Abstract
Objective
Vaginal transmission is crucial to the spread of HIV-1 around the world. It is not yet clear what type(s) of innate defenses against HIV-1 infection are present in the vagina. Here, we aimed to determine whether human vaginal fluid contains exosomes that may possess anti-HIV-1 activity.
Methods
The exosomal fraction was isolated from samples of vaginal fluids. The presence of exosomes was confirmed by flow cytometry and western blotting. The newly discovered exosomes were tested for their ability to block early steps of HIV-1 infection in vitro using established cell culture systems and real time PCR-based methods.
Results
Vaginal fluid contains exosomes expressing CD9, CD63 and CD81 exosomal markers. The exosomal fraction of the fluid reduced transmission of HIV-1 vectors by 60%, the efficiency of reverse transcription step by 58.4%, and the efficiency of integration by 47%. Exosomes had no effect on the entry of HIV-1 vectors.
Conclusion
Human vaginal fluid exosomes are newly discovered female innate defenses that may protect women against HIV-1 infection.
Introduction
Worldwide, around 16 million women are infected with HIV-1, with the majority of infections occurring through heterosexual contact, with vaginal contact accounting for about half of these transmissions [1, 2]. Consequently, a reduction in vaginal transmission will have a major impact on the reduction of new HIV-1 infections around the world. Although 24 potential vaginal microbicides are currently under development, there is no efficient method to eliminate vaginal transmission [3]. At the same time, data have shown that the pooled risk estimate for anal intercourse is 1.7%, which is at least 5–6 times higher than the risk estimate for vaginal sex [4]. These data suggest that there exist natural barriers to HIV-1 infection in the vaginal environment. Some of these barriers have been studied, but our understanding is yet in its infancy [5–10].
Very recently, HIV-1 infection efficiency was tied to the presence of exosomes in biological fluids [11–13]. Exosomes are nano-vesicles that contain proteins, mRNA, and microRNAs (miRNAs) [14]. Recent studies demonstrated that exosomes are secreted by multiple cell types, and a recent study also demonstrated exosomes in mouse oviduct during estrus [14–19]. It has been shown that semen and breast milk contain exosomes that may inhibit HIV-1 transmission [11–13].
We aimed to determine whether vaginal fluid contains exosomes, and whether these may play a role in HIV-1 infection. Our data support the hypothesis that biological fluid exosomes, in this case those discovered by us, in vaginal fluid, present one of the first lines of defense against HIV-1 infection in women.
Materials and Methods
Vaginal Fluid Samples
Raw, unprocessed frozen vaginal fluid and seminal fluid – non-reactive for HIV-1/HCV/HBV by NAT, HBsAg, HCV Ab, HIV 1&2 Ab and RPR by currently approved FDA methods – was purchased from Lee Biosolutions, Maryland Heights, MO (vaginal fluid cat # 991-10-C).
Exosome Isolation and Quantitation
To isolate vaginal fluid exosomes, we used ExoQuick Exosome Precipitation Solution (EXOQ5A-1) from System Biosciences, LLC, and followed the manufacturer’s instructions. The volume of vaginal fluid (VF) was approximately 700 µl/sample following the initial centrifugation step to remove cells and cell debris. The final exosome containing pellets were resuspended in a PBS volume of 100 µl (1/7 the original volume of fluid).
To confirm our results using an alternative method, exosomes were also isolated using qEV size exclusion column (cat # SP1, iZon Science) following the manufacturer’s instructions. Briefly, Several fractions of the column output were collected, concentrated and tested for its ability to inhibit the vector-mediated transduction of the GFP marker, as described in the Figure 2D. After the sample was added to the column, the first 1.5ml through the column was discarded. The second 1.5ml was collected as a control (as this is predicted to be the second half of the void volume according to the manufacturer’s instructions) and we call this fraction fraction 1. The fraction 2 (the next 1.75ml through the column), is where the exosomes are concentrated as per the qEV column protocol, and their presence was confirmed using the NanoSight NS300 utilizing Nanoparticle Tracking Analysis software (Malvern). The presence of exosomes then decreases in the fraction 3 (the following 1.5ml). Fractions were concentrated using Amicon Ultra-4 10kDa nominal molecular weight centrifugal filter units (cat # UFC501024).
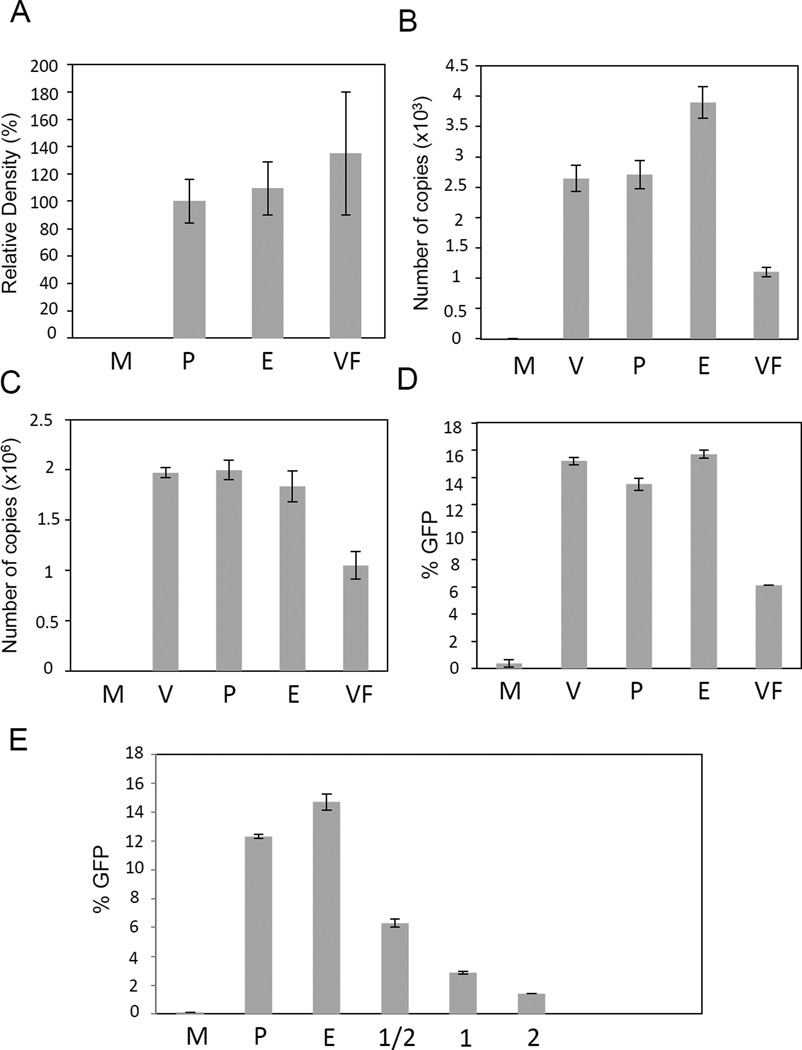
Figure 2.
Analysis of the VFE effect on the steps of the life-cycle of the HIV-1 vector. A. Analysis of the VFE effect on the entry step. This analysis was essentially done as described [12]. Briefly, 293T/17 cells were infected at an m.o.i. of 0.1 with VFE or controls. Cells were then washed and trypsinized to remove extracellular proteins, as described [12]. The intracellular p24 levels were then quantified using western blotting analysis and ImageJ analysis. M – mock-infected cells, P – cells infected with vector and PBS control, E – cells infected with vector and diluted ExoQuick reagent control, VF – cells infected with the vector and VFE. Relative density in %, B. Real-time PCR analysis of total virus DNA copies in infected cells. Cells were infected as described in Methods. One day post-infection DNA was extracted from infected cells and vector DNA copies quantified using real-time PCR. Real-time PCR was performed as described in the Methods. Samples labeled as in A, V – cells infected with vector only. C. Analysis of the integrated virus DNA in VFE-treated cells. Infections were performed as in B. Three days post-infection, genomic DNA was extracted. A nested Alu-PCR technique was conducted as described in the Methods and by us previously [22, 23]. Samples labeled as in B. D. Analysis of the HIV-1 mediated transduction of the GFP marker. Infections were performed as described in Methods. FACS was performed three days post-infection to quantify GFP expression. Samples labeled as in B. Error bars represent standard deviations. E. Repeated analysis of the HIV-1 mediated transduction of the GFP marker in the presence of increasing amounts of VFE. The highest amount is equivalent to double that used in Figure 2D. Samples labeled as in D, except the following: 1 – VFE used at the concentration that was used in Figures 2A–D, ½ - VFE diluted twofold, 2 – concentration of VFE increased twofold in comparison to Figures 2A–2D.
A standard Bradford protein assay was conducted to quantify total protein concentration present in purified exosome samples.
Exosome Characterization
Vaginal fluid exosomes (VFE, isolated as described above) were analyzed for exosomal surface markers by FACS analysis utilizing an Exo-Flow kit from SBI (cat # EXOFLOW150A-1). Following the manufacturer’s instructions, biotinylated capture antibodies (CD9 or CD63, provided with kit, the biotinylated CD81 antibody was not available from the manufacturer) were conjugated to Exo-Flow FACS magnetic streptavidin 9.1 µm beads to allow for the efficient capture of exosomes expressing these surface markers. Then 70 µg exosomes were captured, stained with FITC, and subjected to FACS analysis. As a control, beads were treated with all reagents in the kit, but instead of exosomes, plain PBS was added.
We also performed western blotting to detect the presence of exosomal surface markers. Semen exosome samples were used as a control. Exosome samples were lysed and ca 20 µg of each sample was run on SDS-PAGE, transferred and blotted with exosome marker antibodies CD63 (SBI, Palo Alto, CA, cat # EXOAB-CD63A-1) and CD81 (SBI, Palo Alto, CA, cat # EXOAB-CD81A-1). As a negative and a purity control, filters were blotted with the ER-derived marker calnexin (Santa Cruz Biotechnology, Santa Cruz, CA, cat # sc-11397).
Cells and HIV-1 vector
293T/17 cells were purchased from ATCC. VSV G-pseudotyped HIV-based vector was described previously [20, 21]. Vector DNA was obtained from Addgene (cat # 8455, 8454, and 19319).
Infections and quantitative Analysis of viral entry
This analysis was done following established protocols [13]. Briefly, 293T/17 cells were infected in the presence of either VFE or controls. To prepare for infection, 50 or 90 µl of vaginal fluid exosomal fraction (corresponding to ca. 170 µg of protein, which was isolated from an equivalent of 630 µl of vaginal fluid following removal of cellular debris) or controls (PBS, or the ExoQuick reagent diluted in PBS) were mixed with the GFP-carrying HIV-1-based vector (see above) in a total volume of 0.6 ml with 6 µg DEAE-dextran. After exosome/vector incubation at 37C for one hour, mixtures were applied to cells (plated the day before at a density of 105 cells per well in 12 well plates) for a six-hour infection. For the quantitation experiment in Supplementary Figure 1, the experiment was scaled down for infections in 48 well plates. To quantify the intracellular p24 levels, six hours post-infection cells were harvested and lysed and equivalent amounts of lysate of each sample were then subjected to western blotting analysis with the p24 antibody (Abcam cat # ab63913). Xray images (p24 bands) were then quantified using the ImageJ software (https://imagej.nih.gov/ij).
Real-time PCR analysis of vector DNA copies in infected cells and Alu-PCR
These experiments were performed as described previously [22, 23].
Transmission efficiency
Cells were assayed 3–5 days post infection by fluorescence-activated cell sorting (FACS) using a BD LSRII to quantify GFP reporter gene expression.
Results
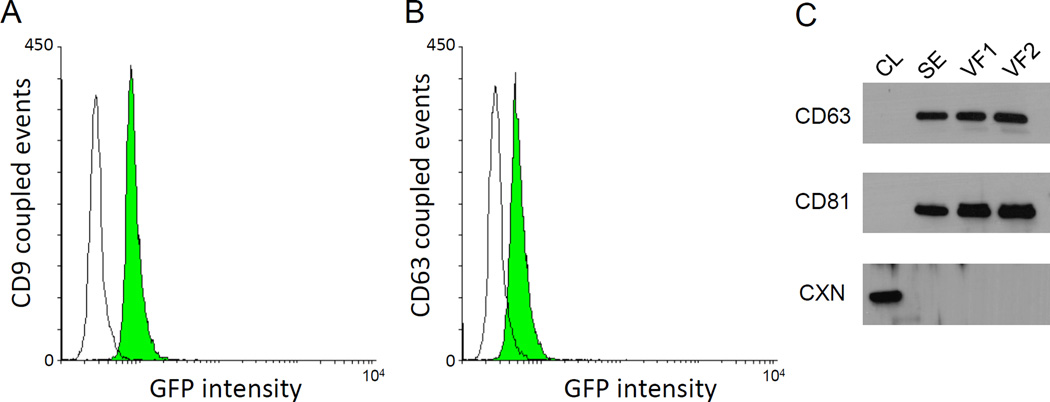
We have isolated the exosomal fraction of human vaginal fluid (VF) samples as described in Methods and [24]. The exosomal fraction was then examined using both flow cytometry and western blotting analysis for the presence of the tetraspanin proteins CD9, CD63, and CD81, which are established exosome markers [25]. As shown in Figure 1A and 1B, the exosomal fraction contains particles containing the human exosomal markers CD9 and CD63 proteins on their surface. These results were confirmed by western blotting, which also showed the presence of CD81 in the exosomal fraction (Figure 1C). In contrast, the samples are devoid of the presence of the endoplasmic reticulum-derived marker calnexin, which would otherwise indicate contamination with cells and cell debris (Figure 1C).
Figure 1.
Analysis of the exosomal fraction of human vaginal fluid (VF). A and B. Detection of exosomal markers on the surface of particles isolated from VF by flow cytometry analysis. Exosomes were purified from VF as described in the Methods, and surface markers were detected using an Exo-Flow Kit from SBI. Biotinylated capture antibodies CD9 (Figure 1A) or CD63 (Figure 1B) were conjugated to streptavidin beads to allow for the efficient capture of exosomes expressing these surface markers. As a control, beads were treated with all reagents in the kit, but instead of exosomes, plain PBS was added. A shift in GFP intensity was observed when VFE were captured with the CD9 antibody (A., green histogram) and with the CD63 antibody (B., green histogram) when compared to PBS controls (white histograms). C. Detection of exosomal markers in the exosomal fraction of VF using western blotting analysis. Exosomes were purified from VF as described in the Methods. As a control, semen samples, which are known to contain exosomes, were also purchased from Lee Biosolutions and exosomes isolated the same way. Samples were lysed and ca 20 µg of each sample was run on the SDS-PAGE, transferred and blotted with exosome marker antibodies CD63 and CD81. As a negative and a purity control, filters were blotted with the ER-derived marker calnexin, which should be present only in cell lysates, and not the exosomal fraction. CL – cell lysate from 293T cells, SE – semen exosomal fraction, VF1 – VF exosomal fraction (sample 1), VF2 –VF exosomal fraction (sample 2, independent purification), CD63 – CD63 protein, CD81 – CD 81 protein, CXN - calnexin.
The exosomal fraction (now termed vaginal fluid exosomes, or VFE) was then evaluated for its ability to affect HIV-1 infection using HIV-1 replication-defective vectors. We have first examined the VFE effect on the entry of the vector particles into cells. As shown in Figure 2A, the efficiency of HIV-1 vector entry into target cells was not significantly different in the presence or absence of VFE, as evaluated by the presence of intracellular p24. We then examined the levels of total viral DNA and integrated viral DNA using our established quantitative real-time PCR assays. We observed that both integrated viral DNA and total viral DNA were decreased by 47% and 58.4% respectively, when compared to controls (Figure 2B and 2C). These latter results correlated with an overall drop in transduction efficiency (Figure 2D). Similar results were obtained with four independently isolated samples of VF, in corresponding independent experiments, and also with semen exosomes (Figure 2E and not shown). We note that in the Figure 2E, we have used increasing amounts of exosomes (increasing by factor 2). We note that at the highest concentration of VFE (column 2, which contains twice as much VFE as used in experiments described in Figure 2A–2D), we have observed that VFE have a cytotoxic effect. We have not observed this effect at lower concentrations of VFE. To determine if our findings were cell type-specific, we also examined VFE effects on HIV-1 transmission in Jurkat Clone E6-1 T cells. We observed that the presence of VFE resulted in a similar reduction of HIV-1 transmission in these cells (data not shown). The latter results are consistent with those obtained on 293T cells (Figure 2D and 2E). One possible issue with VFE is the purity of the exosomes. To purify VFE, we have used the ExoQuick method, which provides high exosome yields, but these also contain significant amounts of impurities [26]. To address this issue, we have employed an alternative purification method, which employs size exclusion columns [26]. We note that this method was demonstrated to yield exosomes of purity higher than the ExoQuick or even density gradient methods [26]. As shown in the Supplementary Figure 1A, we have observed that VFE that were purified by the size exclusion column-based method suppress the vector transmission by 40% compare to negative controls, which is consistent with the ExoQuick results (Figure 2D). In addition, we have observed comparable results when we used this method to purify semen exosomes (Supplementary Figure 1B).
Discussion
HIV-1 infection is a complex process that involves extensive intercellular communications. Very recently, new data appeared to suggest that this intercellular communication network might involve a new component, the exosome [11–13]. Very recent results by other laboratories have shown that human semen and breast milk contain exosomes that have inhibitory effect on HIV-1 replication. The results of our experiments suggest that HIV-1-inhibiting exosomes are also present in the human vagina.
Our results, obtained with replication-defective virus, suggest that VFE blocked a post-entry step, most likely reverse transcription. The observed decrease in the integration efficiency is likely due to the decrease in reverse transcription. We did not observe any effect of exosomes on the entry step of the vector. However, we note that our HIV-1 vector is pseudotyped with the VSV G envelope, and we thus cannot exclude the possibility that VFE also block the entry of wild type HIV-1 virus. We also note that semen exosomes appear to inhibit HIV-1 infection at the reverse transcription step [11, 12]. Extensive analysis will be needed to determine whether VFE represent a species of exosomes different from semen exosomes or breast milk exosomes or whether they represent a common defense against HIV-1 infection.
Finally, vaginal transmission plays a crucial role in the spread of HIV-1. Consequently, a number of vaginal microbicides are under development. The presence of vaginal natural defenses, such as VFE, suggests that these new drugs may need to be tested for their ability to synergize with VFE.
Supplementary Material
Effect of VFE and semen exosomes that were purified by an alternative method (using qEV size exclusion columns from iZon Science), on the HIV-1 vector-mediated transduction. Following the removal of cellular debris by centrifugation, VFE and semen exosomes were purified with qEV columns as per the manufacturer’s instructions. Several fractions of the column output were collected, concentrated (see Methods), and tested for its ability to inhibit the vector-mediated transduction of the GFP marker, as described in the Figure 2D. Exosomes are concentrated in the fraction 2 (see Methods). A. Analysis of the VFE effect. Infections were performed as described above. FACS was performed five days post-infection to quantify GFP expression. M – mock-infected cells, P – cells infected with vector and PBS control, 1 – cells infected with vector in the presence of the fraction 1, 2 – cells infected with vector in the presence of the fraction 2, 3 – cells infected with vector in the presence of the fraction 3. B. Analysis of the effect of semen exosomes. Infection and FACS were performed as in A. Samples were labeled as in A.
Acknowledgments
Funding/Support: This study was supported by the NIH R21AI102684 grant to R.D.
Footnotes
Conflicts of interest
The authors have no commercial or other association that might pose conflicts of interest.
Author’s Contributions: All experiments were performed by J.S., within the exception of the western blotting analysis, which was performed by R.D. The study design: R.D. Manuscript was written by both J.S. and R.D.
References
- 1. http://www.who.int/gho/hiv/epidemic_status/cases_adults_women_children/en/. In.
- 2.Quinn TC, Wawer MJ, Sewankambo N, Serwadda D, Li C, Wabwire-Mangen F, et al. Viral load and heterosexual transmission of human immunodeficiency virus type 1. Rakai Project Study Group. N Engl J Med. 2000;342:921–929. doi: 10.1056/NEJM200003303421303. [DOI] [PubMed] [Google Scholar]
- 3.Antimisiaris SG, Mourtas S. Recent advances on anti-HIV vaginal delivery systems development. Adv Drug Deliv Rev. 2015;92:123–145. doi: 10.1016/j.addr.2015.03.015. [DOI] [PubMed] [Google Scholar]
- 4.Boily MC, Baggaley RF, Wang L, Masse B, White RG, Hayes RJ, et al. Heterosexual risk of HIV-1 infection per sexual act: systematic review and meta-analysis of observational studies. Lancet Infect Dis. 2009;9:118–129. doi: 10.1016/S1473-3099(09)70021-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Allen SA, Carias AM, Anderson MR, Okocha EA, Benning L, McRaven MD, et al. Characterization of the Influence of Semen-Derived Enhancer of Virus Infection on the Interaction of HIV-1 with Female Reproductive Tract Tissues. J Virol. 2015;89:5569–5580. doi: 10.1128/JVI.00309-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Archary D, Liebenberg LJ, Werner L, Tulsi S, Majola N, Naicker N, et al. Randomized Cross-Sectional Study to Compare HIV-1 Specific Antibody and Cytokine Concentrations in Female Genital Secretions Obtained by Menstrual Cup and Cervicovaginal Lavage. PLoS One. 2015;10:e0131906. doi: 10.1371/journal.pone.0131906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fuller DH, Richert-Spuhler LE, Klatt NR. HIV vaccine trial exploits a dual and central role for innate immunity. J Virol. 2014;88:11640–11643. doi: 10.1128/JVI.02140-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li Q, Zeng M, Duan L, Voss JE, Smith AJ, Pambuccian S, et al. Live simian immunodeficiency virus vaccine correlate of protection: local antibody production and concentration on the path of virus entry. J Immunol. 2014;193:3113–3125. doi: 10.4049/jimmunol.1400820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Politch JA, Marathe J, Anderson DJ. Characteristics and quantities of HIV host cells in human genital tract secretions. J Infect Dis. 2014;210(Suppl 3):S609–S615. doi: 10.1093/infdis/jiu390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smith JD, Garber GE. Trichomonas vaginalis infection induces vaginal CD4+ T-cell infiltration in a mouse model: a vaccine strategy to reduce vaginal infection and HIV transmission. J Infect Dis. 2015;212:285–293. doi: 10.1093/infdis/jiv036. [DOI] [PubMed] [Google Scholar]
- 11.Madison MN, Jones PH, Okeoma CM. Exosomes in human semen restrict HIV-1 transmission by vaginal cells and block intravaginal replication of LP-BM5 murine AIDS virus complex. Virology. 2015;482:189–201. doi: 10.1016/j.virol.2015.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Madison MN, Roller RJ, Okeoma CM. Human semen contains exosomes with potent anti-HIV-1 activity. Retrovirology. 2014;11:102. doi: 10.1186/s12977-014-0102-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Naslund TI, Paquin-Proulx D, Paredes PT, Vallhov H, Sandberg JK, Gabrielsson S. Exosomes from breast milk inhibit HIV-1 infection of dendritic cells and subsequent viral transfer to CD4+ T cells. AIDS. 2014;28:171–180. doi: 10.1097/QAD.0000000000000159. [DOI] [PubMed] [Google Scholar]
- 14.Tang MK, Wong AS. Exosomes: Emerging biomarkers and targets for ovarian cancer. Cancer Lett. 2015;367:26–33. doi: 10.1016/j.canlet.2015.07.014. [DOI] [PubMed] [Google Scholar]
- 15.Melo SA, Sugimoto H, O'Connell JT, Kato N, Villanueva A, Vidal A, et al. Cancer exosomes perform cell-independent microRNA biogenesis and promote tumorigenesis. Cancer Cell. 2014;26:707–721. doi: 10.1016/j.ccell.2014.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Teng X, Chen L, Chen W, Yang J, Yang Z, Shen Z. Mesenchymal Stem Cell-Derived Exosomes Improve the Microenvironment of Infarcted Myocardium Contributing to Angiogenesis and Anti-Inflammation. Cell Physiol Biochem. 2015;37:2415–2424. doi: 10.1159/000438594. [DOI] [PubMed] [Google Scholar]
- 17.Tietje A, Maron KN, Wei Y, Feliciano DM. Cerebrospinal fluid extracellular vesicles undergo age dependent declines and contain known and novel non-coding RNAs. PLoS One. 2014;9:e113116. doi: 10.1371/journal.pone.0113116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang J, Wei F, Schafer C, Wong DT. Detection of tumor cell-specific mRNA and protein in exosome-like microvesicles from blood and saliva. PLoS One. 2014;9:e110641. doi: 10.1371/journal.pone.0110641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Al-Dossary AA, Strehler EE, Martin-Deleon PA. Expression and secretion of plasma membrane Ca2+-ATPase 4a (PMCA4a) during murine estrus: association with oviductal exosomes and uptake in sperm. PLoS One. 2013;8:e80181. doi: 10.1371/journal.pone.0080181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Daniel R, Greger JG, Katz RA, Taganov KD, Wu X, Kappes JC, et al. Evidence that stable retroviral transduction and cell survival following DNA integration depend on components of the nonhomologous end joining repair pathway. J Virol. 2004;78:8573–8581. doi: 10.1128/JVI.78.16.8573-8581.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Naldini L, Blomer U, Gallay P, Ory D, Mulligan R, Gage FH, et al. In vivo gene delivery and stable transduction of nondividing cells by a lentiviral vector. Science. 1996;272:263–267. doi: 10.1126/science.272.5259.263. [DOI] [PubMed] [Google Scholar]
- 22.Smith JA, Yeung J, Kao GD, Daniel R. A role for the histone deacetylase HDAC4 in the life-cycle of HIV-1-based vectors. Virol J. 2010;7:237. doi: 10.1186/1743-422X-7-237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smith JA, Daniel R. Up-regulation of HIV-1 transduction in nondividing cells by double-strand DNA break-inducing agents. Biotechnol Lett. 2011;33:243–252. doi: 10.1007/s10529-010-0449-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. https://www.systembio.com/microrna-research/exoquick-exosomes/overview?gclid=CI_zhuewhMoCFQsjHwod7bYL8g. [Google Scholar]
- 25.Thery C, Zitvogel L, Amigorena S. Exosomes: composition, biogenesis and function. Nat Rev Immunol. 2002;2:569–579. doi: 10.1038/nri855. [DOI] [PubMed] [Google Scholar]
- 26.Lobb RJ, Becker M, Wen SW, Wong CS, Wiegmans AP, Leimgruber A, et al. Optimized exosome isolation protocol for cell culture supernatant and human plasma. J Extracell Vesicles. 2015;4:27031. doi: 10.3402/jev.v4.27031. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Effect of VFE and semen exosomes that were purified by an alternative method (using qEV size exclusion columns from iZon Science), on the HIV-1 vector-mediated transduction. Following the removal of cellular debris by centrifugation, VFE and semen exosomes were purified with qEV columns as per the manufacturer’s instructions. Several fractions of the column output were collected, concentrated (see Methods), and tested for its ability to inhibit the vector-mediated transduction of the GFP marker, as described in the Figure 2D. Exosomes are concentrated in the fraction 2 (see Methods). A. Analysis of the VFE effect. Infections were performed as described above. FACS was performed five days post-infection to quantify GFP expression. M – mock-infected cells, P – cells infected with vector and PBS control, 1 – cells infected with vector in the presence of the fraction 1, 2 – cells infected with vector in the presence of the fraction 2, 3 – cells infected with vector in the presence of the fraction 3. B. Analysis of the effect of semen exosomes. Infection and FACS were performed as in A. Samples were labeled as in A.