Abstract
Skeletal fragility is a major complication of type 2 diabetes mellitus (T2D), but there is a poor understanding of mechanisms underlying T2D skeletal fragility. The increased fracture risk has been suggested to result from deteriorated bone microarchitecture or poor bone quality due to accumulation of advanced glycation end-products (AGEs). We conducted a clinical study to determine whether: 1) bone microarchitecture, AGEs, and bone biomechanical properties are altered in T2D bone, 2) bone AGEs are related to bone biomechanical properties, and 3) serum AGE levels reflect those in bone. To do so, we collected serum and proximal femur specimens from T2D (n = 20) and non-diabetic (n = 33) subjects undergoing total hip replacement surgery. A section from the femoral neck was imaged by microcomputed tomography (microCT), tested by cyclic reference point indentation, and quantified for AGE content. A trabecular core taken from the femoral head was imaged by microCT and subjected to uniaxial unconfined compression tests. T2D subjects had greater HbA1c (+23%, p ≤ 0.0001), but no difference in cortical tissue mineral density, cortical porosity, or trabecular microarchitecture compared to non-diabetics. Cyclic reference point indentation revealed that creep indentation distance (+18%, p ≤ 0.05) and indentation distance increase (+20%, p ≤ 0.05) were greater in cortical bone from T2D than in non-diabetics, but no other indentation variables differed. Trabecular bone mechanical properties were similar in both groups, except for yield stress, which tended to be lower in T2D than in non-diabetics. Neither serum pentosidine nor serum total AGEs were different between groups. Cortical, but not trabecular, bone AGEs tended to be higher in T2D subjects (21%, p = 0.09). Serum AGEs and pentosidine were positively correlated with cortical and trabecular bone AGEs. Our study presents new data on biomechanical properties and AGEs in adults with T2D, which are needed to better understand mechanisms contributing to diabetic skeletal fragility.
Keywords: Type 2 diabetes, bone, microarchitecture, porosity, advanced glycation end-products, reference point indentation
1. Introduction
Individuals with type 2 diabetes mellitus (T2D) have an increased risk of fracture, despite having normal or high bone mineral density (BMD) [1-5]. While falls are more common among T2D patients, fracture risk remains increased even after accounting for the higher incidence of falls within this group [6]. Thus, it has been suggested that the increased fracture risk seen in T2D may be due to altered bone microarchitecture and/or poor bone quality (i.e. matrix properties) [7, 8]. Notably, some but not all, studies report altered cortical bone microarchitecture in T2D [7-9]. However, very little is known about the contribution of poor bone quality to reduced bone strength in T2D. Thus, mechanisms underlying diabetic skeletal fragility are poorly understood, making it difficult to develop appropriate strategies to diagnose and prevent fractures in this population.
Specifically, the accumulation of advanced glycation end-products (AGEs) by non-enzymatic glycation, a spontaneous reaction between amino acid residues on collagen fibers and extracellular sugars [10, 11], can lead to poor bone tissue matrix composition. Literature indicates that AGEs can adversely affect mechanical properties, which may ultimately contribute to increased skeletal fragility [12-14]. However, the limited data available regarding the effect of AGEs on bone mechanical properties is contradictory. For example, one study showed that human trabecular bone specimens with AGEs induced by in vitro incubation had lower post-yield strain energy compared to vehicle-incubated specimens [15], but there was no difference in post-yield strain energy due to induced AGEs in bovine cortical bone [16]. Further, two ex vivo studies in human trabecular and cortical bone showed negative relationships between AGE content and ultimate strain and stress [17, 18], while a study in human trabecular bone reported no relationships between AGEs and biomechanical properties [19]. Therefore, the effect of AGEs on bone mechanical properties, and especially in diabetic bone, remains unclear.
Further, a few studies report increased pentosidine (an AGE) in urine or serum of individuals with T2D who experience fractures compared to those without fractures [20, 21]. One study reported that in bone retrieved during total knee replacement, pentosidine content was higher in patients with T2D than in non-diabetics [22]. However, this study was conducted in a small, homogenous sample of men, and did not assess bone biomechanical properties. Moreover, given that pentosidine composes only 1% of total fluorescent AGEs [23], it may be important to assess the total amount of AGEs in bone. Furthermore, it is not known whether the amount of AGEs in other biological sources (e.g. serum) are associated with the amount of bone AGEs and/or bone biomechanical properties.
Thus, our goals were to: 1) investigate whether bone microarchitecture, AGEs, and bone biomechanical properties are altered in diabetic bone, 2) determine if bone AGEs relate to bone biomechanical properties, and 3) determine whether serum AGEs reflect those in bone. We hypothesized that bone specimens from patients with T2D would have increased cortical porosity but similar trabecular microarchitecture, increased AGE content, and deteriorated biomechanical properties compared to non-diabetic specimens. We also hypothesized that higher HbA1c and bone AGEs would be associated with worse bone biomechanical properties, and that bone and serum AGE content would be associated with each other.
2. Methods
2.1 Subject Recruitment and Specimen Collection
We sequentially recruited patients undergoing elective total hip replacement surgery at Beth Israel Deaconess Medical Center (BIDMC) in Boston, MA, USA. The protocol was approved by the BIDMC Institutional Review Board, and all subjects provided written informed consent prior to participation. Subjects were considered to have T2D if: 1) they had an HbA1c ≥ 6.5% in their medical record within the past 2 years; 2) they had an HbA1c ≥ 6.5% more than 2 years ago and are currently using T2D medication; 3) they had a fasting blood glucose measurement ≥ 125 mg/dL recorded in their medical record within the past 2 years; 4) they had a fasting blood glucose measurement ≥ 125 mg/dL more than 2 years ago and are currently using T2D medication; or 5) they were currently using T2D medication other than metformin.
Exclusion criteria included abnormalities in bone and mineral metabolism, current use of hormone replacement therapy, current use of medications known to negatively impact bone (e.g. glucocorticoids, anti-retroviral medications), use of osteoporosis medications within the past 12 months (i.e. bisphosphonates, teriparatide, and denosumab), current or prior use of thiazolidinediones, and/or use of glucocorticoids within the past 3 months.
We enrolled 20 subjects with T2D and 33 non-diabetic controls. Medications used by the 20 subjects with T2D included monotherapy with metformin (n=11), insulin (n=2), or second generation sulfonylurea (n=2), and combination therapies of second generation sulfonylurea and GLP1 receptor agonist (n=1); metformin and SGLT2 inhibitor (n=1); insulin and bromocriptine (n=1); insulin, metformin and second generation sulfonylurea (n=1); and insulin, metformin, second generation sulfonylurea and GLP1 receptor agonist (n=1).
Serum samples were collected prior to surgery and stored at −80°C until use. Discarded femoral head and neck surgical specimens were collected fresh, grossly sectioned, reviewed by the pathology department, and stored without any fixative at 4°C until collection by our study team within 24-48 hours after surgery. Femoral specimens were wrapped in saline soaked gauze and stored at −20°C until use.
2.2 Sample Preparation
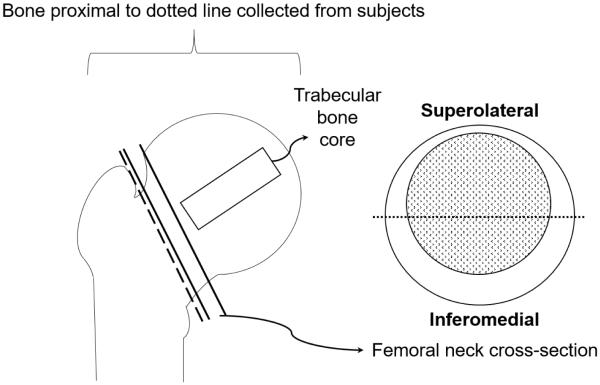
We cut a 3 mm thick cross-section from the posterior-medial half of the femoral neck to use for microcomputed tomography (microCT) imaging, cyclic reference point indentation (cRPI), and quantification of AGEs as described below (Figure 1). We also excised a trabecular core (8mm diameter × 25mm length) from femoral heads along the direction of the principal trabeculae, which was subsequently imaged by microCT and subjected to uniaxial unconfined compression testing as described below.
Figure 1.
The inferomedial half of the femoral neck cross-section was used for microcomputed tomography, cyclic reference point indentation, and AGE quantification. The extracted trabecular core from the femoral head was used for microcomputed tomography and compression testing.
2.3 Tissue Mineral Density, Cortical Porosity, and Trabecular Microarchitecture
Femoral neck cross-sections were imaged by microCT (μCT40, Scanco Medical AG, Brüttisellen, Switzerland) to assess cortical tissue mineral density (Ct.TMD, mgHA/cm3) and cortical porosity (Ct.Po, %). Specifically, images were acquired at 15 μm voxel nominal resolution (X-ray tube current 114 μA, effective energy 70 kV, 300 ms integration time) and segmented using a threshold of 601.8 mgHA/cm3. Femoral head trabecular cores were also imaged to assess bone volume fraction (BV/TV, %), connectivity density (Conn.D. 1/mm3), structural model index (SMI), trabecular number (Tb.N, 1/mm), trabecular thickness (Tb.Th, mm) and trabecular separation (Tb.Sp, mm) using the same microCT device with the same scanning parameters. Trabecular cores were segmented using a threshold of 443.4 mgHA/cm3.
2.4 Cortical Bone Properties by Cyclic Reference Point Indentation
Femoral neck specimens were thawed to room temperature and then tested using cRPI (Biodent Hfc, Active Life Scientific, Santa Barbara, CA) with a probe assembly featuring a beveled reference probe with blunted end (~5 mm cannula length) and test probe with spherical tip (2.5 μm radius point) that tapers from a 90° cone shape to cylindrical shaft (BP2 probes, Active Life Scientific, Santa Barbara, CA). Indentation tests were conducted parallel to the longitudinal axis of the femoral neck. We made 8 indentations per specimen ~1 mm apart at 6 N, 2 Hz, for 20 cycles. Outcomes from the 8 indentations per bone sample were averaged. The following variables were measured: indentation distance (ID, μm [indentation distance into the bone during the first cycle]), creep indentation distance (CID, μm [total indentation distance during the hold step of the first cycle]), total indentation distance (TID, μm [total indentation distance into the bone across all cycles]), indentation distance increase (IDI, μm [difference in indentation distance into the bone between the first and last cycles]), average energy dissipated (avg ED, μJ [area enclosed by the test’s hysteresis loop from the third to last cycle]), average unloading slope (avg US, N/μm [average unloading slope from 3rd to last cycle]), and average loading slope (avg LS, N/μm [average loading slope from 3rd to last cycle]).
2.5 Trabecular Bone Biomechanical Properties by Compression Testing
Radiographs were taken of both the anterior and posterior halves of the femoral heads to determine the primary angle of orientation for the trabeculae. Halves with the most available bone were chosen for coring to ensure cores of suitable length could be obtained (24 anterior, 2 posterior). Trabecular cores were extracted along the direction of the primary trabeculae using an 8 mm diamond tipped coring bit. From the extracted cores, a ~23 mm length section was cut for testing using a low-speed saw (Isomet 1000, Buehler, Lake Bluff, IL). Cores were prepared using a shallow end-capping method intended to reduce end-artifacts during compression testing [24]. Circular wells (3 mm deep, ~8 mm diameter) were machined into the top of brass end-caps (12.5 mm diameter). Each end of the core was embedded in an end-cap by filling the well in the end-cap with poly-methyl-methacrylate (PMMA) and then inserting the end of the core into the well. A custom jig was used to ensure the end-caps on each end of the core were aligned. The gage-length of each sample was measured as the distance between the end-caps.
Compression testing was performed on a servo-hydraulic testing system (Model 8511, Instron, Norwood, MA) with a 2000 N load cell. The samples were inserted into the testing system with the top end-cap clamped into a three-jaw chuck, attached to the load cell on the actuator, and the bottom end-cap resting horizontally and un-confined on a flat steel platen attached to the base of the load frame. A pre-load was applied to 10 N and then compression testing was conducted at a fixed strain rate of 0.5% strain/s until the sample was strained to 10%. Force and displacement from actuator LVDT were recorded at 100 Hz. Force and displacement data were used to calculate the following structural properties: maximum force (N), work to maximum force (mJ), stiffness (N/mm), and yield strain (mm/mm). Additionally, cross-sectional area measured by calipers were coupled with the mechanical testing data to calculate the following apparent material properties: toughness to yield (mJ/mm3), toughness to maximum force (mJ/mm3), toughness to 3% strain (mJ/mm3), post-yield toughness (mJ/mm3), apparent compressive modulus (MPa), and yield stress (MPa).
2.6 Advanced Glycation End-Products in Bone
AGE content was assessed separately in cortical and trabecular bone pieces from the femoral neck using a fluorometric assay, as previously published [15, 16, 18]. Cortical bone taken from the neck was previously indented, but trabecular bone from the neck was not mechanically tested before collection for AGE measurement. Specimens were defatted in isopropyl ether (three 15-minute washes under constant agitation), lyophilized overnight using a freeze dryer, and hydrolyzed in 6N hydrochloric acid for 20 hours at 110°C. Hydrolysates were stored in −80°C in complete darkness until use in the assay. Fluorescence was measured for hydrolysates using a microplate reader (Synergy MX, BioTek, Winooski, VT) at 360/460 nm excitation/emission, and normalized to a quinine sulfate standard. Then, a chloramine-T solution was added to the remaining hydrolysates and hydroxyproline standards and incubated for 20 minutes at room temperature to oxidize hydroxyproline. A 3.15 M perchloric acid solution was added and incubated for 5 minutes at room temperature to neutralize residual chloramine-T. Lastly, a p-dimethylaminobenzaldehyde solution was added and incubated for 20 minutes at 60°C. After allowing samples and standards to cool in complete darkness for 5 minutes, absorbance was measured at 570 nm using a microplate reader. Collagen content was calculated based on hydroxyproline content [25], and total fluorescent AGEs were assessed in units of quinine fluorescence per unit collagen.
2.7 Serum Biochemistry
HbA1c was measured by a commercial laboratory via the Harvard Catalyst Clinical Research Center (LabCorp, Newton, MA). Serum levels of pentosidine (Lifeome BioLabs, Oceanside, CA; ELISA kit # CEA264Ge, Intra-Assay CV <10%, Inter-Assay CV <12%) and total AGEs (Cell Biolabs, Inc., San Diego, CA; ELISA kit # STA-817, Intra-Assay CV = 4.5%, Inter-Assay CV = 8%) were both measured using commercially available enzyme linked immuno-sorbent assay kits according to manufacturers’ protocols.
2.8 Statistical Analyses
Distributions for all variables were plotted to identify potential outliers. No outliers were identified and thus all data were included in the statistical analyses. Basic demographics and clinical characteristics were calculated for both groups and compared by Student’s T-Test. Possible differences between groups in bone tissue mineral density, microarchitecture, AGE content and mechanical properties were determined by ANCOVA tests with age, race, sex, and BMI considered as possible confounding variables. Pearson correlation tests were used to determine relationships between variables. All statistical analyses were performed using IBM SPSS Statistics (version 24) with the significance level for all tests set to μ = 0.05.
3. Results
3.1 Sample Size
Several bone specimens (5 T2D, 10 non-T2D) were mishandled or unavailable for use due to logistical issues in the pathology department after surgery, resulting in 15 T2D and 23 non-T2D specimens available for RPI and AGE measurement. Additional specimens were excluded from microCT imaging due to unavailability of the posterior-medial portion of the femoral neck for imaging (3 T2D, 4 non-T2D), resulting in 12 diabetics and 19 non-diabetics whose bone specimens were imaged by microCT. Finally, because some of the femoral head specimens were unusable due to logistical issues (i.e. femoral head was damaged during surgery, femoral head was incorrectly cut by pathology), we were able to obtain trabecular cores from only a subset of the original recruited subjects (12 T2D, 13 non-T2D).
3.2 Basic Demographics
55% of the subjects in both the T2D and control group were men. Subjects with T2D had similar age, height, and weight as non-diabetics, but tended to have a higher BMI in all subjects enrolled (+12%, p = 0.09) and within the subset described above (+15%, p = 0.08) (Table 1). Subjects with T2D had higher HbA1c compared to non-diabetics in all subjects enrolled (+23%, p ≤ 0.0001) and within the subset described above (+32%, p ≤ 0.002) (Table 1).
Table 1. Demographics and clinical characteristics of non-diabetic and T2D subjects enrolled in the study, expressed as mean ± SD for continuous variables and number of subjects for categorical variables.
| All Subjects Enrolled | Subset | |||
|---|---|---|---|---|
|
Non-
Diabetic (n = 33) |
Type 2
Diabetic (n = 20) |
Non-
Diabetic (n = 19) |
Type 2
Diabetic (n = 12) |
|
| Sex | ||||
| Male | 19 (58%) | 11 (55%) | 12 (63%) | 7 (58%) |
| Female | 14 (42%) | 9 (45%) | 7 (37%) | 5 (42%) |
| Race | ||||
| White / Caucasian | 27 (82%) | 16 (80%) | 16 (84%) | 9 (75%) |
| Black / African-American | 5 (15%) | 4 (20%) | 2 (11%) | 3 (25%) |
| Asian | 1 (3%) | 0 | 1 (5%) | 0 |
| Basic Clinical Characteristics | ||||
| Age (yrs) | 64.3 ± 10.9 | 65.9 ± 10.0 | 61.6 ± 11.6 | 63.8 ± 9.7 |
| Height (m) | 1.70 ± 0.11 | 1.68 ± 0.08 | 1.72 ± 0.09 | 1.69 ± 0.08 |
| Weight (kg) | 86.2 ± 23.2 | 94.0 ± 15.2 | 85.2 ± 25.1 | 94.0 ± 16.0 |
| BMI (kg/m2) | 30.0 ± 8.0 | 33.5 ± 5.5φ | 28.7 ± 7.8 | 33.0 ± 5.1φ |
| Diabetic Status | ||||
| HbA1c (at PAT, %) | 5.70 ± 0.24 | 6.99 ± 1.34** | 5.64 ± 0.21 | 7.45 ± 1.51* |
| Diabetes Medication Used | ||||
| Metformin | - | 11 (55%) | 7 (58%) | |
| Insulin | - | 2 (10%) | 4 (33%) | |
| Other# | - | 7 (35%) | 1 (9%) | |
p≤0.05
p≤0.0001
0.05<p≤0.10
Other group represents (a) any subject who took a T2D medication that is not metformin or insulin, and (b) any subject who took multiple T2D medications simultaneously, including insulin, sulfonylureas, GLP-1 receptor agonists, biguanides, dopamine receptor agonists, and SGLT2 inhibitors.
3.3 Bone Microarchitecture, Biomechanical Properties, and AGEs
Cortical TMD and cortical porosity did not differ between groups (Table 2). cRPI revealed higher CID (+17.5%, p ≤ 0.05) and IDI (+20.1%, p ≤ 0.05) in T2D than in non-diabetics (Table 2). None of the other RPI variables differed significantly between groups, but indentation distances trended in the same direction.
Table 2. Mean ± standard deviation for cortical bone morphology, trabecular bone volume fraction and microarchitecture, cortical cyclic indentation outcomes, trabecular compressive biomechanical properties, and AGE measures in the femoral neck and serum from non-diabetic and T2D subjects. All data were adjusted for race, sex, gender, and BMI.
| Non-Diabetic | Type 2 Diabetic | |
|---|---|---|
| Microcomputed Tomography | ||
| Cortical tissue mineral density (mgHA/ccm) | 909 ± 39 | 916 ± 47 |
| Cortical porosity (%) | 16.3 ± 7.2 | 16.8 ± 6.7 |
| Tb.BV/TV (%) | 32.0 ± 5.0 | 32.7 ± 7.7 |
| Tb.N (1/mm) | 1.95 ± 0.19 | 2.01 ± 0.33 |
| Tb.Th (mm) | 0.17 ± 0.02 | 0.16 ± 0.02 |
| Tb.Sp (mm) | 0.352 ± 0.060 | 0.346 ± 0.092 |
| Conn.D (1/mm3) | 10.57 ± 2.57 | 11.41 ± 5.00 |
| Cyclic Reference Point Indentation | ||
| Indentation distance (μm) | 71.4 ± 18.1 | 73.4 ± 16.7 |
| Creep indentation distance (μm) | 5.88 ± 1.31 | 6.91 ± 1.50* |
| Total indentation distance (μm) | 80.0 ± 20.1 | 83.9 ± 19.5 |
| Indentation distance increase (μm) | 13.6 ± 4.3 | 16.4 ± 4.7* |
| Average energy dissipation (μJ) | 19.3 ± 5.4 | 20.0 ± 5.7 |
| Average loading slope (N/μm) | 0.40 ± 0.06 | 0.39 ± 0.06 |
| Average unloading slope (N/μm) | 0.53 ± 0.07 | 0.52 ± 0.07 |
| Compressive Biomechanical Properties | ||
| Apparent compressive modulus (MPa) | 566 ± 174 | 590 ± 243 |
| Yield stress (MPa) | 2.29 ± 1.54 | 1.20 ± 0.90φ |
| Maximum stress (MPa) | 3.36 ± 0.79 | 3.30 ± 0.95 |
| Stress at 3% strain (MPa) | 2.40 ± 0.66 | 2.32 ± 0.69 |
| Toughness to maximum point (mJ/mm3) | 0.014 ± 0.009 | 0.014 ± 0.005 |
| Toughness to 3% strain (mJ/mm3) | 0.071 ± 0.018 | 0.070 ± 0.021 |
| Post-yield toughness (mJ/mm3) | 0.06 ± 0.02 | 0.07 ± 0.02 |
| Advanced Glycation End-Products (AGEs) | ||
| Cortical bone AGEs (ng quinine/mg collagen) | 178 ± 53 | 216 ± 64φ |
| Trabecular bone AGEs (ng quinine/mg collagen) | 211 ± 60 | 224 ± 50 |
| Serum pentosidine (ng/mL) | 46.6 ± 11.5 | 53.5 ± 11.7 |
| Serum AGEs (μg/mL) | 32.3 ± 9.2 | 29.2 ± 7.1 |
p≤0.05
0.05<p≤0.10
For the trabecular cores, there was no difference in trabecular bone volume fraction, TMD, or microarchitecture between groups (Table 2). Pre- and post-yield compressive mechanical testing outcomes also did not differ between groups, with the exception of a trend in yield stress, which was 47.6% lower in T2D than in non-T2D (p = 0.08).
Serum levels of total AGEs or pentosidine did not differ between groups (Table 2). In the bone itself, cortical bone AGEs tended to be higher in T2D (+21.3%, p = 0.09), while trabecular bone AGEs were similar between the two groups.
3.4 Relationships Among Bone Microarchitecture, AGEs, and Biomechanical Properties
HbA1c was not related to cortical TMD or porosity, or any cortical biomechanical properties. The indentation parameters ID (r = 0.33, p = 0.074), CID (r = 0.47, p ≤ 0.05), TID (r = 0.33, p = 0.068), and IDI (r = 0.32, p = 0.082) tended to be positively associated with cortical porosity. There were no relationships between bone AGEs and cRPI variables, but there were negative relationships between serum total AGEs and indentation distances (−0.50 ≤ r ≤ −0.34, p ≤ 0.05).
HbA1c was positively correlated with trabecular bone volume fraction, connectivity density, and trabecular number, and negatively associated with trabecular separation and structural model index, but was not related to any mechanical properties (Table 3). AGE content in trabecular bone was positively correlated with yield stress and yield strain, and negatively associated with post-yield displacement, but was not related to any other compressive mechanical properties or any microarchitectural variables (Table 3).
Table 3. Correlation coefficients for HbA1c, trabecular bone AGEs, trabecular microarchitecture, and compressive biomechanical properties. P-values are listed in parentheses.
| HbA1c | Trabecular bone AGEs (ng quinine/mg collagen) |
|
|---|---|---|
| Microcomputed Tomography | ||
| BV/TV (%) | 0.51 (≤ 0.05) | NS |
| Tb.N. (1/mm) | 0.55 (≤ 0.05) | NS |
| Tb.Th. (mm) | NS | NS |
| Tb.Sp. (mm) | −0.45 (= 0.07) | NS |
| Conn.D. (1/mm3) | 0.55 (≤ 0.05) | NS |
| SMI | −0.59 (≤ 0.05) | NS |
| Compressive Biomechanical Properties | ||
| Apparent compressive modulus (MPa) | NS | NS |
| Yield stress (MPa) | NS | 0.56 (≤ 0.05) |
| Yield strain (%) | NS | 0.68 (≤ 0.05) |
| Maximum stress (MPa) | NS | NS |
| Stress at 3% strain (MPa) | NS | NS |
| Toughness to maximum point (mJ/mm3) | NS | NS |
| Toughness to 3% strain (mJ/mm3) | NS | NS |
| Post-yield toughness (mJ/mm3) | NS | NS |
| Post-yield displacement | NS | −0.66 (≤ 0.05) |
NS = not significant
3.5 Relationship between HbA1c, Serum AGEs, and Bone AGEs
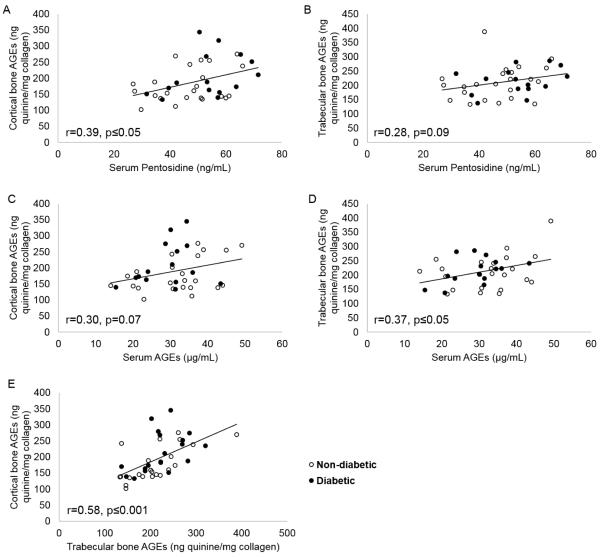
HbA1c was not related to cortical or trabecular AGEs, serum AGEs, or serum pentosidine. Cortical bone AGE content was positively associated with both serum pentosidine (r = 0.39, p ≤ 0.05) and serum total AGEs (r = 0.30, p = 0.07, Figure 2). Trabecular bone AGE content was positively associated with serum pentosidine (r = 0.28, p = 0.09) and total AGEs (r = 0.37, p ≤ 0.05) (Figure 2). Total AGE content of cortical and trabecular bone were strongly associated with each other (r = 0.58, p ≤ 0.001) (Figure 2).
Figure 2.
There were significant positive relationships between serum AGEs and pentosidine and bone AGEs (A-D), and between cortical and trabecular bone AGEs (E).
4. Discussion
Despite increased fracture risk among individuals with T2D, there is limited information on how bone microarchitecture and bone quality components contribute to bone mechanical properties in patients with T2D. By studying bone specimens from the proximal femur, our first objective was to provide new information on bone structure, mechanical properties, and AGE content in patients with and without T2D. Our second goal was to determine if AGE content in bone was related to bone mechanical properties. We found that compared to non-diabetics, bone from patients with T2D had altered cortical bone biomechanical properties, as evidenced by some cyclic reference point indentation (cRPI) properties, and a trend for higher cortical bone AGEs. In contrast, we found no major differences in trabecular bone biomechanical properties and AGE content in those with and without T2D. Our third objective was to compare serum AGE measures with AGE content in bone. We found that cortical and trabecular bone AGEs were weakly positively correlated with serum pentosidine and total AGE content.
Prior studies using high-resolution peripheral computed tomography (HR-pQCT) of the distal radius and tibia have reported increased cortical porosity in those with T2D [7, 8, 26-29], however we found no impact of T2D on cortical porosity at the femoral neck. While discrepant from the several of the HR-pQCT studies, our findings are similar to other reports that showed cortical porosity is lower in diabetics or not different from non-diabetics [30-32]. The discrepancy regarding cortical porosity may be due to differences in measurement sites, as our measurements were taken at the femoral neck, whereas most previous studies report data on cortical microarchitecture at the distal radius and tibia. One other study used in vivo volumetric CT to assess cortical geometry and bone density at the femoral neck in T2D patients, and found lower cortical vBMD and thinner cortices in women with T2D and prevalent fragility fracture, but did not report results on cortical porosity [33].
Despite no differences in cortical microarchitecture between groups, bone specimens from patients with T2D had some altered indentation properties as measured by cRPI suggesting impaired cortical bone tissue properties. This observation is consistent with prior work showing larger indentation distances in cortical bone from rats and mice with diabetes [34, 35]. Specifically, our previous work showed TallyHo mice (early onset T2D) had bone with greater indentation distances with a corresponding trend for increased AGEs compared to controls [35]. A more recent study using adult-onset UCD-T2D rats indicated that the T2D group had reduced whole bone strength with a corresponding increase in AGEs [36].
Our findings are also broadly consistent with reports of lower bone material strength index in patients with T2D compared to non-diabetic controls, as assessed by impact microindentation of the anterior tibia [30, 37, 38]. Of note, comparisons between our study and those reporting data on bone material strength index should be made carefully because impact microindentation and cyclic-based reference point indentation measurements are weakly related to each other and may reflect different aspects of bone’s mechanical behavior [39]. Notably, however, Jenkins, et al. reported that indentation distance increase was negatively associated with fracture toughness in human cortical bone specimens acquired from the femoral neck [40]. Thus, our results suggest that cortical bone specimens from human femoral neck of T2D patients may similarly have some deterioration in fracture toughness, although this needs to be confirmed in future studies through fracture toughness tests of microbeams extracted from the femoral neck.
We found that total AGE content was ~21% higher, though not reaching statistical significance, in the cortical bone of T2D patients than controls. This is in line with a prior study reporting about 30% higher pentosidine content in bone specimens from the proximal tibia in men with diabetes [22]. However, it should be noted that the prior study did not assess total fluorescent AGEs and likely evaluated a mix of cortical and trabecular bone, making it difficult to compare the two studies directly, as AGE content of cortical and trabecular bone differs [41]. Somewhat surprisingly, we did not detect any association between cortical bone AGEs and indentation variables, even though they were assessed at the same site. We originally anticipated that there would be an association between cortical AGEs and cRPI variables such that bone with higher AGE content would have higher indentation distances [42-44]. It is possible that the lack of relationships may be due to the small and heterogeneous sample size and/or the fact that hyperglycemia in our subjects was fairly well-controlled, as evidenced by their relatively low HbA1c values. Future work will need to include more subjects, perhaps with a greater variation in diabetes severity, duration, and/or age, as well as measurement of bone pentosidine and other crosslinks.
There was no statistically significant difference in trabecular bone AGEs between groups although the trend was in the same direction as observed in cortical bone. This finding is consistent with a study that showed no difference in pentosidine content in trabecular bone from femoral heads of diabetic and non-diabetic patients undergoing total hip replacement [45]. However, given that pentosidine content is very poorly associated with total fluorescent AGEs [41], it is difficult to compare results from the two studies directly. We also observed no differences in trabecular microarchitecture between groups, similar to results reported in the distal radius and tibia of adults with and without T2D [29, 30, 32, 46]. Accordingly, there were no differences in trabecular compressive biomechanical properties between T2D and control subjects. Notably, however, trabecular bone AGE content was positively associated with yield stress and yield strain, and negatively associated with post-yield displacement. The latter finding is consistent with prior reports that accumulation of AGEs is associated with reduced energy dissipation and/or toughness [15, 17, 47, 48]. We had expected to see a decrease in toughness in bone specimens from diabetics, in accordance with in vitro studies that incubated trabecular bone in ribose to induce AGEs[15]. However, our measure of in vivo levels of AGEs was much lower than the level induced in the in vitro study (average AGE content in T2D = 220 ng quinine/mg collagen vs ribose-induced AGE content = 322 ng quinine/mg collagen). It is possible that the in vitro study observed changes in post-yield mechanical properties due to higher levels of AGEs, whereas our findings are more physiologically relevant.
We also aimed to determine whether serum measures of AGEs reflected AGE accumulation in bone. We found that both serum pentosidine and total AGE levels were positively, though weakly, correlated to total AGE content in cortical bone. Serum pentosidine and total AGE levels were also positively, but weakly correlated to trabecular bone AGE content. Odetti, et al. also reported a weak positive relationship between plasma pentosidine and cortical bone AGEs, but not with trabecular AGEs [49]. These results infer that serum measures of glycation may not serve as good predictors of non-enzymatic glycation content in bone, emphasizing the importance of utilizing in vivo methods to assess bone quality rather than relying on measurements taken from the serum. For instance, Furst, et al. showed that bone material strength index assessed by impact-microindentation was inversely related to skin AGEs assessed by skin autofluorescence in post-menopausal women with T2D [38]. Further work including more subjects with a wider range of AGEs is necessary to ensure that the weak correlations detected in our study were not due to having a small sample size and small range of AGEs.
Our results should be considered in light of several limitations. As mentioned above, this study included a relatively small sample size with subjects who had a limited age and HbA1c range. Further work with additional subjects, including those with more severe and/or poorly controlled diabetes, is needed. In addition, we assessed cortical bone mechanical properties by cRPI only, which is still a relatively new technique. All subjects recruited in this study were undergoing hip replacement surgery due to osteoarthritis. Patients with osteoarthritis tend to have a localized increase of bone density and/or sclerosis in subchondral bone of the femoral head, but minimal differences in bone density of the femoral neck [50], where we conducted our cRPI tests. Moreover, both our diabetic and non-diabetic subjects were undergoing total hip replacement, and therefore the groups should be comparable. Thus, we do not believe presence of osteoarthritis negatively affected our ability to draw conclusions about diabetes vs. non-diabetes. To date, several AGEs have been identified including pentosidine, imidazolium compounds, crossline, and vesperlysines [44]. However, among these pentosidine is the most commonly quantified individual AGE in bone and has been shown to have some relationships with biomechanical properties. Thus, future work should involve measurement of pentosidine in our specimens.
In conclusion, we found that cortical bone from patients with T2D has worse indentation properties compared to non-diabetics. Results also indicated that higher serum AGEs are associated to deteriorated indentation properties and that AGE content in bone and serum are weakly correlated, with each other, but further work is needed to clarify these relationships. Altogether, these results provide new data on biomechanical properties and AGEs in the proximal femur of adults with T2D, but additional work is needed to confirm these results and identify additional biomechanical mechanisms underlying diabetic skeletal fragility.
Highlights.
Type 2 diabetics have similar cortical and trabecular microarchitecture as non-diabetics in the femoral neck and head
Reference point indentation measures in cortical bone at the femoral neck are worse in type 2 diabetics than in non-diabetics
Advanced glycation end-products (AGEs) in bone are not related to bone biomechanical properties at the femoral neck
Cortical bone AGEs are higher in type 2 diabetics than in non-diabetics
Serum AGEs and pentosidine are positively, but weakly, correlated with bone AGEs
Acknowledgements
We would like to thank Dr. Anne Breggia from Maine Medical Center for measurement of serum AGEs and pentosidine. Financial support for this work was provided by NIH-T32AG023480, a pilot and feasibility grant from the Boston Area Diabetes and Education Center (NIH-P30DK057521), the Harvard Catalyst Early Clinical Data Support for Grant Submissions (NIH-NCRR and NIH-NCATS Award UL1TR001102), NIH-1K01AR069685-01A1, NIH-R21AR070366, and the NIDDK Diabetic Complications Consortium grant DK076169. The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding agencies.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Epstein S, LeRoith D. Diabetes and fragility fractures - a burgeoning epidemic? Bone. 2008 Jul;43:3–6. doi: 10.1016/j.bone.2008.03.017. [DOI] [PubMed] [Google Scholar]
- [2].Schwartz AV. Diabetes Mellitus: Does it Affect Bone? Calcif Tissue Int. 2003 Dec;73:515–9. doi: 10.1007/s00223-003-0023-7. [DOI] [PubMed] [Google Scholar]
- [3].Schwartz AV, Sellmeyer DE. Diabetes, fracture, and bone fragility. Curr Osteoporos Rep. 2007 Sep;5:105–11. doi: 10.1007/s11914-007-0025-x. [DOI] [PubMed] [Google Scholar]
- [4].Schwartz AV, Vittinghoff E, Bauer DC, Hillier TA, Strotmeyer ES, Ensrud KE, et al. Association of BMD and FRAX score with risk of fracture in older adults with type 2 diabetes. JAMA. 2011 Jun;305:2184–92. doi: 10.1001/jama.2011.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Premaor MO, Pilbrow L, Tonkin C, Parker RA, Compston J. Obesity and fractures in postmenopausal women. J Bone Miner Res. 2010 Feb;25:292–7. doi: 10.1359/jbmr.091004. [DOI] [PubMed] [Google Scholar]
- [6].Pijpers E, Ferreira I, de Jongh RT, Deeg DJ, Lips P, Stehouwer CD, et al. Older individuals with diabetes have an increased risk of recurrent falls: analysis of potential mediating factors: the Longitudinal Ageing Study Amsterdam. Age Ageing. 2012 May;41:358–65. doi: 10.1093/ageing/afr145. [DOI] [PubMed] [Google Scholar]
- [7].Burghardt AJ, Issever AS, Schwartz AV, Davis KA, Masharani U, Majumdar S, et al. High-resolution peripheral quantitative computed tomographic imaging of cortical and trabecular bone microarchitecture in patients with type 2 diabetes mellitus. J Clin Endocrinol Metab. 2010 Nov;95:5045–55. doi: 10.1210/jc.2010-0226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Pritchard JM, Giangregorio LM, Atkinson SA, Beattie KA, Inglis D, Ioannidis G, et al. Association of larger holes in the trabecular bone at the distal radius in postmenopausal women with type 2 diabetes mellitus compared to controls. Arthritis Care Res (Hoboken) 2012 Jan;64:83–91. doi: 10.1002/acr.20602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Patsch JM, Burghardt AJ, Yap SP, Baum T, Schwartz AV, Joseph GB, et al. Increased cortical porosity in type-2 diabetic postmenopausal women with fragility fractures. J Bone Miner Res. 2012 Sep 18; doi: 10.1002/jbmr.1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Bailey AJ, Paul RG, Knott L. Mechanisms of maturation and ageing of collagen. Mech Ageing Dev. 1998 Dec;106:1–56. doi: 10.1016/s0047-6374(98)00119-5. [DOI] [PubMed] [Google Scholar]
- [11].Knott L, Bailey AJ. Collagen cross-links in mineralizing tissues: a review of their chemistry, function, and clinical relevance. Bone. 1998 Mar;22:181–7. doi: 10.1016/s8756-3282(97)00279-2. [DOI] [PubMed] [Google Scholar]
- [12].Tang SY, Vashishth D. Non-enzymatic glycation alters microdamage formation in human cancellous bone. Bone. 2010 Jan;46:148–54. doi: 10.1016/j.bone.2009.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Zioupos P. Accumulation of in-vivo fatigue microdamage and its relation to biomechanical properties in ageing human cortical bone. J Microscopy. 2001;201:270–278. [PubMed] [Google Scholar]
- [14].Norman TL, Yeni YN, Brown CU, Wang Z. Influence of microdamage on fracture toughness of the human femur and tibia. Bone. 1998 Sep;23:303–6. doi: 10.1016/s8756-3282(98)00103-3. [DOI] [PubMed] [Google Scholar]
- [15].Tang SY, Zeenath U, Vashishth D. Effects of non-enzymatic glycation on cancellous bone fragility. Bone. 2007 Apr;40:1144–51. doi: 10.1016/j.bone.2006.12.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Vashishth D, Gibson GJ, Khoury JI, Schaffler MB, Kimura J, Fyhrie DP. Influence of nonenzymatic glycation on biomechanical properties of cortical bone. Bone. 2001 Feb;28:195–201. doi: 10.1016/s8756-3282(00)00434-8. [DOI] [PubMed] [Google Scholar]
- [17].Nyman JS, Roy A, Tyler JH, Acuna RL, Gayle HJ, Wang X. Age-related factors affecting the postyield energy dissipation of human cortical bone. J Orthop Res. 2007 May;25:646–55. doi: 10.1002/jor.20337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Karim L, Vashishth D. Heterogeneous glycation of cancellous bone and its association with bone quality and fragility. PLoS One. 2012;7:e35047. doi: 10.1371/journal.pone.0035047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Follet H, Viguet-Carrin S, Pichat B. Burt, Depalle B, Bala Y, Gineyts E, et al. Effects of preexisting microdamage, collagen cross-links, degree of mineralization, age, and architecture on compressive mechanical properties of elderly human vertebral trabecular bone. J Orthop Res. 2011 Apr;29:481–8. doi: 10.1002/jor.21275. [DOI] [PubMed] [Google Scholar]
- [20].Yamamoto M, Yamaguchi T, Yamauchi M, Yano S, Sugimoto T. Serum pentosidine levels are positively associated with the presence of vertebral fractures in postmenopausal women with type 2 diabetes. J Clin Endocrinol Metab. 2008 Mar;93:1013–9. doi: 10.1210/jc.2007-1270. [DOI] [PubMed] [Google Scholar]
- [21].Schwartz AV, Garnero P, Hillier TA, Sellmeyer DE, Strotmeyer ES, Feingold KR, et al. Pentosidine and increased fracture risk in older adults with type 2 diabetes. J Clin Endocrinol Metab. 2009 Jul;94:2380–6. doi: 10.1210/jc.2008-2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Oren TW, Botolin S, Williams A, Bucknell A, King KB. Arthroplasty in veterans: Analysis of cartilage, bone, serum, and synovial fluid reveals differences and similarities in osteoarthritis with and without comorbid diabetes. The Journal of Rehabilitation Research and Development. 2011;48:1195. doi: 10.1682/jrrd.2010.09.0186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Dyer DG, Blackledge JA, Thorpe SR, Baynes JW. Formation of pentosidine during nonenzymatic browning of proteins by glucose. Identification of glucose and other carbohydrates as possible precursors of pentosidine in vivo. J Biol Chem. 1991 Jun 25;266:11654–60. [PubMed] [Google Scholar]
- [24].Bevill G, Eswaran SK, Gupta A, Papadopoulos P, Keaveny TM. Influence of bone volume fraction and architecture on computed large-deformation failure mechanisms in human trabecular bone. Bone. 2006 Dec;39:1218–25. doi: 10.1016/j.bone.2006.06.016. [DOI] [PubMed] [Google Scholar]
- [25].Gross J. Studies on the formation of collagen. I. Properties and fractionation of neutral salt extracts of normal guinea pig connective tissue. J Exp Med. 1958 Feb 1;107:247–63. doi: 10.1084/jem.107.2.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Yu EW, Putman MS, Derrico N, Abrishamanian-Garcia G, Finkelstein JS, Bouxsein ML. Defects in cortical microarchitecture among African-American women with type 2 diabetes. Osteoporos Int. 2015 Feb;26:673–9. doi: 10.1007/s00198-014-2927-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Patsch JM, Burghardt AJ, Yap SP, Baum T, Schwartz AV, Joseph GB, et al. Increased cortical porosity in type 2 diabetic postmenopausal women with fragility fractures. J Bone Miner Res. 2013 Feb;28:313–24. doi: 10.1002/jbmr.1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Shanbhogue VV, Hansen S, Frost M, Jorgensen NR, Hermann AP, Henriksen JE, et al. Compromised cortical bone compartment in type 2 diabetes mellitus patients with microvascular disease. Eur J Endocrinol. 2016 Feb;174:115–24. doi: 10.1530/EJE-15-0860. [DOI] [PubMed] [Google Scholar]
- [29].Samelson EJ, Demissie S, Cupples LA, Zhang X, Xu H, Liu CT, et al. Diabetes and Deficits in Cortical Bone Density, Microarchitecture, and Bone Size: Framingham HR-pQCT Study. J Bone Miner Res. 2018 Jan;33:54–62. doi: 10.1002/jbmr.3240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Nilsson AG, Sundh D, Johansson L, Nilsson M, Mellstrom D, Rudang R, et al. Type 2 Diabetes Mellitus Is Associated With Better Bone Microarchitecture But Lower Bone Material Strength and Poorer Physical Function in Elderly Women: A Population-Based Study. J Bone Miner Res. 2017 May;32:1062–1071. doi: 10.1002/jbmr.3057. [DOI] [PubMed] [Google Scholar]
- [31].Osima M, Kral R, Borgen TT, Hogestol IK, Joakimsen RM, Eriksen EF, et al. Women with type 2 diabetes mellitus have lower cortical porosity of the proximal femoral shaft using low-resolution CT than nondiabetic women, and increasing glucose is associated with reduced cortical porosity. Bone. 2017 Apr;97:252–260. doi: 10.1016/j.bone.2017.01.037. [DOI] [PubMed] [Google Scholar]
- [32].Shu A, Yin MT, Stein E, Cremers S, Dworakowski E, Ives R, et al. Bone structure and turnover in type 2 diabetes mellitus. Osteoporos Int. 2012 Feb;23:635–41. doi: 10.1007/s00198-011-1595-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Heilmeier U, Carpenter DR, Patsch JM, Harnish R, Joseph GB, Burghardt AJ, et al. Volumetric femoral BMD, bone geometry, and serum sclerostin levels differ between type 2 diabetic postmenopausal women with and without fragility fractures. Osteoporos Int. 2015 Apr;26:1283–93. doi: 10.1007/s00198-014-2988-7. [DOI] [PubMed] [Google Scholar]
- [34].Gallant MA, Brown DM, Organ JM, Allen MR, Burr DB. Reference-point indentation correlates with bone toughness assessed using whole-bone traditional mechanical testing. Bone. 2013 Mar;53:301–5. doi: 10.1016/j.bone.2012.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Devlin MJ, Van Vliet M, Motyl K, Karim L, Brooks DJ, Louis L, et al. Early-onset type 2 diabetes impairs skeletal acquisition in the male TALLYHO/JngJ mouse. Endocrinology. 2014 Oct;155:3806–16. doi: 10.1210/en.2014-1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Acevedo C, Sylvia M, Schaible E, Graham JL, Stanhope KL, Metz LN, et al. Contributions of Material Properties and Structure to Increased Bone Fragility for a Given Bone Mass in the UCD-T2DM Rat Model of Type 2 Diabetes. J Bone Miner Res. 2018 Jan 17; doi: 10.1002/jbmr.3393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Farr JN, Drake MT, Amin S, Melton LJ, 3rd, McCready LK, Khosla S. In vivo assessment of bone quality in postmenopausal women with type 2 diabetes. J Bone Miner Res. 2014 Apr;29:787–95. doi: 10.1002/jbmr.2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Furst JR, Bandeira LC, Fan WW, Agarwal S, Nishiyama KK, McMahon DJ, et al. Advanced Glycation Endproducts and Bone Material Strength in Type 2 Diabetes. J Clin Endocrinol Metab. 2016 Jun;101:2502–10. doi: 10.1210/jc.2016-1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Karim L, Van Vliet M, Bouxsein ML. Comparison of cyclic and impact-based reference point indentation measurements in human cadaveric tibia bone. Bone. 2015 doi: 10.1016/j.bone.2015.03.021. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Jenkins T, Katsamenis OL, Andriotis OG, Coutts LV, Carter B, Dunlop DG, et al. The inferomedial femoral neck is compromised by age but not disease: Fracture toughness and the multifactorial mechanisms comprising reference point microindentation. J Mech Behav Biomed Mater. 2017 Nov;75:399–412. doi: 10.1016/j.jmbbm.2017.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Karim L, Tang SY, Sroga GE, Vashishth D. Differences in non-enzymatic glycation and collagen cross-links between human cortical and cancellous bone. Osteoporos Int. 2013 Sep;24:2441–7. doi: 10.1007/s00198-013-2319-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Karim L, Bouxsein ML. Effect of type 2 diabetes-related non-enzymatic glycation on bone biomechanical properties. Bone. 2016 Jan;82:21–7. doi: 10.1016/j.bone.2015.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Vashishth D. The role of the collagen matrix in skeletal fragility. Curr Osteoporos Rep. 2007 Jun;5:62–6. doi: 10.1007/s11914-007-0004-2. [DOI] [PubMed] [Google Scholar]
- [44].Vashishth D. Advanced Glycation End-products and Bone Fractures. IBMS Bonekey. 2009 Aug;6:268–278. doi: 10.1138/20090390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Pritchard JM, Papaioannou A, Schwarcz HP, Willett TL. A Comparison of Collagen Crosslink Content in Bone Specimens from Elective Total Hip Arthroplasty Patients with and without Type 2 Diabetes. J Bone Rep Recomm. 2016;2:14. [Google Scholar]
- [46].Pritchard JM, Papaioannou A, Tomowich C, Giangregorio LM, Atkinson SA, Beattie KA, et al. Bone mineralization is elevated and less heterogeneous in adults with type 2 diabetes and osteoarthritis compared to controls with osteoarthritis alone. Bone. 2013 May;54:76–82. doi: 10.1016/j.bone.2013.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Wang X, Shen X, Li X, Agrawal CM. Age-related changes in the collagen network and toughness of bone. Bone. 2002 Jul;31:1–7. doi: 10.1016/s8756-3282(01)00697-4. [DOI] [PubMed] [Google Scholar]
- [48].Burstein AH, Zika JM, Heiple KG, Klein L. Contribution of collagen and mineral to the elastic-plastic properties of bone. J Bone Joint Surg Am. 1975 Oct;57:956–61. [PubMed] [Google Scholar]
- [49].Odetti P, Rossi S, Monacelli F, Poggi A, Cirnigliaro M, Federici M, et al. Advanced glycation end products and bone loss during aging. Ann N Y Acad Sci. 2005 Jun;1043:710–7. doi: 10.1196/annals.1333.082. [DOI] [PubMed] [Google Scholar]
- [50].Arokoski JP, Arokoski MH, Jurvelin JS, Helminen HJ, Niemitukia LH, Kroger H. Increased bone mineral content and bone size in the femoral neck of men with hip osteoarthritis. Ann Rheum Dis. 2002 Feb;61:145–50. doi: 10.1136/ard.61.2.145. [DOI] [PMC free article] [PubMed] [Google Scholar]