Abstract
The objective of this study was to explore the potential of transmission infrared (TIR) spectroscopy in combination with partial least squares regression (PLSR) for quantification of dairy and beef cow colostral immunoglobulin G (IgG) concentration and assessment of colostrum quality. A total of 430 colostrum samples were collected from dairy (n = 235) and beef (n = 195) cows and tested by a radial immunodiffusion (RID) assay and TIR spectroscopy. Colostral IgG concentrations obtained by the RID assay were linked to the preprocessed spectra and divided into combined and prediction data sets. Three PLSR calibration models were built: one for the dairy cow colostrum only, the second for beef cow colostrum only, and the third for the merged dairy and beef cow colostrum. The predictive performance of each model was evaluated separately using the independent prediction data set. The Pearson correlation coefficients between IgG concentrations as determined by the TIR-based assay and the RID assay were 0.84 for dairy cow colostrum, 0.88 for beef cow colostrum, and 0.92 for the merged set of dairy and beef cow colostrum. The average of the differences between colostral IgG concentrations obtained by the RID- and TIR-based assays were −3.5, 2.7, and 1.4 g/L for dairy, beef, and merged colostrum samples, respectively. Further, the average relative error of the colostral IgG predicted by the TIR spectroscopy from the RID assay was 5% for dairy cow, 1.2% for beef cow, and 0.8% for the merged data set. The average intra-assay CV% of the IgG concentration predicted by the TIR-based method were 3.2%, 2.5%, and 6.9% for dairy cow, beef cow, and merged data set, respectively.
The utility of TIR method for assessment of colostrum quality was evaluated using the entire data set and showed that TIR spectroscopy accurately identified the quality status of 91% of dairy cow colostrum, 95% of beef cow colostrum, and 89% and 93% of the merged dairy and beef cow colostrum samples, respectively. The results showed that TIR spectroscopy demonstrates potential as a simple, rapid, and cost-efficient method for use as an estimate of IgG concentration in dairy and beef cow colostrum samples and assessment of colostrum quality. The results also showed that merging the dairy and beef cow colostrum sample data sets improved the predictive ability of the TIR spectroscopy.
Keywords: beef cow, colostrum, dairy cow, immunoglobulin G, infrared spectroscopy, partial least squares regression
INTRODUCTION
Simple, rapid, and accurate methods for quantification of immunoglobulin G (IgG) concentration in dairy and beef cow colostrum are needed. Measuring IgG content in colostrum before feeding to calves is a useful tool to improve calf health and reduce calf mortality (Filteau et al., 2003). Several methods are in current use to measure colostral IgG content, both directly and indirectly. The direct methods are: radial immunodiffusion (RID) assay (McBeath et al., 1971), ELISA (Gelsinger et al., 2015), chromatography (Abernethy and Otter, 2010), and electrophoresis (Page and Thorpe, 2002). Several indirect on-farm methods such as the colostrometer (Bartier et al., 2015) and Brix refractometers (Bartens et al., 2016) have been used to estimate IgG levels. Recently, transmission infrared (TIR) spectroscopic methods have been used in the dairy industry as a rapid tool to predict milk protein (Rutten et al., 2011), determine feed composition (Ohlsson et al., 2007), and measure serum IgG concentrations (Elsohaby et al., 2014). Although TIR has been used to assess IgG in colostrum from dairy cows (Elsohaby et al, 2016), to our knowledge, there is no previous report of the use of TIR spectroscopy for measuring the IgG concentration in beef cow colostrum, which has a substantially different IgG concentration. The study of the use of TIR in beef colostrum is indicated given the expected differences between beef vs. dairy derived colostrum and previous studies from our group on dairy cow colostrum, which showed that at high IgG concentration the level of agreement between the reference RID and TIR was lower (Elsohaby et al., 2016). We hypothesized that merging of data from dairy and beef cow colostrum would improve the accuracy of TIR spectroscopy testing for IgG in colostrum by training the system over a broader data source. The objectives of the current study were: 1) to explore the potential of TIR spectroscopy in combination with partial least squares regression (PLSR) for quantification of IgG concentration in dairy and beef cow colostrum; and, 2) to investigate the impact of merging dairy and beef cow colostrum data sets on predictive performance of the TIR spectroscopy.
MATERIALS AND METHODS
This study was conducted in accordance with the Canadian Council on Animal Care guidelines (CCAC, 2009) under a protocol approved by the Animal Care Committee at University of Prince Edward Island (protocol #6006206) and the Veterinary Science Animal Care Committee of the University of Calgary (protocols #AC13-0324 and #AC15-0150).
Colostrum Samples
A total of 430 colostrum samples were collected from dairy and beef cows. Of these, 235 samples were collected between February 2014 and November 2015 from Holstein dairy cows on eight commercial dairy farms in New Brunswick, Canada, and the remaining 195 samples were collected between March 2014 and April 2016 from beef cows on six ranches in Alberta, Canada. Colostrum samples (40–50 mL) were collected in screw-top tubes labeled with the farm name, cow identification number, and date of collection, and were frozen until shipping. Samples were shipped on ice to the Maritime Quality Milk Laboratory, University of Prince Edward Island (UPEI), where they were stored at −80 °C. An aliquot of the collected colostrum samples was shipped to The Saskatoon Colostrum Company Ltd. (SCCL; Saskatoon, SK, Canada), where they were tested for IgG concentration using RID assay, as described below.
RID Assay
The reference IgG concentration used in this study were obtained by the Quality Assurance Laboratory of The Saskatoon Colostrum Co. Ltd. (SCCL). The RID assay was performed as originally described by Chelack et al. (1993) with modifications. Colostrum samples were diluted 1:15 in PBS. Antiserum against bovine IgG (heavy and light chains; Jackson Laboratories, West Grove, Pennsylvania, USA) was used. Immunodiffusion plates were prepared from 2% agarose containing 2.5% antiserum in PBS at pH 7.25. Standard curves (1.06 to 8.5 g/L) were produced by means of duplicate samples of a bovine IgG serum calibrator (Midland BioProducts Corporation, Boone, Iowa, USA). Plate validity was assessed with a reference serum from the Center for Veterinary Biologics, Animal and Plant Health Inspection Service, United States Department of Agriculture, Ames, Iowa. All samples were tested in duplicate and incubated in a humid atmosphere at 25 °C for 18 to 19 h. Ring diameters were measured with a computer-assisted plate reader (The Binding Site Group, Birmingham, England) and the values for the samples calculated with a program for linear analysis. Colostrum samples with IgG concentration outside the range of the standard curve were retested at a different dilution. The final IgG concentration for each sample was determined by calculating a single value from the average diameter of the two replicates. The plate was considered acceptable if the coefficient of determination was greater than 0.97 for the standard curve.
Infrared Spectra Collection
Infrared (IR) spectra were acquired for 430 bovine colostrum samples using transmission infrared spectrometer (Tensor 37, Bruker Optics, Milton, ON, Canada) equipped with a deuterium tryglycine sulfate detector and controlled by proprietary software (OPUS ver. 6.5, Bruker Optics, Milton, ON, Canada). Thawed colostrum samples were diluted (1:3) with deionized sterile water and tested in replicates of six by evenly spreading 5 μL aliquots into 5 mm diameter wells within an adhesive-masked, 96-well silicon microplate (Riley et al., 2007). An empty well served as the background reference for each microplate. A total of 2,580 (430 samples × 6 replicates) spectra were collected over the wave number range between 4,000 and 400 cm−1 with a nominal resolution of 4 cm−1, with 512 scans collected for data acquisition. Collected spectra were converted into printable (PRN) format (GRAMS/AI version 7.02, Thermo Fisher Scientific, Waltham, MA, USA), imported into MATLAB (MathWorks R2012b, Natick, MA, USA), and further data analysis was performed.
Spectra Preprocessing
Each IR spectrum was preprocessed using Savitsky–Golay smoothing (second order polynomial function with nine points), first-order derivatization (Savitzky and Golay, 1964), and two different normalization methods (standard normal variate [SNV] and vector normalization) (Barnes et al., 1989; Barnes et al., 2004), followed by spectral regions (3,700–2,600 cm−1 and 1,800–1,300 cm−1) selection. Dixon’s Q-test was applied to the replicate spectra of each sample to detect spectrum outliers (Dean and Dixon, 1951; Rorabacher, 1991). The spectrum was considered as an outlier and excluded from further analysis if more than 50% of absorbance values were outside the 95% confidence level. The average of the replicate spectra for each colostrum sample (after removal of outliers if applicable) was used for subsequent analysis.
PLS Calibration Models Development and Validation
On average, the IgG concentration of dairy cow colostrum is lower than that of beef cows (Guy et al. 1994). Also, dairy and beef cow colostrum might have substantially different chemical composition (Mulvey, 1996). Therefore, a multivariate regression method (PLSR) was used to create three calibration models: one for the 235 dairy cow colostrum samples, a second for the 195 beef cow colostrum samples, and a third for the merged data sets of the 430 dairy and beef cow colostrum samples.
The RID-IgG values obtained from SCCL were linked to their corresponding preprocessed IR spectra, and then split into a prediction and a combined set. The prediction set was selected by ordering all colostrum samples by IgG concentration then selecting the spectrum of every third colostrum sample. Thus, the prediction set encompassed the full range of IgG values. The remaining colostrum samples were assigned to the combined set and were further randomly split into training and validation data sets for model development.
A previously described, a PLSR approach (Elsohaby et al., 2016) was applied to the training set to develop 30 trial calibration models with the number of PLS factors ranging from 1 to 30. Each trial model was used to quantify the IgG concentrations of the validation set of colostrum samples. This procedure was repeated 10,000 times, utilizing randomly assigned splits of the combined data set into new training and validation sets. The root mean squared error for the Monte Carlo cross-validation value (RMMCCV) (Picard and Cook, 1984; Xu and Liang, 2001) was calculated for each of the 30 trial calibration models, and the optimal number of PLS factors was chosen based on the lowest RMMCCV value. Once the number of PLS factors had been determined, the training and validation sets were recombined to build the final calibration model with the optimal number of PLS factors (Elsohaby et al., 2016).
The predictive performance of the PLS calibration models was assessed using the prediction data set. Pearson correlation coefficients, concordance correlation coefficients (Lin, 1989), scatter plots, Bland-Altman plots (Altman and Bland, 1983), and relative error (RE = (IR-IgG value − RID-IgG value)/RID-IgG value) were calculated to assess the level of agreement between IgG concentration measured by the reference RID assay and those predicted by the TIR-based assay. The intra-assay sample precision of the TIR-based assay was evaluated by calculation and plotting of the SD and CV between IgG concentrations of the six replicates predicted by TIR-based assay (Salkind, 2010). For comparison, the intra-assay CV between the duplicate replicates for the same samples using the reference RID method was also calculated.
The ratio of predictive deviation (RPD = SD of RID-IgG/root mean squared error of prediction [RMSEP]) and the range error ratio (RER = range of RID-IgG/RMSEP) were also used to evaluate the performance of the PLS calibration models for the prediction of unknown samples (Williams and Sobering, 1996). The PLS models were classified according to the RPD and RER values into poorly predictive (RPD < 2), adequate for qualitative evaluation or for screening purposes (2 < RPD < 2.5), acceptable for quantification (2.5< RPD <3 or RER >10) and suitable for very accurate quantitative analysis (RPD >3 or RER >20) (Williams and Sobering, 1996). The development and validation of the three PLS calibration models were carried out using the same procedures as previously reported except for the number of colostrum samples assigned to prediction, training, and validation sets.
Assessment of Colostrum Quality
To evaluate the applicability of the PLS calibration models for assessment of colostrum quality, the diagnostic test characteristics (sensitivity, specificity, and accuracy) were calculated in the prediction and entire data sets. Sensitivity (Se) was defined as identification of the proportion of low-quality dairy colostrum with RID-IgG values <50 g/L (Godden, 2008) and of beef cow colostrum with RID-IgG values <100 g/L (Windeyer et al., 2015), that was correctly classified by the TIR assay. Conversely, specificity (Sp) was defined as the proportion of dairy and beef cow colostrum samples with RID-IgG values ≥50 g/L and ≥100 g/L, respectively, that was correctly classified by the TIR assay. Accuracy was defined as the percentage of dairy and beef cow colostrum samples that were correctly classified by the TIR assay.
RESULTS AND DISCUSSION
RID-IgG Values
Descriptive statistics of the RID-IgG concentration of the combined and prediction data sets obtained from SCCL for the dairy and beef cow colostrum samples are summarized in Table 1. In the present study, the average RID-IgG concentrations of the dairy and beef cow colostrum were 65.5 g/L and 143.2 g/L, respectively, which was similar to the average IgG reported previously in dairy cow herds by Elsohaby et al. (2017; 64.7 g/L) and Bartier et al. (2015; 63.7 g/L) and higher than the average IgG reported in beef cow herds by Vandeputte et al. (2014; 95.9 g/L). Further, the average of beef cow colostral IgG was higher than that of dairy cow colostrum, in agreement with the result obtained for beef (113.4 g/L) and dairy (42.7 g/L) cows by Guy et al. (1994). The differences in the colostral IgG concentration between beef and dairy cows could be attributed to the genetic differences, management, and dilution effects (Godden, 2008; Baumrucker et al., 2010).
Table 1.
Descriptive statistics of the reference IgG values obtained by radial immunodiffusion assay
| Colostrum | Combined seta | Prediction seta | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | Mean | SD | Min | Max | N | Mean | SD | Min | Max | |
| Dairy cow | 157 | 65.7 | 32.5 | 3.6 | 154.2 | 78 | 65.1 | 31.9 | 6.4 | 153.3 |
| Beef cow | 130 | 143.3 | 40.6 | 2.8 | 251.6 | 65 | 142.9 | 40.9 | 3.8 | 225.4 |
| Dairy and beef cow | 287 | 100.9 | 53.3 | 2.8 | 251.6 | 143 | 100.4 | 52.7 | 3.6 | 223.5 |
a N = number of colostrum samples; SD = standard deviation (g/L); Min = minimum (g/L); Max = maximum (g/L).
The RID assay indicated that the prevalence of poor quality colostrum in dairy cows (IgG <50 g/L) involved in the study was 35% (83/235), which was similar to that recently reported by Bartens et al. (2016; 34.7%) and Bartier et al. (2015; 29.1%). Only 13% (26/195) of beef cows collected for this study had poor quality colostrum (IgG <100 g/L), which similar to previously reported by Mulvey (1996; 12%).
Infrared Spectra
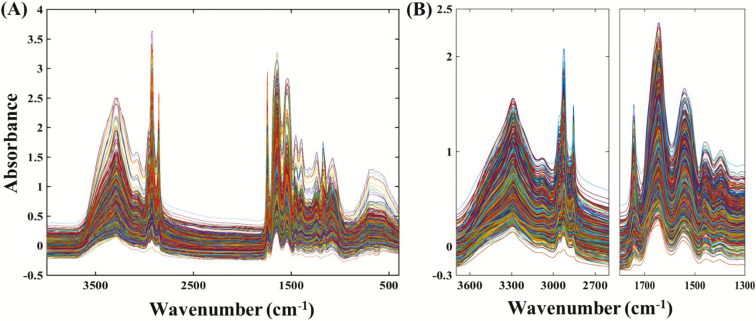
Figure 1A shows the IR spectra of the dairy and beef cow colostrum samples employed in this work over the wave number of 4,000–400 cm−1. All the colostrum spectra obtained have a similar profile, but with complex absorption patterns which arise from the compositional complexity and variability of the colostrum constituents (Kehoe et al., 2007). The intense bands centered around 3,300 cm−1 were attributed to protein N-H (Amid A) stretching vibrations. The region between 1,650 and 1,550 cm−1 contain intense bands corresponding to protein C=O (Amid I) stretching vibrations and protein N=H (Amid II) bending vibrations, respectively. Fat dominates in the region between 2,920 and 2,850 cm−1 (CH2 stretching modes) and at ~1,745 cm−1 (C=O stretch) (Norris, 2001). The two-protein intense spectral regions (3,700–2,600 cm−1) and (1,800–1,300 cm−1) were used for further data preprocessing (Figure 1B) and PLS calibration model development. The spectral region between 2,600–1,800 cm−1 showed the weak absorption band of atmospheric CO2 and was excluded from the development of PLS models (Shaw and Mantsch, 1999).
Figure 1.
Infrared spectra of dairy and beef cow colostrum samples; (A) Raw spectra in the region from 4,000 to 400 cm−1; (B) Preprocessed truncated spectra.
PLS Calibration for Dairy Cow Colostrum Samples
The number of colostrum samples assigned to the training, validation, and prediction sets were 79, 78, and 78, respectively. The number of PLS factors was determined to be 14, based on the lowest RMMCCV (13.75 g/L) (Table 2). The selected PLS model was based on the first-order derivatives spectra with nine points and SNV normalization. This 14-PLS calibration model has a similar number of PLS factors to that previously built by the authors for dairy cow colostrum (Elsohaby et al., 2016) and higher than that built for near infrared (NIR) spectroscopy (Rivero et al., 2012, 2016).
Table 2.
Calibration and prediction statistics of the partial least squares models for quantification of dairy and beef cow colostrum IgG concentrations
| Colostrum | PLS calibration models characteristicsa | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PLS factors | RMMCCV (g/L) | Combined set | Prediction set | |||||||||||
| N | r | ccc | RMSEC (g/L) | N | r | ccc | RMSEP (g/L) | RE (%) | CV (%) | RPD | RER | |||
| Dairy cow | 14 | 13.75 | 157 | 0.96 | 0.96 | 9.8 | 78 | 0.84 | 0.83 | 19.7 | 5 | 3.2 | 1.6 | 7.5 |
| Beef cow | 14 | 21.2 | 130 | 0.93 | 0.92 | 16.4 | 65 | 0.88 | 0.88 | 20.9 | 1.2 | 2.5 | 2 | 10.6 |
| Dairy and beef cow | 14 | 21 | 287 | 0.95 | 0.94 | 17.9 | 143 | 0.92 | 0.92 | 20.6 | 0.8 | 6.9 | 2.6 | 10.7 |
aPLS factors = number of partial least squares factors; RMMCCV = root mean squared error for the Monte Carlo cross-validation value (g/L); N = number of colostrum samples; r = Pearson correlation coefficient; ccc = concordance correlation coefficient; RMSEC = root mean squared error of calibration (g/L); RMSEP = root mean squared error of prediction; RE% = relative error % ((TIR-IgG value − RID-IgG value)/RID-IgG value × 100); CV% = intra-assay CV % ((SD/mean) × 100); RPD = ratio of predictive deviation (SD of RID-IgG/RMSEP); RER = range error ratio (range of RID-IgG/RMSEP).
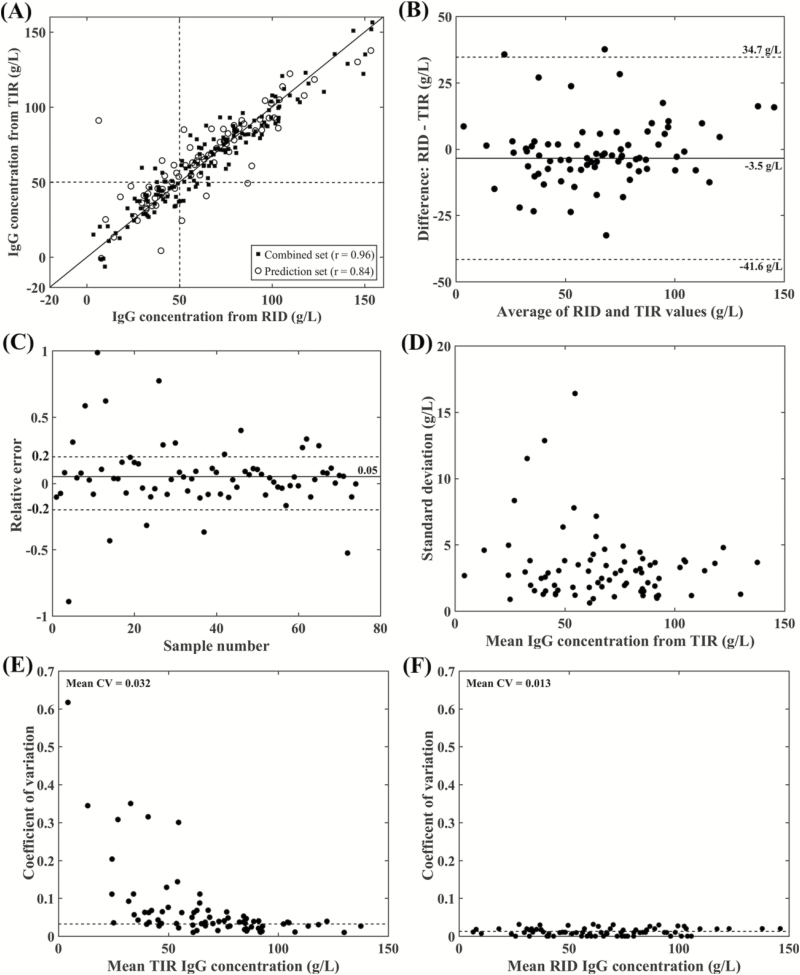
The scatter plot (Figure 2A) and Pearson correlation coefficients between colostral IgG concentration obtained by the reference RID assay and TIR-based assay for the combined and prediction sets were 0.96 and 0.84, respectively. The plots of the combined and prediction sets show similar dispersion with no apparent underfitting or overfitting problems. However, there is a relatively smaller correlation coefficient for colostrum samples with high IgG concentration (over 100 g/L). This is similar to the finding reported in a previous study (Elsohaby et al., 2016). The correlation coefficient of 0.84 reported in this study was similar to the value of 0.85 reported by Løkke et al. (2016) between colostral IgG measured by ELISA and TIR spectroscopy and lower than the 0.97 reported by Rivero et al. (2012) between colostral IgG obtained by RID and NIR spectroscopy. Variations in the correlation coefficients in these studies could be explained by the differences in number and source of colostrum. Further, the Pearson correlation between IgG concentration obtained by RID- and TIR-based methods was higher than that reported for colostrometer (r = 0.77) and Brix refractometer (r = 0.64) (Bartier et al., 2015).
Figure 2.
Results of the PLS calibration for the spectra from dairy cow samples. (A) Scatter plots comparing the reference RID-IgG values to those measured by the TIR-based method for the combined (r = 0.96) and prediction (r = 0.84) data sets. (B) Bland–Altman plot of the difference in IgG concentration obtained by RID assay and the TIR-based method for the prediction data set (average of the difference = −3.5 g/L). (C) Relative error plot, the average of relative error from the reference RID value for the prediction set was 0.05. (D) SD of the IgG concentrations measured by the TIR-based method for the prediction set. (E) CV plot, the average intra-assay CV of IgG concentrations between the six replicates measured by the TIR-based method for the prediction set was 0.032. (F) CV plot, the average intra-assay CV of IgG concentrations between the duplicate samples measured by reference RID assay for the prediction set was 0.013.
The Bland–Altman plot (Figure 2B) revealed that the average of the difference between colostral IgG concentration obtained by the RID- and TIR-based method for the prediction set was −3.5 g/L, which indicating no obvious bias between two methods. The 95% confidence interval ranged from −41.6 g/L to 37.7 g/L, which is relatively small in comparison with the colostral IgG concentrations in this study. The predictive accuracy of the TIR-based method was further assessed by calculating the RE of the TIR predicted IgG concentration from the reference RID values (Figure 2C). The average RE% was 5% (Table 2), which lies within the acceptable accuracy range (±20%) according to the quality control standards of the US Food and Drug Administration Agency (US Food and Drug Administration, 2001). The RE plot shows that 16 of 78 tested samples lie outside of the range which means that 80% of samples tested by the TIR-based method lies within the acceptable accuracy range.
The variability among the replicates when using the TIR-based method for measuring the IgG content of dairy cow colostrum samples of the prediction data set is presented graphically in Figure 2D and E. Mostly, the SD and CV are below 5 g/L and 10%, respectively. The average intra-assay CV of the TIR-based method among the replicates was 3.2% (Table 2), which is higher than that of the reference RID method (Figure 2F; 1.3%). The TIR-based method is considered reliable if the average intra-assay CV is lower than 10% (Albanell et al., 1999).
The utility of the TIR-based method for prediction of colostral IgG concentration in unknown dairy cow colostrum samples were assessed using RPD and RER values. In the current study, the RPD and RER values were 1.6 and 7.5, respectively (Table 2), indicating inadequate quantitative predictive ability of TIR-based method (Williams and Sobering, 1996). Further, these values were lower than that reported by Rivero et al., (2012) for prediction of dairy cow colostral IgG concentration using NIR spectroscopy.
PLS Calibration for Beef Cow Colostrum Samples
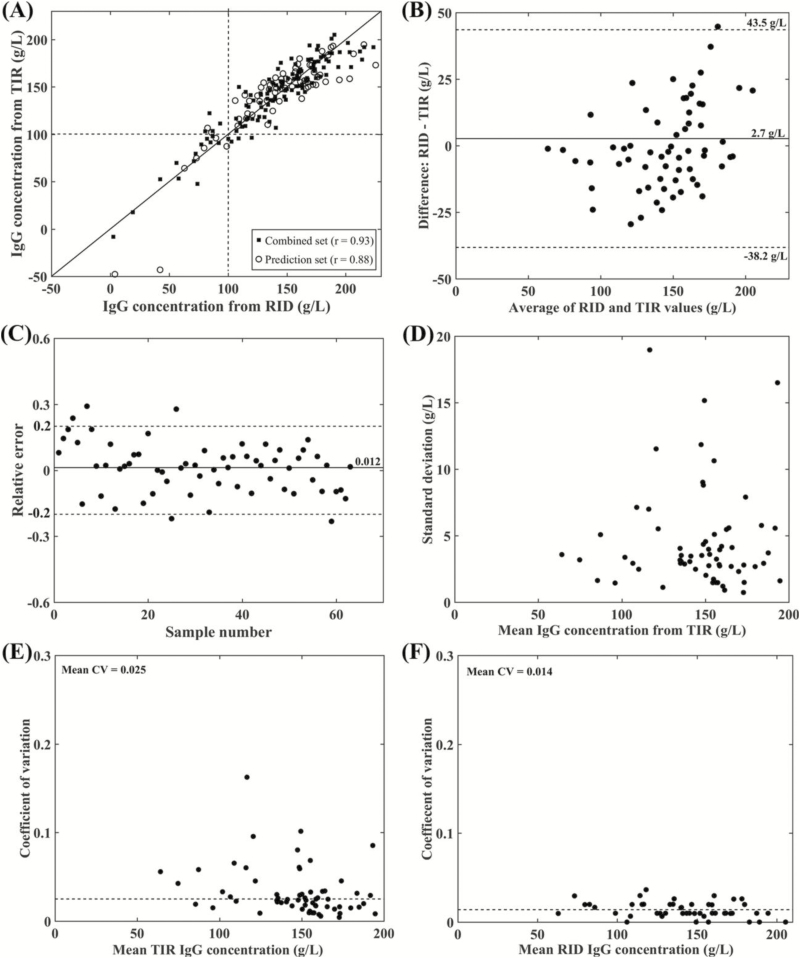
For the beef colostrum samples, 65 samples each were used for the training, validation, and predication sets. The PLS calibration model including 14 PLS factors was selected as optimal based on the lowest RMMCCV (21.2 g/L). This model was based on the smoothed spectra with nine points and vector normalization. The scatter plots for the combined and prediction data sets (Figure 3A) shows similar dispersion with no underfitting or overfitting problems, indicting that the model is well-determined. The Pearson correlation coefficient between IgG concentration obtained by the reference RID- and TIR-based method for the combined set was 0.93 and for the prediction set was 0.88 (Table 2), which is higher than the correlation of 0.80 reported by Vandeputte et al. (2014) between RID and digital Brix refractometer. The plots (Figure 3A) show that at high IgG concentrations, the correlation coefficient between RID- and TIR-based methods is less than that at low concentrations. The lower number of beef cow colostrum samples that have IgG concentration less than 100 g/L (26 of 195) could explain this variation since the correlation coefficient is highly sensitive to the concentration range (Altman and Bland, 1983).
Figure 3.
Results of the PLS calibration for the spectra from beef cow colostrum samples. (A) Scatter plots for the combined and prediction data sets with Pearson correlation coefficients (r) of 0.93 and 0.88, respectively. (B) Bland–Altman plot for the prediction set, the average of the difference between RID and TIR-based methods was 2.7 g/L. (C) The relative error plot for the prediction set with a mean relative error of 0.012. (D) SD plot of the IgG concentrations measured by the TIR-based method for the prediction set. (E) Intra-assay CV of the IgG concentrations between the six replicates measured by the TIR-based method for the prediction set (average CV = 0.025). (F) Average intra-assay CV between the IgG concentrations of the duplicate samples measured by RID assay for the prediction set was 0.014.
Agreement between the RID- and TIR-based method was further assessed using Bland–Altman plot (Figure 3B), which shows no obvious systematic bias between two methods. The average value of the difference was 2.7 g/L and the 95% confidence interval ranged from −38.2 g/L to 43.5 g/L. Figure 3C shows that the RE from the reference RID values for each sample in the prediction set. The average of the RE% was 1.2%, which lies within the acceptable accuracy criterion (±20%) for analytical methods (US Food and Drug Administration, 2001). The RE plot shows that only 5 of 65 samples were out of this range, indicating that the TIR-based method accurately measures 92% of the test samples.
The SD and intra-assay CV for the beef cow colostral IgG concentrations obtained by the TIR-based method among the six replicates of the prediction data set are represented graphically in Figure 3D and E, respectively. SD among the replicates of each sample are small with a value less than 5 g/L and the average intra-assay CV% was 2.5%, which is higher than that for the same samples RID results (Figure 3F, 1.4%) and lower than that of the dairy cow colostrum. The RPD and RER values of the beef cow colostrum PLS calibration model were 2 and 10.6 (Table 2), indicating that the model is adequate for qualitative and screening evaluation of beef cow colostrum IgG concentration (Williams and Sobering, 1996).
PLS Calibration for Merged Dairy and Beef Cow Colostrum Samples
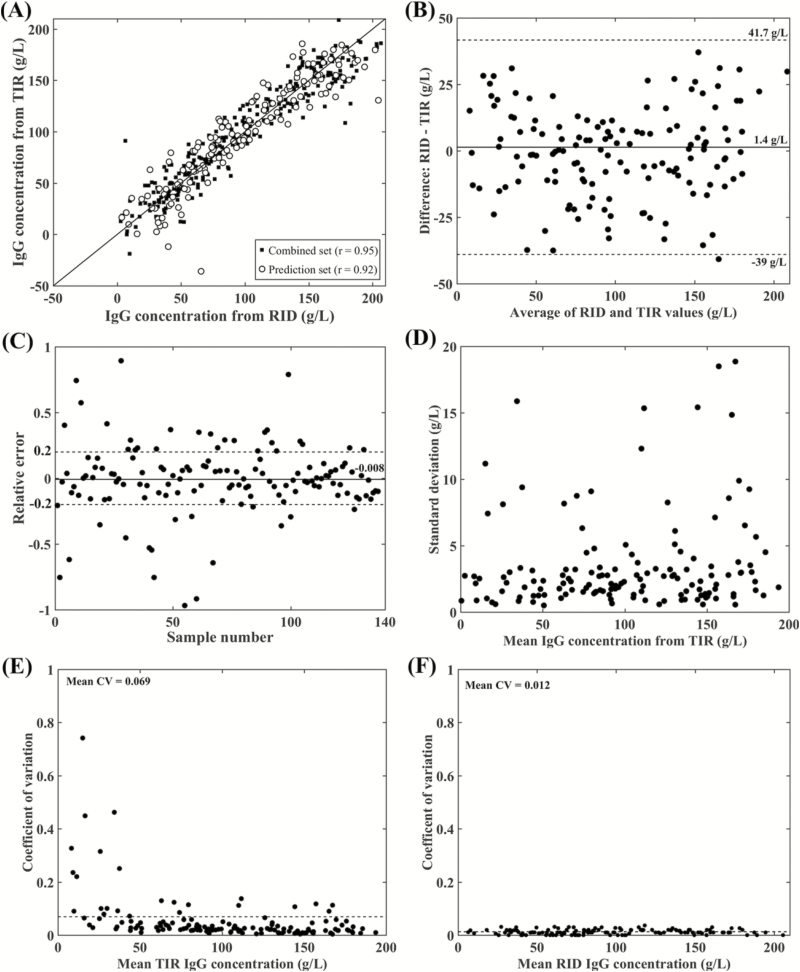
To build a PLS calibration model with a high predictive ability, the data set should have a wide and evenly distributed composition (Murray, 1986). Therefore, the dairy and beef cow colostrum samples were merged and then split into the training (n = 144), validation (n = 143), and prediction (n = 143) sets. The number of PLS factors determined based on the lowest RMMCCV (21 g/L) was 14 (Table 2). The PLS calibration model was built using first-order derivatives spectra with nine points and vector normalization. The scatter plots for the combined and prediction sets are shown in Figure 4A, with Pearson correlation coefficients of 0.95 and 0.92, respectively. The dispersions for the combined and prediction sets are similar with no evidence of underfitting or overfitting. Further, combining the dairy and beef cow colostrum in one PLS calibration model increased the range of IgG concentration predicted by the TIR-based method and overcame the low agreement between RID- and TIR-based methods at high IgG concentrations reported for the dairy and beef cow colostrum models.
Figure 4.
Results of the PLS calibration for the spectra from the merged dairy and beef cow colostrum samples. (A) Scatter plots and Pearson correlation coefficients of the combined (r = 0.95) and prediction (r = 0.92) data sets. (B) Bland–Altman plot for the prediction set, the average of the difference was 1.4 g/L. (C) The relative error plot for the prediction set with an average relative error of −0.008. (D) SD for the IgG concentrations measured by the TIR-based method for the prediction set. (E) Intra-assay CV of the IgG concentrations between the six replicates measured by the TIR-based method for the prediction set (average CV = 0.069). (F) Average intra-assay CV between the IgG concentrations of the duplicate samples measured by the RID assay for the prediction set was 0.012.
The Bland–Altman plot (Figure 4B) does not show any apparent systematic bias and the mean of the difference between IgG obtained by the RID- and TIR-based method was 1.4 g/L. The 95% confidence interval range was −39 to 41.7 g/L; which is small enough to suggest that the PLS calibration model of the combined dairy and beef cow colostrum has the accuracy that is similar to the PLS calibration models built separately for dairy and beef cow colostrum.
The average RE% from the reference values was 0.8% (Table 2), with most of the tested samples (>75%) have an RE% within the acceptable ±20% range (Figure 4C). The SD plot for the IgG concentration predicted by the TIR-based method for replicate spectra against the average IgG concentration shows that most of the samples with SD below 5 g/L (Figure 4D). The average intra-assay CV% for the IgG concentration among the six replicates predicted by the TIR-based method for the prediction set was 6.9% (Figure 4E), which higher than the intra-assay CV% of 1.2% reported between the duplicate replicates for the same samples using the reference RID method (Figure 4F). The PLS calibration model of the combined dairy and beef cow colostrum was acceptable for quantification of IgG concentration in both dairy and beef cow colostrum based on the RPD value (2.6) and RER value (10.7) (Williams and Sobering, 1996).
Comparing the predictive performance of the PLS calibration model for the merged dairy and beef cow colostrum (RMSEP = 20.6 g/L) and the PLS model separately built for the dairy cow colostrum (RMSEP = 19.7 g/L) and beef cow colostrum (RMSEP = 20.9 g/L) showed that the PLS model for merged colostrum samples has better accuracy and comparable precision to that of the models built separately for quantification of dairy and beef cow colostral IgG concentration.
TIR Assay Sensitivity and Specificity for Detection of Colostrum Quality
The sensitivity, specificity, and accuracy of the TIR spectroscopic method for assessment of dairy and beef cow colostrum quality using RID-IgG values <50 g/L and 100 g/L, respectively, as the cutoff values for poor quality colostrum are shown in Table 3. The Se (86%) and Sp (94%) of TIR spectroscopic method for assessment of dairy cow colostrum were higher than the Se (79%, 84% and 66%) and Sp (87%, 77% and 83%) recently reported for the TIR spectroscopy, colostrometer, and digital Brix refractometer, respectively (Bartier et al., 2015; Løkke et al., 2016). Whereas, for beef cow colostrum, TIR spectroscopic method showed lower Se (77%) and higher Sp (98%) than those (Se = 94.4% and Sp = 86.1%) reported by Vandeputte et al. (2014) for digital Brix refractometer. The TIR spectroscopic method based on the merged data set correctly classified the majority of dairy and beef cow colostrum and misclassified 11% (26 of 430) of dairy colostrum and 7% (14 of 195) of beef colostrum samples (Table 3). The false negative (6%) and false positive (5%) of the dairy colostrum samples have RID-IgG concentrations that ranged between (37–49.2 g/L) and (50.2–65.7 g/L), respectively, which are close to the cutoff value of 50 g/L. For beef colostrum, the false negative (5%) and false positive (2.6%) samples have RID-IgG concentrations that ranged between (80.6–93.5 g/L) and (100.3–112 g/L), respectively, which are close to the cutoff value of 100 g/L.
Table 3.
Diagnostic test characteristics of transmission infrared spectroscopy for assessment of dairy and beef cow colostrum quality using radial immunodiffusion assay IgG concentration as the reference method
| Test characteristics | PLS calibration models | |||||||
|---|---|---|---|---|---|---|---|---|
| Dairy cow model | Beef cow model | Merged dairy and beef cow model | ||||||
| Prediction set | All data | Prediction set | All data | Prediction set (dairy) | All data (dairy) | Prediction set (beef) | All data (beef) | |
| Cut-point (g/L) | 50 | 50 | 100 | 100 | 50 | 50 | 100 | 100 |
| Number | 78 | 235 | 65 | 195 | 73 | 235 | 70 | 195 |
| True positives | 22 | 71 | 7 | 20 | 23 | 69 | 8 | 17 |
| False positives | 3 | 9 | 0 | 4 | 5 | 12 | 0 | 5 |
| True negatives | 47 | 143 | 56 | 165 | 41 | 140 | 59 | 164 |
| False negatives | 6 | 12 | 2 | 6 | 4 | 14 | 3 | 9 |
| Sensitivity | 79% | 86% | 78% | 77% | 85% | 83% | 73% | 65% |
| Specificity | 94% | 94% | 100% | 98% | 89% | 92% | 100% | 97% |
| Accuracy | 89% | 91% | 97% | 95% | 88% | 89% | 96% | 93% |
In conclusion, the PLS calibration model in combination with TIR spectroscopy shows potential as a rapid, accurate, precise, reagent-free, and inexpensive method for estimation of dairy and beef cows colostrum IgG concentration and assessment of colostrum quality. Further, merging of dairy and beef cow colostrum samples improved the predictive performance of the TIR-based method for measuring colostral IgG concentration.
Acknowledgments
The authors thank participating dairy and beef farmers and veterinarians, as well as Natasha Robinson (Maritime Quality Milk Laboratory, Charlottetown, PE, Canada) and Chantel deBeurs (Department of Production Animal Health, University of Calgary, Calgary, AB, Canada) for assisting in data collection. The authors also thank the management and staff at the Saskatoon Colostrum Company for their technical assistance. This research was funded by MILK 2020 (Fredericton, NB, Canada), the Atlantic Canada Opportunities Agency (Charlottetown, PE, Canada), the Simpson Ranch Beef Cattle Health Endowment (Cochrane, AB, Canada), and the University of Calgary (Calgary, AB, Canada).
LITERATURE CITED
- Abernethy G., and Otter D.. 2010. Determination of immunoglobulin G in bovine colostrum and milk powders, and in dietary supplements of bovine origin by protein G affinity liquid chromatography: collaborative study. J. AOAC Int. 93:622–627. [PubMed] [Google Scholar]
- Albanell E., Caceres P., Caja G., Molina E., and Gargouri A.. 1999. Determination of fat, protein, and total solids in ovine milk by near-infrared spectroscopy. J. AOAC Int. 82:753–758. [PubMed] [Google Scholar]
- Altman D.G., and Bland J.M.. 1983. Measurement in medicine: the analysis of method comparison studies. Statistician. 32:307–317. doi:10.2307/2987937 [Google Scholar]
- Barnes R., Dhanoa M., and Lister S.. 2004. Letter: correction to the description of standard normal variate (SNV) and de-trend (DT) transformations in practical spectroscopy with applications in food and beverage Analysis–2nd edition. J. Near Infrared Spec. 1:185–186. [Google Scholar]
- Barnes R., Dhanoa M., and Lister S.J.. 1989. Standard normal variate transformation and de-trending of near-infrared diffuse reflectance spectra. Appl. Spectrosc. 43:772–777. [Google Scholar]
- Bartens M., Drillich M., Rychli K., Iwersen M., Arnholdt T., Meyer L., and Klein-Jöbstl D.. 2016. Assessment of different methods to estimate bovine colostrum quality on farm. N. Z. Vet. J. 64:263–270. doi:10.1080/00480169.2016.1184109 [DOI] [PubMed] [Google Scholar]
- Bartier A.L., Windeyer M.C., and Doepel L.. 2015. Evaluation of on-farm tools for colostrum quality measurement. J. Dairy Sci. 98:1878–1884. doi:10.3168/jds.2014-8415 [DOI] [PubMed] [Google Scholar]
- Baumrucker C.R., Burkett A.M., Magliaro-Macrina A.L., and Dechow C.D.. 2010. Colostrogenesis: mass transfer of immunoglobulin G1 into colostrum. J. Dairy Sci. 93:3031–3038. doi:10.3168/jds.2009–2963 [DOI] [PubMed] [Google Scholar]
- CCAC.. 2009. The care and use of farm animals in research, teaching and testing. Ottawa (ON): Canadian Council on Animal Care (CCAC); p. 12–15. [Google Scholar]
- Chelack B.J., Morley P.S., and Haines D.M.. 1993. Evaluation of methods for dehydration of bovine colostrum for total replacement of normal colostrum in calves. Can. Vet. J. 34:407–412. [PMC free article] [PubMed] [Google Scholar]
- Dean R., and Dixon W.. 1951. Simplified statistics for small numbers of observations. Anal. Chem. 23:636–638. doi:10.1021/ac60052a025 [Google Scholar]
- Elsohaby I., Riley C.B., Hou S., McClure J.T., Shaw R.A., and Keefe G.P.. 2014. Measurement of serum immunoglobulin G in dairy cattle using Fourier-transform infrared spectroscopy: a reagent free approach. The Veterinary J. 202:510–515. doi:10.1016/j.tvjl.2014.09.014 [DOI] [PubMed] [Google Scholar]
- Elsohaby I., McClure J.T., Cameron M., Heider L.C., and Keefe G.P.. 2017. Rapid assessment of bovine colostrum quality: how reliable are transmission infrared spectroscopy and digital and optical refractometers?J. Dairy Sci. 100:1427–1435. doi:10.3168/jds.2016-11824 [DOI] [PubMed] [Google Scholar]
- Elsohaby I., McClure J.T., Hou S., Riley C.B., Shaw R.A., and Keefe G.P.. 2016. A novel method for the quantification of bovine colostral immunoglobulin G using infrared spectroscopy. Int. Dairy J. 52:35–41. doi:10.1016/j.idairyj.2015.08.004 [Google Scholar]
- Filteau V., Bouchard É., Fecteau G., Dutil L., and DuTremblay D.. 2003. Health status and risk factors associated with failure of passive transfer of immunity in newborn beef calves in Quebec. Can. Vet. J. 44:907. [PMC free article] [PubMed] [Google Scholar]
- Gelsinger S., Smith A., Jones C., and Heinrichs A.. 2015. Technical note:Comparison of radial immunodiffusion and ELISA for quantification of bovine immunoglobulin G in colostrum and plasma. J. Dairy Sci. 98:4084–4089. doi:10.3168/jds.2014-8491 [DOI] [PubMed] [Google Scholar]
- Godden S. 2008. Colostrum management for dairy calves. Vet. Clin. North Am. Food Anim. Pract. 24:19–39. doi:10.1016/j.cvfa.2007.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guy M.A., McFadden T.B., Cockrell D.C., and Besser T.E.. 1994. Regulation of colostrum formation in beef and dairy cows. J. Dairy Sci. 77:3002–3007. doi:10.3168/jds.S0022-0302(94)77241-6 [DOI] [PubMed] [Google Scholar]
- Kehoe S.I., Jayarao B.M., and Heinrichs A.J.. 2007. A survey of bovine colostrum composition and colostrum management practices on Pennsylvania dairy farms. J. Dairy Sci. 90:4108–4116. doi:10.3168/jds.2007-0040 [DOI] [PubMed] [Google Scholar]
- Lin L.I. 1989. A concordance correlation coefficient to evaluate reproducibility. Biometrics 45:255–268. doi:10.2307/2532051 [PubMed] [Google Scholar]
- Løkke M.M., Engelbrecht R., and Wiking L.. 2016. Covariance structures of fat and protein influence the estimation of IgG in bovine colostrum. J. Dairy Res. 83:58–66. doi:10.1017/S0022029915000734 [DOI] [PubMed] [Google Scholar]
- McBeath D.G., Penhale W.J., and Logan E.F.. 1971. An examination of the influence of husbandry on the plasma immunoglobulin level of the newborn calf, using a rapid refractometer test for assessing immunoglobulin content. Vet. Rec. 88:266–270. [DOI] [PubMed] [Google Scholar]
- Mulvey J. 1996. The concentrations of immunoglobulin G in the colostrum of beef cows and in the sera of suckler calves and of calves fed a colostrum substitute before suckling. Ir. Vet. J. 49:348–352. [Google Scholar]
- Murray I. 1986. Near infrared reflectance analysis of forages. Recent advances in animal nutrition. London (UK): Butterworths; p. 141–156. [Google Scholar]
- Norris K.H. 2001. Understanding and correcting the factors which affect diffuse transmittance spectra. NIR News. 12:6–9. [Google Scholar]
- Ohlsson C., Houmøller L.P., Weisbjerg M.R., Lund P., and Hvelplund T.. 2007. Effective rumen degradation of dry matter, crude protein and neutral detergent fibre in forage determined by near infrared reflectance spectroscopy. J. Anim. Physiol. Anim. Nutr. 91:498–507. doi:10.1111/j.1439-0396.2007.00683.x [DOI] [PubMed] [Google Scholar]
- Page M., and Thorpe R.. 2002. Analysis of IgG fractions by electrophoresis. The Protein Protocols Handbook. Totowa (NJ): Springer Humana Press; p. 1005–1007. [Google Scholar]
- Picard R.R. and Cook R.D.. 1984. Cross-validation of regression models. J. Amer. Statist. Assoc. 79:575–583. doi:10.2307/2288403 [Google Scholar]
- Riley C.B., McClure J.T., Low-Ying S., and Shaw R.A.. 2007. Use of Fourier-transform infrared spectroscopy for the diagnosis of failure of transfer of passive immunity and measurement of immunoglobulin concentrations in horses. J. Vet. Intern. Med. 21:828–834. doi:10.1111/j.1939-1676.2007.tb03028.x [DOI] [PubMed] [Google Scholar]
- Rivero M.J., Alomar D., Valderrama X., Le Cozler Y., Velásquez A., and Haines D.. 2016. Prediction efficiency by near-infrared spectroscopy of immunoglobulin G in liquid and dried bovine colostrum samples. J. Dairy Res. 83:345–351. doi:10.1017/S0022029916000364 [DOI] [PubMed] [Google Scholar]
- Rivero M.J., Valderrama X., Haines D., and Alomar D.. 2012. Prediction of immunoglobulin G content in bovine colostrum by near-infrared spectroscopy. J. Dairy Sci. 95:1410–1418. doi:10.3168/jds.2011-4532 [DOI] [PubMed] [Google Scholar]
- Rorabacher D.B. 1991. Statistical treatment for rejection of deviant values: critical values of dixon’s” Q” parameter and related subrange ratios at the 95% confidence level. Anal. Chem. 63:139–146. doi:10.1021/ac00002a010 [Google Scholar]
- Rutten M., Bovenhuis H., Heck J., and van Arendonk J.. 2011. Predicting bovine milk protein composition based on Fourier transform infrared spectra. J. Dairy Sci. 94:5683–5690. doi:10.3168/jds.2011-4520 [DOI] [PubMed] [Google Scholar]
- Salkind N.J. 2010. Encyclopedia of research design. Thousand Oaks (CA): SAGE Publications, Inc. [Google Scholar]
- Savitzky A., and Golay M.J.. 1964. Smoothing and differentiation of data by simplified least squares procedures. Anal. Chem. 36:1627–1639. doi:10.1021/ac60214a047 [Google Scholar]
- Shaw R.A., and Mantsch H.H.. 1999. Vibrational biospectroscopy: from plants to animals to humans. A historical perspective. J. Mol. Struct. 480–481:1–13. doi:10.1016/S0022-2860(98)00648-6 [Google Scholar]
- US Food and Drug Administration.. 2001. Guidance for industry, bioanalytical methods validation https://www.fda.gov/downloads/Drugs/Guidance/ucm070107.pdf.
- Vandeputte S., Detilleux J., and Rollin F.. 2014. Investigation of colostrum quality in beef cattle by radial immunodiffusion and brix refractometry. Vet. Rec. 175:353 doi:10.1136/vr.101590 [DOI] [PubMed] [Google Scholar]
- Williams P.C., and Sobering D.C.. 1996. How do we do it: a brief summary of the methods we use in developing near infrared calibration. In: Davis, A.M.C., and P. Williams, editors. Near Infrared Spectroscopy: The Future Waves, NIR Publications, Chichester. p. 185–188. [Google Scholar]
- Windeyer M.C., Grover M., Homerosky E., Murray C., Pajor E.A., Lewis R., Haines D. 2015. Evaluation of digital Brix refractometry in assessing maternal colostrum quality and the transfer of passive immunity in beef cattle. In The 48th Annual Conference of the American Association of Bovine Practitioners (AABP); September 17–19, 2015;. New Orleans (LA)p. 255–256 [Google Scholar]
- Xu Q., and Liang Y.. 2001. Monte Carlo cross validation. Chemometrics Intellig. Lab. Syst. 56:1–11. doi:10.1016/S0169-7439(00)00122-2 [Google Scholar]