Abstract
Caffeine has been shown to be a robust uncompetitive inhibitor of glucose uptake in erythrocytes. It preferentially binds to the nucleotide-binding site on GLUT1 in its tetrameric form and mimics the inhibitory action of ATP. Here we demonstrate that caffeine is also a dose-dependent, uncompetitive inhibitor of 2-deoxyglucose (2DG) uptake in L929 fibroblasts. The inhibitory effect on 2DG uptake in these cells was reversible with a rapid onset and was additive to the competitive inhibitory effects of glucose itself, confirming that caffeine does not interfere with glucose binding. We also report for the first time that caffeine inhibition was additive to inhibition by curcumin, suggesting distinct binding sites for curcumin and caffeine. In contrast, caffeine inhibition was not additive to that of cytochalasin B, consistent with previous data that reported that these two inhibitors have overlapping binding sites. More importantly, we show that the magnitude of maximal caffeine inhibition in L929 cells is much lower than in erythrocytes (35% compared to 90%). Two epithelial cell lines, HCLE and HK2, have both higher concentrations of GLUT1 and increased basal 2DG uptake (3e4 fold) compared to L929 cells, and subsequently display greater maximal inhibition by caffeine (66e70%). Interestingly, activation of 2DG uptake (3-fold) in L929 cells by glucose deprivation shifted the responsiveness of these cells to caffeine inhibition (35%e70%) without a change in total GLUT1 concentration. These data indicate that the inhibition of caffeine is dependent on the activity state of GLUT1, not merely on the concentration.
Keywords: GLUT1, Caffeine, L929 fibroblast, Glucose uptake, Curcumin
1. Introduction
The facilitated glucose transporter, GLUT1 (SLC2A1), is expressed in a wide variety of cell types and is generally thought to be responsible for basal uptake of glucose, though it responds to changing metabolic conditions as well [1]. Chronic exposure to cell stressorsdsuch as hypoxia, hypoglycemia, and AMP kinase activationdincrease GLUT1 protein expression [2–4]. In addition, this transporter appears to be overexpressed in a number of cancers, especially those driven by KRAS mutations, thereby accelerating glucose uptake [5–9]. However, there is increasing evidence that the transport activity of GLUT1 can also be regulated acutely in the absence of new protein synthesis. This acute regulation is distinctly different from, and possibly more varied than, the more widely understood transporter, GLUT4, where exposure to insulin in insulin-sensitive tissues and increased contractile activity in myocytes both enhance glucose uptake by stimulating the translocation of GLUT4 transporters from internal stores to the cell surface [10]. The complexity of the acute GLUT1 regulation depends, at least in part, on cell type. For instance, hypoglycemia stimulates GLUT1-mediated glucose uptake in brain capillary endothelial cells via activation of AMP kinase, leading to an increase in the membrane concentrations of GLUT1 [11]. In contrast, hypoglycemia and other cell stressorsdsuch as azide treatment or hyper-osmolaritydincreased glucose uptake in clone 9 cells, also via activation of AMP kinase, but without a detectable change in concentration of cell surface GLUT1 [12,13]. The change in activity was attributed to a change in the membrane or lipid environment of the transporter [12,14–17]. L929 fibroblast cells, which express GLUT1 at a relatively low concentration, are particularly sensitive to a variety of small molecule activators with different kinetic patterns, suggesting that there may be multiple mechanisms for the acute activation of GLUT1 in this cell line [18–20].
The structure and activity of GLUT1 has been most intensively studied in erythrocytes, in which this transporter makes up 10e20% of membrane protein content. Carruthers has shown that, while GLUT1 can exist and transport glucose as monomers, dimers and tetramers, in erythrocytes GLUT1 exists predominately as homotetrameric complexes. These tetramers are stabilized by an internal disulfide bond within each GLUT1 monomer and have greater transport activity than the monomeric or dimeric forms [1,21]. The self-association of GLUT1 has also been demonstrated in kidney cells where it is dependent, at least in part, on GLUT1 concentration [22]. Thus, stimulated association of GLUT1 into tetramers is an appealing mechanism to explain the acute activation of glucose uptake in L929 cells, but this dynamic multimerization has yet to be directly demonstrated. Given the sensitivity of acute GLUT1 activation to cysteine chemistry, however, it remains possible that the activation of glucose uptake in L929 cells involves an increase in tetramer formation driven by a dynamic regulation of disulfide bond chemistry within individual GLUT1 proteins [23–27].
One indirect means of demonstrating the presence of tetramers within a given cell line is to test its response to an inhibitor of glucose transport that preferentially binds to and inhibits the tetramer form of GLUT1. One such inhibitor is the nucleotide triphosphate ATP, which acts as an uncompetitive inhibitor of GLUT1. The nucleotide-binding site maps to an endofacial site that is only available in tetramers [28,29], and is lost when tetrameric structure is disrupted [30,31]. Recently, it was shown that caffeine mimics ATP by binding to this nucleotide-binding site and eliciting a similar conformational change in GLUT1 that is induced by ATP [32,33].
Therefore, the purpose of this study was to determine whether the inhibitory efficacy of caffeine changes in cells with variable GLUT1 activities. Cells such as L929 cells, have low concentrations of GLUT1 with low basal glucose uptakes, while HCLE and HK2 cells have higher concentrations of GLUT1 and higher basal glucose uptakes. In addition, a brief period of glucose-deprivation activates uptake in L929 cells without a change in GLUT1 concentration. Comparison of the effects of caffeine in L929, HK2, HCLE and glucose-deprived L929 cells will help us determine if caffeine inhibition is simply a function of GLUT1 content or a function of the activity state of GLUT1.
2. Materials and methods
2.1. Chemicals and antibodies
Curcumin, cytochalasin B, and caffeine were purchased from the Sigma-Aldrich Chemical Company (St. Louis, MO, USA) and 2-deoxy-D-glucose- [1,2-3H] (2DG) from Moravek Biochemicals (Brea, CA, USA). Antibodies to GLUT1 and b-actin were purchased from Epitomics/Abcam (Burlingam, CA) and Cell Signaling Technology (Danvers, MA), respectively.
2.2. Cell culture
L929 mouse fibroblast cells and HK2 (human kidney) cells were obtained from the American Type Culture Collection. The immortalized human cornealelimbal epithelial (HCLE) cell line was obtained from Dr. Ilene Gipson (Department of Ophthalmology, Harvard Medical School) and maintained as monolayer cultures in Keratinocyte-Serum Free medium (K-SFM) (Invitrogen, Carlsbad, CA), as previously described [34]. To initiate each experiment, a 24-well plate was seeded either 1 or 2 days prior to experimentation. The cells were grown at 37 °C in an incubator supplied with humidified room air with 5% CO2. Uptake experiments were done with cells near confluency, which is about 1.0 105 cells per well for HCLE and HK2 cells and 3.0 105 for L929 fibroblast cells.
2.3. Glucose uptake assay
Glucose uptake was measured using the radiolabeled glucose analog 2-deoxyglucose (2DG) using a modified procedure from previous studies [35]. Briefly, the media was replaced with 0.2 mL of glucose-free HEPES buffer (140 mM NaCl, 5 mM KCl, 20 mM HEPES/Na pH ¼ 7.4, 2.5 mM MgSO4, 1 mM CaCl2, 2 mM NaPyruvate, 1 mM mannitol) supplemented with 1.0 mM (0.3 mCi/mL) 2DG (1,2-3H). 1.0 mM 2DG is below the Km of transport, 6e8 mM, and allows us to monitor linear uptake for longer times. After a 15-min incubation, cells were washed twice with cold glucose-free HEPES. The cells were digested in 0.25 mL of 0.3 M NaOH and the 3H-2DG was measured using scintillation spectrometry. In previous studies, we had included 14C-mannitol in the uptake media as way to detect radioactivity trapped in interstitial spaces. However, we have found that in monolayered tissue culture cells, the extracellular radioactivity is efficiently removed by the subsequent washes. Thus, as a cost saving measure, we no longer include radiolabeled mannitol in the uptake media.
The uptake media was supplemented with caffeine from a 75 mM stock solution in HEPES to a final concentration indicated in the figure legends. For the kinetics experiment (Fig. 1B), the concentration of 2DG in the uptake solution was varied as indicated in the figure legend. For experiments investigating the effects of time (Fig. 2), cells were incubated in DMEM containing 10 mM caffeine for either a varied length of time prior to measurement of uptake (Fig. 2A) or for 20 min followed by replacement with DMEM without caffeine for a varied amount of time (Fig. 2B). For experiments measuring the combined effects of inhibitors (Fig. 3) curcumin, cytochalasin B, and glucose were supplied from a 200 stock solution in DMSO, ethanol and water respectively. Activation of L929 cells (Fig. 4) was accomplished by incubating cells in DMEM without glucose for 30 min prior to measurement of 2DG uptake as previously described [19].
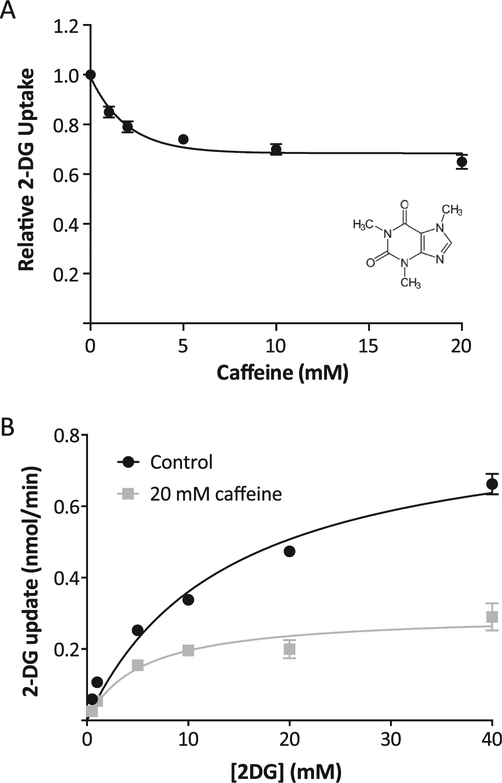
Fig. 1.
Effects of caffeine in L929 fibroblast cells. Panel A: Dose-dependent effects. 2DG uptake was measured at various concentrations of caffeine, ranging from 0 to 20 mM. Data (n ¼ 20) were normalized to basal uptake (0 mM caffeine), and the relative up-takes are expressed as means ± S.E. with a best-fit line to simple decay. All caffeine treatments were significantly lower at P < 0.01. Inset shows structure of caffeine. Panel B: Kinetics of 2DG uptake. 2DG uptake was measured at 0.5, 1.0, 5.0, 10, 20, and 40 mM 2DG in the absence or presence of 20 mM caffeine and reported as nmol/min/well. Data are means ± S.E. of quadruplicate samples from a representative experiment with a best-fit line to Michaelis-Menten kinetics.
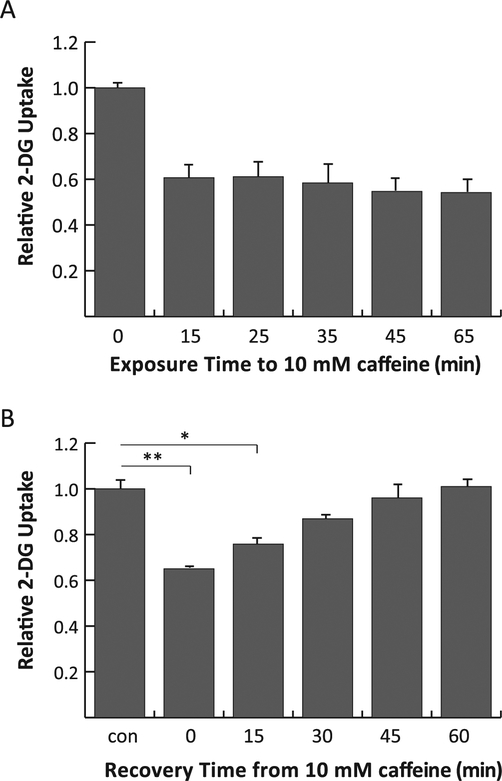
Fig. 2.
Effects of time on the inhibitory effects of caffeine. Panel A: Exposure time to curcumin. L929 fibroblast cells were treated with 10 mM caffeine during the 2DG uptake only (15 min) or to additional times prior to 2DG uptake. The total exposure time to caffeine is shown on the legend. 2DG uptakes were measured, normalized to control and expressed as means of relative uptake ± S.E. All caffeine effects were significantly lower than control at P < 0.01. Panel B: Recovery from caffeine effects. Cells were treated with media containing 10 mM caffeine for 20 min. The media was replaced with caffeine-free media to recover for 0, 15, 30, 45, or 60 min 2DG uptakes were measured, normalized to control and expressed as means of relative uptake ± S.E.*Significant from control at P < 0.05.**Significant from control at P < 0.01.
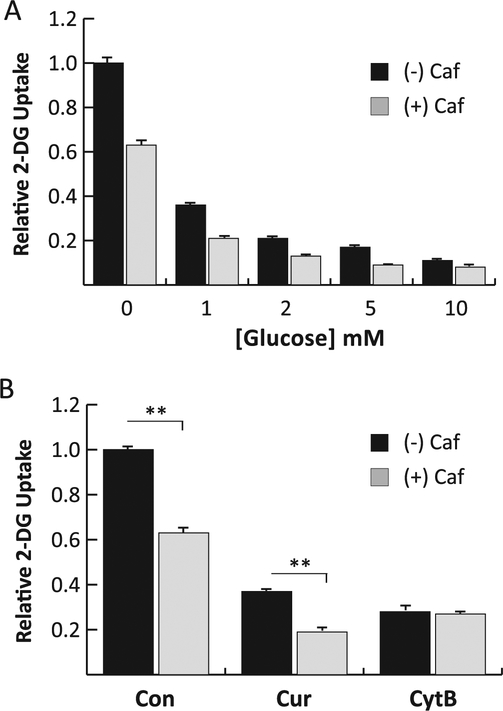
Fig. 3.
Combined effects of caffeine with other inhibitors of glucose uptake. Panel A: Effects of glucose. 2DG was measured in L929 cells in the presence of increasing concentrations of glucose with or without 20 mM caffeine. 2DG uptakes in caffeine treated cells were significantly lower at P < 0.01 at all concentrations of glucose. Panel B: Effects of curcumin and cytochalasin B. 2DG was measured in L929 cells in the absence or presence of 20 mM caffeine, either alone (Con) or with the addition of 100 mM curcumin (Cur) or 20 mM cytochalasin B (CytB).**Significant from control at P < 0.01.
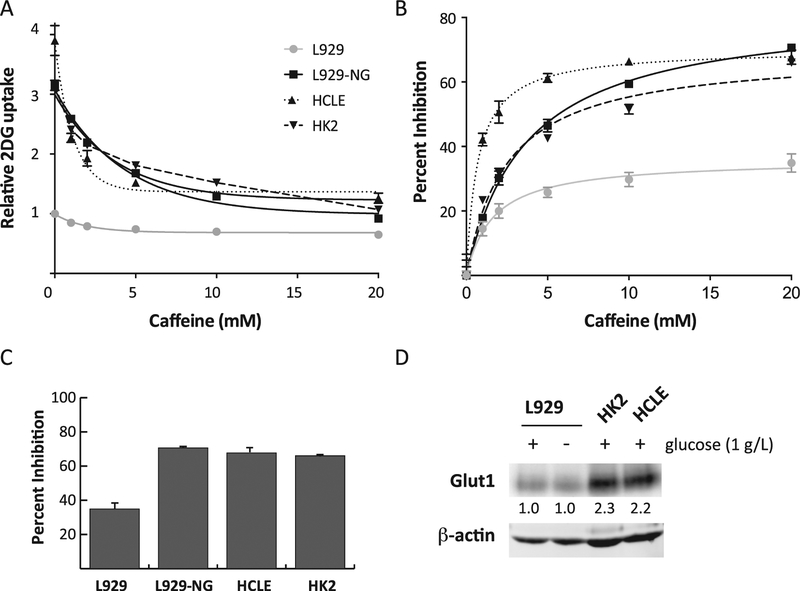
Fig. 4.
Caffeine inhibition in cells with higher GLUT1 activity. Panel A: Caffeine’s effect on 2DG uptake. 2DG uptake was measured in L929 cells, L929 cells activated by glucose deprivation (L929-NG), HCLE cells, and HK2 cells in the presence of 0, 1, 2, 5, 10, or 20 mM caffeine, and the results were normalized to L929 control cells at 0 mM caffeine and expressed as means of relative uptake ± S.E with best fit line to simple decay. Panel B: Percent inhibition. The uptake data from each cell type in Panel A were each normalized to their respective uptake at 0 mM caffeine, expressed as percent inhibition, and displayed as means ± S.E with best fit line. Panel C: Maximum effects of caffeine. Bar graph shows the percent inhibition ± S.E. of 2DG uptake at 20 mM caffeine. Panel D: GLUT1 Western blot. 75 mg of protein from cell extracts treated with PNGaseF of L929 cells treated with or without glucose, HCLE cells, and HK2 cells were separated by electrophoresis and probed with anti-GLUT1 and anti-actin antibodies as described. Numbers indicate fold change in GLUT1 normalized to actin compared to control (L929 + glucose).
2.4. Western blotting
HCLE, HK2 or L929 cells from confluent 6-cm dishes were rinsed with PBS and scraped directly into 200 mL of lysis buffer (20 mM Tris [pH 7.5], 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, 2.5 mM sodium pyrophosphate, 1 mM sodium glycerophosphate, 1 mM sodium orthovanadate, 0.5% NP40, 0.1% Brij35, 0.1% sodium deoxycholate) supplemented with mammalian cell protease inhibitor cocktail (Sigma-Aldrich), and homogenized by brief sonication. The concentration of cleared whole cell lysate was determined by Bradford assay along with a two-fold serial dilution of 10 mg/mL BSA to generate a standard curve. Equal amounts of protein lysate (75 μg) were treated with recombinant PNGaseF to deglycosylate GLUT1 (prepared from E. coli using the pOPH6 vector from Dr. Shaun Lott, obtained via Addgene, #40315) [36], separated on a 8% SDS-PAGE gel and transferred overnight to nitrocellulose membrane using a traditional wet transfer apparatus (TE62 model; Hoefer, Holliston, MA). The blots were blocked with 3% non-fat dry milk in Tris-buffered saline containing 0.05% Tween-20 (TBST), and then probed overnight at 4 °C with an anti-GLUT1 rabbit monoclonal antibody (1:1000) and an anti-b-actin mouse monoclonal antibody (1:3000). After washing off unbound primary antibody, the membranes were incubated for 1 h at room temperature with goat anti-rabbit-IRDye™800 and goat anti-mouse-IRDye™680 secondary antibodies (LiCor, Lincoln, NE) and then imaged with an Odyssey scanner (LiCor). The signal from each band was quantified using the Odyssey Infrared Imaging System software (version 3.0.25)
2.5. Statistical analysis
Each experiment with quadruplicate samples was repeated a minimum of three times to ensure that results could be replicated. 2DG uptake data were measured as nmol/15 min/well ±standard error, normalized to control conditions and reported as relative 2DG uptake. Statistical significance was determined by a two-tailed t-test and is reported at P < 0.05 or P < 0.01. The software program, Prism v 6.0f, was used to fit the data and determine parameters such as Km, Vmax and IC50.
3. Results
3.1. Caffeine is an uncompetitive inhibitor of 2DG uptake in L929 fibroblast cells
It has been recently reported that caffeine is an uncompetitive inhibitor of glucose uptake in human erythrocytes [32]. The evidence indicates that caffeine binds to the nucleotide-binding site located on the endofacial side of the transporter and mimics ATP in its inhibition of GLUT1 [32]. Erythrocytes have an abnormally high concentration of GLUT1 and the dominant structure of GLUT1 is a homotetramer, which is required to generate the nucleotide-binding site [1,28,30]. However, the effect of caffeine on glucose uptake in cells where the concentration of the GLUT1 is significantly lower than in erythrocytes is unknown. We therefore measured the effects of caffeine (varied from 0 to 20 mM) on 2DG uptake in L929 fibroblast cells, which exclusively express GLUT1 at a relatively low concentration [37]. The results, plotted in Fig. 1A with the calculated best fit line, indicate that caffeine inhibits 2DG uptake in a dose-dependent manner achieving a maximal inhibition by 10 mM of about 35% and an IC50 of about 1.4 mM. Either 10 or 20 mM caffeine was used in subsequent experiments as a maximally effective concentration. The maximum inhibition by 20 mM caffeine is markedly less than the approximately 90% inhibition observed in erythrocytes [32]. The kinetics of 2DG uptake was measured in the presence and absence of 20 mM caffeine with results displayed in Fig. 1B. Treatment with caffeine triggered a 65% decrease in the Vmax of uptake (0.86e0.30 nmol/min) and a 62% decrease in the Km (13.8e5.3 mM). This parallel decrease in both the Vmax and Km is indicative of uncompetitive inhibition, which recapitulates the mode of the kinetic effects of caffeine in erythrocytes [32].
3.2. Inhibition of caffeine is immediate and reversible
To determine the onset of inhibition, L929 cells were incubated in media containing 20 mM caffeine during the 15-min 2DG uptake measurement alone, or with a specified pre-treatment period just prior to the uptake assay. The results, shown in Fig. 2A, report 2DG uptake as a function of the total exposure time to caffeine. These data demonstrate that inhibition occurs within the 15-min time-frame required for the measurement of 2DG uptake, and that the magnitude of inhibition does not increase with different times of pre-treatment.
We also measured the reversibility of the inhibitory effects by exposing L929 cells to 20 mM caffeine for 20 min and then chasing them in caffeine-free media for increasing lengths of time prior to performing 2DG uptake assays. The results, shown in Fig. 2B, indicate that the inhibitory effect of caffeine is reversed within 30 min.
3.3. Combined effects of caffeine with other inhibitors
Neither the binding of ATP nor caffeine is sensitive to glucose concentration, which predicts that the inhibitory effect of glucose on 2DG uptake should be additive to both of these compounds in L929 cells [28,32,33]. To confirm this property of caffeine in L929 cells, we measured 2DG uptake in the presence of increasing concentrations of glucose with or without 20 mM caffeine. The results shown in Fig. 3A clearly demonstrate that glucose reduces 2DG uptake in a dose-dependent manner, and that caffeine adds to the inhibitory effects of glucose at all doses along this titration.
We were also interested in measuring the combined inhibitory effects of caffeine with other GLUT1 inhibitors, cytochalasin B and curcumin, as a means to confirm the binding sites of these inhibitors relative to that of caffeine. We predicted that if the binding sites of the inhibitors overlap, the maximum inhibitory effects should not be additive since binding of the compounds would be mutually exclusive. Cytochalasin B is an inhibitor that binds to an endofacial site on GLUT1 that overlaps the nucleotide-binding site, and therefore the combined inhibitory effects should not be additive [32]. In contrast, the binding site of curcumin has not been clearly mapped onto GLUT1, though it inhibits cytochalasin B binding and is not sensitive to glucose concentration [38,39]. To determine the combined effects of inhibitors, we measured 2DG uptake in L929 cells in the presence of maximum effective concentrations of either cytochalasin B (20 mM), or curcumin (100 mM) without or with 20 mM caffeine. The results displayed in Fig. 3B show that inhibition by cytochalasin B, as predicted, is not increased by addition of caffeine, confirming previous results in erythrocytes [32]. However, the effects of caffeine and curcumin do appear to be additive, suggesting that these inhibitors likely bind at distinct sites on GLUT1. Alternatively, it is also possible that these inhibitors target distinct populations of GLUT1 in the tetrameric and monomeric form and therefore show additive effects because they effectively block the activity of both species when used in combination.
3.4. Caffeine is a more robust inhibitor in cells with highly active GLUT1
One clear difference between the effects of caffeine on GLUT1 in erythrocytes compared to L929 cells is the magnitude of inhibition at 20 mM (90% vs 35% respectively). It is not clear if this difference is merely a function of the concentration of GLUT1 or more closely correlated to the activity state of GLUT1. In erythrocytes GLUT1 makes up 10e20% of the total membrane protein and at this concentration the dominant transporter structure is homotetramers, which leads to effective caffeine binding and inhibition [1,21]. It has also been demonstrated in engineered 293FT-HEK cells that an inducible increase in the expression of GLUT1 leads to an increased aggregation of the transporter [22]. In contrast, at a lower concentration of GLUT1 (as in L929 cells) more of the transporter may be in monomeric or dimeric structures with a lesser amount in the tetrameric form, and therefore have a lower magnitude of caffeine inhibition. Cells with expression levels of GLUT1 that are intermediate to erythrocytes and L929 fibroblasts would be expected to fall somewhere within this spectrum of caffeine efficacy. Along the same line of reasoning, “activated” L929 cells would be expected to show increased sensitivity to caffeine if the activation process involved a dynamic association of monomer and dimers forms of GLUT1 into tetramers.
To determine whether the efficacy of caffeine inhibition on 2DG uptake is a function of GLUT1 content or of activity level, we measured the effects of caffeine on 2DG uptake in HK2 and HCLE cells, which display an elevated expression of GLUT1 compared to L929 cells. At the same time, we also measured the effect of caffeine on 2DG uptake in L929 cells activated by glucose deprivation. The dose-dependent effects of caffeine on 2DG uptake in HCLE, HK2 and activated L929 cells were normalized to untreated L929 cells without caffeine, and are shown in Fig. 4A. Data from untreated L929 cells (Fig. 1A) are included for comparison. Basal 2DG uptakes (0 mM caffeine) in HCLE, HK2, and activated L929 cells are 3.9 and3.1 and 3.1-fold higher than the control, respectively, and are dose-dependently inhibited by caffeine.
To better demonstrate differences in the magnitude of caffeine inhibition on 2DG uptake, we normalized the data from each cell type to its respective basal uptake at 0 mM caffeine and then expressed the uptake as a percent inhibition (Fig. 4B). HCLE cells were the most sensitive to caffeine inhibition at lower concentrations but displayed a maximal inhibition similar to that of HK2 and activated L929 cells. Data summarized in Fig. 4C clearly show the similarity between maximal effects at 20 mM caffeine in these cellular models (all near 70%), which is significantly greater than the 35% inhibition observed in basal L929 cells. Western blots of 75 mg of protein from extracts of L929 cells, activated L929 cells, HCLE cells, and HK2 cells, shown in Fig. 4D. Interestingly, the epithelial cell lines, HK2 and HCLE, appear to express higher concentrations of actin than the L929 fibroblast cell line. GLUT1 was isolated as a 32 kD protein, which is the size of deglycosylated GLUT1. The GLUT1 concentration was normalized to the actin content in the extract and the results indicate that HK2 and HCLE cells have a 2.3 and 2.2 higher GLUT1 concentrations than L929 cells. Activation of glucose uptake in L929 cells does not alter the concentration of GLUT1. This provides evidence that the efficacy of caffeine is not merely related to the absolute amount of GLUT1 present in cells, but also to the activation state of GLUT1.
4. Discussion
Caffeine binds to the ATP binding site located on the endofacial side of GLUT1, and like ATP, is a potent uncompetitive inhibitor of GLUT1 activity [32,33]. However, this work was done in erythrocytes where the concentration of GLUT1 is particularly high (10e20% of membrane protein) and thus the effects of caffeine may be unique to these cells.
In erythrocytes the dominant structure of GLUT1 is a homotetramer, which has greater transport activity than the dimer and monomer forms of GLUT1 [1,40]. The tetramer is stabilized by the formation of an internal disulfide bond between Cys347 and Cys421 within each subunit [21,40]. ATP binds to the tetramer form, and thus exposure of erythrocytes to a reducing agent leads to the transition of GLUT1 tetramers to dimers and the subsequent loss of ATP binding and inhibition [30,31]. Caffeine binds to the same site as ATP and induces a similar conformational change in GLUT1. Specifically, both ATP and caffeine appear to trigger a conformational change that brings the C-terminus of GLUT1 closer to the intracellular loop connecting transmembrane helices 6e7, creating a ‘cage-like’ structure around the endofacial exit site for glucose transport that likely impedes the release of glucose into the cytoplasmic space [32]. This does not affect glucose exchange through the transporter but does lead to uncompetitive inhibition of net glucose uptake. These data suggest that caffeine inhibition may be an indirect probe for the tetrameric structure of GLUT1. Therefore, the purpose of this study was to determine if caffeine inhibits glucose uptake in other GLUT1 expressing cells and if the inhibition correlates with the content level and/or activity level of the transporter.
The L929 fibroblast cell line, which we have used to characterize glucose uptake by GLUT1, presents a unique cellular model in contrast to erythrocytes. These cells express exclusively GLUT1, but at a much lower level than in erythrocytes [37]. We show that caffeine inhibits 2DG uptake in L929 cells in a dose-dependent manner with uncompetitive kinetics, which mirrors its effects in erythrocytes. The effects of caffeine are immediate and reversible, and are also additive to the competitive inhibitory effects of glucose, which is consistent with previous work showing that caffeine does not interfere with glucose binding sites [32]. We also show that the inhibition by caffeine is not additive to the effects of cytochalasin B. This is again consistent to the results from erythrocytes, where the high affinity binding sites for caffeine and cytochalasin B map to overlapping sites and caffeine can inhibit specific 3H-cytochalasin B binding [32]. Finally, it had been suggested by an earlier study that curcumin might share a binding site with caffeine based on the observation that both inhibitors compete with cytochalasin B binding but not with glucose [38]. However, contrary to that suggestion, we show that the inhibitory effects of the maximally effective concentrations of caffeine and curcumin are additive, indicating that these inhibitors do not share binding sites.
In spite of the similarities of caffeine’s effects in L929 cells and erythrocytes, a major difference can be identified in the magnitude of inhibition (35% versus 90%). It initially seems reasonable to conclude from this finding that the reduced magnitude of caffeine inhibition in L929 cells is simply a function of the lower concentration of GLUT1. It is reasonable to expect that as the concentration of GLUT1 in the membrane increases, the close proximity of the transporters would produce more GLUT1 tetramers, and therefore, be more responsive to inhibition by caffeine. This is in fact what is observed in erythrocytes. These cells have high GLUT1 concentrations and aggregate as tetramers, and as a result, are highly responsive to inhibition by caffeine [1,21]. In addition, increasing the expression of GLUT1 in kidney cells, leads to a dose dependent aggregation of GLUT1 [22]. Therefore, to test if GLUT1 concentration is important, we measured caffeine’s effects in HCLE and HK2 cells, both of which express significantly more GLUT1 than L929 cells [41]. As expected, both HCLE and HK2 cells also have higher basal rates of 2DG uptake. In line with these observations, we found that both cell types are much more responsive to caffeine, with maximal inhibition at about 70%, which approaches the 90% inhibition observed in erythrocytes.
While the data in HK2 and HCLE cells supports a role for GLUT1 abundance in cellular response to caffeine, our parallel study with “activated” L929 cells demonstrates that the maximal effect of caffeine on 2DG transport is not simply a function of cellular GLUT1 content. L929 cells deprived of glucose for 20 min display 2DG uptakes greater than 3-fold that of basal cells, but show no change in cellular GLUT1 content. Interestingly, the stimulated uptake rate in L929 cells is similar in magnitude to the basal uptake rates in HCLE and HK2 cells, and in this context L929 cells are similarly inhibited by caffeine (Fig. 4A). As such, the inhibitory effect of caffeine appears to be more sensitive to the activity state of GLUT1 than to the total GLUT1 content.
If caffeine binds only to GLUT1 tetramers in L929 cellsdas has been reported to be the case in erythrocytesdit seems likely that glucose deprivation stimulates tetramer formation by GLUT1 in L929 cells. Though this supposition has yet to be proven formally by biochemical isolation of tetramers in L929 cells, it is consistent with a model in which acute activation of glucose uptake is driven by dynamic changes in the thiol chemistry of GLUT1, shifting its quaternary structure to favor the tetrameric structure. Previous work by our lab has shown that the activation of 2DG uptake in L929 cells depends on thiol chemistry [23–27]. For example, nitroxyl (HNO), which promotes disulfide bond formation especially in hydrophobic environments [42–44], stimulates 2DG up-take 5-fold within 2 min and this activation is blocked by thioreactive compounds such as pretreatment with iodoacetamide[25]. This is consistent with the notion that nitroxyl chemically promotes a disulfide formation within GLUT1 that leads to stabilization of the tetramer structure. However, future work, beyond the scope of this study, needs to be done to directly detect formation of GLUT1 tetramers within the membrane if this mechanism is to be confirmed.
5. Conclusions
This study reports that caffeine directly inhibits 2DG uptake in a dose-dependent manner in multiple GLUT1 expressing cells. The inhibition is immediate and reversible, and the magnitude of inhibition correlates with the activation level of GLUT1 rather than simply with the expression level of the transporter. The data are consistent with a model of activation of GLUT1 that involves an active multimerization of the transporter.
Acknowledgements
This research was supported by a NIH R15 grant (DK08193–1A1). Special thanks go to the Ubels lab for supplying the HCLE cells.
References
- [1].Carruthers A, DeZutter J, Ganguly A, Devaskar SU, Will the original glucose transporter isoform please stand up!, Am. J. Physiol. Endocrinol. Metab 297 (2009) E836–E848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Boado RJ, Pardridge WM, Glucose deprivation and hypoxia increase the expression of the GLUT1 glucose transporter via a specific mRNA cis-acting regulatory element, J. Neurochem 80 (2002) 552–554. [DOI] [PubMed] [Google Scholar]
- [3].Wertheimer E, Sasson S, Cerasi E, Ben-Neriah Y, The ubiquitous glucose transporter GLUT-1 belongs to the glucose-regulated protein family of stress-inducible proteins, Proc. Natl. Acad. Sci. U. S. A 88 (1991) 2525–2529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Kitzman HH Jr., McMahon RJ, Williams MG, Frost SC , Effect of glucose deprivation of GLUT 1 expression in 3T3–L1 adipocytes, J. Biol. Chem 268 (1993) 1320–1325. [PubMed] [Google Scholar]
- [5].Macheda ML, Rogers S, Best JD, Molecular and cellular regulation of glucose transporter (GLUT) proteins in cancer, J. Cell. Physiol 202 (2005) 654–662. [DOI] [PubMed] [Google Scholar]
- [6].Furuta E, Okuda H, Kobayashi A, Watabe K, Metabolic genes in cancer: their roles in tumor progression and clinical implications, Biochim. Biophys. Acta 1805 (2010) 141–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Szablewski L, Expression of glucose transporters in cancers, Biochim. Biophys. Acta 1835 (2013) 164–169. [DOI] [PubMed] [Google Scholar]
- [8].Yun J, Mullarky E, Lu C, Bosch KN, Kavalier A, Rivera K, Roper J, Chio II, Giannopoulou EG, Rago C, Muley A, Asara JM, Paik J, Elemento O, Chen Z, Pappin DJ, Dow LE, Papadopoulos N, Gross SS, Cantley LC, Vitamin C selectively kills KRAS and BRAF mutant colorectal cancer cells by targeting GAPDH, Science 350 (2015) 1391–1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Yun J, Rago C, Cheong I, Pagliarini R, Angenendt P, Rajagopalan H,Schmidt K, Willson JK, Markowitz S, Zhou S, Diaz LA Jr., Velculescu VE,Lengauer C, Kinzler KW, Vogelstein B, Papadopoulos N, Glucose deprivation contributes to the development of KRAS pathway mutations in tumor cells, Science 325 (2009) 1555–1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Govers R, Molecular mechanisms of GLUT4 regulation in adipocytes, Diabetes Metab. 40 (2014) 400–410. [DOI] [PubMed] [Google Scholar]
- [11].Cura AJ, Carruthers A, AMP kinase regulation of sugar transport in brain capillary endothelial cells during acute metabolic stress, Am. J. Physiol. Cell Physiol 303 (2012) C806–C814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Barnes K, Ingram JC, Porras OH, Barros LF, Hudson ER, Fryer LG,Foufelle F, Carling D, Hardie DG, Baldwin SA, Activation of GLUT1 by metabolic and osmotic stress: potential involvement of AMP-activated protein kinase (AMPK), J. Cell Sci 115 (2002) 2433–2442. [DOI] [PubMed] [Google Scholar]
- [13].Shetty M, Loeb JN, Vikstrom K, Ismail-Beigi F, Rapid activation of GLUT-1 glucose transporter following inhibition of oxidative phosphorylation in clone 9 cells, J. Biol. Chem 268 (1993) 17225–17232. [PubMed] [Google Scholar]
- [14].Rubin D, Ismail-Beigi F, Distribution of Glut1 in detergent-resistant membranes (DRMs) and non-DRM domains: effect of treatment with azide, Am. J. Physiol. Cell Physiol 285 (2003) C377–C383. [DOI] [PubMed] [Google Scholar]
- [15].Rubin D, Ismail-Beigi F, Differential accumulation of Glut1 in the non-DRM domain of the plasma membrane in response to the inhibition of oxidative phosphorylation, Arch. Biochem. Biophys 431 (2004) 224–232. [DOI] [PubMed] [Google Scholar]
- [16].Barnes K, Ingram JC, Bennett MD, Stewart GW, Baldwin SA, Methyl-beta-cyclodextrin stimulates glucose uptake in Clone 9 cells: a possible role for lipid rafts, Biochem. J 378 (2004) 343–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Barros LF, Barnes K, Ingram JC, Castro J, Porras OH, Baldwin SA, Hyper-osmotic shock induces both activation and translocation of glucose transporters in mammalian cells, Pflugers Arch. Eur. J. Physiol 442 (2001) 614–621. [DOI] [PubMed] [Google Scholar]
- [18].Cok A, Plaisier C, Salie MJ, Oram DS, Chenge J, Louters LL, Berberine acutely activates the glucose transport activity of GLUT1, Biochimie 93 (2011) 1187–1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Roelofs B, Tidball A, Lindborg AE, TenHarmsel A, Vander Kooy TO,Louters LL, Acute activation of glucose uptake by glucose deprivation in L929 fibroblast cells, Biochimie 88 (2006) 1941–1946. [DOI] [PubMed] [Google Scholar]
- [20].Louters LL, Dyste SG, Frieswyk D, Tenharmsel A, Vander Kooy TO,Walters L, Whalen T, Methylene blue stimulates 2-deoxyglucose uptake in L929 fibroblast cells, Life Sci. 78 (2006) 586–591. [DOI] [PubMed] [Google Scholar]
- [21].Zottola RJ, Cloherty EK, Coderre PE, Hansen A, Hebert DN, Carruthers A, Glucose transporter function is controlled by transporter oligomeric structure. A single, intramolecular disulfide promotes GLUT1 tetramerization, Biochemistry 34 (1995) 9734–9747. [DOI] [PubMed] [Google Scholar]
- [22].Looyenga B, VanOpstall C, Lee Z, Bell J, Lodge E, Wrobel K, Arnoys E,Louters L, Determination of GLUT1 oligomerization parameters using bioluminescent forster resonance energy transfer, Sci. Rep 6 (2016) 29130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Gunnink SM, Kerk SA, Kuiper BD, Alabi OD, Kuipers DP, Praamsma RC, Wrobel KE, Louters LL, Alkaline pH activates the transport activity of GLUT1 in L929 fibroblast cells, Biochimie 99 (2014) 189–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Louters LL, Scripture JP, Kuipers DP, Gunnink SM, Kuiper BD, Alabi OD, Hydroxylamine acutely activates glucose uptake in L929 fibroblast cells, Biochimie 95 (2013) 787–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Salie MJ, Oram DS, Kuipers DP, Scripture JP, Chenge J, MacDonald GJ,Louters LL, Nitroxyl (HNO) acutely activates the glucose uptake activity of GLUT1, Biochimie 94 (2012) 864–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Scott J, Opejin A, Tidball A, Stehouwer N, Rekman J, Louters LL, Dual action of phenylarsine oxide on the glucose transport activity of GLUT1, Chem. Biol. Interact 182 (2009) 199–203. [DOI] [PubMed] [Google Scholar]
- [27].Plaisier C, Cok A, Scott J, Opejin A, Bushhouse KT, Salie MJ, Louters LL, Effects of cinnamaldehyde on the glucose transport activity of GLUT1, Biochimie 93 (2011) 339–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Blodgett DM, De Zutter JK, Levine KB, Karim P, Carruthers A, Structural basis of GLUT1 inhibition by cytoplasmic ATP, J. General Physiol 130 (2007) 157–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Levine KB, Cloherty EK, Hamill S, Carruthers A, Molecular determinants of sugar transport regulation by ATP, Biochemistry 41 (2002) 12629–12638. [DOI] [PubMed] [Google Scholar]
- [30].Heard KS, Fidyk N, Carruthers A, ATP-dependent substrate occlusion by the human erythrocyte sugar transporter, Biochemistry 39 (2000) 3005–3014. [DOI] [PubMed] [Google Scholar]
- [31].Levine KB, Cloherty EK, Fidyk NJ, Carruthers A, Structural and physiologic determinants of human erythrocyte sugar transport regulation by adenosine triphosphate, Biochemistry 37 (1998) 12221–12232. [DOI] [PubMed] [Google Scholar]
- [32].Sage JM, Cura AJ, Lloyd KP, Carruthers A, Caffeine inhibits glucose transport by binding at the GLUT1 nucleotide-binding site, Am. J. Physiol. Cell Physiol 308 (2015) C827–C834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Ojeda PG, Perez AA, Ojeda L, Vargas-Uribe M, Rivas CI, Salas M, Vera JC, Reyes AM, Non-competitive blocking of human Glut1 Hexose transporter by methylxanthines reveals an exofacial regulatory binding site, Am. J. Physiol. Cell Physiol 303 (2012) C530–C539. [DOI] [PubMed] [Google Scholar]
- [34].Gipson IK, Spurr-Michaud S, Argueso P, Tisdale A, Ng TF, Russo CL, Mucin gene expression in immortalized human corneal-limbal and conjunctival epithelial cell lines, Investig. Ophthalmol. Vis. Sci 44 (2003) 2496–2506. [DOI] [PubMed] [Google Scholar]
- [35].Van Dyke DA, Walters L, Frieswyk D, Kokmeyer D, Louters LL, Acute effects of troglitazone and nitric oxide on glucose uptake in L929 fibroblast cells, Life Sci. 72 (2003) 2321–2327. [DOI] [PubMed] [Google Scholar]
- [36].Loo T, Patchett ML, Norris GE, Lott JS, Using secretion to solve a solubility problem: high-yield expression in Escherichia coli and purification of the bacterial glycoamidase PNGase F, Protein Expr. Purif 24 (2002) 90–98. [DOI] [PubMed] [Google Scholar]
- [37].Liong E, Kong SK, Au KK, Li JY, Xu GY, Lee YL, Kwok TT, Choy YM, Lee CY, Fung KP, Inhibition of glucose uptake and suppression of glucose transporter 1 mRNA expression in L929 cells by tumour necrosis factor-alpha, Life Sci. 65 (1999) PL215–220. [DOI] [PubMed] [Google Scholar]
- [38].Gunnink LK, Alabi OD, Kuiper BD, Gunnink SM, Schuiteman SJ,Strohbehn LE, Hamilton KE, Wrobel KE, Louters LL, Curcumin directly inhibits the transport activity of GLUT1, Biochimie 125 (2016) 179–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Green A, Krause J, Rumberger JM, Curcumin is a direct inhibitor of glucose transport in adipocytes, Phytomed. Int. J. Phytother. Phytopharm 21 (2014) 118–122. [DOI] [PubMed] [Google Scholar]
- [40].Hebert DN, Carruthers A, Glucose transporter oligomeric structure determines transporter function. Reversible redox-dependent interconversions of tetrameric and dimeric GLUT1, J. Biol. Chem 267 (1992) 23829–23838. [PubMed] [Google Scholar]
- [41].Kuipers DP, Scripture JP, Gunnink SM, Salie MJ, Schotanus MP, Ubels JL,Louters LL, Differential regulation of GLUT1 activity in human corneal limbal epithelial cells and fibroblasts, Biochimie 95 (2013) 258–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Sherman MP, Grither WR, McCulla RD, Computational investigation of the reaction mechanisms of nitroxyl and thiols, J. Org. Chem 75 (2010) 4014–4024. [DOI] [PubMed] [Google Scholar]
- [43].Paolocci N, Jackson MI, Lopez BE, Miranda K, Tocchetti CG, Wink DA, Hobbs AJ, Fukuto JM, The pharmacology of nitroxyl (HNO) and its therapeutic potential: not just the Janus face of NO, Pharmacol. Ther 113 (2007) 442–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Fukuto JM, Carrington SJ, HNO signaling mechanisms, Antioxid. Redox Signal 14 (2011) 1649–1657. [DOI] [PubMed] [Google Scholar]