Abstract
Research in growth and development, accumulation of lean, and fat metabolism in farm animals was gaining attention principally from a carcass perspective by meat scientists and animal nutritionists about a century ago. Under the auspices of the USDA Cooperative State Research Service, State Agricultural Experiment Stations, and the Land Grant University system, researchers from various universities embarked on forming combined regional research projects (across states) with unifying specific aims. In the North Central region, this included states in the upper and lower Mid-West region. For those interested in improving production and eating quality of meats, initially a single multistate committee was formed in the North Central region which was active for many years. However, these efforts were later split into two committees with one addressing lipids and the other muscle biology. Herein we reviewed research of workers in the North Central region in the 1940s and 1950s and to a limited extent in the 2000s on meat animal’s lipid metabolism. We further reviewed the history of meat animal carcass composition research and the influence of the Word War II (WWII) period on porcine carcass composition. The development and utilization of adipocyte cellularity research methodology in meat animals was demonstrated. The history of the progression of adipose tissue metabolism research in meat animals was also reviewed. Finally, the history of research on lipid deposition in muscle that ultimately precipitated the expanded marbling and the intramuscular research was delineated. By the 1970s, great interest had emerged on how to curtail excessive fat deposition in meat-producing animals. Thus, for some segments of the animal lipid metabolism community, the focus then shifted to exploring the processes of lipogenesis and lipolysis in farm animals. These efforts morphed into research efforts in fat cell biology and cellularity. Today adipocyte biology is studied by many in the biomedical and agricultural-life sciences communities. In this article, we present a history of this research and notable achievements up to the 1980s. Herein we revisit these research efforts and results that have become an important knowledge base for growth and development, nutrition, and meat science research.
Keywords: adipocyte, adipose tissue, history, meat animals
INTRODUCTION
Important classical research in adipose tissue accretion, growth and development, and fat metabolism in farm animals was gaining attention over a 75-yr period (Hammond, 1932; McMeekan, 1940; Fortin, 1980). During this time, meat science and animal nutrition researchers were organized into regional research committees under the auspices of the USDA Cooperative Research Service and Land Grant University system. In the 1940s and 1980s, workers in the North Central region were particularly interested in the role of lipids in animal foods, the pattern of fat tissue accretion and fat depot development, and the role of lipids in the organoleptic properties of meat and its relation to quality scores. Furthermore, the priority of nutrient utilization by various tissues and factors that influenced fat deposition during the finishing phase was of considerable interest. In this review, we chronicled the history of adipose tissue and adipocyte research to indicate when and how this research began and the subsequent fruits of the regional research committees.
THE INFLUENCE OF WORLD WAR II ON PORCINE CARCASS COMPOSITION
The history of adipocyte and adipose tissue research in meat animals is associated with several factors including the drastic changes in the degree of fat in pigs from before to well after World War II (WWII). During WWII, households were encouraged to save bacon drippings and grease to return to their butchers for use in munitions used in WWII (see Turning Bacon into Bombs, http://www.theatlantic.com/health/archive/2014/04/reluctantly-turning-bacon-into-bombs-during-world-war-ii/360298/; Bombs and Butter; http://www.shorpy.com/node/ 3645/; and Rise and Fall of the Great American Hog; https://quizlet.com/132497054/intro-to-animal- sciences-220001-pigs-flash-cards/). Lard was commonly used for baking and other cooking purposes during this time because vegetable shortenings had not been developed yet. Furthermore, it was also considered acceptable to use lard because a lot of the energy was expended in intensive human labor on farms and in other industries. During this time period, pig selection was based on visual appraisal, so selection pressure was exerted for pigs with a high percentage of fat. The strong patriotic fever during WWII helped to drive the production of lard (see Rise and Fall of the Great American Hog, https://quizlet.com/132497054/intro-to-animal-sciences-220001-pigs-flash-cards/). Rapid changes can be made in carcass fat because body type in pigs is highly heritable, and pigs have a short generation interval and a high rate of prolificacy. A poignant example is the lean and obese USDA pigs generated by Hetzer and Harvey (1967) based on selection for low or high back fat thicknesses.
After WWII, fat pigs became less desirable because consumers began to discriminate against lard and fatty cuts when they selected pork at the retail level. The advent of vegetable shortenings also hastened the decline in use of lard. Because the need for animal fat by consumers diminished after WWII, the livestock industry decided the body composition of the animals. Considerable pig improvement took place between 1958 and 1967 resulting in an average loss of 4.04 kg of lard or adipose tissue which was replaced most likely by muscle. The 4.04 kg of adipose tissue replaced by muscle would have cost $1.70 on the live animal basis (Allen, 1968) but in the form of lard would have been worth only $0.52, which amounted to a $1.18 loss in value. As a result, a savings of over $98 million was realized when applied to all pigs marketed in 1967(Allen, 1968).
The history of beef carcass composition was not influenced by WWII as was pork carcass composition. The earliest account of corn feeding and cattle “fattening” was documented in historical proceedings in the late 1800s (Ball, 1998), which was concurrent with the arrival of British breeds. For several more decades, almost all of the beef consumed in the United States was from grass-fed cattle obtained directly off grass or 200 to 240 kg calves directly off the cow (reviewed in Corah, 2008). In fact, the impact of feeding corn to weaned calves or yearlings did not appear in the literature until the 1950s (Butler et al., 1956; Corah, 2008). Corah (2008) reviewed a 1956 study that reported for the first time an all-corn grain diet for fattening steers.
The first step in conducting meat animal adipocyte research occurred long before WWII. Early investigator, such as Moulton, Haeker, and McMeekan among others in the 1920s to 1940, examined the proportional growth of adipose tissue and ether extract material during meat animal growth. Several of these studies represented cooperative efforts between the North Central agricultural experiment stations and the USDA. The adipocyte research programs were focused on gaining insight into adipose tissue accretion because they realized that the efficiency of production was reduced with increase fat deposition. At the beginning of this research, there was little known about the role or influence of fat tissue during meat animal growth and development.
THE HISTORY OF MEAT ANIMAL CARCASS COMPOSITION
The growth of meat animals and their body and carcass compositions have been studied extensively over the years. Initially, important compositional data were gathered by animal scientists. Such information was collected as early as the 1860s but was based on crude dissection and extraction techniques (Mitchell, 2007). Demonstration and discussion of techniques for quantitative and qualitative evaluation of carcass composition and meat quality were provided in the proceedings of the Reciprocal Meats Conference (RMC; http://www.meatscience.org/publications-resources/rmc-proceedings) beginning in 1948. In particular, the 1951 RMC proceedings contained numerous papers on slaughtering procedures, carcass evaluation, and meat quality procedures. Consequently, refined dissection and chemical techniques of meat animal composition became available in subsequent years. Improved carcass composition analysis was deemed necessary to eventually study nutritional, pharmacological, and genetic factors that could affect the growth and composition of meat animals.
The North Central-58 (NC-58) and North Central-91 (NC-91, later NC-97) research groups (1920s to 1980) were focused on the determination of body, carcass and chemical composition and development, evaluation, and improvement of methods for these purposes. It became very clear that with increased age or body weight, the amount of fat or ether extract in the meat animal body increased faster than muscle or protein and continued to accumulate after muscle and bone accretion had plateaued (Hedrick et al., 1967). Body composition studies of meat animals were focused primarily on changes that occurred during growth of the animal when at which they were marketed. Body type was deemed an important consideration because it influenced growth rate and mature body size that accounted for animal composition at a given body weight or level of physiological maturity (Allen et al., 1976). Many studies also compared body composition traits of beef cattle with dairy cattle. Carcass composition was different between Herefords and Holsteins because of differences in growth rate and extent of fat deposition (Berg and Butterfield, 1968). Carcasses from steers of matings of Hereford and Angus cows to Hereford, Angus, Jersey, and several lean European breed sires were compared at a constant age, a constant weight, and constant percentage of fat in the longissimus muscle (Koch et al., 1976, 1982). A negative association between growth rate of breed groups and percentage of fat trim was observed. Furthermore, they had different longissimus fat at similar average carcass weights (Koch et al., 1976, 1982). However, when finish in different beef and dairy breeds were assessed, there was little difference in carcass composition if cattles were slaughtered at the same degree of chemical maturity. Other studies demonstrated the relationship between cattle body weight and total body fat (Allen et al., 1976).
A number of approaches were evaluated to estimate the fatness of animals (Warner and Elis, 1934). These approaches included the following: chemical analysis of a single-representative cut; the weight of fat or lean cuts in relation to the weight of the carcass; measurements of thickness of the fat on the ham, shoulder, and back; and the chemical composition of the 9th, 10th, and 11th rib (Hankins and Howe, 1946). The weight of fat or lean cuts in relation to the carcass weight was more practical and more accurate estimate of fatness than the other approaches. Furthermore, early work on porcine back fat by Warner and Ellis (1934) and Hankins and Titus (1939) demonstrated the practical use of the thickness of backfat as a reliable indicator of the fatness, weight, firmness, and other carcass characteristics. Backfat thickness of hog carcasses was found to be a reliable index of total fat. A correlation coefficient of 0.84 between the average thickness of backfat and the percentage of fat in the edible carcass was determined and was the best coefficient found between fatness and other physical characteristics at the time. Later, a probe technique for measuring backfat on live hogs was developed (Hazel and Kline, 1952, 1959). The method involved placing probes into the backfat very quickly causing little discomfort to the pigs. The correlation between the average of four backfat measurements taken on carcasses and on live hogs was 0.81. Measurements with backfat probes made on 96 live hogs were slightly more accurate as indicators of leanness and percentage primal cuts than carcass measurements of backfat thickness. These studies precipitated future studies on the development of noninvasive methods to measure backfat thickness.
Location has a major influence on adipose tissue composition. For instance, s.c., intermuscular, and i.m. adipose tissues have markedly different lipid-to-moisture ratios (Lee and Kauffman, 1973). Furthermore, the i.m. adipocytes constitute one-half of the body’s adipocyte population yet extractable lipid is 10% or less. The outer s.c. layer has a greater proportion of unsaturated fat than does the middle or inner s.c. layers (Villegas et al., 1973). Fatty acid composition also distinguishes the perirenal and s.c. adipose tissues (Villegas et al., 1973). A number of other factors will reduce adipose tissue lipids such as stress and a number of environmental factors (Allen et al., 1968).
The lack of energy in submaintenance diets reduces lipogenesis and enhances lipolysis, which decreases triglyceride levels and adipose tissue depots. Carcass composition was not influenced when feed dry matter intake or energy levels were significantly reduced in ruminants or pigs slaughtered at either a constant weight or constant age end point (Allen et al., 1976). In a particularly striking study by Lee and Kauffman (1974), feed intake of pigs was severely restricted during the suckling period, and comparisons were made at either a constant body weight of 80 kg or a constant age. The underfed pigs gained one-seventh of the body weight of the controls during the first 4 wk of age. Carcass composition was not influenced when undernourished and control pigs were compared at a constant weight point with no influence on fat, muscle, or bone deposition. As expected, the undernourished pigs were much older when they were compared with control pigs (Lee and Kauffman, 1974).
Early researchers also examined the influence of feeding frequency on total extractable fat in pigs (Allen et al., 1968). This and other studies showed that single feeding compared with multiple feedings (five to six) per day significantly reduced the quantity of leaf fat and total extractable carcass fat. The voluntary consumption of feed per day was similar for the feeding groups (Allen et al., 1968). The results in pigs were in direct contrast to the response obtained in the rat where multiple rather than single feedings caused a reduction in fat accumulation (Cohn and Joseph, 1960). Pigs fasted 24 h have an increased rate of fatty acid oxidation indicating that single meal-fed pigs mobilize fat for energy resulting in less accumulation of carcass fat compared with multiple meal-fed pigs. This is another example of a species-dependent phenomena related to fat deposition.
Early on, assessments of body composition of live animals were accomplished primarily by visual appraisal (Mitchell, 2007). It was apparent that a suitable means for measuring composition of the live animal were needed to progress in nutritional and genetic improvements in body composition. The use of x-ray measurements to estimate composition of the live animal was developed in Germany in the 1930s and was used successfully to measure the thickness of pig s.c. and belly fat. It was never clear why but the x-ray approach was never widely adopted, and research on the use of ultrasonics to measure body composition of pigs began in the mid-1950s (Hazel et al., 1959) Ultrasonics became the commonly used method for estimating live body composition in cattle, pigs, and sheep, and it has been extensively validated in pigs. Real-time ultrasound scans consist of s.c. fat thickness and the cross-sectional area of the longissimus muscle. The current and future potential use of ultrasound for evaluating carcass traits in feeding and finishing animals was reviewed by Houghton and Turlington (1992). Furthermore, genetic studies have been greatly improved with the application of ultrasound technology (Wilson, 1992).
HISTORY OF ADIPOCYTE CELLULARITY RESEARCH IN MEAT ANIMALS
Excess adipose tissue growth is energetically wasteful; considerable nutrients are used for the accretion of a tissue (and constituent fat) that now is not wanted nor needed by consumers. Conducting research to study cellular mechanisms of meat animal adipose tissue growth and development was imperative to develop strategies for decreasing adipose tissue accretion.
A relatively small cohort of animal scientists in the 1970s began exploring the cellular mechanisms involved in the regulation of adipose tissue growth and the metabolic events that were involved in the control of nutrition uptake and utilization for lipid accretion in cells (adipocytes) resident in adipose tissue (Allen et al., 1976). The goal was to develop strategies that would reduce adipose tissue accretion, divert (or repartition) nutrients to muscle growth to result in increased growth rate of this tissue, and, hence, result in leaner, more efficient meat animals.
Inherent to a historical accounting of science is the benefit of looking back to assess what happened vs. what the research objectives were. When the founding scientists of NC-97 began their pursuit to learn more about the cellular basis of adipose tissue growth in meat animals, very little was known about the cellular and biological mechanisms that regulated adipose tissue growth. It was widely understood at the time that adipose tissue was the preeminent tissue site for energy deposition in meat animals; however, the biological mechanisms that accounted for tissue growth via hyperplasia and hypertrophy were only rudimentarily understood. In addition, while it was recognized that hypertrophy of adipocytes was a direct function of lipid (largely triglyceride) deposition in the cell, it was not clear how lipid synthesis and degradation (lipolysis) were regulated. Thus, another undertaking of the NC-97 group was to better understand regulation of nutrient flow into and out of adipocytes, and how the endocrine system regulated this. An early hypothesis was that a better understanding of how the endocrine system regulated lipid synthesis and degradation in the adipocyte could lead to strategies that provided ways to decrease adipocyte hypertrophy and reduce adipose tissue accretion in growing meat animals (Bergen and Merkel, 1991a, 1991b; Dunshea, 1993; Etherton and Bauman, 1998; Etherton, 2000).
Early Studies
Before research conducted in the 1960s and 1970s, few adipose tissue cellularity studies had been conducted in meat animals. The early seminal studies of Bell (1909a, 1909b) were important because they provided findings that turned out to prescient. In his article “On the histogenesis of the adipose tissue of the ox” (Bell, 1909b), it was observed that “the mass of adipose tissue increases in amount in fattening 1) by the increase in the size of its cells, 2) by the formation and filling of new cells in the interior of the lobule, 3) by the formation of new lobules.” Thus, the initial idea that adipose tissue mass of meat animals expanded by hyperplasia and hypertrophy was appreciated over 107 yr ago! While these findings are important because of their insightful historical perspective about the cellular aspects of adipose tissue growth, they also provided the impetus for a group of ‘growth biologists’ in animal science to begin looking at the questions about the regulation of adipocyte hyperplasia, what was the embryonic and postnatal source of ‘new’ adipocytes, how was adipocyte hypertrophy regulated, and what was the relative contribution of changes in cell number and cell size to changes in adipose tissue mass?
The recognition that adipocyte hypertrophy was important for adipose tissue growth in meat animals was actively pursued during this time by several animal scientists, including Allen, Anderson, Kauffmann, Hood, and Mersmann, among others (reviewed by Allen, 1976; also see Anderson and Kauffman, 1973; Hood and Allen, 1973, 1977; Mersmann et al., 1973, 1975; Lee and Kauffman, 1974; Etherton et al., 1977). Early on it was recognized that there was a maximum diameter that adipocytes could attain. Interestingly, as some proportion of cells attained this maximum diameter, another population of smaller adipocytes emerged, resulting in what was referred to as a biphasic adipocyte diameter distribution, with a minimum diameter of 20 μm. The emergence of this population of smaller cells became a focus of research with the questions being asked focusing on the mechanism that accounted for this. Did this reflect proliferation of preadipocytes (stem cells) that subsequently differentiated so that lipid accumulation began with the cells becoming large enough to be detected as adipocytes? Alternatively, were there cell populations that were committed to becoming adipocytes but had not begun lipid filling?
It was evident at the time that adipose tissue accretion continued long after skeletal muscle growth rates declined in meat animals (Allen, 1976). A conclusion was that the ability to ‘recruit’ a population of cells that could undergo lipid filling after some proportion of larger cells had reached a maximum size provided a cellular explanation for the enormous plasticity that adipose tissue has to expand in mammals. Although the work done during this time indicated that adipocyte number increased postnatally in meat animals, differences in adipose tissue growth rate appeared to be due to different rates of adipocyte hypertrophy. Hood and Allen (1977) were the first to report that the ratio of carcass adipocyte number to muscle mass was not different between pigs that varied considerably in their adiposity. Their findings indicated that differences in cell size accounted for the different mass of carcass adipose tissue. This was subsequently confirmed in pigs that diverged even more in carcass adiposity (Etherton et al. 1982).
The idea that there was a biphasic adipocyte diameter distribution was exciting. It also came with the recognition that the primary method used at the time to size and count adipocytes was limited by “certain technical” problems. The method used involved sizing and counting osmium-fixed adipocytes with a Coulter Counter. At the time, it was recognized, however, that it was difficult to accurately ascertain cell size and particularly cell number of small adipocytes when connective tissue debris from the isolation process was present (Etherton et al., 1977). The process to size and count osmium-fixed adipocytes was laborious! In brief, it entailed fixing adipose tissue slices with osmium and then ‘liberating’ the cells from the connective tissue matrix and collecting cells on nylon screens of various sizes. The problem was that there was always some connective debris that was present (Etherton et al., 1977), which led to the question of whether the debris was being counted as though it was small adipocytes. A method was developed that employed the use of 8 M urea to solubilize the connective tissue matrix resulting in a cell suspension that was devoid of debris. It was quickly shown that this reduced (but did not eliminate) the presence of small adipocytes in the 20 to 40 μm range (Etherton et al., 1977). The advent of this method largely eliminated the problem of connective tissue debris being counted as adipocytes and paved the way for future adipocyte cellularity studies beyond the timeline of the current review.
Nutrient Partitioning
The recognition that hypertrophy accounted for differences in adipose tissue growth was the impetus for subsequent research designed to evaluate strategies that would slow down postnatal adipose tissue growth with a resultant improvement in carcass composition (decrease in fat content). With respect to adipocyte hypertrophy, it was recognized early that lipid deposition rates in the cell were a function of the difference between lipid synthesis (lipogenesis) and degradation (lipolysis), and that the rate of lipid accumulation in an adipocyte would determine rate of hypertrophy. There was great interest in the concept of regulating nutrient partitioning between skeletal muscle and adipose tissue to divert nutrients to muscle growth and away from adipose tissue deposition. Also, there was keen interest in understanding nutrient uptake and utilization in the adipocyte with the idea that a better understanding of this might lead to strategies that would partition nutrients away from adipose tissue to muscle growth.
The progress realized in the 1960s and 1970s provided the framework for the early and pioneering research on the endocrine regulation of meat animal growth in the 1980s and subsequently. The hypothesis developed and tested was that the endocrine system could be ‘manipulated’ to increase muscle growth and inhibit adipose tissue growth. The advent of the modern era of biotechnology in late 1970s and early 1980s was timely because it provided the ability to synthesize biologically active recombinant hormones that could be used in experiments with growing animals to test this hypothesis. Over the next decade, there was an explosion in research that involved administration of recombinant GH (somatotropin; ST) and beta-adrenergic agonists (BAR) to growing meat animals, especially pigs. ST administration resulted in unprecedented increases in muscle growth with a concurrent decrease in adipose tissue, whereas effects on lean deposition and fattening by BAR were less robust, but significant. What was striking was the extent to which adipose tissue accretion could be blunted by recombinant porcine ST (rpST). In pigs, the maximally effective doses of rpST can decrease lipid accretion rates by as much as 70% (reviewed in Etherton and Bauman, 1998). The effects of rpST on muscle and adipose tissue growth (and underlying mechanisms of action) in meat animals have been discussed extensively, and the interested reader is referred to reviews on this topic (Bergen and Merkel, 1991a, 1991b; Dunshea, 1993; Etherton and Bauman, 1998; Etherton, 2000).
The period of time between 1980 and 2010 can be characterized as a time when our understanding of the mechanisms that regulated adipocyte hypertrophy became better understood. This largely reflected findings from numerous studies that were conducted to evaluate the mechanistic effects of rpST and BAR. For an in-depth perspective of this research, there are excellent reviews that summarize this literature (Smith and Smith, 1995; Etherton and Bauman, 1998; Mersmann, 1998b; Etherton, 2009). With respect to rpST decreasing adipose tissue accretion, it was evident early that this reflected a marked decrease in the rate of adipocyte hypertrophy during animal growth. An impressive body of evidence accumulated showed that glucose carbon flow to lipogenesis and subsequent adipocyte hypertrophy was greatly decreased. This reflected a variety of biological changes that included decreases in glucose uptake, lipogenesis, and the effects of insulin on stimulating these biological events (i.e., insulin sensitivity). The reduction in carbon flow into adipocytes for de novo lipogenesis and subsequent storage of triglycerides was paralleled by an increase in catecholamine-stimulated lipolysis. The decrease in adipocyte glucose uptake was related to a reduction in translocation of glucose transporters to the plasma membrane (primarily glucose transporter 4) and a marked diminution in the activity of a variety of important lipogenic enzymes, including fatty acid synthase (FAS). It was subsequently shown that transcription of the FAS was decreased by rpST and recombinant bovine ST, respectively, in pigs and cattle (Donkin et al., 1996; Schlegel, 1999). Therefore, of all the lipogenic genes, FAS may be the critical or target gene in efforts to reduce lipid accretion.
By the end of this era (i.e., 2010), one of the interesting unanswered questions was what was the mechanism(s) that accounted for gene transcription being reduced? In an effort that involved the use cultured 3T3-F442A adipocytes, Yin et al. (1998) demonstrated that ST decreased FAS mRNA as the result of a marked decrease in both transcription of the FAS gene and stability of the FAS mRNA. Unfortunately, there have not been any studies subsequent to this that have further examined how lipogenic enzyme genes are downregulated by ST in adipose tissue or adipocytes from meat animals.
Likewise other workers from the NC-91 multistate group studied the regulatory effects of BAR on pig lean and fat deposition (Bergen and Merkel, 1991a, 1991b). An adipogenic cell line TA1 assay system was established to ascertain the effects of a BAR (ractopamine) at the cellular level. The results with TA1 cells showed that FAS expression is reduced and triacylglycerol hydrolysis is enhanced (Weber et al., 1992). In addition, further work showed that ractopamine effects on lipolysis were similar to the BAR isoproterenol, and that propranolol (a beta antagonist) can reverse the BAR effect. Finally, it was shown that ractopamine and cAMP analogues dibutyryl-cAMP and 8-(chlorophenylthio)-cAMP all inhibited FASN enzyme activity (per milligram protein) in FAS cells (Bergen, 2001). Later this group showed that BAR reduces acetyl CoA carboxylase (ACC), FAS, and PPARγ, whereas PPARα and acyl-CoA dehydrogenase were enhanced (Halsey et al., 2011).
HISTORY OF ADIPOSE TISSUE METABOLISM RESEARCH IN MEAT ANIMALS
By the 1970s, great interest had emerged in how to curtail excessive fat deposition in meat-producing animals and for years the dairy industry wanted to insure adequate mammary fatty acid synthesis to negate milk fat depressions in high producing dairy cows. Thus, for some segments of the animal lipid metabolism community, the focus shifted to exploring the processes of lipogenesis and lipolysis in farm animals.
The process of de novo fatty acid synthesis (DNL) in animals was first studied with rodents (Wakil, 1962), and it became clear that dietary carbohydrates are the principal precursors of DNL (Dole, 1965; Hanson and Ballard, 1967; Ballard et al., 1969). At this time, lipid metabolism research also came to the forefront in meat animal research. While monogastric farm species (pigs and poultry) were directly utilizing carbohydrates as the principal precursor for DNL, the issue of carbon source for DNL was less clear in ruminants. In these animals, carbohydrates are fermented in the rumen to short-chain fatty acids (SCFA; principally acetic, propionic, and butyric acids). These acids are the principal combined energy source for ruminants (Hungate, 1964); however, glucose has to be produced via gluconeogenesis in ruminants. Gluconeogenesis requires specific precursors, and for the SCFA, only the three-carbon propionic acid can directly contribute to glucose production (Annison and Lindsay, 1961), although amino acid carbon skeletons can also be converted into glucose synthesis. Generally then, two- and four-carbon SCFA, but not glucose, are most likely DNL precursors in ruminants (Annison and Lindsay, 1961). By the early 1980s, new data indicated that gluconeogenic precursors and glucose may be utilized for lipogenesis in some lipid depots, especially intramuscular fat in ruminants (Smith and Crouse, 1984). The pattern and extent of fattening in beef cattle had been an important area of meats research, and this was followed by explorations into lipogenesis and lipolysis basically using enzyme assays.
In a wide array of tissues (e.g., liver, muscle, and numerous fat depots) in pigs, cattle, sheep, goats, and poultry, activities of malic enzyme, NADP-isocitrate dehydrogenase, pentose cycle enzymes glucose-6 phosphate dehydrogenase, and 6-phosphogluconate dehydrogenase were assayed to monitor generation of reducing equivalents. ACC, FAS, and stearoyl CoA desaturase activities were assayed specifically for DNL, and ATP citrate lyase (ACL), and acetyl-CoA synthetase were assayed to monitor source of carbon skeletons (glucose or acetate, respectively) for DNL in ruminants. Numerous experiments were performed by collected researchers from the North Central region, representing multiple NC-91 committee members as of 1976. The carbon source, reducing equivalents, and species characteristics were all described by this group of workers. The overall conclusions for these NC-91 experiments were summarized by Allen et al. (1976). They found that 1) in all farm animal species, DNL occurs principally in the adipose tissues (poultry liver only); 2) there were two main aspects of DNL, generation of reducing potential as NADPH + H and assembly of the carbon long-chained backbone from two carbon units; 3) glucose users (primarily nonruminants) for DNL rely on malic enzyme and the pentose-phosphate pathway to generate NADPH + H; 4) acetate users for DNL (primarily ruminants) rely on cytosolic NADP-isocitrate dehydrogenase and the pentose-phosphate pathway to generate NADPH + H; 5) feeding high-fat diets and (or) fasting with pigs markedly attenuated DNL in the adipose; 6) subcutaneous adipose depots have greater DNL activity per cell than internal fat depots (perirenal and i.m.); and 7) in most cases, ACL was not important in ruminant DNL.
Another important issue that arose during this time was the realization that fat cells vary from small to large and that the proportional protein content is less in mature fat cells (factors controlling hyperplasia and hypertrophy of adipocytes were addressed previously). This impacted the expression of enzyme activity data. The usual expression of activity/100 mg protein was not realistic, and approaches to measure size and number of fat cells in a given sample were developed (Hood and Allen, 1973; Etherton et al., 1977; Robelin 1981). Thereafter lipogenic enzyme activities in multiple fat depots were preferentially reported on a cell number basis (Anderson et al., 1972; Anderson and Kauffman, 1973; Hood and Allen, 1973; Allen et al., 1976). Among important conclusions by the late 1970s were that most likely ACC was the rate-limiting enzyme in DNL, while the remainder of the DNL enzymes were adaptable to energy intake (Allen et al., 1976).
Intramuscular adipose tissue has several metabolic features that distinguish it from other depots. Intramuscular adipocytes are smaller (25 to 50 μm diameter) than s.c. adipocytes (50 to 200 μm diameter in beef and pork; Allen et al., 1976; Smith and Crouse, 1984). Metabolism also appears to differ between i.m. and s.c. adipose tissues. The rate of acetate incorporation into fatty acids greatly exceeds the rate of glucose incorporation into fatty acids in vitro in s.c. adipose tissue (Smith and Prior, 1982; Smith, 1983; Smith and Crouse, 1984). Acetate provides 70% to 80% of the acetyl units to in vitro lipogenesis in s.c. adipose tissue, but only 10% to 25% in i.m. adipose tissue (Smith and Crouse, 1984). Conversely, glucose provides 1% to10% of the acetyl units in s.c adipose tissue, but 50% to 75% of the acetyl units in i.m. adipose tissue. Additionally, glucose incorporation into fatty acids is greater in i.m. adipose tissue than in s.c. adipose tissue (Smith and Crouse, 1984), although another study (Miller et al., 1991) demonstrated greater rates of fatty acid synthesis from glucose in s.c. than in i.m. adipose tissue. Regardless, numerous studies have demonstrated that glucose provided a greater proportion of acetyl units to fatty acid biosynthesis in i.m. adipose tissue than in s.c. adipose tissue using in vitro approaches (Smith and Crouse, 1984; Smith, 1995). More recently, in vivo stable isotope infusions of glucose and acetate and fractional synthesis rates measures were used to determine the extent of acetate or glucose use in i.m. or s.c. adipose DNL (Nayananjalie et al., 2015). This work showed that in both the i.m. or s.c. adipose depots, glucose as a carbon precursor accounted for only 10% of the palmitate synthesized (Nayananjalie et al., 2015). This mirrors earlier reports by Allen et al. (1976). Obviously, there are differences between researchers on the topic of preferential carbon sources for i.m and s.c. adipose DNL. Further information on the glucose carbon preference for DNL by i.m. adipose can be evaluated from Choi et al., (2014); Smith, (1983); Smith and Crouse, (1984); and Smith and Prior, (1984, 1986).
Finished carcasses had been utilized in the past to assess the effect of breed, sex, and dietary energy and protein content on overall fat and protein gain. Many of the earlier studies were done with U.S. cattle more typical of those available in the 1930s to 1950s and a reexamination of these aspects of cattle finishing was needed (Schroeder et al., 1987). In North American beef cattle, daily protein gains had increased from 50 to 200 g while fat deposition remained at 400 to 500 g from 1950 through the 1990s (Bergen and Merkel, 1991a, 1991b). When such results were expressed on a basis of protein to fat ratio, it could be concluded that cattle were getting leaner by the 1990s. However, much of this was due to increasing lean gains while fat deposition stayed constant. The increase in the lean to fat ratio over those 40 yr was a consequence of restructuring in the cattle industry from small-framed, early-maturing to later-maturing, larger framed cattle. Thus, the protein to fat ratios increased industry wide as a consequence of utilizing these larger framed, later-maturing cattle, which were slaughtered at similar body weight end points as small-framed, early-maturing cattle (Bergen and Merkel, 1991a). When later-maturing, large-framed cattle were fed to the same final finish end point, their carcass lean to fat ratios declined. Since daily fat deposition had apparently not changed much with the larger framed cattle, additional work was done in the 1980s to quantify daily protein gains (accretion) and fat gains. To examine composition of gain in live cattle, Byers (1979) championed a D2O infusion technique to determine sizes of skeletal muscle and fat in animals while Hankins and Howe (1946) had used the protein and fat composition of the 9th to 11th rib in cattle to estimate body composition. These methods, as well as differential slaughter, were utilized to determine daily fat and protein gain in cattle representative of the last quarter of the 20th century (Spivey, 1980; Eversole et al., 1981; McCarthy et al., 1983; Mulvaney et al., 1985; Schroeder et al., 1987; Anderson et al., 1988).
A key point in studies on comparative fat and protein gains was that animals were fed either to the same age or body weight (Bergen and Merkel, 1991a, 1991b). Since intact males have greater lean deposition, further work on the role sex and(or) steroid implants also was completed. Comparing large-frame bulls and steers fed to the same age or body weight indicated that dietary protein level (10% to 14%) increased ADG and protein accretion, but fat deposition was not different (Anderson et al., 1988). When Simmental and Angus bulls were fed a high corn silage vs. a high-grain diet, the high-grain diet had the highest energy content and increased rate of fat accretion in both breeds. The protein gains were greater for the larger framed Simmentals. McCarthy et al. (1983) found that while fractional protein synthesis rates (FSR) and fractional breakdown rates (FBR) did not differ between small- and large-framed steers, absolute rates of protein synthesis, accretion, and breakdown were related to frame size. Observed protein deposition was around 75 g/d for small-framed and >100 g/d in heavier large-framed cattle. This work did not uncover physiological reasons for difference in protein deposition other than frame size. Similar relationships between energy and protein intake and protein and fat synthesis were also noted in pigs (Bergen and Merkel, 1991a). With the emergence, after intense selection, of much larger, leaner and more rapidly growing pigs and cattle, actual daily production of fat has likely declined, but there are few data on contemporary beef cattle. The dynamics of whole body lipid metabolism among different organs also received considerable attention. In particular, lipolysis in storage adipose deposits in energy repartitioning and generation of fat synthesis precursors and the putative role and regulation of lipoprotein lipase and was of interest to participants in the multistate committees NC-91 and NC-97. Early work by Jeanrenaud et al. (1970) and Rodbell (1997) focused on the role of hormones, in particular epinephrine, on lipolysis in rodent fat cells. The now very familiar roles of epinephrine (isoproterenol and beta-adrenergic agonists), G-protein-coupled (beta) receptors, cAMP production, and protein kinase A in lipolysis have been well documented and are now textbook knowledge in biochemistry (Rodbell, 1997). Work with rodents was soon followed by work with farm animals. Cunningham et al. (1963) used subcutaneous injections of epinephrine and noted increased body nitrogen retention and decreased fat deposition in pigs. Epinephrine stimulated lipolysis in vitro in incubated backfat and omental adipose tissues from beef cattle above basal lipolysis in both fat depots (Pothoven et al., 1975). Similar work on lipolysis was done by Sidhu et al. (1973), Peterla and Scanes (1990), Scanes et al. (1994), and McNeel et al. (2000). Soon thereafter, pharmaceutical companies started to investigate effects of proprietary catecholamines, beta-adrenergic agonists, and(or) repartitioning agents on lipogenesis and lipolysis in farm animals (Dalrymple et al., 1984; Bergen and Merkel, 1991b; Silliance, 2004; Mersmann, 1998b). When originally developed (1980–1990), beta-adrenergic agonists decreased lipogenesis and increased lipolysis in fat depots of pigs (Halsey et al., 2011). As U.S. farm animals (particularly pigs) became leaner by 2010, enhanced lipolysis and decreased lipogenesis became much less relevant in animal production. What remained of relevance was the positive effect on lean tissue and feed efficiency (Bergen et al., 1989; Bergen and Merkel, 1991a, 1991b). Presently, there are three FDA-approved repartitioning agents for use in U.S. production of beef and pork (i.e., Optaflex, Paylean, and Xilmax).
HISTORY OF MEAT ANIMAL MARBLING RESEARCH
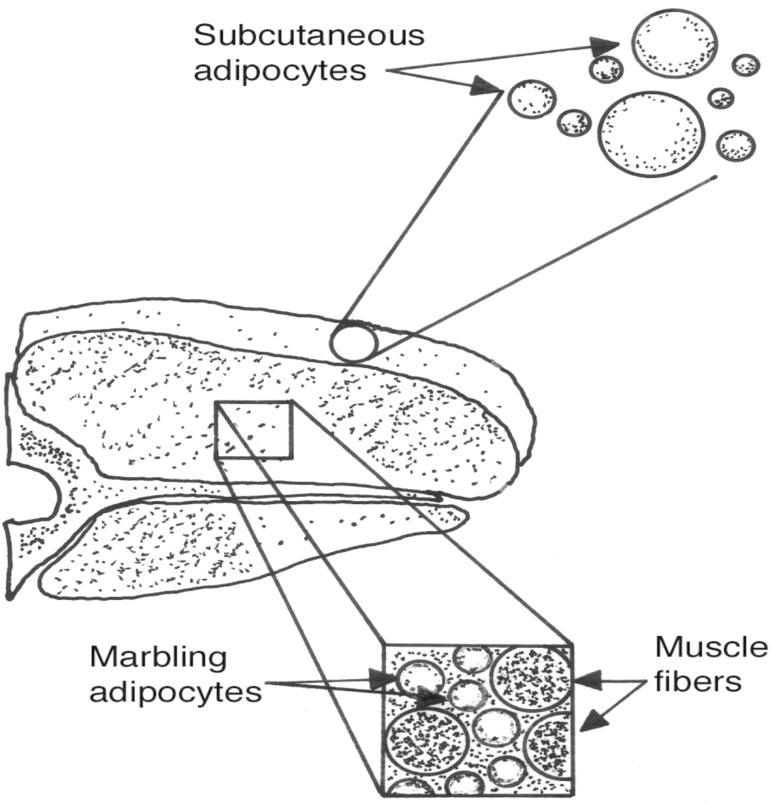
There are four sources of lipid in meat: 1) the muscle fibers; 2) s.c. adipose tissue; 3) intermuscular (seam) adipose tissue between muscle groups; and 4) i.m. adipose tissue (Figure 1). Marbling adipose tissue (i.m. adipose tissue) represents a unique depot. It can be distinguished from other fat depots by its location within perimysial connective tissues alongside myofibers (Moody and Cassens, 1968; Brooks et al., 2011). The localization of marbling adipocytes to the perimysium in most breed types indicates that marbling may arise primarily from fibroblasts associated with perimysial connective tissue (Moody and Cassens, 1968; Brooks et al., 2011). Subsequent studies utilized molecular markers to identify bovine i.m. preadipocytes and confirmed the position of small clusters of adipocytes that ultimately gave rise to marbling flecks (Albrecht et al., 2015). Very lean beef, pork, or lamb, in which all s.c. and intermuscular adipose tissues have been removed, contain approximately 1% extractable lipid. At the other extreme, closely trimmed beef from Japanese Black (Wagyu) cattle can contain over 50% extractable lipid. This extraordinary concentration of lipid can be attributed solely to lipid in i.m. adipose tissue.
Figure 1.
Sources of lipid in meat. Subcutaneous adipose tissue and intermuscular (seam) adipose tissue (not shown) contribute as much as 30% to the total lipid content of meat. Lipid associated with muscle fibers contributes approximately 1%, whereas intramuscular adipose tissue (marbling) can contribute from less than 1% to over 35% of the lipid in meat in certain breeds of cattle.
Early researchers concluded that the chemical composition of the animal body changes with age in a species- and individual-dependent manner. However, Moulton (1923) discovered that these changes were not coincident with physiological maturity. Subsequently, much work was done to examine interrelationships between physiological maturity and chemical changes in the body. In this regard, Moulton (1923) concluded that “chemical maturity” occurred at the age at which relationships among protein, water, and total mineral content (fat not included) became stabilized. Physiological aging or maturity was characterized by continual decreases in water, increases in solid matter during aging, and a continuous increase in ash content most rapidly during early life (Guenther, 1967). Physiological maturity has generally been thought from early times until now to indicate the point when ether-extractable material or fat in muscle begins to accumulate in earnest (Guenther, 1967).
It was noted that fat was normally deposited at widely varying rates in different parts of the body, resulting in marked variation in the proportions of fat throughout the body (Hankins and Titus, 1939). Fat appeared in the younger animals around viscera and kidney, and in older animals with adequate caloric intake, fat was evident between the muscles (i.e., intermuscular fat), beneath the skin (s.c. fat), and lastly between muscle fibers (i.m. fat; Callow, 1948; Zinn, 1967).
Callow (1947, 1948) and Lawrie (1956a, 1956b) reported that young animals had less chemical fat in muscle tissues than would be expected based on their degree of fatness. They found that breed influenced the age when amount of chemical fat begins to increase as in early-maturing cattle like the Aberdeen Angus, which have more i.m. fat than did later-maturing cattle. They also noted that sex influences both the nature and the quality of fat deposited. The percentage of chemical fat in tissues increased as fattening increased, but a considerable variation between individual animals was evident (Callow, 1948). Laurie (1963) examined nine different muscles in pigs and found significant differences in the i.m. fat content among muscles. The longissimus muscle had the greatest i.m. fat content and the sartorious muscle had the least. The researchers concluded that in addition to the general level of fatness, other factors influence the amount of i.m. fat in muscle and chemical composition of muscular tissues in general. Other early studies with cattle demonstrated that heifers had the greatest percentage of ether extract in the longissimus muscle compared with yearling bulls, bull calves, and steers (Smith, 1974). However, studies on pigs showed that barrows consistently have more i.m. fat than gilts. (Allen et al., 1976)
The relationship between backfat thickness and ether-extractable lipid in longissimus muscle was examined in gilts and barrow Yorkshire, Duroc, and high-fat and low-fat pigs (Allen et al., 1976). Duroc and high-fat pigs had the greatest backfat thickness and the greatest amount of extractable longissimus muscle lipid demonstrating a positive relationship between backfat thickness and muscle lipid. A study of ether-extractable lipid in two muscles from Duroc and Hampshire pigs showed that, when expressed on a percentage of wet weight basis, muscle lipid increased slowly for the first 8 wk and then increased with age in both breeds, with a greater increase in Duroc pigs (Lee and Kauffman, 1974). During this time, the Duroc breed became known for greater muscle lipid accretion than other pig breeds.
The longissimus muscle of cattle produced in the United States can accumulate up to 20% i.m. lipid (Lunt et al., 1993), primarily as triglycerides. Thus, i.m. adipocytes would provide the greatest amount of fatty acids in meat. A landmark publication by Moody and Cassens (1968) documented with histochemical studies that i.m. adipocytes increase by the dual processes of hyperplasia and hypertrophy. The accumulation of lipids in adipose tissue is regulated by the stage of differentiation of adipocytes. Intramuscular fat cells in bovine muscle differentiate as clusters of various sizes. Intramuscular fat cell size is dependent on the size of the fat cell clusters; small clusters or masses contain smaller fat cells than do larger clusters or masses. Preadipocytes first proliferate, followed by induction of the genes responsible for lipid filling (Uezumi et al., 2010). Thus, both the apparent number of adipocytes and the size of adipocytes increase with animal growth. In the case of i.m. adipose tissue, adipocytes initially are visible as linear strings of small, lipid-filled cells embedded in perimyseal adipose tissue. Even after 4 mo of grazing on pasture, adipocytes do not enlarge measurably nor does their apparent number increase. After being fed a grain-based finishing diet, both the size and the apparent number of adipocytes increase substantially.
A major contribution to meat animal adipocyte study was realized after the development of the Coulter counter approach to quantitate fat cell size and number, as discussed previously. Subsequently, the cellular development of adipose tissue in meat animals was revealed, and relationships between adipose cellularity and adipocyte development patterns were demonstrated for the first time. Cellular studies on cattle and pig muscle found that increases in fat cell number with minimal changes in fat cell size characterized developing i.m. adipose tissue (Hood and Allen, 1973; Lee and Kauffman, 1974). Comparing adipose cell size distributions from i.m. adipose tissue of four bovine muscles and the number distributions found in s.c. and perirenal tissue from the same animals demonstrated that the i.m. adipocytes were smaller than s.c. and perirenal adipocytes (Hood and Allen, 1973). Furthermore, bovine muscles with the most i.m. fat consistently had the greater number of fat cells (Hood and Allen, 1975). Pig i.m. fat also had greater numbers of fat cells coupled with smaller fat cell size compared with s.c. adipose tissue (Lee and Kauffman., 1974). Measures of i.m. fat cell size in two pig muscles throughout growth showed that fat cell size was similar for the semitendinosus and trapezius muscles, whereas fat cell number in the semitendinosus muscle greatly exceeded that in the trapezius muscle (Lee and Kauffman, 1974). This cellular characteristic was associated with greater i.m. adipose tissue in the semitendinosus muscle. Clearly, the i.m. fat depot is a later developing depot in meat animals.
Past work indicated that the only characteristic among sensory traits that related significantly to taste was marbling (Campion et al., 1975). Unfortunately almost all strategies to reduce total carcass fat affect marbling negatively. We are now having a paradox in that animal products are leaner but there appears to be a decline in taste (Schwab et al., 2010).
SUMMARY AND CONCLUSIONS
It was our goal to review the early contributions of adipocyte and adipose tissue researchers and, in so doing, acknowledge the great efforts made by the members of North Central region committees and other scientists through the years. Their pioneering research on carcass composition, adipocyte anabolic and catabolic lipid metabolism, and fatty acid composition inspired subsequent research. Subsequently, they focused on nutritional, pharmacological, and endocrine strategies to change body composition. More recently, their research has revealed factors that control adipocyte hyperplasia, differentiation, and hypertrophy, and effects of ST and BAR were tested for influences on adipocyte hypertrophy. It is a tribute to their earlier work that adipocyte and adipose tissue development problems are now studied using cutting edge biology and molecular biology for the 21st century.
Conflict of interest statement. None declared.
REFERENCES
- Albrecht E., Kuzinski J., Komolka K., Gotoh T., and Maak S.. 2015. Localization and abundance of early markers of fat cell differentiation in the skeletal muscle of cattle during growth—are DLK1-positive cells the origin of marbling flecks. Meat Sci. 100:237–245. doi:10.2527/jas.2011-4642 [DOI] [PubMed] [Google Scholar]
- Allen C.E. 1968. Fat deposition in the porcine animal. In: Proc. Reciprocal Meat Conf.; p. 306–315. [Google Scholar]
- Allen C.E. 1976. Cellularity of adipose tissue in meat animals. Federation Proc. 35:2302–2307. [PubMed] [Google Scholar]
- Allen C.E., Beitz D.C., Cramer D.A., and Kauffman R.G.. 1976. Biology of fat in meat animals. North Central Regional Publication No.234. Research Division, College of Agriculture and Life Sciences. Madison: University of Wisconsin Press. [Google Scholar]
- Anderson P.T., Hawkins D.R., Bergen W.G., and Merkel R.A.. 1988. A note on dry-matter intake and composition of gain of Simmental bulls and steers fed to the same age or weight. J. Anim. Sci. 47:493–496. [Google Scholar]
- Anderson D.B., and Kauffman R.G.. 1973. Cellular and enzymatic changes in porcine adipose tissue during growth. J. Lipid Res. 14:160–168. [PubMed] [Google Scholar]
- Anderson D.B., Kauffman R.G., and Kastenschmidt L.L.. 1972. Lipogenic enzyme activities and cellularity of porcine adipose tissue from various anatomical locations. J. Lipid Res. 13:593–599. [PubMed] [Google Scholar]
- Annison E.F., and Lindsay D.B.. 1961. Acetate utilization in sheep. Biochem. J. 78:777–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball C.E. 1998. Building the beef industry. New York (NY): Saratoga Publishing Group, Saratoga Springs; p. 1–296. [Google Scholar]
- Ballard F.J., Hanson R.W., and Kronfeld D.S.. 1969. Gluconeogenesis and lipogenesis in tissue from ruminant and nonruminant animals. Fed. Proc. 28:218–231. [PubMed] [Google Scholar]
- Bell E.T. 1909a. I. On the occurrence of fat in the epithelium, cartilage, and muscle fibers for the ox. Am. J. Pathol. 9:401–412. [Google Scholar]
- Bell E.T. 1909b. I. On the histogenesis of the adipose tissue of the ox. Am. J. Pathol. 9:412–442. [Google Scholar]
- Berg R.T., and Butterfield R.M.. 1968. Growth patterns of bovine muscle, fat and bone. J. Anim. Sci. 27:611–619. [Google Scholar]
- Bergen W.G. 2001. The role of cyclic AMP elevating agents and somatotropin in pre and posttranslational regulation of lipogenesis and lipolysis in Bos taurus and Sus srofa. Recent Res. Devel. Lipids 5:47–59. [Google Scholar]
- Bergen W.G., and Merkel R.A.. 1991a. Body composition in animals treated with partitioning agents; implication for human health. FASEB J. 5:2951–2957. [DOI] [PubMed] [Google Scholar]
- Bergen W.G., and Merkel R.A.. 1991b. Protein accretion. In: Pearson A.M., and T.R. Dutson, editors. Adv Meat Sci Research; vol. 7 London (UK) and New York (NY): Elsevier Applied Science; p. 169–202. [Google Scholar]
- Bergen W.G., Mulvaney D.R., Skjaerlund D.M., Babbiker A.A.S., Ames N.K., Merkel R.A., and Anderson D.B.. 1989. Muscle protein metabolism in finishing pigs fed ractopamine. J. Anim. Sci. 67:2255–2262. [DOI] [PubMed] [Google Scholar]
- Bickerstaffe R., Annison E., and Linzell J.. 1974. The metabolism of glucose, acetate, lipids and amino acids in lactating dairy cows. J. Agr. Sci. 82:71–85. [Google Scholar]
- Brooks M.A., Choi C.W., Lunt D.K., Miller R.K., Choi C.B., and Smith S.B.. 2011. Carcass and meat characteristics and M. longissimus thoracis histology of beef from calf-fed and yearling-fed Angus steers. Prof. Anim. Sci. 27:385–393. [Google Scholar]
- Butler O.D., Warwick B.L., and Cartwright T.C.. 1956. Slaughter and carcass characteristics of short fed yearling, Hereford, and Brahman × Hereford steers. J. Anim. Sci. 15:93–96. [Google Scholar]
- Byers F.M. 1979. Measurement of protein and fat accretion in cattle through isotope dilution procedures. Ohio Agric Res Dev Center Animal, Animal Sci. Ser. 79-1, 36. [Google Scholar]
- Callow E.H. 1947. Comparative studies of meat. I. The chemical composition of fatty and muscular tissue in relation to growth and fattening. J. Agr. Sci. 37:113–129. [Google Scholar]
- Callow E.H. 1948. Comparative studies of meat. The changes in the carcass during growth and fattening, and their relation to the chemical composition of the fatty and muscular tissue. J. Agr. Sci. 38:174–199. [Google Scholar]
- Campion D.R., Crouse J.D., and Dikeman M.E.. 1975. Predictive value of USDA beef quality grade factors for cooked meat palatability. J. Food. Sci. 40:1225–1228. [Google Scholar]
- Choi S.H., Silvey D.T., Johnson B.J., Doumit M.E., Chung K.Y., Sawyer J.E., Go G.W., and Smith S.B.. 2014. Conjugated linoleic acid (t-10, c-12) reduces fatty acid synthesis de novo, but not expression of genes for lipid metabolism in bovine adipose tissue ex vivo. Lipids 49:15–24. doi:10.1007/s11845-013-3864-0 [DOI] [PubMed] [Google Scholar]
- Cohn C., andD Joseph. 1960. Role of rate of ingestion of diet on regulation of intermediary metabolism (“meal eating” vs. “nibbling”). Metabolism. 9:492–500. [PubMed] [Google Scholar]
- Corah L.R. 2008. ASAS centennial paper: development of a corn-based beef industry. J. Anim. Sci. 86:3635–3639. doi:10.2527/jas.2008-0935 [DOI] [PubMed] [Google Scholar]
- Cramer D.A., Hecker A.L., and Cornforth D.P.. 1934. Distribution of fat in beef cattle. J. Anim. Sci. 37:258 (Abstr.). [Google Scholar]
- Cunningham H.M., Friend D.W., and Nicholson J.W.G.. 1963. Effect of epinephrine on nitrogen and fat deposition of pigs. J. Anim. Sci. 22:632–636. [Google Scholar]
- Dalrymple R.H., Baker B.K., Gingher P.E., Ingle D.L., Pensack J.M., and Ricks C.A.. 1984. A repartitioning agent to improve performance and carcass composition of broilers. Poult. Sci. 63:2376–2383. [DOI] [PubMed] [Google Scholar]
- Dickerson-Weber P.S., Merkel R.A., and Bergen W.G.. 1992. Adipogenic cell line TA1: a suitable model to study effects of β-adrenergic agonist on lipid metabolism. Proc. Soc. Expl. Biol. Med. 201:47–53. [DOI] [PubMed] [Google Scholar]
- Dole V.I. 1965. Energy Storage. In: Renold A.E., and G.F. Cahill, Jr, editors. Handbook of physiology: adipose tissue, section 5. Baltimore (MD): Williams and Wilkins Co; p. 13. [Google Scholar]
- Donkin S.S., Chiu P.Y., Yin D., Louveau I., Swencki B., Vockroth J., Evock-Clover C.M., Peters J.L., and Etherton T.D.. 1996. Porcine somatotropin differentially down-regulates expression of the GLUT4 and fatty acid synthase genes in pig adipose tissue. J. Nutr. 126:2568–2577. [DOI] [PubMed] [Google Scholar]
- Dunshea F.R. 1993. Effect of metabolism modifiers on lipid metabolism in the pig. J. Anim. Sci. 71:1966–1977. [DOI] [PubMed] [Google Scholar]
- Etherton T.D. 2000. The biology of somatotropin in adipose tissue growth and nutrient partitioning. J. Nutr. 130:2623–2625. [DOI] [PubMed] [Google Scholar]
- Etherton T.D. 2009. ASAS centennial paper: animal growth and development research: historical perspectives. J. Anim. Sci. 87:3060–3064. doi:10.2527/jas.2009-1805 [DOI] [PubMed] [Google Scholar]
- Etherton T.D., and Bauman D.E.. 1998. Biology of somatotropin in growth and lactation of domestic animals. Physiol. Rev. 78:745–761. Available from 10.1152/physrev.1998.78.3.745 [DOI] [PubMed] [Google Scholar]
- Etherton T.D., Wangsness P.J., Hammers V.M., and Ziegler J.H.. 1982. Effect of dietary restriction on carcass composition and adipocyte cellularity of swine with different propensities for obesity. J. Nutr. 112:2314–2323. [DOI] [PubMed] [Google Scholar]
- Etherton T.D., Thompson E.H., and Allen C.E.. 1977. Improved techniques for studies of adipocyte cellularity and metabolism. J. Lipid Res. 18:552–557. [PubMed] [Google Scholar]
- Eversole D.E., Bergen W.G., Merkel R.A., Magee W.T., and Harpster H.W.. 1981. Growth and muscle development of feedlot cattle of different genetic back-grounds. J. Anim. Sci. 53:91–101. [DOI] [PubMed] [Google Scholar]
- Flatt J.P., and Ball E.G.. 1966. Studies on the metabolism of adipose tissue. J. Biol. Chem. 211:2862–2869. [PubMed] [Google Scholar]
- Fortin A., Simpfendorfer S., Reid J.T., Ayala H.J., Anrique R., and Kertz A.F.. 1980. Effect of level of energy intake and influence of breed and sex on the chemical composition of cattle. J. Anim. Sci. 51:604–614. [DOI] [PubMed] [Google Scholar]
- Guenther J.J. 1967. Qualitative characteristics of meat animals as influenced by physiological material equivalents. In: Proc. Reciprocal Meat Conf.; p. 88–96
- Halsey C.H., Weber P.S., Reiter S.S., Stronach B.N., Bartosh J.L., and Bergen W.G.. 2011. The effect of ractopamine hydrochloride on gene expression in adipose tissues of finishing pigs. J. Anim. Sci. 89:1011–1019. doi:10.2527/jas.2010-3269 [DOI] [PubMed] [Google Scholar]
- Hammond J. 1932. Growth and the development of mutton qualities in the sheep. London (UK): Oliver and Boyd, Ltd. [Google Scholar]
- Hankins O.G., and Howe P.E.. 1946. Estimation of the composition of beef carcasses and cuts. USDA Tech. Bulletin 926:1–20. [Google Scholar]
- Hankins O.G., and Titus H.W.. 1939. Growth, fattening and meat product. U.S.D.A, Yearbook; pp. 450–468. [Google Scholar]
- Hanson R.W. and Ballard F.J.. 1967. The relative significance of acetate and gluose as precursors for lipid synthesis in liver adipose tissue from ruminants. Biochem. J. 105:529–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazel L.N., and Kline E.A.. 1952. Mechanical measures of fatness and carcass value on live hogs. J. Anim. Sci. 11:3–18. [Google Scholar]
- Hazel L.N. and Kline E.A.. 1959. Ultrasonic measures of fatness in swine. J. Anim. Sci. 18:815–819. [Google Scholar]
- Hedrick H.B., Zinn D.W., Zobrisky S.F., and Bailey M.F.. 1967. Quantitative relationships of meat animal composition during growth and development. In: Proc. Reciprocal Meat Conf.; p. 55–87.
- Hetzer H.O. and Harvey W.R.. 1967. Selection for high and low fatness in swine. J. Anim. Sci. 26:1244–1251. [Google Scholar]
- Hiner R.L. and Bond J.. 1971. Growth of muscle and fat in beef steers from 6 to 36 months age. J. Anim. Sci. 32:225–232. [Google Scholar]
- Hood R.L. and Allen C.E.. 1973. Cellularity of bovine adipose tissue. J. Lipid Res. 14:605–610. [PubMed] [Google Scholar]
- Hood R.L. and Allen C.E.. 1977. Cellularity of porcine adipose tissue: effects of growth and adiposity. J. Lipid Res. 18:275–284. [PubMed] [Google Scholar]
- Houghton P.L. and Turlington L.M.. 1992. Application of ultrasound for feeding and finishing animals: a review. J. Anim. Sci. 70:930–41. [DOI] [PubMed] [Google Scholar]
- Hudson N.J., Reverter A., Greenwood P.L., Guo B., Cafe L.M., and Dalrymple B.P.. 2015. Longitudinal muscle gene expression patterns associated with differential intramuscular fat in cattle. Animal 9:650–659. doi:10.1017/S1751731114002754 [DOI] [PubMed] [Google Scholar]
- Hungate R.E. 1964. The rumen and its microbes. New York (NY): Academic Press. [Google Scholar]
- Ireland J.J., Roberts R.M., Palmer G.H., Bauman D.E., and Bazer F.W.. 2008. A commentary on domestic animals as dual-purpose models that benefit agricultural and biomedical research. J. Anim. Sci. 86:2797–2805. doi:10:2527/jas. 2008-1088 [DOI] [PubMed] [Google Scholar]
- Jeanrenaud B., Hepp D., and Renold A.E.. 1970. The influence of lipolysis on energy metabolism of isolated fat cells. Horm. Metab. Res. 2(Suppl. 2):76–79. [PubMed] [Google Scholar]
- Katz J. and Wals P.A.. 1974. Lipogenesis from lactate in rat adipose tissue. Biochem. Biophys. Acta 348:344–356. [DOI] [PubMed] [Google Scholar]
- Koch R.M., Dikeman M.E., Allen D.M., Crouse J.D., and Campion D.R.. 1976. Characterization of biological types of cattle 1. Carcass composition, quality and palatability. J. Anim. Sci. 43:48–62. [Google Scholar]
- Koch R.M., Dikeman M.E., and Crouse J.D.. 1982. Characterization of biological types of cattle (Cycle III). III. Carcass composition, quality and palatability. J. Anim. Sci. 54:35–45. [Google Scholar]
- Lawrie R.A. 1961a. Studies on the muscles of meat animals. I. Differences in composition of beef longissimus dorsi muscles determined by age and anatomical location. J. Agr. Sci. 56:249. [Google Scholar]
- Lawrie R.A. 1961b. Systematic analytical differences between psoas major and longissims dorsi muscles of cattle. Br. J. Nutr. 15:453–456. [DOI] [PubMed] [Google Scholar]
- Lawrie R.A., Pomeroy R.W., and Cuthbertson A.. 1963. Studies on the muscles of meat animals. 111. Comparative composition of various muscles in pigs of three weight groups. J. Agr. Sci. 60:195–209. [Google Scholar]
- Lee Y.B., Kauffman R.G., and Grummer R.H.. 1973. Effect of Early Nutrition on the Development of Adipose Tissue in the Pig. II. Weight Constant Basis. J. Anim. Sci. 37:1319–1325. doi:10.2527/jas1973.3761319x [Google Scholar]
- Lee Y.B. and Kauffman R.G.. 1974. Cellular and enzymatic changes with animal growth in porcine intramuscular adipose tissue. J. Anim. Sci. 38:532–537. [DOI] [PubMed] [Google Scholar]
- Lin K.C., Cross H.R., and Smith S.B.. 1992. Esterification of fatty acids by bovine intramuscular and subcutaneous adipose tissue. Lipids 27:111–116. [DOI] [PubMed] [Google Scholar]
- Lunt D.K., Riley R.R., and Smith S.B.. 1993. Growth and carcass characteristics of Angus and American Wagyu steers. Meat Sci. 34:327–334. doi:10.1016/0309-1740(93)90081-R [DOI] [PubMed] [Google Scholar]
- McCarthy F.D., Bergen W.G., and Hawkins D.R.. 1983. Muscle protein turnover in cattle of different genetic backgrounds as measured by Nτ-methyl histidine. J. Nutr. 113:2455–2463. [DOI] [PubMed] [Google Scholar]
- McMeekan C.P. 1940. Growth and development in the pig, with special reference to carcass quality characteristics. J Agricultural Sci. 30:387–511. [Google Scholar]
- McMeekan C.P. 1959. Principles of animal production. Whitcombe and Tombe, Ltd. [Google Scholar]
- McNeel R.L., Ding S.T., Smith E.O., and Mersmann H.J.. 2000. Expression of porcine adipocyte transcripts during differentiation in vitro and in vivo. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 126:291–302. [DOI] [PubMed] [Google Scholar]
- Mersmann H.J. 1998a. Lipoprotein and hormone-sensitive lipases in porcine adipose tissue. J. Anim. Sci.76:1396–1404. [DOI] [PubMed] [Google Scholar]
- Mersmann H.J. 1998b. Overview of the effects of beta-adrenergic receptor agonists on animal growth including mechanisms of action. J. Anim. Sci. 76:160–172. [DOI] [PubMed] [Google Scholar]
- Mersmann H.J., Goodman J.R., and Brown L.J.. 1975.. Development of swine adipose tissue: morphology and chemical composition. J. Lipid Res. 16:269–279. [PubMed] [Google Scholar]
- Mersmann H.J., Underwood M.C., Brown L.J., and Houk J.M.. 1973. Adipose tissue composition and lipogenic capacity in developing swine. Am. J. Physiol. 224:1130–1135. [DOI] [PubMed] [Google Scholar]
- Miller P.S., Reis B., Calvert C., DePeters E., and Baldwin R.. 1991. Patterns of nutrient uptake by the mammary glands of lactating dairy cows. J. Dairy Sci. 74:3791–3799. doi:10.3168/jds.S0022-0302(91)78571-8 [DOI] [PubMed] [Google Scholar]
- Mitchell A.D. 2007. Impact of research with cattle, pigs, and sheep on nutritional concepts: body composition and growth. J. Nutr. 137:711–714. [DOI] [PubMed] [Google Scholar]
- Moody W.B. and Cassens R.G.. 1968. A quantitative and morphological study of bovine longissimus fat cells. J. Food Sci. 33:47–55. [Google Scholar]
- Moulton C.R. 1923. Age and chemical development in mammals. J. Biol. Chem. 57:79–97. [Google Scholar]
- Mulvaney D.R., Merkel R.A., and Bergen W.G.. 1985. Skeletal muscle protein turnover in young male pigs. J. Nutr. 115:1057–1064. [DOI] [PubMed] [Google Scholar]
- Nayananjalie W.A., Wiles T.R., Gerrard D.E., McCann M.A., and Hanigan M.D.. 2015. Acetate and glucose incorporation into subcutaneous, intramuscular, and visceral fat of finishing steers. J. Anim. Sci. 93:2451–2459. doi:10:2527/jas.2014–83 [DOI] [PubMed] [Google Scholar]
- Peterla T.A. and Scanes C.G.. 1990. Effect of beta-adrenergic agonists on lipolysis and lipogenesis by porcine adipose tissue in vitro. J. Anim. Sci. 68:1024–1029. [DOI] [PubMed] [Google Scholar]
- Pothoven M.A. and Beitz D.C.. 1973. Effect of adipose tissue site, animal weight, long-term fasting on lipogenesis in the bovine. J. Nutr. 103:468–475. [DOI] [PubMed] [Google Scholar]
- Pothoven M.A., Beitz D.C., and Thornton J.H.. 1975. Lipogenesis and lipolysis in adipose tissue of ad libitum and restricted-fed cattle during growth. J. Anim. Sci. 40:957–962. [DOI] [PubMed] [Google Scholar]
- Prior R.L., Smith S.B., and Jacobson J.J.. 1981. Metabolic pathways involved in lipogenesis from lactate and acetate in bovine adipose tissue: effects of metabolic inhibitors. Arch. Biochem. Biophys. 211:202–210. [DOI] [PubMed] [Google Scholar]
- Reynolds S.P., Ireland J.J., Caton J.S., Bauman D.A., and Davis T.A.. 2009. Commentary on domestic animals in agricultural and biomedical research: an endangered enterprise. J. Nutr. 139:427–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robelin J. 1981. Cellularity of bovine adipose tissues; developmental changes from 15 to 65 percent mature weight. J. Lipid Res. 22:452–457. [PubMed] [Google Scholar]
- Rodbell M. 1997. The complex regulation of receptor-coupled G-proteins. Adv. Enzyme Regul. 37:427–435. [DOI] [PubMed] [Google Scholar]
- Scanes C.G., Peterla T.A., and Campbell R.M.. 1994. Influence of adenosine or adrenergic agonists on growth hormone stimulated lipolysis by chicken adipose tissue in vitro. Comp. Biochem. Physiol. Pharmacol. Toxicol. Endocrinol. 107:243–248. [DOI] [PubMed] [Google Scholar]
- Schlegel M.L. 1999. Influence of bovine somatotropin administration to Holstein steers on growth, lipid metabolism and carcass characteristics [PhD dissertation]. East Lansing (MI): Michigan State University. [Google Scholar]
- Schroeder A.L., Bergen W.G., Stachiw M.A., and Merkel R.A.. 1987. Comparisons of commonly used methods for estimating beef carcass composition. J. Anim. Sci. 64(Suppl. 1):260. [Google Scholar]
- Schwab C.R., Baas T.J., and Stalder K.J.. 2010. Results from six generations of selection for intramuscular fat in Duroc swine using real-time ultrasound. II. Genetic parameters and trends. J. Anim. Sci. 88:69–79. [DOI] [PubMed] [Google Scholar]
- Shirouchi B., E Albrecht, G Nuernberg, S Maak, Olavanh S., Y Nakamura, M Sato, M.T Gotoh, and K Nuernberg. 2014. Fatty acid profiles and adipogenic gene expression of various fat depots in Japanese Black and Holstein steers. Meat Sci. 96:157–164. doi:10.1016/j.meatsci.2013.06.027 [DOI] [PubMed] [Google Scholar]
- Sidhu K.S., Emery R.S., Parr A.F., and Merkel R.A.. 1973. Fat mobilizing lipase in relation to fatness in lambs. J. Anim. Sci. 136:658–662. doi:10.2527/jas.2008-1336 [DOI] [PubMed] [Google Scholar]
- Sillience M.N. 2004. Technologies for the control of fat and lean deposition in livestock. Vet. J. 167:242–257. doi:10.1016/j.tvjl.2003.10.020 [DOI] [PubMed] [Google Scholar]
- Smith S.B. 1974. The effect of market weight and ration concentrate level on carcass traits of bulls, heifers, and steers [MS thesis]. Brookings (SD): South Dakota State University. [Google Scholar]
- Smith S.B. 1983. Contribution of the pentose cycle to lipogenesis in bovine adipose tissue. Arch. Biochem. Biophys. 221:46–56. [DOI] [PubMed] [Google Scholar]
- Smith S.B. 1995. Substrate utilization in ruminant adipose tissues. In: Smith S.B., and D.R. Smith, editors. The biology of fat in meat animals: current advances. Savoy (IL): American Society of Animal Science; p. 166–188. [Google Scholar]
- Smith S.B. and Crouse J.D.. 1984. Relative contributions of acetate, lactate and glucose to lipogenesis in bovine intramuscular and subcutaneous adipose tissue. J. Nutr. 114:792–800. [DOI] [PubMed] [Google Scholar]
- Smith S.B. and Johnson B.J.. 2016. Marbling: management of cattle to maximize the deposition of intramuscular adipose tissue. J. Anim. Sci. 94(E-Suppl):794. [Google Scholar]
- Smith S.B., Kawachi H., Choi C.B., Choi C.W., Wu G., and Sawyer J.E.. 2009. Cellular regulation of bovine intramuscular adipose tissue development and composition. J. Anim. Sci. 87(E Suppl):E72–E82. [DOI] [PubMed] [Google Scholar]
- Smith S.B. and Prior R.L.. 1981. Evidence for a functional ATP-citrate lyase:NADP-malate dehydrogenase pathway in bovine adipose tissue: enzyme and metabolite levels. Arch. Biochem. Biophys. 211:192–201. doi:10.2527/jas.2008-1340 [DOI] [PubMed] [Google Scholar]
- Smith S.B. and Prior R.L.. 1982. The effect of 3-mercaptopicolinic acid and substrate interactions on the incorporation of lipogenic precursors into glyceride-glycerol, glyceride-fatty acids and nonesterified fatty acids in bovine adipose tissue. Biochim. Biophys. Acta 712:365–373. [DOI] [PubMed] [Google Scholar]
- Smith S.B. and Prior R.L.. 1984. Pentose cycle flux and fatty acid synthesis in bovine adipose tissue slices incubated with 6-aminonicotinamide. Proc. Soc. Exp. Biol. Med. 175:98–105. [DOI] [PubMed] [Google Scholar]
- Smith S.B. and Prior R.L.. 1986. Comparisons of lipogenesis and glucose metabolism between ovine and bovine adipose tissues. J. Nutr. 116:1279–1286. [DOI] [PubMed] [Google Scholar]
- Smith S.B., Prior R.L., Ferrell C.L., and Mersmann H.J.. 1984. Interrelationships among diet, age, fat deposition and lipid metabolism in growing steers. J. Nutr. 114:153–162. [DOI] [PubMed] [Google Scholar]
- Smith S.B., and Smith D.R.,editors. 1995. The biology of fat in meat animals – current advances. Champaign (IL): ASAS. [Google Scholar]
- Spivey S.P. 1980. The effect of frame-size and diet on daily protein accretion and fat deposition in young growing bulls [MS thesis]. East Lansing (MI): Michigan State University. [Google Scholar]
- Uezumi A.S., Fukada S., Yamamoto N., Takeda S., and Tsuchida K.. 2010. Mesenchymal progenitors distinct from satellite cells contribute to ectopic fat cell formation in skeletal muscle. Nat. Cell Biol. 12:143–152. doi:1038/ncb2014 [DOI] [PubMed] [Google Scholar]
- Villegas F.J., Hedrick H., Veum T.L., McFate K.L., Thomas W.K., and Bailey M.E.. 1973. Effect of breed and diet containing cooked soybeans vs. extracted soybean meal on pork carcass characteristics. J. Anim. Sci. 37:443–449. [Google Scholar]
- Wakil S.J. 1962. Enzymatic synthesis of fatty acids. Comp. Biochem. Physiol. 4:123–158. [DOI] [PubMed] [Google Scholar]
- Warner K.F., and Ellis N.R.. 1934. Cutting yields of hogs and index of fatness. J. Agr. Res. 48:241–255. [Google Scholar]
- Weber P.S., Merkel R.A., and Bergen W.G.. 1992. Adipogenic cell line TA1: A suitable model to study effects of β-adrenergic agonist on lipid metabolism. Pro. Soc. Expl. Biol. Med (PSEBM). 201:47–53. [DOI] [PubMed] [Google Scholar]
- Wilson D.E. 1992. Application of ultrasound for genetic improvement. J. Anim. Sci. 70:973–983. [DOI] [PubMed] [Google Scholar]
- Yin D., Clarke S.D., Peters J.L., and Etherton T.D.. 1998. Somatotropin-dependent decrease in fatty acid synthase mRNA abundance in 3T3-F442A adipocytes is the result of a decrease in both gene transcription and mRNA stability. Biochem. J. 331:815–820. [DOI] [PMC free article] [PubMed] [Google Scholar]