP 44 and 5 × 10−9 to 1 × 10−4 M for all other agonists. LP 44, BW, L-694, TCB-2, and BIMU-8 were prepared in dimethylsulfoxide (DMSO) (472301; Sigma Chemical Co.). The 5-HT1B agonist CP 93129 was prepared in H2O. Eight standard additions of LP 44 were prepared based on limited available stock of LP 44 (1 × 10−11, 1 × 10−10, 1 × 10−9, 1 × 10−8, 1 × 10−7, 1 × 10−6, 1 × 10−5, and 1 × 10−4 M). For all other agonists, there were a total of 10 standard additions (5 × 10−9, 1 × 10−8, 5 × 10−8, 1 × 10−7, 5 × 10−7, 1 × 10−6, 5 × 10−6, 1 × 10−5, 5 × 10−4, and 1 × 10−4 M). Standard addition concentration ranges were prepared based on previous bovine vascular bioassay research using 5-HT agonists (Klotz et al., 2013; Klotz et al., 2016). All additions were added to tissue chambers in increasing order of concentration in volumes that did not exceed 0.5% of the total buffer.
Table 1.
Agonists, function, and distribution of serotonin (5-HT) receptor subtypes
| 5-HT receptor | Agonist | Receptor function | Distribution | Sources |
|---|---|---|---|---|
| 5-HT1B | CP 93129 dihydrochloride | Mood, locomotion, vasoconstriction | CNS, blood vessels | Weinshank et al. (1992) |
| 5-HT1D | L-694, 247 | Locomotion, vasoconstriction | CNS, blood vessels | Hamel et al. (1993) |
| 5-HT2A | TCB-2 | Appetite, thermoregulation, smooth muscle contractions, vasodilation, vasoconstriction | CNS, smooth muscles, blood vessels, platelets | Morecroft and MacLean (1998) |
| 5-HT2B | BW 723C86 hydrochloride | Appetite, vasoconstriction, CV function, GI motility | CNS, blood vessels, CV system, GI tract | Ullmer et al. (1995) |
| 5-HT4 | BIMU-8 | Mood, appetite, GI motility | CNS, GI tract | Dumuis et al. (1989) |
| 5-HT7 | LP 44 | Behavior, vasoconstriction | CNS, blood vessels | Ullmer et al. (1995) |
CNS = central nervous system; CV = cardiovascular.
Myograph Protocol
Mesenteric and ruminal artery and vein cross sections used to generate individual agonist dose–response curves were collected from n = 5 to n = 7 different animals depending on tissue viability and availability. Vessels were mounted onto luminal supports in individual wells of a multimyograph (DMT 610M, Danish Myo Technology, Atlanta, GA) containing 5 mL Krebs–Henseleit buffer subject to constant gassing (95% O2/5% CO2; pH = 7.4; 37 °C). The incubation buffer was the same composition as the transport buffer plus 3 × 10−5 M desipramine (D3900; Sigma Chemical Co.) to inactivate neuronal catecholamine uptake and 1 × 10−6 M propranolol (P0844; Sigma Chemical Co.) to block β-adrenergic receptors. Vessels were allowed to equilibrate under the above conditions for 90 min with buffer changes occurring every 15 min to allow a vessel to equilibrate at a resting tension of approximately 1 g. Experimental tissues were exposed to a reference addition of 120 mM KCl (Sigma Chemical Co.) for 15 min to confirm tissue responsiveness and viability. Vessels were rinsed with new incubation buffer every 15 min until approximately 1 g of tension was reached. Once vessels had returned to resting tension, standard additions were added for contractile response experiments. Additions were added in 15-min cycles in order of increased agonist concentration. Each cycle consisted of a 9-min incubation period, two 2.5-min buffer washes, a third buffer replacement, and 1-min recovery period before the next standard addition. Vessels were again exposed to 120 mM KCl once the experiment was completed to verify vessel viability for the entire experiment.
Isometric contractions in mesenteric and ruminal vessels to KCl, CP 93129, L-694, 247, TCB-2, BW 723C86, BIMU-8, and LP 44 were digitized and recorded in grams of tension using PowerLab16/35 and Chart software (version 7.3, ADInstruments, Colorado Springs, CO). Baseline tension was recorded before addition of 120 mM KCl. For all contractile response data, the maximum tension (measured in g) during the 9-min incubation was recorded as the contractile response and then corrected for baseline tension. Due to variation between animals and tissues, contractile response data were normalized as a percentage of the maximum grams of tension induced by the reference addition of KCl to compensate for differences in vessel responsiveness. Vessel contractile response data are reported as the percentage mean contractile response ± SEM of the maximum contractile response produced by the 120 mM KCl reference addition. Sigmoidal concentration response curves of ruminal and mesenteric vasculature to 5-HT agonists were calculated and plotted using nonlinear regression with fixed slope (GraphPad Prism 7, GraphPad Software Inc., La Jolla, CA). Data were plotted and calculated using a 3-parameter equation:
where y represents contractile response, x represents agonist concentration, top and bottom are the percentage of 120 mM KCl maximum contractile response at the plateaus, and EC50 is the molar concentration of the agonist producing 50% of the maximum response of KCl.
Statistical Analysis
All data were analyzed using the MIXED model of SAS (SAS 9.3, SAS Inst. Inc., Cary, NC). Contractile response and EC50 data for each agonist were analyzed as a completely randomized design for effect of agonist concentration and vessel type. The animal from which all 4 vessel types were collected was the experimental unit. A vessel × agonist concentration interaction was analyzed with vessel and agonist concentration being the main effects. Mean separation was conducted for data if the probability of a greater F-statistic in the ANOVA was significant for the effect of agonist concentration. Individual mean differences were evaluated by using the LSD feature of SAS. Data were analyzed for deviations from normality and homogeneity. Results are considered significant if probabilities are P < 0.05, unless reported otherwise.
RESULTS AND DISCUSSION
Presence of 5-HT2A Receptors: Responses to TCB-2
To our knowledge, this is the first study to profile the 5-HT receptor subtypes present in bovine ruminal and mesenteric vasculature. Because there are numerous 5-HT receptor subtypes located throughout the body, the biological responses to 5-HT are complex and not fully characterized. Serotonin can act as a vasodilator or vasoconstrictor depending on the animal species, blood vessel type, or status of the endothelial cell layer (Ni and Watts, 2006). Serotonin has been previously shown to be vasoactive in bovine ruminal artery and vein (Klotz et al., 2011) and mesenteric artery and vein (Egert et al., 2014). The 5-HT2A subtype is the primary receptor involved in vasoconstrictive responses. The current study found that there was a significant contractile response for mesenteric and ruminal vasculature to the 5-HT2A agonist TCB-2 (P < 0.05) (Fig. 1). Klotz et al. (2013) had previously identified the presence of 5-HT2A receptors in bovine lateral saphenous veins using the antagonist ketanserin. The onset of the contractile response was observed at the addition of 5 × 10−5 M TCB-2 for the MA, MV, and RV (P < 0.05), whereas the RA reached a maximal contractile response when exposed to a lower concentration, 5 × 10−6 M TCB-2 (P < 0.05). This is an indication of the presence of this serotonin receptor subtype in the blood vessels evaluated in the current study. Klotz et al. (2016) also demonstrated contractile responses in lateral saphenous veins in response to TCB-2 concentrations in a range similar to the concentration range used in this study.
Figure 1.
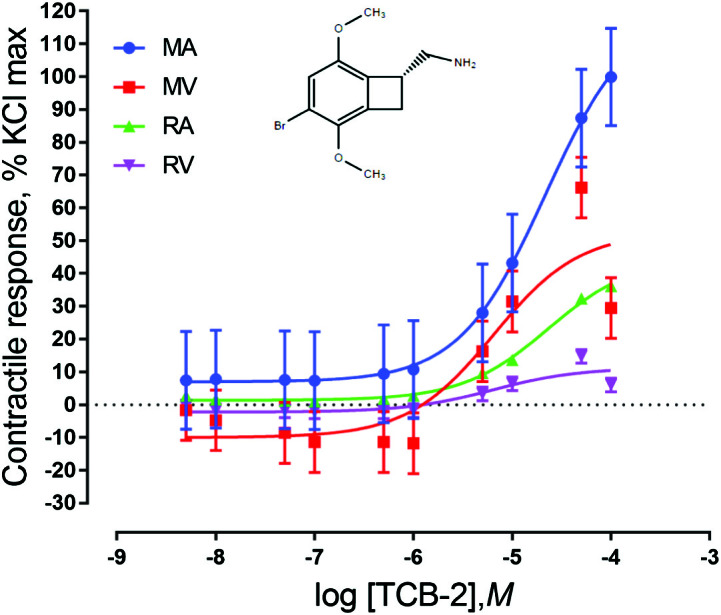
Chemical structure of the 5-HT2A agonist TCB-2 and mean contractile responses of mesenteric artery (MA; n = 7), mesenteric vein (MV; n = 7), ruminal artery (RA; n = 6), and ruminal vein (RV; n = 6) to increasing concentrations of TCB-2. Graphical points represent the mean and bars represent the SEM.
For EC50 values, only data regarding 5-HT2A were considered as all other experimental agonists failed to produce a positive sigmoidal contractile response. Because the units of expression for EC50 values are logarithmic, the larger the EC50 value, the lower the TCB-2 concentration needed to achieve 50% of total response and the more sensitive a vessel is to TCB-2. Thus, when comparing EC50 values of vessels exposed to TCB-2 (Table 2), the EC50 value of MA was higher than that of MV (P = 0.0047) and RV (P = 0.0025) but did not differ from RA (P = 0.266). Ruminal arteries also had higher EC50 values than MV (P = 0.0004) and RV (P = 0.0002). While MV and RV were different from MA and RA, they did not differ from each other (P = 0.7018). As RV and MV had lower EC50 values than MA and RA, it could be suggested that TCB-2 reached 50% of the maximum contractile response at a lower concentration, suggesting that RV and MV are more sensitive to TCB-2 than MA and RA. Egert et al. (2014) reported similar findings when exposing mesenteric vasculature to 5-HT as MA was found to be less sensitive to 5-HT exposure than MV.
Table 2.
Log EC50 values in response to TCB-2 for mesenteric arteries (MA), mesenteric veins (MV), ruminal arteries (RA), and ruminal veins (RV)1,2
| Myograph treatment | Vessel | Log EC50, M |
|---|---|---|
| TCB-2, M | MA | −4.79 ± 0.09 (7)3a |
| MV | −5.23 ± 0.09 (7)b | |
| RA | −4.63 ± 0.11 (6)a | |
| RV | −5.29 ± 0.11 (6)b |
Values are expressed as the mean ± SEM.
a,bWithin a column for Log EC50, means without a common superscript differ (P < 0.05).
1Log EC50 = measure of the potency of a drug or agonist that induces a response midway (50%) between the baseline and maximum after a certain time of exposure; expressed as the molar concentration of the agonist, in this instance, 120 mM KCl.
2EC50 values were only considered for vessels exposed to TCB-2 as TCB-2 was the only agonist that elicited a vasoconstrictive response.
3Values in parentheses are the number of animals used in each experiment.
A significant vessel type × concentration interaction was observed for vessels exposed to TCB-2 (P = 0.0005; Fig. 1). This indicates that the contractile response seen with TCB-2 depends on both the concentration of the agonist the vessel is exposed to and the type of blood vessel being used. Mesenteric arteries and RA exposed to TCB-2 differ from RV and MV, exhibiting a larger contractile response, which is supported by the lower EC50 values associated with the MA and RA responses. This evidence supports the idea that MA and RA may have a larger amount of functional 5-HT2A receptors present than RV and MV. This understanding is similar to the findings of Egert et al. (2014), where maximal response to 5-HT appeared greater in the MA than the MV (no direct comparison across vessel type was conducted in this study).
Previous myograph experiments using bovine vasculature have also shown varied sensitivity in response to agonist treatments. Larger contractile responses in arteries may be explained by the differential expression of the 5-HT2A receptor. Using rats, Kato et al. (1999) demonstrated that the expression of 5-HT2A receptors is significantly increased in the aorta compared to the vena cava. Watts (2002) also found that 5-HT2A receptors are the primary contractile receptor in mesenteric arteries of hypertensive rats. These findings indicate that there may be a larger quantity of 5-HT2A receptors in arteries than in veins. Regarding the larger responses seen in ruminal vasculature as compared to mesenteric vasculature, differences could be due to the type of blood vessel (e.g., ruminal vessels vs. mesenteric vessels) and a corresponding difference in 5-HT2A receptor population numbers.
Presence of 5-HT1B, 5-HT4, and 5-HT7 Receptors: Responses to CP 93129, BIMU-8, and LP 44
Ruminal and mesenteric vessels exposed to CP 93129, LP 44, and BIMU-8 did not exhibit contractile responses (P > 0.05) (Figs. 2–4). These results indicate that the 5-HT receptor subtypes 5-HT1B, 5-HT7, and 5-HT4 are not present or do not play a role in EA-induced vasoconstriction in bovine mesenteric or ruminal vessels. It is possible that these receptors are present but not in concentrations high enough to be detected by the bioassays used in the current, or they may be present, but not functional. For example, Banes and Watts (2003) showed that the 5-HT1B agonist CP 93129 failed to cause a contraction in the aorta of rats. However, molecular expression of 5-HT1B receptors was demonstrated in rats, indicating that although these receptors were present, they were not functional or involved in aortic contractions.
Figure 2.
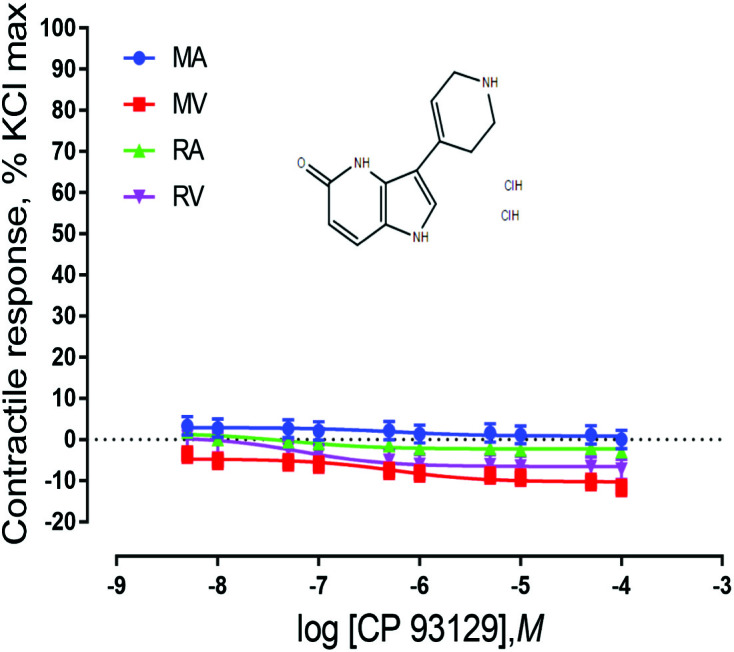
Chemical structure of the 5-HT1B agonist CP 93129 dihydrochloride and mean contractile responses of mesenteric artery (MA; n = 6), mesenteric vein (MV; n = 6), ruminal artery (RA; n = 6), and ruminal vein (RV; n = 6) to increasing concentrations of CP 93129 dihydrochloride. Graphical points represent the mean and bars represent the SEM.
Figure 3.
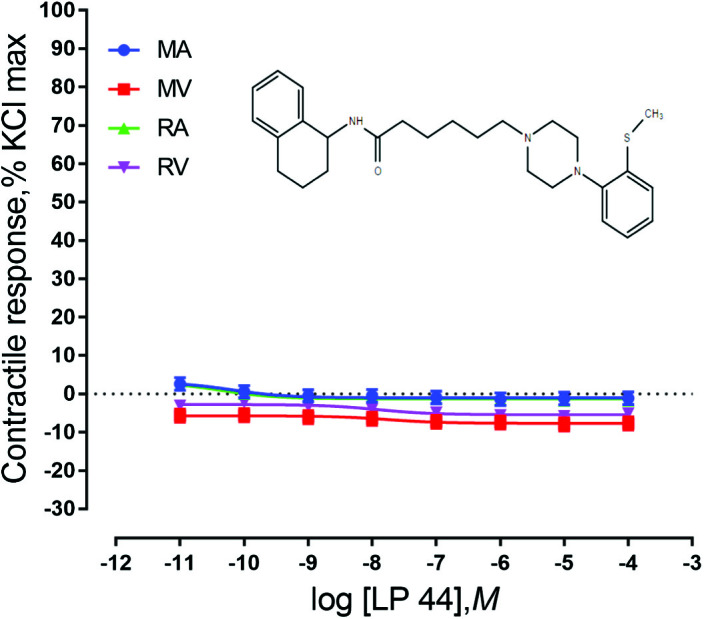
Chemical structure of the 5-HT7 agonist LP 44 and mean contractile responses of mesenteric artery (MA; n = 5), mesenteric vein (MV; n = 5), ruminal artery (RA; n = 5), and ruminal vein (RV; n = 5) to increasing concentrations of LP 44. Graphical points represent the mean and bars represent the SEM.
Figure 4.
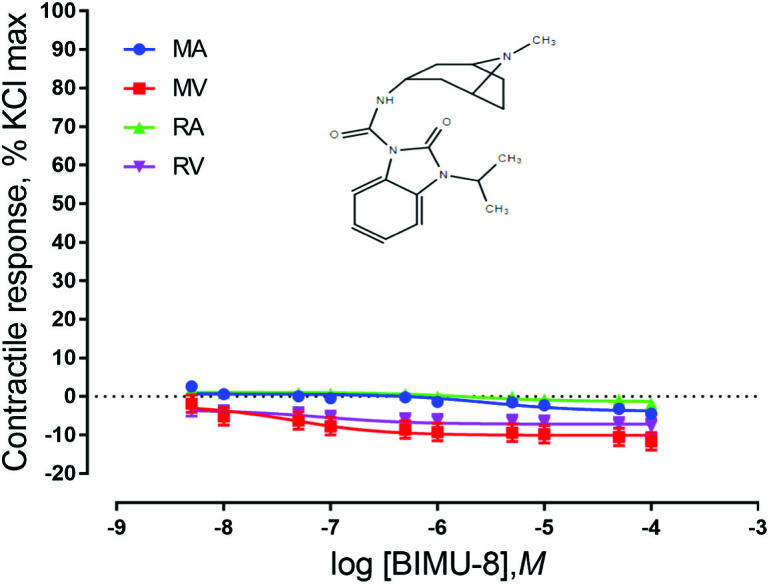
Chemical structure of the 5-HT4 agonist BIMU-8 and mean contractile responses of mesenteric artery (MA; n = 5), mesenteric vein (MV; n = 5), ruminal artery (RA; n = 5), and ruminal vein (RV; n = 5) to increasing concentrations of BIMU-8. Graphical points represent the mean and bars represent the SEM.
There was not a vessel × concentration interaction for vessels exposed to receptor agonists for 5-HT1B (P = 1.0; Fig. 2), 5-HT7 (P = 1.0; Fig. 3), or 5-HT4 (P = 0.99; Fig. 4). However, these vessels did exhibit significant responses based on both vessel type and concentration of agonist. Vessels exposed to 5-HT7 agonist exhibited a vessel effect (P < 0.0001) and an agonist concentration effect (P = 0.014). Mesenteric artery and RA responses were not different, but relaxed less than both MV and RV. Vessels also responded differentially to 5-HT4 agonist exposure (P < 0.0001) as well as responding to agonist concentration (P < 0.0001). Similar to vessels exposed to 5-HT7 agonist, MA and RA did not exhibit differing responses from each other but relaxed less than both RV and MV. Vessels exposed to 5-HT1B agonist exhibited a vessel effect (P < 0.0001) and an agonist concentration main effect (P = 0.0002). MA, MV, RA, and RV are all significantly different from one another (P < 0.0001). For both 5-HT1B and 5-HT4 agonists, MA relaxed less than MV and RA relaxed less than RV. In vessels exposed to CP 93129, MA had a stronger response than RV and RA had a stronger response than MV. This finding and the fact that MA and RA relaxed less in the presence of an agonist than MV and RV indicate that the location from which the vessel came from (mesenteric vs. ruminal) may not be as important as the type of vessel (artery vs. vein).
The 5-hydroxytryptamine 4 (5-HT4) receptor is coupled to adenylate cyclase and is distributed throughout the central nervous system and peripheral tissues, specifically in those tissues and organs that contain smooth muscle, such as the bladder and the alimentary tract. As mentioned previously, it was found in this experiment that 5-HT4 did not elicit a constrictive or relaxation response to the agonist BIMU-8 in ruminal or mesenteric vessels. 5-HT4 receptors have previously been found to induce endothelium-independent relaxation of bovine mesenteric lymphatics (Miyahara et al., 1994). However, little information is available about the location of 5-HT4 receptors in core body vasculature. Ullmer et al. (1995) demonstrated the diversity of receptor subtypes expressed in various blood vessels. Reverse transcription (RT)–PCR was used to distinguish mRNAs for G-protein-coupled 5-HT receptors expressed in rat and pig blood vessels. Most blood vessels expressed mRNA receptors for 5-HT1D, 5-HT2A, 5-HT2B, 5-HT4, and 5-HT7 mRNA although they were expressed at differing levels. Similar analogous RT–PCR assays were performed using smooth muscle and endothelial cells from human pulmonary artery, aorta, coronary artery, and umbilical vein. Of the known G-protein-coupled 5-HT receptors, only 5 were found to be expressed in the various blood vessels tested. Endothelial cells were found to express receptor mRNA for 5-HT1D, 5-HT2B, and 5-HT4, whereas smooth muscle cells expressed 5-HT1D, 5-HT2A, and 5-HT7. This could explain why no response was seen in vessels exposed to BIMU-8 in our study. 5-HT4 may be present in endothelial cells but absent in smooth muscle cells, meaning that contraction of the vessel cannot occur via direct acting vasoconstrictors. Ontsouka et al. (2006) compared the levels of mRNA and 5-HT4 receptor binding sites in smooth muscle layers from the gastrointestinal (GI) tract of dairy cows using real-time PCR to verify if mRNA and protein expression were correlated. It was found that mRNA levels of the 5-HT4 receptor were affected by location along the GI tract. Levels in the external loop of the spiral colon and ileum were greater than those in the proximal loop of the ascending colon (mesenteric blood vessels utilized in the current study were collected from mesentery supporting the ileum). However, these levels of mRNA were similar to levels of receptors in the fundus abomasa, pylorus, and cecum. It could be concluded from this study that mRNA and 5-HT4 receptor binding sites were present in the smooth muscle layer of the entire GI tract but that they were not correlated with each other. Although Ontsouka et al. (2006) studied receptors in smooth muscle rather than blood vessels, the findings were similar to that of Ullmer et al. (1995) and suggest that 5-HT receptors may be present within a tissue but may not play an active role or a role that has yet to be identified.
Presence of 5-HT2B and 5-HT1D Receptors: Responses to BW 723C86 and L-694, 247
When exposed to the agonists BW 723C86 or L-694, 247, ruminal and mesenteric vasculature did not show a contractile response (P > 0.05). However, MV relaxed in response to both agonists (P < 0.05) (Figs. 5 and 6). This indicates that 5-HT1D and 5-HT2B receptors may be present in MV and contribute to vasorelaxation. A significant vessel × concentration interaction was observed (P = 0.0012) in vessels exposed to the 5-HT1D agonist, indicating that the vasorelaxation response seen depends on the concentration of the agonist that the vessel is exposed to and the type of vessel being used. The response of MV differs from RV for the 5 × 10−8 M addition onward, and is different from the RA from 1 × 10−8 M onward.
Figure 5.
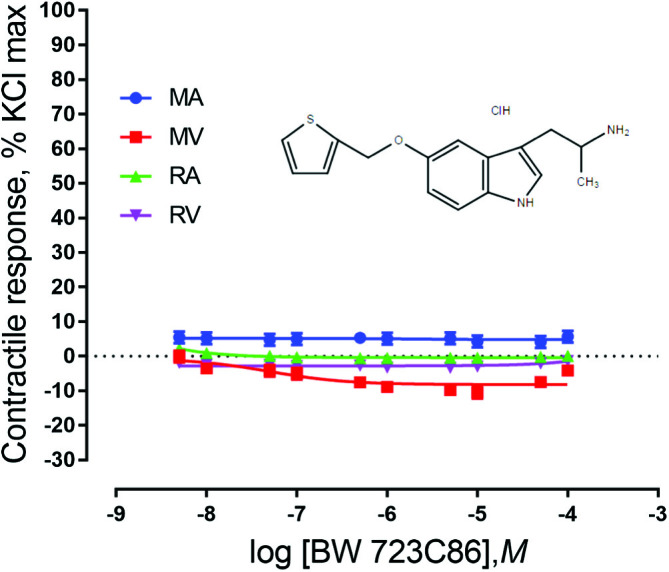
Chemical structure of the 5-HT2B agonist BW 723C86 and mean contractile responses of mesenteric artery (MA; n = 6), mesenteric vein (MV; n = 6), ruminal artery (RA; n = 5), and ruminal vein (RV; n = 5) to increasing concentrations of BW 723C86. Graphical points represent the mean and bars represent the SEM.
Figure 6.
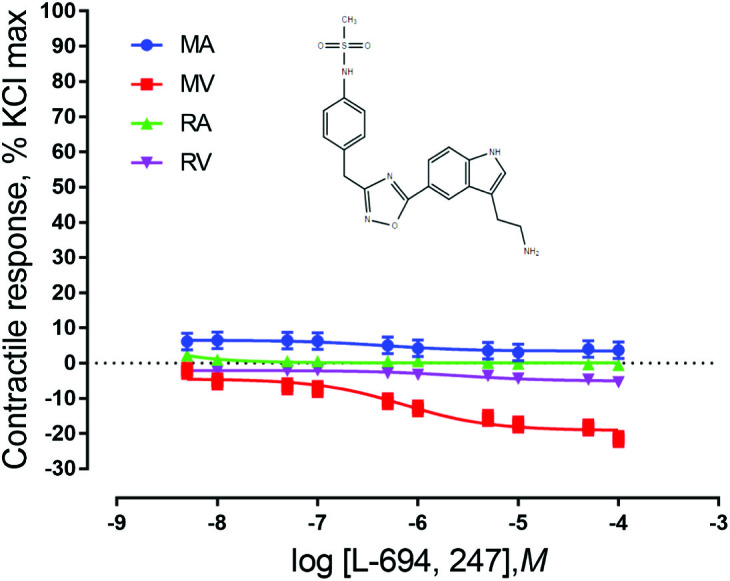
Chemical structure of the 5-HT1D agonist L-694, 247 and mean contractile responses of mesenteric artery (MA; n = 6), mesenteric vein (MV; n = 6), ruminal artery (RA; n = 5), and ruminal vein (RV; n = 5) to increasing concentrations of L-694,247. Graphical points represent the mean and bars represent the SEM.
There was not a vessel × concentration interaction for vessels exposed to 5-HT2B agonist (P = 0.15; Fig. 5). Using the same agonist (BW 723C86), Klotz et al. (2012) also showed no presence of 5-HT2B receptors in bovine lateral saphenous veins. Vessels did, however, exhibit significant responses based on both vessel type (P < 0.0001) and concentration of agonist (P = 0.0013). Similar to vessels exposed to an agonist for the 5-HT1D receptor, MA had a stronger response than RV and RA had a stronger response than MV. This indicates that the type of vessel does have an effect on the type and intensity of the response seen.
The existence of 5-HT2B receptors has been reported to cause endothelium-dependent vasorelaxation in rabbit and rat jugular vein and the porcine vena cava and pulmonary artery (Watts and Cohen, 1999). The existence of 5-HT7 has also been demonstrated in the femoral vein and pulmonary arteries of rabbits (Martin and Wilson, 1995; Morecroft and MacLean, 1998) and in the coronary and cerebral arteries of dogs (Cushing et al., 1996; Terrón, 1996; Terrón and Falcón-Neri, 1999). Although Ullmer et al. (1995) and Bhalla et al. (2002) used RT–PCR to identify mRNA for 5-HT2B and 5-HT7 in porcine pulmonary arteries, the presence of mRNA is not indicative of whether the receptor in functional or active in producing a response, as noted previously. Jähnichen et al. (2005) further characterized the vasorelaxative responses in pulmonary arteries precontracted to prostaglandin F2α in weaned pigs. By using the 5-HT2B receptor agonist BW 723C86, the selective 5-HT2C receptor antagonist SB 242084, and the 5-HT2B/2C agonist SB 206553, it was demonstrated that 5-HT in weaned pigs induced an endothelium-dependent relaxation response mediated by 5-HT2B receptors and a direct relaxation response by 5-HT7 receptors. However, these findings are in direct contrast to the response seen in slaughter-age pigs. In slaughter-age pigs, it was found that pulmonary artery vasorelaxation only is mediated by 5-HT2B receptors. These results indicate that as the pig ages, it acquires a greater population of 5-HT2B receptors that contribute to vasorelaxation more so than 5-HT7. While our current study only found a vasorelaxation response in mesenteric veins in response to 5-HT1D and 5-HT2B receptor agonists, the findings of Jähnichen et al. (2005) provide insight into the responses observed. It is possible that as ruminants age, they too rely more heavily on 5-HT2B than 5-HT7.
CONCLUSIONS
The findings of this study indicate that 5-HT2A is present in bovine ruminal and mesenteric vasculature, plays a role in vasoconstriction, and could be influenced by EA exposure as has been demonstrated in peripheral blood vessels. In contrast, the 5-HT1D and 5-HT2B receptor subtypes do not contribute to vasoconstriction in any evaluated blood vessel, but may be present in mesenteric veins and contribute to vasorelaxation. Further research with 5-HT2A should be directed toward defining the relationship this receptor has with EA and associated vasoconstriction. Additionally, as this study was confined to mesenteric and ruminal vasculature, it may be possible to expand this study to include blood vessels in different anatomic locations.
ACKNOWLEDGMENTS
The authors acknowledge Adam J. Barnes of the Forage-Animal Production Research Unit, the University of Kentucky Meats Lab, and Hi-View Meats for their hard work and collaboration toward the completion of this experiment. Mention of trade name, proprietary product of specified equipment does not constitute a guarantee or warranty by the USDA and does not imply approval to the exclusion of other products that may be available.
LITERATURE CITED
- Banes A. K., and Watts S. W.. 2003. Arterial expression of 5-HT 2B and 5-HT 1B receptors during development of DOCA-salt hypertension. BMC Pharmacol. 3:12. doi:10.1186/1471-2210-3-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berde B. 1980. Ergot compounds: a synopsis. In: M. Goldstein A. Lieberman D. B. Calne, and M. O. Thorner, editors, Ergot compounds and brain function: neuroendocrine and neuropsychiatric aspects. Raven Press, New York, NY: p. 4–23. [Google Scholar]
- Bhalla P., Saxena P. R., and Sharma H. S.. 2002. Molecular cloning and tissue distribution of mRNA encoding procine 5-HT 7 receptor and its comparison with the structure of other species. Mol. Cell. Biochem. 238:81–88. doi:10.1023/A:1019959121602 [DOI] [PubMed] [Google Scholar]
- Cushing D. J., Zgombick J. M., Nelson D. L., and Cohen M. L.. 1996. LY215840, a high-affinity 5-HT7 receptor ligand, blocks serotonin-induced relaxation in canine coronary artery. J. Pharmacol. Exp. Ther. 277:1560–1566. [PubMed] [Google Scholar]
- Dumuis A., Sebben M., and Bockaert J.. 1989. The gastrointestinal prokinetic benzamide derivatives are agonists at the non-classical 5-HT receptor (5-HT 4) positively coupled to adenylate cyclase in neurons. Naunyn Schmiedebergs Arch. Pharmacol. 340(4):403–410. [DOI] [PubMed] [Google Scholar]
- Dyer D. C. 1993. Evidence that ergovaline acts on serotonin receptors. Life Sci. 53:PL223–PL228. doi:10.1016/0024-3205(93)90555-H [DOI] [PubMed] [Google Scholar]
- Egert A. M., Kim D. H., Schrick F. N., Harmon D. L., and Klotz J. L.. 2014. Dietary exposure to ergot alkaloids decreases contractility of bovine mesenteric vasculature. J. Anim. Sci. 92:1768–1779. doi:10.2527/jas2013-7141 [DOI] [PubMed] [Google Scholar]
- Foote A. P., Harmon D. L., Strickland J. R., Bush L. P., and Klotz J. L.. 2011. Effect of ergot alkaloids on contractility of bovine right ruminal artery and vein. J. Anim. Sci. 89:2944–2949. doi:10.2527/jas.2010-3626 [DOI] [PubMed] [Google Scholar]
- Hamel E., Fan E., Linville D., Ting V., Villemure J. G., and Chia L. S.. 1993. Expression of mRNA for the serotonin 5-hydroxytryptamine1D beta receptor subtype in human and bovine cerebral arteries. Mol. Pharm. 44:242–246. [PubMed] [Google Scholar]
- Hoyer D., Clarke D. E., Fozard J. R., Hartig P. R., Martin G. R., Mylecharane E. J., Saxena P. R., and Humphrey P. P.. 1994. International Union of Pharmacology classification of receptors for 5-hydroxytryptamine (serotonin). Pharmacol. Rev. 46:157–203. [PubMed] [Google Scholar]
- Jähnichen S., Horowski R., and Pertz H. H.. 2005. Agonism at 5-HT 2B receptors is not a class effect of the ergolines. Eur. J. Pharmacol. 513:225–228. doi:10.1016/j.ejphar.2005.03.010 [DOI] [PubMed] [Google Scholar]
- Kato S., Kumamoto H., Hirano M., Akiyama H., and Kaneko N.. 1999. Expression of 5-HT2A and 5-HT1B receptor mRNA in blood vessels. Mol. Cell. Biochem. 199:57–62. [DOI] [PubMed] [Google Scholar]
- Klotz J. L., Aiken G. E., Bussard J. R., Foote A. P., Harmon D. L., Goff B. M., Schrick F. N., and Strickland J. R.. 2016. Vasoactivity and vasoconstriction changes in cattle related to time off endophyte-infected tall fescue. Toxins 8:271. doi:10.3390/toxins8100271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klotz J. L., Aiken G. E., Johnson J. M., Brown K. R., Bush L. P., and Strickland J. R.. 2013. Antagonism of lateral saphenous vein serotonin receptors from steers grazing endophyte-free, wild-type, or novel endophyte-infected tall fescue. J. Anim. Sci. 91:4492–4500. doi:10.2527/jas.2012-5896 [DOI] [PubMed] [Google Scholar]
- Klotz J. L., and Barnes A. J.. 2014. Isolating and using sections of bovine mesenteric artery and vein as a bioassay to test for vasoactivity in the small intestine. J. Vis. Exp. 92:e52020. doi:10.3791/52020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klotz J. L., Brown K. R., Xue Y., Matthews J. C., Boling J. A., Burris W. R., Bush L. P., and Strickland J. R.. 2012. Alterations in serotonin receptor-induced contractility of bovine lateral saphenous vein in cattle grazing endophyte-infected tall fescue. J. Anim. Sci. 90:682–693. doi:10.2527/jas.2011-4323 [DOI] [PubMed] [Google Scholar]
- Klotz J. L., Bush L. P., and Strickland J. R.. 2011. A vascular contractility bioassay using bovine right ruminal artery and vein. J. Anim. Sci. 89:1944–1951. doi:10.2527/jas.2010-3532 [DOI] [PubMed] [Google Scholar]
- Klotz J. L., Kirch B. H., Aiken G. E., Bush L. P., and Strickland J. R.. 2008. Effects of selected combinations of tall fescue alkaloids on the vasoconstrictive capacity of fescue-naive bovine lateral saphenous veins. J. Anim. Sci. 86:1021–1028. doi:10.2527/jas.2007-0576 [DOI] [PubMed] [Google Scholar]
- Klotz J. L., Kirch B. H., Aiken G. E., Bush L. P., and Strickland J. R.. 2010. Contractile response of fescue-naive bovine lateral saphenous veins to increasing concentrations of tall fescue alkaloids. J. Anim. Sci. 88:408–415. doi:10.2527/jas.2009-2243 [DOI] [PubMed] [Google Scholar]
- Martin G. R., and Wilson R. J.. 1995. Operational characteristics of a 5-HT receptor mediating direct vascular relaxation: identity with the 5-ht7 receptor?Br. J. Pharmacol. 114:383P.7881738 [Google Scholar]
- Miyahara H., Kawai Y., and Ohhashi T.. 1994. 5-Hydroxytryptamine-2 and-4 receptors located on bovine isolated mesenteric lymphatics. J. Pharmacol. Exp. Ther. 271:379–385. [PubMed] [Google Scholar]
- Morecroft I., and MacLean M. R.. 1998. 5‐Hydroxytryptamine receptors mediating vasoconstriction and vasodilation in perinatal and adult rabbit small pulmonary arteries. Br. J. Pharmacol. 125:69–78. doi:10.1038/sj.bjp.0702055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni W., and Watts S. W.. 2006. 5‐hydroxytryptamine in the cardiovascular system: focus on the serotonin transporter (SERT). Clin. Exp. Pharmacol. Physiol. 33:575–583. doi:10.1111/j.1440-1681.2006.04410.x [DOI] [PubMed] [Google Scholar]
- Ontsouka E. C., Blum J. W., Steiner A., and Meylan M.. 2006. 5-Hydroxytryptamine-4 receptor messenger ribonucleic acid levels and densities in gastrointestinal muscle layers from healthy dairy cows. J. Anim. Sci. 84:3277–3284. doi:10.2527/jas.2006-228 [DOI] [PubMed] [Google Scholar]
- Rhodes M. T., Paterson J. A., Kerley M. S., Garner H. E., and Laughlin M. H.. 1991. Reduced blood flow to peripheral and core body tissues in sheep and cattle induced by endophyte-infected tall fescue. J. Anim. Sci. 69:2033–2043. [DOI] [PubMed] [Google Scholar]
- Terron J. A. 1996. The relaxant 5‐HT receptor in the dog coronary artery smooth muscle: pharmacological resemblance to the cloned 5‐ht7 receptor subtype. Br. J. Pharmacol. 118:1421–1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terrón J. A., and Falcón‐Neri A.. 1999. Pharmacological evidence for the 5‐HT7 receptor mediating smooth muscle relaxation in canine cerebral arteries. Br. J. Pharmacol. 127:609–616. doi:10.1038/sj.bjp.0702580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullmer C., Schmuck K., Kalkman H. O., and Lübbert H.. 1995. Expression of serotonin receptor mRNAs in blood vessels. FEBS Lett. 370:215–221. [DOI] [PubMed] [Google Scholar]
- Watts S. W. 2002. Serotonin-induced contraction in mesenteric resistance arteries. Hypertension 39:825–829. [DOI] [PubMed] [Google Scholar]
- Watts S. W., and Cohen M. L.. 1999. Vascular 5-HT receptors: pharmacology and pathophysiology of 5-HT1B, 5-HT1D, 5-HT1F, 5-HT2B and 5-HT7 receptors. Neurotransmissions 15:3–15. doi:10.2174/1389557516666160728121050 [Google Scholar]
- Weinshank R. L., Zgombick J. M., Macchi M., Branchek T. A., and P. R Hartig. 1992. The human serotonin 1D receptor is encoded by a subfamily of two distinct genes: 5-HT1Dα and 5-HT1Dβ. Proc. Natl. Acad. Sci. U.S.A: 89:3630–3634. [DOI] [PMC free article] [PubMed] [Google Scholar]


