Abstract
Pregnancy loss is a major contributing factor to reproductive inefficiency in both the beef and dairy industries. Sires can have a significant influence on the amount of pregnancy loss; however, this relationship is still poorly investigated. The primary objective of this study was to identify sires associated with high or low incidence of pregnancy loss (between d 30 and 100 of gestation) and investigate their effect on concentration of circulating pregnancy-associated glycoproteins (PAGs). Postpartum multiparous Nelore cows were inseminated artificially at a fixed time (FTAI, d 0) after synchronization of ovulation. A total of 736 cows were assigned randomly to be inseminated with semen from either of 6 Angus sires, whereas a separate subset of 492 cows were inseminated randomly with semen from either of 3 Nelore (n = 235) or either of 2 Angus sires (n = 257). Estrus expression was evaluated on d 0 using Estrotect Heat Detector patches. Blood samples were collected on d 30 of gestation for quantification of PAGs and pregnancy diagnosis was performed by ultrasound on d 30 and 100 after FTAI. Cows diagnosed pregnant at the first examination but not pregnant at the second were defined to have pregnancy loss. Overall pregnancy rate at d 30 was 54% (660/1,228) and pregnancy loss was 6.21% (41/660). Cows receiving semen from Nelore sires had greater (P < 0.001) pregnancy rate, greater (P = 0.014) pregnancy loss, and lesser (P = 0.002) PAG concentrations at d 30 of gestation compared with cows receiving Angus semen. Circulating PAG concentrations were lower (P = 0.008) in cows that had pregnancy loss (9.76 ± 0.25 vs. 7.41 ± 1.02 ng/mL). Angus sires were retrospectively classified according to percentage of pregnancy loss as either high pregnancy loss (mean of 7.25% or 67% of total) or low pregnancy loss (mean of 3.93% or 33% of total). Cows receiving semen from high pregnancy loss sires had 1.9 times greater (P = 0.123) rate of pregnancy loss and had lower (P = 0.059) PAG concentrations at d 30 of gestation compared with cows mated to low pregnancy loss sires. In summary, PAG concentrations reflected probability of pregnancy maintenance and were influenced by both sire and sire breed used at FTAI. Variation in the incidence of pregnancy loss was detected among sires that could not be predicted with standard semen fertility evaluations. Exploring the relationship of sire and PAG production might be promising to improve sire selection with regard to pregnancy loss.
Keywords: beef cows, embryonic and fetal mortality, pregnancy-associated glycoproteins, ruminant, sire fertility, trophoblast
INTRODUCTION
Pregnancy loss (between d 30 and 100 of gestation) contributes to significant adverse impacts on production and economic efficiency in cattle systems. Even though all mechanisms causing late embryonic/early fetal mortality are unknown, it may be related to insufficient placental development. Bovine pregnancy-associated glycoproteins (PAGs) are secreted from binucleate trophoblast cells of the placenta and have been used to monitor embryonic or fetal viability as well as placental function in cattle (Perry et al., 2005; Thompson et al., 2010; Pohler et al., 2013; Pohler et al., 2016a). Cows having pregnancy loss during the late embryonic/early fetal period had significantly reduced PAG concentrations compared with cows that maintained pregnancy (Pohler et al., 2016b). Limited information has been reported on the influence of sire on circulating PAGs, but based on the large influence that the sire has on placental formation, there is considerable interest in this relationship. Different studies have shown that parthenogenomes (Kaufman et al., 1977; Surani et al., 1987) and gynogenones (Surani et al., 1984; Barton et al., 1985), which are embryos with maternal genome only, develop with poor placental proliferation but a reasonably well-developed embryo. In turn, androgenones, embryos derived from a paternal genome only, result in inadequate embryo development but form a robust placenta (McGrath and Solter, 1984; Surani et al., 1984; Barton et al., 1985). Moreover, substantial variation in the amount of pregnancy loss exists between sires with comparable initial pregnancy rate (López-Gatius et al., 2002; Starbuck et al., 2004). Therefore, the objectives of these experiments were to identify sires associated with high or low pregnancy loss and to investigate their effect on circulating concentrations of PAG. We hypothesized that pregnancies sired by high pregnancy loss sires will have decreased circulating PAGs at d 30 of gestation compared with pregnancies sired by low pregnancy loss sires.
MATERIALS AND METHODS
This study was conducted on a commercial beef farm in Mato Grosso, Brazil following the recommendations of the “Guide for the Care and Use of Agricultural Animals in Agricultural Research and Teaching” (FASS, 1999). This study included a total of 1,228 suckled multiparous Nelore cows (average BCS = 3.24), separated in 10 management groups with approximately 120 cows in each, maintained on pastures of Brachiaria brizantha with water and mineral salt ad libitum. All cows were at least 20-d postpartum (average 51 d; ranging from 22 to 79 d) when subjected to the following estrus synchronization protocol: an intravaginal progesterone (P4) insert containing 1.9 g of P4 (CIDR; Zoetis, São Paulo, Brazil), and 2.0 mg (i.m.) estradiol benzoate (2.0 mL of Gonadiol; Zoetis, São Paulo, Brazil) on d-11, CIDR withdrawal, 12.5 mg (i.m.) dinoprost tromethamine (PGF; 2.5 mL of Lutalyse; Zoetis, São Paulo, Brazil), 300 IU of equine chronic gonadotropin (1.5 mL of Novormon; Zoetis, São Paulo, Brazil), and 0.6 mg (i.m.) of estradiol cypionate (0.3 mL of E.C.P.; Zoetis, São Paulo, Brazil) on d-2, and fixed-time artificial insemination (FTAI) on d 0 (Meneghetti et al., 2009). Estrotect Heat detector patches were placed at d-2 and scored at the time of AI on a scale of 0 to 4 (0, lost patch; 1, <25% activated; 2, <50% activated; 3, <75% activated; and 4, >75% activated) as described by Pohler et al. (2016b). Cows with patch scores 1 and 2 were defined to not be in estrus, whereas patch scores of 3 and 4 signified estrus had occurred. Cows with patch scores of 0 were removed from the analysis that evaluated estrus expression.
After FTAI, all cows underwent pregnancy diagnosis at d 30 and 100 of gestation by transrectal ultrasonography (Aloka 500V, Aloka, Wallingford, CT) with a 7.5-MHz transrectal linear probe. Positive pregnancy status was based on the presence of a viable embryo with a heartbeat. Cows diagnosed pregnant at the first examination (d 30) but nonpregnant at d 100 were defined to have pregnancy loss.
Blood Sampling
Blood samples were collected from cows diagnosed as pregnant at d 30 post FTAI by tail venipuncture into a 10-mL vacutainer tube (BD Vacutainer, Becton, Dickinson and Company, NJ) and allowed to clot at room temperature for 1 h before being placed in a 4 °C refrigerator for approximately 24 h. Samples were centrifuged at 1,500 × g for 15 min and stored at −20 °C until measurement of PAGs.
Sire Distribution
A majority of cows (6 management groups, n = 736) were assigned randomly to be inseminated with semen from either of 6 Angus sires, whereas another subset of cows (4 management groups, n = 492) were inseminated randomly with either semen from either of 3 Nelore (n = 235) or either of 2 Angus sires (n = 257) to assess the effects of sire breed on incidence of pregnancy loss and PAG concentrations 30 d after FTAI. To ensure randomization, sires were alternated every 10 cows and 4 different sires were used in each management group. Mean number of inseminations per sire was 122 for first subset and 98 for second subset, ranging from 53 to 131. Straws of frozen semen were purchased from major semen companies that follow CSS (Certified Semen Services) and all health, ethical, and animal welfare NAAB (National Association of Animal Breeders) guidelines. All semen passed pre-freezing quality tests with a minimum of 30% progressive motility and 70% normal morphology, as well as post-thaw testing at each company before reaching the farm.
Assay Procedure
Serum concentration of PAGs was determined by a monoclonal-based PAG ELISA similar to that described by Green et al. (2005) using a polyclonal antibody (Ab 63) as described by Reese et al. (2017) to quantify PAGs secreted early in gestation, with a sensitivity of 0.28 ng/mL. Each assay was run with duplicates of each sample, a standard curve, a sample from a pregnant cow approximately 60 d in gestation, and a pooled sample from nonpregnant cows as controls. Intra- and inter-assay coefficients of variation were less than 10%.
Statistical Analyses
One-way ANOVA (GLM procedure, SAS 9.4, Institute Inc., Cary, NC) was used to test differences in the dependent variables pregnancy rate, pregnancy loss, and PAG concentrations. For pregnancy rate and pregnancy loss, fixed effects included sire breed (Angus or Nelore), sire fertility (high or low pregnancy loss), and estrus expression (yes or no). For PAG concentrations, fixed effects included pregnancy status (maintenance or loss), sire breed, and sire fertility. Cow BCS, age, days postpartum, management group, semen collection number, and PAG plate were included as random variables. All data were analyzed using cow as the experimental unit and means were separated using LSMEANS and adjusted according to the Tukey–Kramer test. Frequency of pregnancy rate and pregnancy loss was compared between variables using odds ratio (FREQ procedure, SAS 9.4, Institute Inc., Cary, NC). Probability for prediction of pregnancy maintenance by circulating PAGs concentration was determined according to the following equation: Probability = (elogistic equation)/(1 + elogistic equation) using GENMOD procedure (SAS 9.4, Institute Inc., Cary, NC). For all analyses, significance was set at P ≤ 0.05 and tendencies were determined when 0.05 < P ≤ 0.15, results are presented as mean ± SEM.
RESULTS
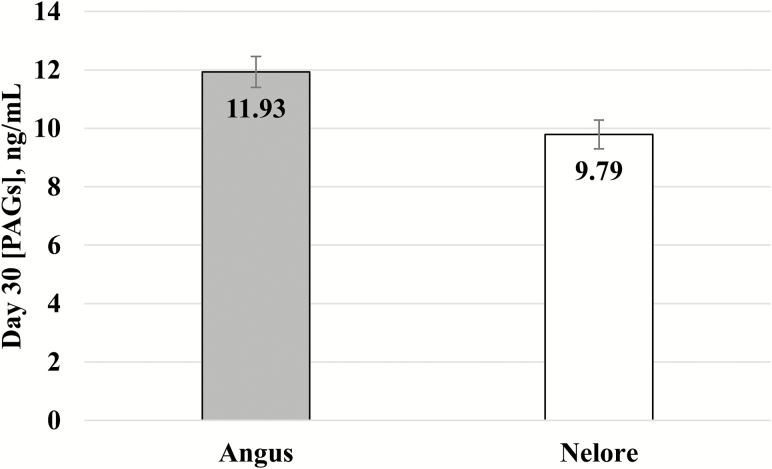
Overall pregnancy rate following FTAI at d 30 was 54% (660/1,228) and pregnancy loss between d 30 and 100 was 6.21% (41/660). Cows inseminated with semen from Angus sires had decreased (P < 0.001) pregnancy rate at d 30 (50.95 ± 1.57% vs. 65.53 ± 3.23%) and decreased (P = 0.014) incidence of pregnancy loss (4.94 ± 0.09% vs. 10.39 ± 2.46%) compared with cows inseminated with semen from Nelore sires. In addition, the breed of sire had a significant effect on circulating concentrations of PAG at d 30 of gestation. Pregnant Nelore cows inseminated with Angus semen had greater (P = 0.003) serum concentration of PAGs on d 30 compared with cows with Nelore sired pregnancies (11.99 ± 0.53 vs. 9.73 ± 0.49 ng/mL; Fig. 1).
Figure 1.
Effect of sire breed on circulating concentration of PAGs (mean ± SEM) in pregnant cows that were inseminated with Angus (n = 131) or Nelore (n = 138) semen and had a viable pregnancy up to d 100 of gestation. Viable pregnancies by Angus sires had greater PAGs concentration (P = 0.003) compared with Nelore pregnancies.
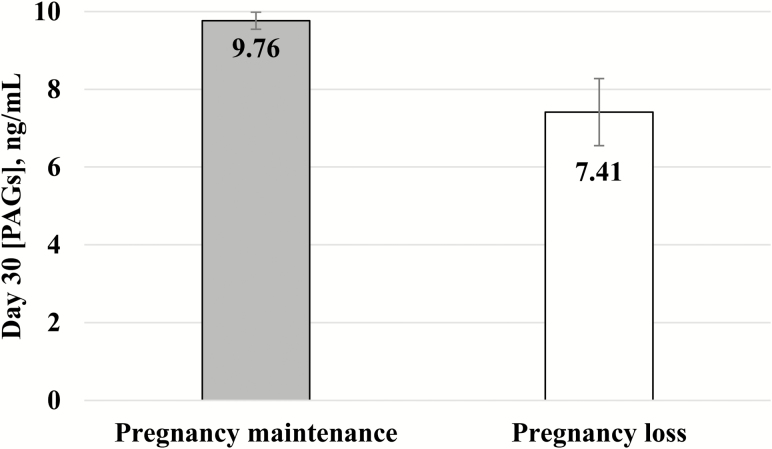
Cows that had a viable pregnancy on d 30, but diagnosed nonpregnant on d 100 of gestation were classified as having pregnancy loss and had decreased (P = 0.008) circulating concentration of PAGs on d 30 compared with cows that maintained pregnancy until d 100 (9.76 ± 0.25 vs. 7.41 ± 1.02 ng/mL; Fig. 2). For each 1 ng/mL increase in circulating concentration of PAGs at d 30, the odds of pregnancy maintenance to d 100 of gestation increased (P = 0.006) by 11%.
Figure 2.
Serum concentrations of PAGs (mean ± SEM) in cows that had a viable embryo on d 30 of gestation (n = 660) and either maintained (pregnancy maintenance; n = 619) or experienced pregnancy loss (pregnancy loss; n = 41). Cows that experienced pregnancy loss by d 100 of gestation had decreased (P = 0.008) circulating concentrations of PAGs on d 30 compared with cows that maintained pregnancy until d 100.
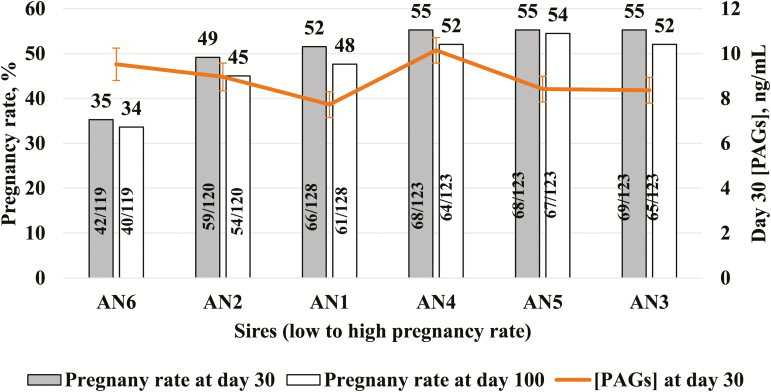
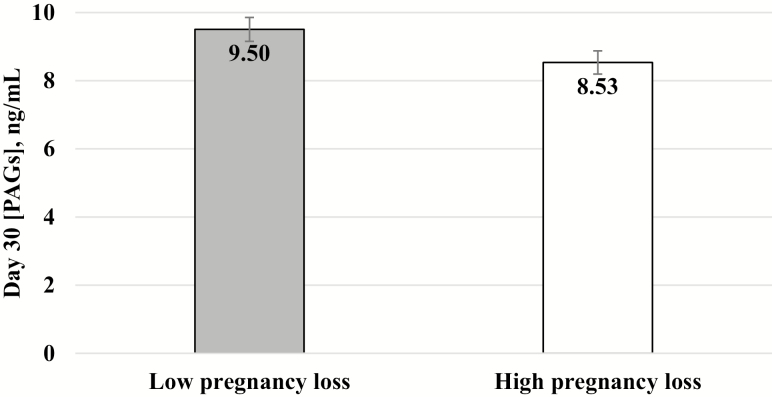
Pregnancy rate at d 30 did not differ among Angus sires (P > 0.15; range 49% to 55%), except sire AN6 which had a lower (P = 0.006) d 30 pregnancy rate (35%); however, no clear relationship was detected between pregnancy rate at d 30 or 100 post FTAI and circulating concentration of PAGs at d 30 (Fig. 3). Angus sires used at FTAI were retrospectively classified according to percentage of pregnancy loss between d 30 and 100 of gestation as high pregnancy loss (3 sires with the greatest pregnancy loss, 7.25% mean) or low pregnancy loss (3 sires with the least pregnancy loss, 3.93% mean; Table 1). High pregnancy loss sires accounted for 66% of the total pregnancy loss, and cows mated to these sires had 1.9 times greater rate of pregnancy loss than cows mated with low pregnancy loss sires (statistical tendency; P = 0.123). After removing data from cows that had pregnancy loss, circulating PAG concentrations in pregnancies sired by the high pregnancy loss sires tended to decrease (P = 0.059) on d 30 of gestation compared with pregnancies sired by low pregnancy loss sires (8.53 ± 0.35 vs. 9.49 ± 0.36 ng/mL; Fig. 4).
Figure 3.
Relationship between circulating concentrations of PAGs and pregnancy rate at d 30 and 100 after fixed-time AI by sire. Six Angus (AN) sires are shown in order of low to high pregnancy rate (left to right). Sire AN6 was the only sire with lower (P = 0.006) pregnancy rate at d 30 and 100 compared with all other sires. Circulating PAGs concentrations from viable pregnancies from each sire are represented in secondary axis (mean ± SEM).
Table 1.
Classification of Angus sire fertility according to percentage of pregnancy loss (n = 371 pregnancies)
| Embryonic loss classification | Sires | Mean pregnancy loss (% of pregnancies) | % of total pregnancy loss |
|---|---|---|---|
| High | AN1, AN2, AN3 | 7.25 (n = 193) | 66.67 |
| Low | AN4, AN5, AN6 | 3.93 (n = 178) | 33.33 |
Figure 4.
Serum concentrations of PAGs (mean ± SEM) in cows inseminated with semen from 1 of 6 Angus sires (n = 371) that had a viable pregnancy up to d 100 of gestation. Three sires accounted for 66.7% of the pregnancy loss and were classified as high pregnancy loss, and their viable pregnancies (after removing data from pregnancy loss) tended to have lower circulating concentrations of PAG on d 30 (P = 0.059) compared with the other 3 sires classified as low pregnancy loss.
Estrus expression before FTAI influenced reproductive results. In general, cows that expressed estrus had greater (P < 0.001) pregnancy rate at d 30 (64.84% vs. 43.25%) compared with those that did not express estrus. Odds ratio test revealed that cows which expressed estrus had 2.7 times greater (P < 0.001) odds of being pregnant at d 30 and 2.6 times greater (P < 0.001) odds of being pregnant up to d 100 compared with those that did not express estrus. The percentage increase in pregnancy rate when cows expressed estrus was highly variable between sires (4% to 51%; Table 2), even though the percentage of cows expressing estrus was not different (P = 0.413) among sires (range 44% to 68%). Pregnancy rate at d 30 from 5 of the 6 Angus sires was lower when cows did not express estrus at the time of FTAI compared with cows that exhibited estrus. Estrus expression did not have an influence on pregnancy rate as strongly in cows inseminated with semen from to Nelore sires compared with cows inseminated with Angus semen (Table 2). Overall, the incidence of pregnancy loss (cows diagnosed pregnant on d 30 and nonpregnant on d 100) was similar (5.77% vs. 7.20%, P = 0.475) when cows expressed estrus (25/438) compared with no estrus expression before FTAI (16/222).
Table 2.
Effect of estrus expression near the time of AI on pregnancy rate at d 30 by sire
| Sirea | Pregnancy rate at d 30, % | P-value | |
|---|---|---|---|
| Estrus (n = 679) | No estrus (n = 549) | ||
| AN1 | 60.46 ± 5.14 | 33.33 ± 7.36 | 0.0026 |
| AN2 | 62.50 ± 4.09 | 33.04 ± 4.44 | <0.0001 |
| AN3 | 72.59 ± 4.10 | 35.96 ± 4.46 | <0.0001 |
| AN4 | 64.93 ± 5.43 | 39.13 ± 7.03 | 0.0038 |
| AN5 | 65.33 ± 5.50 | 39.58 ± 6.88 | 0.0036 |
| AN6 | 40.00 ± 6.15 | 30.50 ± 6.21 | 0.2782 |
| NEL7 | 78.26 ± 9.94 | 60.00 ± 8.71 | 0.1676 |
| NEL8 | 61.11 ± 7.95 | 58.62 ± 8.86 | 0.8343 |
| NEL9 | 78.43 ± 6.68 | 59.09 ± 5.87 | 0.0299 |
aAN represents Angus sires and NEL represents Nelore sires.
DISCUSSION
Bovine PAGs are products of binucleate trophoblast cells in the bovine placenta and can be detected in the maternal circulation beginning 24 to 26 d after insemination (Green et al., 2005; Pohler et al., 2013; Reese et al., 2017). Even though their physiological role and mechanism of potential action remain unclear, it has been hypothesized that PAGs may act in camouflaging fetal or placental antigens from the maternal immune system, process growth factors, or facilitate adhesion actions at the fetal maternal interface (Wallace et al., 2015). It has been well established that circulating PAG concentrations are an effective method of pregnancy diagnosis in cattle and sheep using either blood or milk samples (Zoli et al., 1992; Green et al., 2005; Karen et al., 2015; Wallace et al., 2015; Reese et al., 2017). Moreover, multiple reports have associated concentration of PAGs with probability of embryonic mortality or pregnancy maintenance in cattle (Perry et al., 2005; Breukelman et al., 2012; Pohler et al., 2013; Pohler et al., 2014; Pohler et al., 2016a, 2016b). In the present study, similar results were observed in which circulating concentration of PAGs was decreased in cows that had pregnancy loss between d 30 and 100 of gestation. For each 1 ng/mL increase in circulating PAG concentration at d 30, the odds of pregnancy maintenance up to d 100 of gestation increased by 11%. An additional finding was that with a newly validated PAG antibody (Ab 63; Reese et al., 2017), we were able to predict late embryonic mortality, which has previously been reported to be antibody and PAG specific (Gatea et al., 2018).
The mechanism behind late embryonic/early fetal loss has not been well established, but it has been suggested to be related to a deficiency in placentation around the time of embryo attachment to the uterus (d 21 through 42 of gestation; Stice et al., 1996; Hill et al., 2000). Using PAGs as a marker of placental function and pregnancy maintenance, we started investigating the possible cause of late embryonic/early fetal loss in cattle by exploring the factors that significantly affect circulating PAGs. Initially, it was hypothesized that PAG production was driven by embryo development itself, where greater circulating concentrations of PAGs would be correlated to a more robust or larger embryo and decreased PAG concentrations would correlate to a smaller embryo. In contrast, Pohler et al. (2014) showed that there was no correlation between crown rump length, embryonic width, or embryonic volume at d 35 or 56 of gestation and d 35 or 56 circulating PAGs, indicating that decreased PAGs concentration is not merely a result of a smaller, potentially developmentally delayed embryo. Furthermore, maternal factors do not seem to be the sole driver of circulating PAGs because no repeatability was detected in circulating PAG concentrations between successive pregnancies in the same maternal environment (Reese et al., 2016). These studies together indicate that other factors play a role in PAG secretion during a single pregnancy.
The primary objective of this study was to explore the paternal contribution to pregnancy loss and PAG secretion. Our interest in this potential relationship stems from the large influence paternal genetics have on placenta formation (Barton et al., 1985; Surani et al., 1987), and from previous studies showing differences between bulls’ ability to sire successful pregnancies (Markusfeld-Nir, 1997; López-Gatius et al., 2002; Pegorer et al., 2007). Even when bulls are specifically selected for AI and pass all standard semen evaluations, significant differences exist in the sire’s ability to generate and maintain successful pregnancies that cannot be explained by variation in visual semen analysis. Sires used in this experiment passed standard commercial semen analysis and meet all minimum requirements for use in the field. From the subset of cows inseminated with Angus semen, pregnancy rate at d 30 did not differ among sires, with the exception of sire AN6 which was significantly lower than the others. Even without much variation in initial pregnancy rate at d 30 post AI, there was a large variation in pregnancy loss between sires that drastically affects final pregnancy rate (33.6% to 54.5%). Similar results have been reported in dairy cows, where pregnancy loss (between d 38 and 90 of gestation) was reported from 3.2% to 17.6% across sires used for AI (López-Gatius et al., 2002). Markusfeld-Nir (1997) did an epidemiological study combining more than 50,000 dairy cow pregnancies sired by 233 different bulls and reported that pregnancy loss (from d 45 of gestation until calving) was significantly greater in cows inseminated with semen from 8 specific bulls. Although the average of pregnancy loss of all cows was 5.9%, the incidence of pregnancy loss for these 8 bulls ranged from 10.6% to 17.9%.
In the present study, there was considerable variance in incidence of pregnancy loss among cows mated with different sires that allowed sire classification as either high or low pregnancy loss. Cows receiving semen from high pregnancy loss sires had 1.9 times greater rate of pregnancy loss, corroborating with results found by López-Gatius et al. (2002) in dairy herds in which cows receiving semen from high pregnancy loss sires had 3.4 times greater rate of pregnancy loss compared with low pregnancy loss sires during the late embryonic/early fetal period. Pregnancies sired by high pregnancy loss sires also had lower circulating PAG concentrations at d 30 of gestation, even after removing from the analysis pregnancies that were not maintained and are known to have decreased circulating concentrations of PAGs. These findings provide evidence that paternal genetics have considerable influence on pregnancy maintenance in cattle and contribute to the difference in early gestation secretion of PAGs. Investigation of the presence or absence of PAG genes in specific sires may provide clarity to the causes and variance of pregnancy loss, and help develop a selection tool to identify bulls with low embryonic loss.
There are several reports in the literature comparing development of purebred and crossbred embryos. The incorporation of Bos indicus genetics has been advantageous to increase the ability of an embryo to tolerate heat stress, specifically in dairy herds, whereas other studies utilize Bos taurus genetics in B. indicus herds to increase heterosis and improve embryo development. There is some discrepancy whether these effects stem from the contribution of the oocyte, the spermatozoa, or both. Most of the studies are focused on early development of in vitro produced embryos and limited data are reported comparing the effects of different breed matings in pregnancy maintenance after d 30 of gestation, specifically in beef herds. In the present study, we analyzed pregnancy rate and pregnancy loss using FTAI with Angus and Nelore sires in commercial Nelore cows. Overall pregnancy rate and incidence of late embryonic/early fetal loss (54% and 6.21%, respectively) were comparable with other studies utilizing multiparous Nelore cows exposed to estradiol- and progesterone-based FTAI protocols in Brazilian cow-calf operations (Filho et al., 2009; Aono et al., 2013; Pohler et al., 2016a, 2016b). Nelore cows inseminated with Nelore semen had greater pregnancy rate at d 30 compared with cows inseminated with Angus semen. These results are supported by the idea that B. indicus × B. indicus embryos are better able to survive elevated temperatures at early stages of development compared with B. indicus × B. taurus embryos (Barros et al., 2002), given that these cows are very likely to be exposed to heat stress during the breeding season in this region and time of year in Brazil. In contrast, other studies have shown no effect of the genetic background of spermatozoa in regard to the embryo’s ability to respond to heat stress (Block et al., 2002). Another explanation for these results is that Nelore sires may have exhibited superior fertility that may have potentially biased pregnancy rate results. This second explanation is not likely because of the equivalent fertility testing at the bull stud facilities. In addition, cows mated with Angus semen had lower pregnancy loss and greater circulating PAGs concentration, indicating a beneficial effect of heterosis in embryo development and pregnancy maintenance in these animals, as seen in previous studies (Pegorer et al., 2007). Still, these results differ from another study showing the maternal breed effect on PAGs secretion, where cows with B. indicus genetics had increased circulating PAG concentration compared with B. taurus cows (Mercadante et al., 2013). These results led us to believe that PAGs secretion might be related to the embryo genetic composition itself, rather than maternal genotype, but the present study was not primarily designed to address this observation. Additionally, the antibodies used in each of these experiments are most likely detecting different PAGs, making it difficult to reach a conclusion about these specific differences.
Estrus expression near the time of FTAI is correlated with pregnancy success, with positive impacts on both ovarian function and uterine environment which affects embryo development and pregnancy maintenance (Sá Filho et al., 2010; Davoodi et al., 2016; Pereira et al., 2016; Pohler et al., 2016b). In this study, estrus expression was evaluated for effects on PAGs concentration, and moreover, to test if pregnancy rate differs among sires when cows expressed estrus before FTAI. Preovulatory estradiol directly affects pregnancy establishment and maintenance through several physiological events including gamete transport and preparation of uterine environment (Hawk and Cooper, 1975; Buhi, 2002; Pohler et al., 2012; Pereira et al., 2016). Our data demonstrated that cows which expressed estrus had 2.7 times greater chance of being pregnant at d 30 than those that did not expressed estrus, similar to previous studies reporting increased fertility when B. taurus cows (Perry et al., 2005; Perry et al., 2007; Thomas et al., 2017) and B. indicus-influenced heifers (Thomas et al., 2017) exhibit estrus before FTAI. Large variation existed among sires with regard to the effect of estrus on pregnancy rate. For example, cows inseminated with semen from sire AN3 had a 50% increase in pregnancy rate at d 30 when they expressed estrus before or at the time of FTAI, whereas cows inseminated with semen from sire NEL8, the percentage increase was only 4%. In general, cows inseminated with Nelore semen had greater pregnancy rate, independent of estrus expression while pregnancy rate in cows sired by Angus was significantly lower in the absence of estrus before AI. It has been reported that changes in the uterine environment including changes in pH following estrus expression affect sperm transport and longevity (Perry and Perry, 2008), increasing sperm viability until the time of ovulation. Even though there is not a clear reason for this variation among sires, we believe differences may exist in sperm characteristics (genetic or molecular) that explain why some sires seem to be more resilient to changes in the uterine environment compared with others, which may be related with changes in sperm longevity. Although no significant association was detected between estrus score and incidence of pregnancy loss and PAG concentration, previous studies have shown that estrus intensity before FTAI is positively correlated with PAGs concentration during gestation (Pohler et al., 2016b) and future studies would be interesting to address if changes in uterine environment, due to estrus expression, can affect pregnancy maintenance during this period.
In summary, the variation in pregnancy rate and pregnancy loss between sires used for FTAI is significant, yet poorly understood. Cows inseminated with Nelore sires had greater initial pregnancy rate, but had greater pregnancy loss and lesser circulating concentration of PAGs. Pregnancies by high pregnancy loss Angus sires had lower circulating concentration of PAGs at d 30 of gestation. Exploring the genomic characteristics of these specific sires is the next step in understanding these differences with regard to sire fertility and PAG secretion. Results from this present study may lead toward developing methods to select sires with high pregnancy rate, but low pregnancy loss thus increasing overall reproductive efficiency in the herd.
ACKNOWLEDGMENTS
The authors thank Select Sires, Inc. (Plain City, OH) and USDA-NIFA Hatch/Multistate Project W3112-TEN00506—Reproductive performance in domestic animals for financial support. Agropecuária Fazenda Brasil for providing the animals and facilities and ESTROTECT, Inc. (Spring Valley, WI) for their donation of Estrotect Heat Detector patches used in the experiment.
LITERATURE CITED
- Aono F., Cooke R. F., Alfieri A., and Vasconcelos J. L. M.. 2013. Effects of vaccination against reproductive diseases on reproductive performance of beef cows submitted to fixed-timed AI in Brazilian cow-calf operations. Theriogenology 79:242–248. doi:10.1016/j.theriogenology.2012.08.008 [DOI] [PubMed] [Google Scholar]
- Barros C. M., Monteiro F. M., Mello D. S., Carvalho L. M., Teixeira A. B., Trinca L. A., and Freitas E. C.. 2002. Resistance of Bos indicus to heat shock, compared to crossbred or Bos taurus, at early stages of in vitro embryo development. In: Proceedings of the International Symposium on Reproduction in Domestic Ruminants, Crieff, Scotland p. A4. [Google Scholar]
- Barton S. C., Adams C. A., Norris M., and Surani M.. 1985. Development of gynogenetic and parthenogenetic inner cell mass and trophectoderm tissues in reconstituted blastocysts in the mouse. Development 90:267–285. [PubMed] [Google Scholar]
- Block J., Chase C. C., and Hansen P. J.. 2002. Inheritance of resistance of bovine preimplantation embryos to heat shock: relative importance of the maternal versus paternal contribution. Mol. Reprod. Dev. 63:32–37. doi:10.1002/mrd.10160 [DOI] [PubMed] [Google Scholar]
- Breukelman S. P., Perenyi Z., Taverne M. A., Jonker H., van der Weijden G. C., Vos P. L., de Ruigh L., Dieleman S. J., Beckers J. F., and Szenci O.. 2012. Characterisation of pregnancy losses after embryo transfer by measuring plasma progesterone and bovine pregnancy-associated glycoprotein-1 concentrations. Vet. J. 194:71–76. doi:10.1016/j.tvjl.2012.02.020 [DOI] [PubMed] [Google Scholar]
- Buhi W. C. 2002. Characterization and biological roles of oviduct-specific, oestrogen-dependent glycoprotein. Reproduction 123:355–362. doi:10.1530/rep.0.1230355 [DOI] [PubMed] [Google Scholar]
- Davoodi S., Cooke R. F., Fernandes A. C. C., Cappellozza B. I., Vasconcelos J. L. M., and Cerri R. L. A.. 2016. Expression of estrus modifies the gene expression profile in reproductive tissues on day 19 of gestation in beef cows. Theriogenology 85:645–655. doi:10.1016/j.theriogenology.2015.10.002 [DOI] [PubMed] [Google Scholar]
- FASS 1999. Guide for the care and use of agricultural animals in agricultural research and teaching. Consortium for Developing a Guide for the Care and Use of Agricultural Animals in Agricultural Research, and Teaching. [Google Scholar]
- Filho O. G. S., Meneghetti M., Peres R. F. G., Lamb G. C., and Vasconcelos J. L. M.. 2009. Fixed-time artificial insemination with estradiol and progesterone for Bos indicus cows II: strategies and factors affecting fertility. Theriogenology 72:210–218. doi:10.1016/j.theriogenology.2009.02.008 [DOI] [PubMed] [Google Scholar]
- Frade M. C., Frade C., Cordeiro M. B., Sá Filho M. F., Mesquita F. S., Nogueira G. P., Binelli M., and Membrive C. M. B.. 2014. Manifestation of estrous behavior and subsequent progesterone concentration at timed-embryo transfer in cattle are positively associated with pregnancy success of recipients. Anim. Reprod. Sci. 151:85–90.doi:10.1016/j.anireprosci.2014.09.005 [DOI] [PubMed] [Google Scholar]
- Gatea A. O., Smith M. F., Pohler K. G., Egen T., Pereira M. H., Vasconselos J. L. M., Lawrence J. C. and Green J. A.. 2018. The ability to predict pregnancy loss in cattle with ELISAs that detect pregnancy associated glycoproteins is antibody dependent. Theriogenology 108:269–276. doi:10.1016/j.theriogenology.2017.12.021 [DOI] [PubMed] [Google Scholar]
- Green J. A., Parks T. E., Avalle M. P., Telugu B. P., McLain A. L., Peterson A. J., McMillan W., Mathialagan N., Hook R. R., Xie S. et al. 2005. The establishment of an ELISA for the detection of pregnancy-associated glycoproteins (PAGs) in the serum of pregnant cows and heifers. Theriogenology 63:1481–1503. doi:10.1016/j.theriogenology.2004.07.011 [DOI] [PubMed] [Google Scholar]
- Hawk H. W., and Cooper B. S.. 1975. Improvement of sperm transport by the administration of estradiol to estrous ewes. J. Anim. Sci. 41:1400–1406. doi:10.2527/jas1975.4151400x [DOI] [PubMed] [Google Scholar]
- Hill J. R., Burghardt R. C., Jones K., Long C. R., Looney C. R., Shin T., Spencer T. E., Thompson J. A., Winger Q. A., and Westhusin M. E.. 2000. Evidence for placental abnormality as the major cause of mortality in first-trimester somatic cell cloned bovine fetuses. Biol. Reprod. 63:1787–1794. doi:10.1095/biolreprod63.6.1787 [DOI] [PubMed] [Google Scholar]
- Karen A., Sousa N. M., Beckers J. F., Bajcsy A. C., Tibold J., Madl I., and Szenci O.. 2015. Comparison of a commercial bovine pregnancy-associated glycoprotein ELISA test and a pregnancy-associated glycoprotein radiomimmunoassay test for early pregnancy diagnosis in dairy cattle. Anim. Reprod. Sci. 159:31–37. doi:10.1016/j.anireprosci.2015.05.005 [DOI] [PubMed] [Google Scholar]
- Kaufman M. H., Barton S. C., and Surani M. A. H.. 1977. Normal postimplantation development of mouse parthenogenetic embryos to the forelimb bud stage. Nature 265:53–55. [DOI] [PubMed] [Google Scholar]
- López-Gatius F., Santolaria P., Yaniz J., Rutllant J., and López-Béjar M.. 2002. Factors affecting pregnancy loss from gestation day 38 to 90 in lactating dairy cows from a single herd. Theriogenology 57:1251–1261. doi:10.1016/S0093-691X(01)00715-4 [DOI] [PubMed] [Google Scholar]
- Markusfeld-Nir O. 1997. Epidemiology of bovine abortions in Israeli dairy herds. Prev. Vet. Med. 31:245–255. [DOI] [PubMed] [Google Scholar]
- McGrath J., and Solter D.. 1984. Completion of mouse embryogenesis requires both the maternal and paternal genomes. Cell 37:179–183. [DOI] [PubMed] [Google Scholar]
- Meneghetti M., Filho O. G. S., Peres R. F. G., Lamb G. C., and Vasconcelos J. L. M.. 2009. Fixed-time artificial insemination with estradiol and progesterone for Bos indicus cows I: basis for development of protocols. Theriogenology 72:179–189. doi:10.1016/j.theriogenology.2009.02.010 [DOI] [PubMed] [Google Scholar]
- Mercadante P. M., Waters K. M., Mercadante V. R. G., Lamb G. C., Elzo M. A., Johnson S. E., Rae D. O., Yellich J. V., and Ealy A. D.. 2013. Subspecies differences in early fetal development and plasma pregnancy associated glycoprotein concentrations in cattle. J. Anim. Sci. 91:3693–3701. doi:10.2527/jas.2012-6130 [DOI] [PubMed] [Google Scholar]
- Pegorer M. F., Vasconcelos J. L. M., Trinca L. A., Hansen P. J., and Barros C. M.. 2007. Influence of sire and sire breed (Gyr versus Holstein) on establishment of pregnancy and embryonic loss in lactating Holstein cows during summer heat stress. Theriogenology 67:692–697. doi:10.1016/j.theriogenology.2006.09.042 [DOI] [PubMed] [Google Scholar]
- Pereira M. H. C., Wiltbank M. C., and Vasconcelos J. L. M.. 2016. Expression of estrus improves fertility and decreases pregnancy losses in lactating dairy cows that receive artificial insemination or embryo transfer. J. Dairy. Sci. 99:2237–2247. doi:10.3168/jds.2015-9903 [DOI] [PubMed] [Google Scholar]
- Perry G. A., and Perry B.. 2008. Effect of preovulatory concentrations of estradiol and initiation of standing estrus on uterine pH in beef cows. Domest. Anim. Endocrinol. 34:333–338. doi:10.1016/j.domaniend.2007.09.003 [DOI] [PubMed] [Google Scholar]
- Perry G. A., Smith M. F., Lucy M. C., Green J. A., Parks T. E., MacNeil M. D., Roberts A. J., and Geary T. W.. 2005. Relationship between follicle size at insemination and pregnancy success. Proc. Natl. Acad. Sci. USA 102:5268–5273. doi:10.1073/pnas.0501700102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry G. A., Smith M. F., Roberts A. J., MacNeil M. D., and Geary T. W.. 2007. Relationship between size of the ovulatory follicle and pregnancy success in beef heifers1. J. Anim. Sci. 85:684–689. [DOI] [PubMed] [Google Scholar]
- Pohler K. G., Geary T. W., Atkins J. A., Perry G. A., Jinks E. M., and Smith M. F.. 2012. Follicular determinants of pregnancy establishment and maintenance. Cell Tissue Res. 349:649–664. [DOI] [PubMed] [Google Scholar]
- Pohler K. G., Geary T. W., Johnson C. C., Atkins J. A., Jinks E. M., Busch D. C., Green J. A., MacNeil M. D., and Smith M. F.. 2013. Circulating bovine pregnancy associated glycoproteins are associated with late embryonic/fetal survival but not ovulatory follicle size in suckled beef cows. J. Anim. Sci. 91:4158–4167. doi:10.2527/jas.2013-6348 [DOI] [PubMed] [Google Scholar]
- Pohler K. G., Green J. A., Moley L. A., Doran K. M., Graff H. B., Peres R. F. G., Vasconcelos J. L. M., and Smith M. F.. 2014. The effect of embryonic size and sire on circulating concentrations of bovine pregnancy associated glycoproteins in beef cattle. In: Juengal, J. L., A. Miyamoto, C. Price, L. P. Reynolds, M. F. Smith and R. Webb, editors, Proceedings of the International Symposium on Reproduction in Domestic Ruminants, Obihiro, Hokkaido, Japan p. 563 [Google Scholar]
- Pohler K. G., Pereira M. H. C., Lopes F. R., Lawrence J. C., Keisler D. H., Smith M. F., Vasconcelos J. L. M., and Green J. A.. 2016a. Circulating concentrations of bovine pregnancy-associated glycoproteins and late embryonic mortality in lactating dairy herds. J. Dairy. Sci. 99:1584–1594. doi:10.3168/jds.2015-10192 [DOI] [PubMed] [Google Scholar]
- Pohler K. G., Peres R. F. G., Green J. A., Graff H. B., Martins T., Vasconcelos J. L. M., and Smith M. F.. 2016b. Use of bovine pregnancy-associated glycoproteins to predict late embryonic mortality in postpartum Nelore beef cows. Theriogenology 85:1652–1659. doi:10.1016/j.theriogenology.2016.01.026 [DOI] [PubMed] [Google Scholar]
- Reese S. T., Geary T. W., Franco G. A., Wehrman M. E., Hansen P. J., Spencer T. E., and Pohler K. G.. 2016. Pregnancy associated glycoproteins (PAGs) in high vs subfertility heifers. Society for the Study of Reproduction Annual Meeting, San Diego, CA. [Google Scholar]
- Reese S. T., Pereira M. H. C., Edwards J. L., Vasconcelos J. L. M., and Pohler K. G.. 2018. Pregnancy diagnosis in cattle using pregnancy associated glycoprotein concentration in circulation at day 24 of gestation. Theriogenology. 106:178–185. doi:10.1016/j.theriogenology.2017.10.020 [DOI] [PubMed] [Google Scholar]
- Sá Filho M. F., Crespilho A., Santos J., Perry G. A., and Baruselli P. S.. 2010. Ovarian follicle diameter at timed insemination and estrous response influence likelihood of ovulation and pregnancy after estrous synchronization with progesterone or progestin-based protocols in suckled Bos indicus cows. Anim. Reprod. Sci. 120:23–30. doi:10.1016/j.anireprosci.2010.03.007 [DOI] [PubMed] [Google Scholar]
- Starbuck M. J., Dailey R. A., and Inskeep E. K.. 2004. Factors affecting retention of early pregnancy in dairy cattle. Anim. Reprod. Sci. 84:27–39. doi:10.1016/j.anireprosci.2003.12.009 [DOI] [PubMed] [Google Scholar]
- Stice S. L., Strelchenko N. S., Keefer C. L., and Matthews L.. 1996. Pluripotent bovine embryonic cell lines direct embryonic development following nuclear transfer. Biol. Reprod. 54:100–110. doi:10.1095/biolreprod54.1.100 [DOI] [PubMed] [Google Scholar]
- Surani M. A., Barton S. C., and Norris M. L.. 1984. Development of reconstituted mouse eggs suggests imprinting of the genome during gametogenesis. Nature 308:548–550. doi:10.1038/308548a0 [DOI] [PubMed] [Google Scholar]
- Surani M. A., Barton S. C., and Norris M. L.. 1987. Influence of parental chromosomes on spatial specificity in androgenetic—parthenogenetic chimaeras in the mouse. Nature 326:395–397. doi:10.1038/326395a0 [DOI] [PubMed] [Google Scholar]
- Thomas J. M., Locke J. W. C., Bishop B. E., Abel J. M., Ellersieck M. R., Yelich J. V., Poock S. E., Smith M. F., and Patterson D. J.. 2017. Evaluation of the 14-d CIDR-PG and 9-d CIDR-PG protocols for synchronization of estrus in Bos indicus-influenced and Bos taurus beef heifers. Theriogenology 92:190–196. doi:10.1016/j.theriogenology.2017.01.020 [DOI] [PubMed] [Google Scholar]
- Thompson I. M., Cerri R. L., Kim I. H., Green J. A., Santos J. E., and Thatcher W. W.. 2010. Effects of resynchronization programs on pregnancy per artificial insemination, progesterone, and pregnancy-associated glycoproteins in plasma of lactating dairy cows. J. Dairy Sci. 93:4006–4018. doi:10.3168/jds.2009-2941 [DOI] [PubMed] [Google Scholar]
- Wallace R. M., Pohler K. G., Smith M. F., and Green J. A.. 2015. Placental PAGs: gene origins, expression patterns, and use as markers of pregnancy. Reproduction 149:R115–R126. doi:10.1530/REP-14-0485 [DOI] [PubMed] [Google Scholar]
- Zoli A. P., Guilbault L. A., Delahaut P., Ortiz W. B., and Beckers J. F.. 1992. Radioimmunoassay of a bovine pregnancy-associated glycoprotein in serum: its application for pregnancy diagnosis. Biol. Reprod. 46:83–92. doi:10.1095/biolreprod46.1.83 [DOI] [PubMed] [Google Scholar]