Abstract
Thalamic degenerations or dementias are poorly understood conditions. The familial forms are (1) selective thalamic degenerations and (2) thalamic degenerations associated with multiple system atrophy. Selective thalamic degenerations share clinical and pathologic features with fatal familial insomnia, an autosomal dominant disease linked to a mutation at codon 178 of the prion protein (PrP) gene that causes the substitution of asparagine for aspartic acid (178Asn mutation). We amplified the carboxyl terminal coding region of the PrP gene from subjects with selective thalamic dementia or thalamic dementia associated with multiple system atrophy. Three of the four kindreds with selective thalamic dementia and none of the three kindreds with thalamic dementia associated with multiple system atrophy had the PrP 178Asn mutation. Thus, analysis of the PrP gene may be useful in diagnosing the subtypes of thalamic dementia. Moreover, since selective thalamic dementia with the PrP 178Asn mutation and fatal familial insomnia share clinical and histopathologic features, we propose that they are the same disease.
Thalamic degenerations or dementias comprise a group of ill-defined conditions commonly subdivided into three types.1,2 The first type, “selective thalamic degeneration,” includes conditions characterized by severe symmetric thalamic atrophy and minimal or no extrathalamic pathology. The second type, identified as “thalamic degeneration associated with multiple system atrophies,” includes more complex conditions such as Friedreich’s ataxia, spinocerebellar degenerations, and Werdnig-Hoffman spinal muscular atrophy.1 In a third type, thalamic degeneration is associated with a significant amount of gliosis or spongiosis in the cerebral cortex and probably corresponds to the “thalamic type” of Creutzfeldt-Jakob disease.1 Distinguishing types 1 and 3 is difficult, if not impossible, with clinical and histopathologic criteria alone. Types 1 and 2 include sporadic and familial forms, whereas, to our knowledge, there are no reports of a familial form of the thalamic type of Creutzfeldt-Jakob disease.
Thalamic degeneration is the histopathologic hallmark of fatal familial insomnia (FFI), an autosomal dominant prion disease linked to a mutation in codon 178 of the prion protein (PrP) gene.3-5 We analyzed the PrP gene in members of seven unrelated kindreds previously classified as having thalamic degenerations of types 1 and 2.
Methods.
Kindreds examined.
We reviewed the records of six kindreds previously published as having either selective thalamic degeneration or thalamic degeneration associated with multiple system atrophy,2,6-10 and one unpublished kindred (identified by L. Berg) classified as having selective thalamic degeneration. The pedigree of the previously unpublished kindred is shown in figure 1. The original classifications were retained with the exception of the one for the subject described by Oda.10 This subject had been classified in either type of thalamic degeneration due to the mild extrathalamic histopathology.2,10 However, he also had sclerosis of the white matter in the centrum ovale. In addition, a cousin who died after a 7-year history of dementia and motor signs had thalamic atrophy associated with “sudanophilic leukodystrophy.”11 Therefore, this kindred is currently classified in the group of the thalamic degenerations associated with multiple system atrophy (J.J. Martin, personal communication). The first three of the four kindreds with selective thalamic degeneration had clinical features similar to those observed in the two kindreds with previously published FFI (table 1), including age of onset, course, and motor and memory impairments. Moreover, dysautonomia and lack of EEG sleep patterns were demonstrated in subject III-2 of the first kindred (table 1), and an overnight polysomnogram also showed the absence of EEG sleep patterns in subject IV-4 of the second kindred (table 1). Both subjects of the third kindred had sleep disturbances, and EEG recording during sleep in one of them “revealed an atypical stage 5 sleep pattern.” The thalamic and extrathalamic lesions present in the subjects from these three kindreds who had histopathologic examination were also similar to those of FFI. None of these cases had widespread spongiosis. However, subject IV-4 of Berg’s kindred had a single microscopic area of spongiosis.
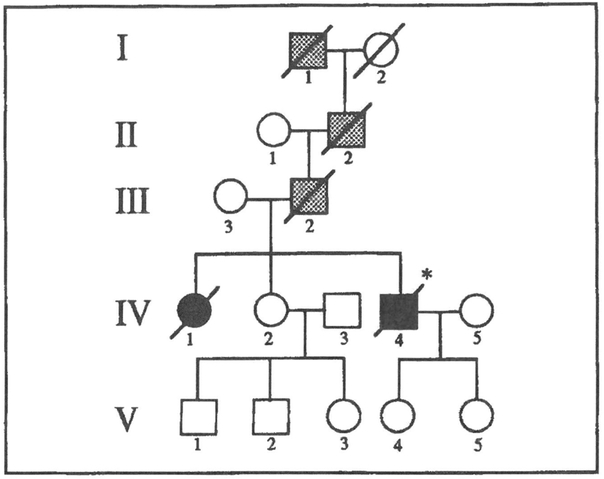
Figure 1.
Pedigree of the unpublished kindred (table 1, Berg). Squares represent males, circles females, and slashes indicate the deceased. Stippled symbols denote those individuals probably affected; solid symbols indicate those individuals examined clinically and demonstrated to be affected histologically in this study.
Table 1.
Selective thalamic degeneration
| Pedigree (ancestry) |
Case identification |
Onset/ duration |
Clinical presentation |
Midcourse | Terminal conditions |
EEG | Histopathology* |
|---|---|---|---|---|---|---|---|
| Julien et al6 (French) | III-1 | 50 yr/8 mo | Apathy, disorientation | Insomnia with nocturnal agitation, abnormal movements, memory deficit, dysarthria, myoclonus, decreased attention | Total insomnia, severe cognitive impairment | Slowing | Thalamus: severe atrophy of AV, MD Other structures: severe atrophy of olives. No spongiosis |
| III-2 | 45 yr/11 mo | Insomnia with nocturnal agitation, memory deficit | Ataxia, myoclonus, enacted dreams, confusion, severe memory deficit, temporal disorientation | Total insomnia, dysautonomia, increased catecholamines, myoclonus, confabulations | Slowing, absence of sleep pattern in 24-hr recording | Thalamus: severe atrophy of AV, MD, CM, P Other structures: moderate gliosis of cerebral cortex, severe atrophy of olives. No spongiosis |
|
| Berg (unpublished) (American/ German) | IV-4 | 51 yr/13 mo | Tiredness, low fever, sleep disturbances with nocturnal agitation and vivid dreams, decreased memory | Dementia, dysarthria, ataxia, myoclonus, absence of cortisol circadian rhythm | Weakness, respiratory difficulties, coma | Normal, absence of sleep pattern in overnight recording | Thalamus: severe atrophy of AV, MD, CM, LD, P, VLp, mild atrophy of other nuclei Other structures: moderate gliosis of entorhinal cortex and caudate; severe gliosis of sup. collie; focal spongiosis in entorhinal cortex |
| IV-1 | 52 yr/9 mo | Altered vision, decreased memory, insomnia, hallucinations | Enacted dreams, gait disorder, myoclonus, dementia, lethargy | Stupor | Slowing | Thalamus: severe atrophy of AV, MD, P Other structures: moderate gliosis of entorhinal cortex and caudate. No spongiosis |
|
| Little et al7 (American/ British) | IV-7 | 46 yr/17 mo | Somnolence, bizarre movements, hallucinations | Speech impairment, decreased memory, sleep episodes with agitation, disorientation, insomnia myoclonus | Coma | “Consistent with narcolepsy,” atypical stage 5 sleep pattern during “sleep episodes” | Thalamus: atrophy of MD, P, VPI, CM Other structures: mild gliosis of cerebral cortex and basal ganglia; severe atrophy of olives. No spongiosis |
| V-14 | 25 yr/12 mo | Ataxia, somnolence | Increased ataxia, dysarthria, decreased memory, hallucinations, myoclonus | Respiratory distress | Unremarkable | Thalamus: severe atrophy of AV, MD, VA, VPI, LD, P Other structures: as IV-7 |
|
| Martin et al3 (Chinese) | NA | 18 yr/3 yr | Memory impairment | Abnormal behavior, apathy, shivering, sweating, urine incontinence, severe tachycardia, memory loss, tremor, no gait disturbances | Coma | NA | Thalamus: severe atrophy of AV, MD, less severe of P, R, VA, LD, CM Other structures: gliosis of vestibular nuclei, bulbar reticular formation, olives. No spongiosis |
See table 2 for abbreviations.
Attempts to detect protease-resistant prion protein on brain tissue from the Berg kindred, case IV-I, using an immunochemical assay, and to transmit the disease to susceptible animals with brain tissue from members of the Little et al kindred, have been unsuccessful (L. Berg, personal communication, and reference 7).
The subject in the Martin et al kindred of this group differed from those in the other kindreds in several respects (table 1). Although she suffered from memory impairment, tremor, and possibly dysautonomia, she did not manifest ataxia or myoclonus. Histopathologically, however, this case is very similar to those of the other kindreds in this group except for the lack of spongiosis, which has always been found in FFI of greater than 2 years’ duration.
The subjects of the three kindreds having thalamic degeneration with multiple system atrophy had heterogeneous clinical and histopathologic features distinct from those of the selective thalamic degenerations except for the aforementioned subject reported by Oda10 (table 2). In this group, transmission to susceptible animals was unsuccessfully attempted with the subject of Deymeer et al.8
Table 2.
Thalamic degeneration associated with multiple system atrophy
| Pedigree | Case identification |
Onset/ duration |
Clinical presentation |
Midcourse | Terminal conditions |
EEG | Histopathology |
|---|---|---|---|---|---|---|---|
| Deymeer etal8 | NA | 46 yr/30 mo | Abnormal behavior, personality change, paranoid ideas | Dementia, dysphasia pyramidal signs, dysphagia | Paresis, muscular atrophy secondary to denervation | Normal | Thalamus: serve atrophy of MD. VA, CM Other structures: mild cortical atrophy with gliosis of cerebral cortex, white matter, hippocampus, and amygdala; marked atrophy of XII nucleus, of anterior horns and corticospinal tracts of spinal cord; neurogenic atrophy of muscle |
| Katz et al9 | 3 | 7 yr/23 yr | Intellectual deterioration, clumsiness | Severe dementia, inappropriate behavior, optic atrophy, spastic paraparesis | Vegetative state, spasticity | Continuous generalized bursts of rhythmic delta | Thalamus: severe atrophy of MD, LD, CM Other structures: marked gliosis of white matter and neostriatum, optic atrophy, cerebellar atrophy with torpedoes, pallor of corticospinal tracts, severe atrophy of olives |
| Oda10 | 1 | 18 yr/14 mo | Memory impairment, dreamy state, gait disturbance, tremor, ataxia, dysautonomia | Continued mental deterioration, pyramidal signs | Coma | NA | Thalamus: severe atrophy of AV, MD, CM, VA, VLp, P, LD, VLa, VM, PV Other structures: gliosis of white matter, atrophy of substantia nigra, olives, globus pallidus, vestibular nuclei, superior collicula, and reticular formation |
NA Not available.
AV Anterior ventral.
MD Mediodorsal.
CM Central median.
VA Ventral anterior.
VLp Ventral lateral posterior
P Pulvinar
LD Lateral dorsal.
R Reticular.
VPI Ventral posterior inferior.
VLa Ventral lateral anterior.
VM Ventral medial.
PV Paraventricular.
DNA analysis.
One or two affected members from the seven kindreds with thalamic degeneration were analyzed. Genomic DNA was extracted from peripheral blood, fresh or fixed tissue, according to procedures previously described.12,13 The codon 178 mutation of the prion protein gene was detected by analysis of the carboxyl terminal coding region. We amplified this region of the gene using the following primers: 5′-CCGTTACTATCGTGAAAACATGCA-3′ and 5′-AAGGATCCCTCAAGCTGGAAAAAGA-3′. The amplification program was as follows: one cycle at 94 °C for 5 minutes; 45 cycles at 94 °C for 1 minute, 60 °C for 1 minute, and 72 °C for 1 minute; and one cycle at 72 °C for 5 minutes. To search for a mutation in the PrP gene codon 178, the amplified DNA was restricted with TthIII 1 and analyzed by agarose gel electrophoresis. Confirmation of the GAC-→AAC mutation in codon 178 was achieved by allele-specific oligonucleotide hybridization utilizing an oligonucleotide, with the 3′ end labeled with digoxigenin-conjugated ddUTP, homologous to either the wild-type sequence, 5′-TTGTGCACGACTGCGTC-3′, or to the mutant sequence, 5′- TTGTGCACAACTGCGTC-3′, applied to slot blots of the PCR products. The hybrids were detected using the Boehringer-Mannheim Genius system.
Linkage analysis.
Linkage between the phenotype of selective thalamic degeneration (or FFI) and the codon 178 mutation was tested with the MLINK program.14 The presence of the codon 178 mutation in the prion protein gene was investigated in 58 nonaffected members of the kindreds reported by Julien et al,6 Berg, and Little et al7 using genomic DNA extracted from peripheral blood, as above. Twenty-six members of the Julien et al6 kindreds were included in the analysis. Five carried the mutation (two affected [table 1] and three asymptomatic), 12 members had no mutation and were asymptomatic, and nine members were uninformative for the mutation (seven asymptomatic and two with undetermined status). The 20 members examined in the Berg kindred included two who carried the mutation and were affected (table 1), 14 with no mutation and asymptomatic, and four uninformative for the mutation (two asymptomatic, two with undetermined status). Of the 12 members examined in the kindred of Little et al,7 four carried the mutation and were affected (two are shown in table 1), eight were uninformative for the mutation (four were affected and four were asymptomatic). The three pedigrees were analyzed using age-dependent penetrance estimated on the basis of the age of onset of the disease. A total of 13 penetrance classes were used, with zero penetrance for members 0 to 19 years old and complete penetrance for those 75 or older. Members were classified as “affected” when the presence of the disease was supported by histologic or clinical examination, “undetermined status” when no medical information was available on the disease, and “asymptomatic” when there was no evidence of thalamic dementia (or FFI). The frequency of the 178 codon mutation in the normal population and the frequency of the disease were as in the previous study. 3
Results and Discussion.
We examined four kindreds previously classified as having selective thalamic degeneration and three classified as having thalamic degeneration associated with multiple system atrophy. Analysis of the amplified DNA with the restriction enzyme TthIII 1 and hybridization with allele-specific probes demonstrated a mutation at codon 178 of the PrP gene, resulting in the substitution of asparagine for aspartic acid (178Asn mutation) in the kindreds of Julien et al,6 Berg, and Little et al7 (figure 2, table 1). The 178Asn mutation is identical to that previously demonstrated in FFI kindreds3,5 and, using these three families, is linked to the disease with a maximal combined lod score of 2.7 (table 3). The combined lod score, including the previous kindreds,3 is 6.5 (unpublished data). In contrast, this mutation was not present in any of the kindreds with thalamic degeneration associated with multiple system atrophy (data not shown). Thus, analysis of the PrP gene, in conjunction with clinical data, is a useful test in differentiating type 1 and type 2 thalamic dementias.
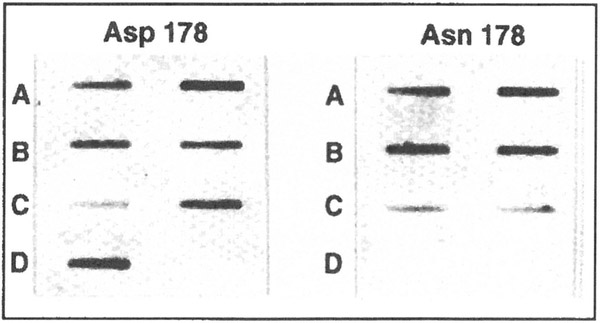
Figure 2.
Demonstration of codon 178 mutation by allele-specific oligonucleotide hybridization. Duplicate filters were hybridized with oligonucleotides that recognize either the wild type allele, Asp 178 on the left, or the mutant allele, Asn 178 on the right. (A-C) The two columns show hybridization of the oligonucleotides to DNA amplified from two affected members with the Asn 178 mutation from the first three kindreds in table 1.(D) An unaffected member from the Berg kindred (IV-2, figure 1).
Table 3.
Lod scores for linkage between disease and codon 178 mutation in three kindreds*
| Recombination fraction† | |||||
|---|---|---|---|---|---|
| p | 0.0 | 0.05 | 0.10 | 0.15 | 0.20 |
| 0.022 | 0.614 | 0.537 | 0.460 | 0.382 | 0.304 |
| 0.590 | 0.511 | 0.433 | 0.355 | 0.279 | |
| 1.342 | 1.192 | 1.036 | 0.875 | 0.710 | |
| 2.546 | 2.240 | 1.929 | 1.612 | 1.293 | |
| 0.001 | 0.613 | 0.537 | 0.459 | 0.381 | 0.304 |
| 0.590 | 0.511 | 0.433 | 0.355 | 0.279 | |
| 1.497 | 1.341 | 1.178 | 1.007 | 0.830 | |
| 2.700 | 2.389 | 2.070 | 1.743 | 1.413 | |
| 10−7 | 0.613 | 0.537 | 0.459 | 0.381 | 0.304 |
| 0.590 | 0.511 | 0.433 | 0.355 | 0.279 | |
| 1.505 | 1.349 | 1.185 | 1.014 | 0.836 | |
| 2.708 | 2.397 | 2.077 | 1.750 | 1.419 | |
The subject with selective thalamic degeneration and lacking the 178Asn mutation had clinical features distinct from those of the subjects with the mutation: (1) an earlier onset, (2) a longer course, and (3) no ataxia or myoclonus. In contrast, clinically and histopathologically, subjects with the 178Asn mutation appeared virtually identical to those previously described as having FFI. The combination of genotypic identity and phenotypic similarity demonstrates that the familial form of selective thalamic degeneration with the 178Asn mutation and FFI are one and the same disease. Thus, most, but not all, of the known kindreds with selective thalamic degeneration suffer from FFI.
Brown et al recently reported six kindreds with the PrP gene 178Asn mutation that differ from FFI patients in several features.15 Despite a similar age of onset and duration of the disease, they apparently lack abnormalities of sleep and autonomic functions, do not show preferential involvement of the thalamus, and have widespread spongiosis of the cerebral cortex and subcortical structures regardless of the duration of the disease. In contrast to FFI, transmission of the disease has been successful in all six kindreds tested. These differences may be explained by postulating a slower rate or a more focal site of formation of the protease-resistant PrP isoform in the FFI kindreds. This is supported by the presence of protease-resistant PrP and spongiosis only in subjects with FFI of long duration,3-5 whereas in the kindreds of Brown et al, these features are present regardless of the duration of the disease.15 A slower rate or more focal formation of the protease-resistant PrP isoform would explain the infrequent spongiosis and lack of transmissibility in FFI. The severe involvement of the thalamus that causes untreatable, progressive insomnia and dysautonomia incompatible with a long survival may explain the relatively rapid course of FFI, despite the more focal pathology.
Clinical and pathologic features of scrapie and human prion diseases appear to be genetically regulated.16-20 The differences between the kindreds expressing FFI and those expressing the Creutzfeldt-Jakob phenotype with the PrP gene 178 mutation are likely due to genotypic variations.
Addendum.
After this study was completed, Goldfarb et al (Ann Neurol 1992;31:274-281) reported finding the 178Asn mutation in the Little et al kindred (our table 1), which they refer to as the McK family.
Acknowledgments
We are indebted to Drs. Shinsaku Oyanagi, Tomohiko Mizutani, Dennis Dickson, and Peter Davies for providing tissue from cases of thalamic degeneration. We also thank the families who participated in this study for their cooperation. Ms. Sandra Bowen provided expert assistance in assembling the manuscript.
Supported by NINCDS NS 14509-13, NIA ADRC AG-08012-02, NIH NIA 1 R01 AGNS08155-Q, the Britton Fund, and NIH HG 0008 (J.O.).
Contributor Information
Dr R.B. Petersen, Division of Neuropathology, Institute of Pathology, Case Western Reserve University, Cleveland, OH.
Dr M. Tabaton, Division of Neuropathology, Institute of Pathology, Case Western Reserve University, Cleveland, OH.
Dr L. Berg, Department of Neurology, Washington University School of Medicine, St. Louis, MO.
Dr B. Schrank, Department of Genetics, Washington University School of Medicine, St. Louis, MO.
Dr R.M. Torack, Department of Pathology, Washington University School of Medicine, St. Louis, MO.
Dr S. Leal, Department of Genetics and Development, Columbia University, New York, NY.
Dr J. Julien, Department of Neurology, University of Bordeaux Medical School, Bordeaux, France.
Dr C. Vital, Department of Pathology, University of Bordeaux Medical School, Bordeaux, France.
Dr B. Deleplanque, Department of Neurology, University of Bordeaux Medical School, Bordeaux, France.
Dr W.W. Pendlebury, Department of Pathology, University of Vermont, Burlington, VT.
Dr D. Drachman, Department of Neurology, University of Massachusetts, Worcester, MA.
Dr T.W. Smith, Department of Neurology, University of Massachusetts, Worcester, MA.
Dr J.J. Martin, Laboratory of Neuropathology, Universitaire Instelling Antwerpen, Wilrijk, Belgium.
Dr M. Oda, Division of Pathology, Tokyo Metropolitan Neurological Hospital, Tokyo, Japan.
Dr P. Montagna, Neurological Institute, University of Bologna, Bologna, Italy..
Dr J. Ott, Department of Genetics and Development, Columbia University, New York, NY.
Dr L. Autilio-Gambetti, Division of Neuropathology, Institute of Pathology, Case Western Reserve University, Cleveland, OH.
Dr E. Lugaresi, Neurological Institute, University of Bologna, Bologna, Italy..
Dr P. Gambetti, Division of Neuropathology, Institute of Pathology, Case Western Reserve University, Cleveland, OH.
References
- 1.Martin JJ. Thalamic degeneration In: Vinken PJ, Bruyn GW, eds. Handbook of clinical neurology, vol 21 Amsterdam: North-Holland, 1975:587–604. [Google Scholar]
- 2.Martin JJ, Yap M, Nei IP, Tan TE. Selective thalamic degeneration—report of a case with memory and mental disturbances. Clin Neuropathol 1983;2:156–162. [PubMed] [Google Scholar]
- 3.Medori R, Tritschler HJ, LeBlanc A, et al. Fatal familial insomnia is a prion disease with a mutation at codon 178 of the prion protein gene. N Engl J Med 1992;326:444–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Manetto V, Medori R, Cortelli P, et al. Fatal familial insomnia: clinical and pathologic study of five new cases. Neurology 1992;42:312–319. [DOI] [PubMed] [Google Scholar]
- 5.Medori R, Montagna P, Tritschler HJ, et al. Fatal familial insomnia: a second kindred with mutation of prion protein gene at codon 178. Neurology 1992;42:669–670. [DOI] [PubMed] [Google Scholar]
- 6.Julien J, Vital C, Deleplanque B, Lagueny A, Ferrer X. Atrophie thalamique subaiguë familiale. Troubles mnésiques et insomnie totale. Rev Neurol (Paris) 1990;146:173–178. [PubMed] [Google Scholar]
- 7.Little BW, Brown PW, Rodgers-Johnson P, Perl DP, Gajdusek DC. Familial myoclonic dementia masquerading as Creutzfeldt-Jakob disease. Ann Neurol 1986;20:231–239. [DOI] [PubMed] [Google Scholar]
- 8.Deymeer F, Smith TW, DeGirolami U, Drachman DA. Thalamic dementia and motor neuron disease. Neurology 1989;39:58–61. [DOI] [PubMed] [Google Scholar]
- 9.Katz DA, Naseem A, Horoupian DS, Rothner AD, Davies P. Familial multisystem atrophy with possible thalamic dementia. Neurology 1984;34:1213–1217. [DOI] [PubMed] [Google Scholar]
- 10.Oda M Thalamus degeneration in Japan: a review from clinical and pathological viewpoints. Appl Neurophysiol 1976/77;39:178–198. [PubMed] [Google Scholar]
- 11.Kato N, Oyanagi S, Abe T, et al. An autopsied case of familial sudanophilic leukodystrophy, with reference to its autopsied cousin case having thalamus degeneration. Adv Neurol Sci 1980;24:330–339. [Google Scholar]
- 12.Nichols WC, Gregg RE, Brewer HB Jr, Benson MD. A mutation in apolipoprotein A-1 in the Iowa type of familial amyloidotic polyneuropathy. Genomics 1990;8:318–323. [DOI] [PubMed] [Google Scholar]
- 13.Maniatis T, Fritsch EF, Sambrook J. Molecular cloning: a laboratory manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory, 1982. [Google Scholar]
- 14.Lathrop GM, Lalouel JM, Julier C, Ott J. Multilocus linkage analysis in humans: detection of linkage and estimation of recombination. Am J Hum Genet 1985;37:482–498. [PMC free article] [PubMed] [Google Scholar]
- 15.Brown P, Goldfarb LG, Gibbs CJ, Gajdusek DC. The phenotypic expression of different mutations in transmissible familial Creutzfeldt-Jakob disease. Eur J Epidemiol 1991;7:469–476. [DOI] [PubMed] [Google Scholar]
- 16.Prusiner SB. Molecular biology of prion diseases. Science 1991;252:1515–1522. [DOI] [PubMed] [Google Scholar]
- 17.Lowenstein DH, Butler DA, Westaway D, McKinley MP, DeArmond SJ, Prusiner SB. Three hamster species with different scrapie incubation times and neuropathological features encode distinct prion proteins. Mol Cell Biol 1990;10:1153–1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Palmer MS, Dryden AJ, Hughes JT, Collinge J. Homozygous prion protein genotype predisposes to sporadic Creutzfeldt-Jakob disease. Nature 1991;352:340–342. [DOI] [PubMed] [Google Scholar]
- 19.Baker HF, Poulter M, Crow TJ, et al. Aminoacid polymorphism in human prion protein and age at death in inherited prion disease. Lancet 1991;337:1286. [DOI] [PubMed] [Google Scholar]
- 20.Doh-Ura K, Kitamoto T, Sakaki Y, Tateishi J. CJD discrepancy. Nature 1991;31:353. [DOI] [PubMed] [Google Scholar]