Abstract
The kappa opioid receptor (KOR) plays a role in stress responsivity, opiate withdrawal and responses to cocaine. KOR activation by its endogenous ligand dynorphin A(1–17) decreases basal and drug-induced striatal levels of dopamine. The complete structure of the human KOR gene (hOPRK1) has not been previously determined. This study:(i) characterized the genomic structure of the hOPRK1 gene; (ii) identified single nucleotide polymorphisms (SNPs) in the hOPRK1 gene; and (iii) investigated possible associations of these variants with vulnerability to develop heroin addiction. Analysis of 5’-RACE cDNA clones revealed the presence of a novel exon 1 ranging in length from 167 to 251 nucleotides in the 5’ 5’-untranslated region of the hOPRK1 mRNA. We found that the hOPRK1 gene has four major exons and three introns, similar to rodent OPRK1 genes. Direct sequencing of amplified DNA containing all four exons and intron 1 of the hOPRK1 gene were evaluated for polymorphisms in 291 subjects (145 former heroin addicts and 146 controls). Twelve SNPs were identified, nine novel variants and three previously reported SNPs. Using logistic regression with opioid dependence as the dependent variable, the 36G>T SNP exhibited a point-wise significant association (P 0.016) with disease status. The number of haplotypes seen in the three ethnic groups were nine, six and five for African-Americans, Caucasians, and Hispanics, respectively, with corresponding significance levels for differences in haplotype frequencies between cases and controls of P 0.0742, 0.1015 and 0.0041. Combining ethnicities by Fisher’s method yields an empirical significance level of P 0.0020. Pharmacogenetics 14:793–804
Keywords: exon/intron organization, haplotype, human kappa opioid receptor, linkage disequilibrium, opioid dependence, single nucleotide polymorphism, 5’-untranslated region
Introduction
Individual variability to develop drug addictions has both an environmental and a heritable basis. Studies from our laboratory and others have shown that the endogenous opioid system plays a major role in addiction to opioids, cocaine and alcohol [1,2]. The effects of endogenous opioid peptides and exogenous opioid drugs are mediated by binding to one or more of three distinct opioid receptors: the mu opioid receptor (MOR), the delta opioid receptor and the kappa opioid receptor (KOR), each of which is encoded by a separate gene.
Because the human MOR is the primary site of action for heroin and its biotransformation products, including morphine, and MOR mediates the analgesic and rewarding effects of opiates, genetic variations of this receptor and their association with specific addictions have been studied in several populations [3,4]. Existing data suggest that the KOR, when activated by the endogenous ligand dynorphin A(1–17), plays a modula-tory role in opioid, cocaine and other rewarding stimuli, presumably through modulation of basal and drug-induced dopaminergic tone [5].
The ability of dynorphin peptides to modulate dopaminergic tone in humans has been demonstrated in studies conducted in normal volunteers and also in methadone-maintained former heroin addicts. These studies have measured prolactin release, which is under tonic inhibition by dopamine in the tuberoinfundibular region of the hypothalamus. Administration of dynorphin A(1–13), a shortened form of the natural peptide dynorphin A(1–17), to humans leads to an abrupt rise in serum prolactin levels caused by a lower dopaminergic tone through activation of KOR [6]. Of interest, prolactin response to dynorphin A(1–13) was significantly attenuated in methadone (μ-opioid agonist)-maintained volunteers compared to control subjects, suggesting either alterations in the KOR system or, more likely, a lowering of tuberoinfundibular dopaminergic tone in former heroin addicts [7]. Clinical studies have shown that dynorphin A(1–13) attenuates opioid-withdrawal symptoms in humans [8] and augments morphine analgesia in patients with chronic pain [9]. In nonhuman primates, it has been demonstrated that dynorphin A(1–17), but not its non-opioid biotransformation fragment, dynorphin A(2–17), is potent in increasing serum prolactin levels [10].
Studies in rats and mice have shown that an infusion of either synthetic KOR ligands or dynorphin A(1–17) to the striatum reduces basal as well as cocaine-induced levels of dopamine [11–14]. This reduction in dopaminergic tone may be part of the mechanism by which synthetic kappa agonists reduce cocaine reward in rodents [14,15] and primates [16]. Coadministration of synthetic KOR ligands to the striatum of rats [14,17], or dynorphin A(1–17) in mice [13], blocks cocaine-induced conditioned place preference. At the neurochemical level, various schedules of cocaine administration, including acute [18], repeated [19], ‘binge’ pattern [20,21] and self-administration [22], result in increased preprodynorphin mRNA levels. By contrast, only acute, but not repeated, morphine administration led to an elevation of preprodynorphin mRNA in the rat striatum [23]. We hypothesized that dynorphin, acting on the KOR, opposes the effects of cocaine and morphine on reward mechanisms [5] by its established effects of both reducing basal dopamine levels and blocking or attenuating drug-induced elevation in basal dopamine levels [12,13,24]. Importantly, increased levels of preprodynorphin mRNA have been observed in the striatum of human cocaine addicts post mortem [25].
Allelic variations in the promoter region of the preprodynorphin gene have recently been associated with differences in individual vulnerability to develop cocaine addiction [26]. Because KOR is activated by dynorphin peptides, we hypothesize that allelic variations in the OPRK1 gene may alter function or expression of the receptor, which may contribute to individual differences in vulnerability to develop opioid and cocaine addiction.
The human OPRK1 gene is located on chromosome 8q11.2 [27]. By contrast to the well-defined mouse and rat OPRK1 genes [28], the complete exon/intron structure of the 5’-untranslated region (UTR) and transcription initiation sites of the hOPRK1 gene have not been previously reported. Although both the mouse and rat OPRK1 genes contain four exons, only three exons for the hOPRK1 gene have been reported [29–31]. In addition to three exons encoding the KOR protein sequence in the human OPRK1 gene, which are similar in human, mouse and rat, the mouse and rat OPRK1 genes have another exon that encodes most of the 59-UTR [32,33]. In mice, determination of exon/intron structure of the 5’-region of the OPRK1 gene and identification of the transcription initiation sites allowed characterization of a number of functional transcription regulatory elements in the promoter region and in the first intron, between exon 1 and the ATG-containing exon 2 [28,32]. A lack of knowledge of the promoter regions in the hOPRK1 gene has precluded studies that identify and determine the functional significance of SNPs in these regions.
The goals of this study were three-fold. The first goal was to identify the major transcription initiation sites of the hOPRK1 gene using the technique of rapid amplification of cDNA ends (5’- and 3’-RACE) and to characterize the genomic structure of the OPRK1 mRNA in the 5’- and 3’-untranslated regions. The second goal was to identify and characterize sequence variations in the hOPRK1 in all exons, intron 1 and flanking regions of exons. The third goal was to investigate possible associations of any of these variants with opioid addiction.
Materials and methods
Study subjects
A total of 291 subjects (168 men and 123 women) in this study were consecutive volunteers meeting the inclusion criteria, as defined below, drawn from subjects participating in ongoing genetics studies conducted by the Laboratory of the Biology of Addictive Diseases at The Rockefeller University. Subjects participating in the present study were unrelated individuals recruited from several clinical resources in New York City, or through newspaper advertisements, posted notices or referrals. All subjects entered our study between 17 July 1997 and 29 November 1999. Each subject provided their written informed consent, approved by The Rockefeller University Hospital Institutional Review Board. Table 1 summarizes the demographic and drug dependence status of the 291 subjects included in the study. Opioid-dependent subjects were former long-term heroin addicts participating in methadone maintenance treatment. Criteria for inclusion of opioid-dependent subjects (n = 145) in this study were 1 yearor more of daily multiple dose self-administration of heroin, the development of dependence and tolerance, and demonstration of drug-seeking behaviour. All subjects in the control group (n = 146) had no history of previous or current illicit drug abuse or dependence (excluding cannabis), or alcohol abuse or dependence. Additional exclusion criteria were as follows: subjects undergoing clinical management of chronic pain or subjects who had one instance of drinking to intoxication during the previous 30 days. Use of nicotine or caffeine was not included in the exclusion criteria. Toxicological urine analyses were performed for multiple drugs of abuse for all subjects. Blood specimens for DNA extraction were obtained by venipuncture (see below). Subjects included in this study were classified as African-American, Caucasian, Hispanic, Asian-American or Other, based on detailed information provided by the subjects regarding family origin and ethnic background.
Table 1.
Demography and categorization of study subjects
| Controls | Dependent | Total | |
|---|---|---|---|
| Gender | |||
| Female | 68 | 55 | 123 |
| Male | 78 | 90 | 168 |
| Total | 146 | 145 | 291 |
| Ethnicity | |||
| Caucasian | 64 | 65 | 129 |
| African-American | 34 | 36 | 70 |
| Hispanic | 25 | 35 | 60 |
| Asian-American | 18 | 1 | 19 |
| Caucasian-American/Hispanic | 3 | 5 | 8 |
| African-American/Hispanic | 2 | 1 | 3 |
| African-American/Native American | 0 | 1 | 1 |
| Asian-American/Caucasian | 0 | 1 | 1 |
| Total | 146 | 145 | 291 |
5’ and 3’-RACE
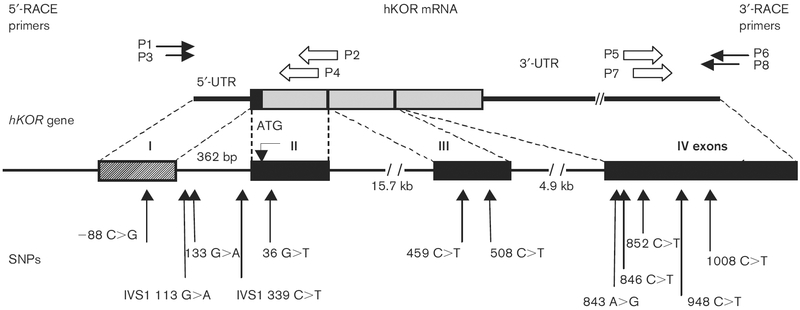
To identify transcription initiation sites of the hOPRK1 gene, the FirstChoice RACE-Ready cDNA Kit (Ambion, Austin, Texas, USA) was employed according to the manufacturer’s protocol. The kit includes cDNAs, with an adaptor at the 5’-end, reverse transcribed from full-length capped poly(A) RNA isolated from human brain. The cDNA was polymerase chain reaction (PCR) amplified with the 5’-RACE outer primer P1 and hOPRK1-specific primer P2 5’-GGGATGGCCGGG GAGAT-3’ (complementary to nucleotides 160–176 relative to ATG codon, GenBank Accession number U17298), and subsequently amplified with the 5’-RACE inner primer P3 and hOPRK1-specific primer P4 5’-GGGAAACCAGGCGCTGCTGTT-3’ (complementary to nucleotides 72–93) (Fig. 1). PCR was performed using Platinum Taq PCRx DNA Polymerase Kit (Invitrogen, Carlsbad, California, USA) designed for amplification of GC-rich DNA templates. The PCR was performed by incubation at 94°C for 3 min, followed by 35 cycles of 94°C for 45 s, 58°C for 30 s, 72°C for 90 s, followed by a final step at 72°C for 7 min. The 5’-RACE DNA products were separated using electrophoresis in a 1% agarose gel. DNA fragments were excised and extracted from the gel using QIAquick Gel Extraction Kit (Qiagen, Valencia, California, USA). The isolated DNA fragments were then cloned into the pCRII vector (Invitrogen).
Fig. 1.
Exon/intron organization of the 5’-UTR of the human OPRK1. The novel exon 1 identified is shown as striped. The human OPRK1-specific primers P2 and P4 were used in 5’-RACE PCR, and P5 and P7 were used in 3’-RACE PCR. Primers P3/P4 were used for PCR amplification and sequencing of exon 2 and its flanking regions. Exons and introns have been numbered to reflect this novel structure of the hOPRK1 gene.
To identify the 3’-terminus of the hOPRK1 mRNA, two rounds of PCR amplification of 3’-RACE Ready cDNA (Ambion) were performed with the hOPRK1-specific primer P5 and the 3’-RACE outer primer P6. A second nested PCR amplification was performed with the hOPRK1-specific primer P7 and the 3’-RACE inner primer P8. The cDNA template was synthesized from human poly(A) RNA primed with an anchored poly(dT) adapter-primer. Primers P5 5’-GGGACCAC ATTGCCATTTATTC-3’ and P7 5’-CAAACCCCT CTGGCTCTCTGA-3’ were located at nucleotides 2660–2681 and 2706–2726 in the sequence of a clone of human chromosome 8 (GenBank Accession number AK023198). PCR amplification, purification of PCR DNA and cloning were performed as described above.
The 5’- and 3’-RACE clones were sequenced at the DNA Sequencing Resource Center of The Rockefeller University using the Big Dye Terminator Cycle Sequencing Kit (ABI, Applied Biosystems, Foster City, California, USA) and an ABI Prism 3700 capillary sequencer. The 5’- and 3’-RACE clones were sequenced with M13 forward and M13 reverse primers. The first base following the 3’-end of the RNA adapter oligonucleotide in the 59-RACE clones corresponds, theoretically, to a transcription start site. A nucleotide in the 3’-UTR sequence at the junction with the anchored poly(dT) adapter is assumed to be a 3’-terminus of human OPRK1 mRNA.
PCR amplification and DNA sequencing of the hOPRK1 intron 1 and exons
DNA was extracted from blood specimens using a salting-out procedure followed by ethanol precipitation and stored at −80°C. Five sets of primers for amplification and sequencing of four exons and intron 1 of the hOPRK1 are shown in Table 2. PCR was performed using the Platinum Taq PCRx DNA Polymerase Kit (Invitrogen) as described above. The size of the PCR products was confirmed on a 1% agarose gel. Amplified PCR fragments were purified using the QIAquick PCR Purification Kit (Qiagen) and subsequently sequenced at the Rockefeller University DNA Sequencing Resource Center. Amplified DNA fragments were sequenced in forward and reverse directions, and the resulting electropherograms were aligned and compared to the prototype sequence using Seqman DNAStar software (DNAStar, Madison, Wisconsin, USA). Geno-types were determined by examination of electropherograms by two investigators blind to the sample’s clinical phenotype.
Table 2.
Oligonucleotide primers used for polymerase chain reaction (PCR) amplification and sequencing of the human KOR
| Region | Primers | Position | Sequence | |
|---|---|---|---|---|
| Exon 1 and intron 1 | F1–1 | −885a | PCR | 5′-ATCTAAATGGCTCAGTCTCAGGT-3′ |
| F1–2 | −613a | Sequence | 5′-CAGAGCCGCCGCCAGTAG-3′ | |
| R1 | −166a | PCR | 5′-TGCGGGAGCGAAAGAACC-3′ | |
| R1–2 | −50a | Sequence | 5′-CAGGACAGGGAGAACGGACTT-3′ | |
| Intron 1 and exon 2 | F2 | −200a | PCR/Seq | 5′-CGGAAAGGCAGCGAGAAGT-3′ |
| R2 | +303b | PCR/Seq | 5′-CTTGCCCTGCGCATAGAGTT-3′ | |
| Exon 3 | F3 | −78c | PCR/Seq | 5′-AAAGGCTATCACAAACACATTCA-3′ |
| R3 | +78b | PCR/Seq | 5′-GCCTACTCACGCTCAAATTAAAA-3′ | |
| Exon 4 | F4 | −61c | PCR/Seq | 5′-TGCAGCTCCACGGTAATAACAA-3′ |
| R4 | +69d | PCR/Seq | 5′-TCAGACTGCAGTAGTGATC-3′ |
Statistical analysis
Deviations from Hardy–Weinberg equilibrium were assessed using the chi-square test. We tested for differences in allele and genotype frequencies among ethnic groups (African-American, Hispanic and Caucasian) in the controls. In addition, with data stratified by ethnicity, we tested for differences in allele and genotype, and haplotype frequencies between opioid-dependent and control groups. Effects of the eight SNPs with allelic frequencies greater than 0.01 were taken into account in two ways: (i) with a logistic regression on the genotypic level and (ii) with haplo-type analysis on the allelic level. For the logistic regression, disease category was the dependent variable, and SNP genotypes and ethnic groups were treated as dummy input variables. Genotype and allele frequencies were compared between case and control individuals for the 36G>T SNP with the likelihood ratio chi-square statistic. The snphap program [34] was used to estimate haplotype frequencies separately for case and control individuals for the eight SNPs with greater than 0.01 allelic frequency, and tests for differences in haplotype frequencies were carried out. Results were evaluated individually for point-wise significance (P < 0.05). Subsequently, results were corrected for multiple testing and were evaluated for experiment-wise significance [35]. Measures of linkage disequilibrium, Δ2 and D’, were calculated using the method implemented in the GOLD software package[36].
Results
Exon/intron structure of the human OPRK1 gene
The 5’-ends of the hOPRK1 mRNA, which correspond to its presumed transcription initiation sites, were determined by amplification, cloning, and sequencing of the FirstChoice RACE-Ready cDNA, prepared by the RNA ligase-mediated 5’-RACE method using mRNA isolated from human brain. A feature of this method is that the RNA adaptor is specifically ligated to the capped end of mRNAs before PCR amplification, thus allowing only those RNAs with capped 5’ ends to be amplified. After nested PCR, using the forward outer P1 and inner 5’-RACE P3 primers and two reverse hOPRK1-specific primers P2 and P4, gel electrophoresis revealed DNA products in the range of 350–450 nucleotides. DNA was purified by excision from the gel and subsequently cloned into the pCRII vector. Sequence analysis of the 5’-RACE cDNA clones showed that the 5’-terminus in 14 out of 20 sequenced clones was located at 238 nucleotides, five clones started at 215 nucleotides, and one clone started at 299 nucleotides upstream of the ATG codon in the hOPRK1 mRNA. These results revealed the length of the 5’-untranslated region of the hOPRK1 mRNA (Fig. 2a). Our data indicate that the hOPRK1 gene has at least three transcription initiation sites. Comparison of the 5’-untranslated region of the hOPRK1 mRNA in these clones with the genomic sequence revealed the presence of a novel exon 1 of 167, 190 or 251 nucleotides (depending on which transcription initiation site was utilized). A 362-nucleotide intron, IVS1, separates exon 1 and the ATG-containing exon 2 (Fig. 2). In this study, all exons and introns shown in Fig. 1 have been renumbered to reflect the novel exon 1 (Fig. 1). Furthermore, the ATG-containing exon 2 has 48 nucleotides of 5’-untranslated sequence and, thus, the total size of exon 2 is 305 nucleotides. Exon 3 has been reported to contain 353 nucleotides [29–31].
Fig. 2.
(a) Nucleotide sequence of 5’-UTR and relative positions of 5’-ends of the human OPRK1 cDNA-RACE clones. Positions of the human OPRK1 exon 1, intron 1, and exon 2 (partial) are shown in comparison with the exon 1/intron 1/exon 2 structure of the mouse OPRK1 gene [32,41]. The exonic regions are in uppercase. The ATG translation initiation codons in the human and mouse OPRK1 mRNA are underlined. The positions of three major transcription start sites are designated by ▼ The location of the SNPs are designated in bold and marked with an asterisk. The functional transcription binding sites for murine Ikaros-1 and E-box are shown as boxed nucleotides [28]. Alignment of human and mouse OPRK1 gene sequences was created using the Multiz program at the UCSC website [52] (www.genome.ucsc). (b) The 3’-end and partial sequence of 3’-UTR (capital letters) of the human OPRK1 with downstream genomic sequence (lower case letters) are shown in comparison with the 3’-end of the mouse OPRK1 mRNA [38] (GenBank Accession number AF490606). Potential polyadenylation sites are shown in bold. Numeration in the sequences designates position of nucleotides by starting from the first nucleotide followed the TGA codon. The sequence of the novel 5’-UTR of the OPRK1 mRNA has been deposited in GenBank (accession number AY466378).
Exon 1/intron 1 boundaries conform to the GT-AG rule. Positions of exon 1/intron 1 and intron 1/exon 2 boundaries in the hOPRK1 gene are similar to those found in the mouse [32] and rat [33] OPRK1 genes. Intron 1 is a G + C-rich region (68%). Sequence analysis of this region using the TRANSFAC database [37] revealed a number of potential binding sites for transcription factors (e.g. AP-1, AP-2, SP-1, GR and Ik-1). Thus, the exon/intron structure of the hOPRK1 gene described herein is similar to that of the mouse and rat OPRK1 genes, consisting of at least four exons. Accordingly, the previously reported ATG-containing exon 1 of the hOPRK1 has been reassigned as exon 2.
Sequence analysis of five clones of 3’-RACE PCR products indicated that the 3’-UTR of human OPRK1 mRNA is 3096 nucleotides in length (Fig. 2b). The length of the identified 3’-UTR is similar to that reported for the 3’-UTR of the mouse OPRK1 gene, 3088 nucleotides [38]. Therefore, exon 4 contains 3621 nucleotides, of which 533 nucleotides are coding (including the termination codon).
Polymorphism identification
By direct sequencing of PCR-amplified fragments containing exon 1, intron 1, exon 2, exon 3 and exon 4 of the OPRK1 gene (Fig. 1), we identified 12 polymorphic sites (Table 3). Among those, nine SNPs are novel and three are previously identified SNPs (36G>T, 459C>T and 843A>G). The SNP 508C>T, found in one subject, alters the amino acid sequence of the receptor protein, substituting a cysteine residue for the prototypic arginine at amino acid position 169. The other identified SNPs in the hKOR are synonymous. Genotype and allele frequencies of eight SNPs that have overall allele frequencies above 0.01 are shown in Table 4. Data are stratified by ethnicity and shown for cases and controls.
Table 3.
Identified polymorphisms in the human kappa opioid receptor gene
| Allelic frequencies | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| SNP | Variant | Location | Chromosome 8 position NCBI AY168006 | refSNPb ID No. | Caucasion (n = 129) | African-American (n = 70) | Hispanic (n = 60) | Asian (n = 19) | Mixed (n = 13) | Total (n = 291) |
| 1 | −88C>G | Exon 1 | 54214007 | 0 | 0.029 | 0 | 0 | 0 | 0.007 | |
| 2 | IVS1113G>A | Intron 1 | 54213855 | 0.047 | 0.088 | 0.060 | 0 | 0.100 | 0.052 | |
| 3 | IVS1133G>A | Intron 1 | 54213835 | 0 | 0.079 | 0.017 | 0 | 0.038 | 0.024 | |
| 4 | IVS1339C>T | Intron 1 | 54213629 | 0.006 | 0.092 | 0.017 | 0 | 0.077 | 0.031 | |
| 5 | 36G>T | Exon 2 | 54213522 | rs1051660 | 0.136 | 0.157 | 0.058 | 0.237 | 0.077 | 0.129 |
| 6 | 459C>T | Exon 3 | 54197430 | rs7815824 | 0.031 | 0.150 | 0.067 | 0 | 0.038 | 0.065 |
| 7 | 508C>Ta | Exon 3 | 54197381 | 0 | 0.007 | 0 | 0 | 0 | 0.002 | |
| 8 | 843A>G | Exon 4 | 54192117 | rs702764 | 0.143 | 0.486 | 0.267 | 0.026 | 0.308 | 0.251 |
| 9 | 846C>T | Exon 4 | 54192114 | 0.050 | 0.150 | 0.033 | 0 | 0.077 | 0.069 | |
| 10 | 852C>T | Exon 4 | 54192108 | 0 | 0.007 | 0 | 0 | 0 | 0.002 | |
| 11 | 948C>T | Exon 4 | 54192012 | 0.004 | 0.043 | 0 | 0 | 0 | 0.012 | |
| 12 | 1008C>T | Exon 4 | 54191952 | 0 | 0 | 0.008 | 0 | 0 | 0.002 | |
Amino acid substitution Arg169Cys (IC loop 2);
refSNP ID from Build 116 of dbSNP as of 19 April 2004. SNP, Single nucleotide polymorphism.
Table 4.
Genotype and allele distribution of OPRK1 single nucleotide polymorphisms (SNPs) stratified by ethnicity and drug status
| Opioid-dependent | Controls | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Genotype | Allele | Genotype | Allele | |||||||
| SNP | No. | No. | No. | No. | No. | No. | No. | No. | No. | No. |
| IVS1113G>A | GG | GA | AA | G | A | GG | GA | AA | G | A |
| Caucasian | 58 (0.89) | 7(0.11) | 0 | 123 | 7 (0.05) | 58 (0.91) | 6 (0.09) | 0 | 122 | 6 (0.05) |
| African-American | 29 (0.81) | 7 (0.1 9) | 0 | 65 | 7 (0.10) | 28 (0.82) | 6 (0.1 8) | 0 | 62 | 6 (0.09) |
| Hispanic | 35 (1.00) | 0 | 0 | 70 | 0 | 22 (0.88) | 3 (0.1 2) | 0 | 47 | 3 (0.06) |
| IVS1133G>A | GG | GA | AA | G | A | GG | GA | AA | G | A |
| Caucasian | 65 (1.00) | 0 | 0 | 130 | 0 | 64 (1.00) | 0 | 0 | 128 | 0 |
| African-American | 29(0.81) | 7 (0.1 9) | 0 | 65 | 7 (0.10) | 30 (0.88) | 4 (0.1 2) | 0 | 64 | 4 (0.06) |
| Hispanic | 35 (1.00) | 0 | 0 | 70 | 0 | 23 (0.92) | 2 (0.08) | 0 | 48 | 2 (0.04) |
| IVS1339C>T | CC | CT | TT | C | T | CC | CT | TT | C | T |
| Caucasian | 65 (1.00) | 0 | 0 | 130 | 0 | 63 (0.98) | 1 (0.02) | 0 | 127 | 1 (0.01) |
| African-American | 29 (0.81) | 7 (0.1 9) | 0 | 65 | 7 (0.10) | 28 (0.82) | 6 (0.1 8) | 0 | 62 | 6 (0.09) |
| Hispanic | 35 (1.00) | 0 | 0 | 70 | 0 | 23 (0.92) | 2 (0.08) | 0 | 48 | 2 (0.04) |
| 36G>T | GG | GT | TT | G | T | GG | GT | TT | G | T |
| Caucasian | 51 (0.78) | 13 (0.20) | 1 (0.02) | 115 | 15 (0.12) | 49 (0.76) | 10 (0.1 6) | 5 (0.08) | 108 | 20 (0.1 6) |
| African-American | 26 (0.72) | 10(0.28) | 0 | 62 | 10(0.14) | 24(0.71) | 8 (0.23) | 2 (0.06) | 56 | 1 2 (0.1 8) |
| Hispanic | 32 (0.91) | 3 (0.09) | 0 | 67 | 3 (0.04) | 22 (0.88) | 2 (0.08) | 1 (0.04) | 46 | 4 (0.08) |
| 459C>T | CC | CT | TT | C | T | CC | CT | TT | C | T |
| Caucasian | 62 (0.95) | 3 (0.05) | 0 | 127 | 3 (0.02) | 59 (0.92) | 5 (0.08) | 0 | 123 | 5 (0.04) |
| African-American | 28 (0.78) | 8 (0.22) | 0 | 64 | 8 (0.11) | 21 (0.62) | 13 (0.38) | 0 | 55 | 13 (0.1 9) |
| Hispanic | 30 (0.86) | 5 (0.14) | 0 | 65 | 5 (0.07) | 22 (0.98) | 3 (0.1 2) | 0 | 47 | 3 (0.06) |
| 843A>G | AA | AG | GG | A | G | AA | AG | GG | A | G |
| Caucasian | 52 (0.80) | 10 (0.15) | 3 (0.05) | 114 | 16 (0.12) | 44 (0.69) | 1 9 (0.30) | 1 (0.01) | 107 | 21 (0.16) |
| African-American | 11 (0.30) | 1 9 (0.53) | 6 (0.1 7) | 41 | 31 (0.43) | 6(0.18) | 1 9 (0.56) | 9 (0.26) | 31 | 37 (0.54) |
| Hispanic | 22 (0.63) | 10(0.28) | 3 (0.09) | 54 | 1 6 (0.23) | 1 2 (0.48) | 10 (0.40) | 3(0.12) | 34 | 1 6 (0.32) |
| 846C>T | CC | CT | TT | C | T | CC | CT | TT | C | T |
| Caucasian | 59(0.91) | 6 (0.09) | 0 | 124 | 6 (0.05) | 57 (0.89) | 7(0.11) | 0 | 121 | 7 (0.05) |
| African-American | 26 (0.72) | 9 (0.25) | 1 (0.03) | 61 | 11 (0.15) | 25 (0.73) | 8 (0.24) | 1 (0.03) | 58 | 10 (0.15) |
| Hispanic | 34 (0.97) | 1 (0.03) | 0 | 69 | 1 (0.01) | 22 (0.88) | 3 (0.1 2) | 0 | 47 | 3 (0.06) |
| 948C>G | CC | CG | GG | C | G | CC | CG | GG | C | G |
| Caucasian | 65 (1.00) | 0 | 0 | 130 | 0 | 63 (0.98) | 1 (0.02) | 0 | 127 | 1 (0.01) |
| African-American | 34 (0.94) | 2 (0.06) | 0 | 70 | 2 (0.03) | 30 (0.88) | 4 (0.1 2) | 0 | 64 | 4 (0.06) |
| Hispanic | 35 (1.00) | 0 | 0 | 70 | 0 | 25 (1.00) | 0 | 0 | 50 | 0 |
Association of SNPs with opiate addiction
Of the 12 SNPs, four have minor allele frequencies below 1%. Thus, only those eight SNPs (no. 2, 3, 4, 5, 6, 8, 9 and 11) with minor allele frequencies ⩾ 0.01 are included in the statistical analysis. Of the five ethnic groups (Asian-American, African-American, Caucasian, Hispanic, Mixed), Asian-Americans comprised 18 controls and only one case individual. Thus, this group was disregarded for further analysis. Furthermore, to ensure as much homogeneity as possible, individuals of mixed ethnicity are not included below. We tested for deviations from Hardy–Weinberg equilibrium (HWE) within control groups for each ethnicity for each of the eight SNPs using Chi-square tests. No significant (P < 0.05) deviations of genotype distribution from HWE were found for SNPs 2, 3, 4, 6, 8, 9 and 11, except 5 (36G>T). Results of separate HWE tests for SNP 5 by ethnicity, gender and disease status are shown in Table 5. The 36G>T SNP significantly deviated from HWE only in the Caucasian female control group (P 0.008). Therefore, this group was removed from further analyses, except the analyses of linkage disequilibrium.
Table 5.
Hardy–Weinberg tests of SNP 5 (36G>T) genotype by ethnicity, gender and disease status
| Genotype | ||||||
|---|---|---|---|---|---|---|
| Ethnicity | Group | Gender | GG | GT | TT | P |
| African-Americans | Control | Male | 12 | 6 | 1 | 0.8301 |
| Female | 11 | 3 | 1 | 0.3225 | ||
| Opiate-dependent | Male | 15 | 7 | 0 | 0.2484 | |
| Female | 11 | 3 | 0 | 0.548 | ||
| Caucasians | Control | Male | 24 | 8 | 3 | 0.1138 |
| Female | 25 | 2 | 2 | 0.0082 | ||
| Opiate-dependent | Male | 33 | 10 | 0 | 0.2507 | |
| Female | 18 | 3 | 1 | 0.2008 | ||
| Hispanics | Control | Male | 10 | 0 | 0 | 1 |
| Female | 11 | 3 | 1 | 0.3225 | ||
| Opiate-dependent | Male | 19 | 1 | 0 | 0.8728 | |
| Female | 13 | 2 | 0 | 0.7053 | ||
Effects of all eight SNPs were taken into account in two different ways, that is, with a logistic regression on the genotype level and with haplotype analysis on the allelic level. For the logistic regression with disease category as the dependent variable, ethnic groups were treated as dummy input variables and genotypes were coded, for example, as AA=1, AG=2, and GG 3 (linear effects alleles). Only one input variable had a P-value less than 0.10: The 36G>T SNP exhibits a point-wise significant association (P=0.016) with disease status given all other input variables (SNPs and ethnicities). The overall regression was not significant (P = 0.0720) but nearly so. For the 36G>T SNP analysed separately, genotype and allele frequencies were not significantly different between cases and controls (P > 0.20).
Haplotype frequencies for the eight SNPs were estimated with the snphap program separately for case and control individuals. When rare haplotypes (less than 5% estimated frequency) were pooled into one group, the number of haplotypes (including the pooled group) seen in the three ethnic groups were 9, 6 and 5 for African-Americans, Caucasians and Hispanics, respectively, with corresponding significance levels for differences in haplotype frequencies between case and control individuals of P = 0.0742, 0.1015 and 0.0041. When these ethnic groups were combined by Fisher’s method [39], the resulting empirical significance level was P = 0.0020, which is highly significant. In Hispanics, the result is mainly due to the following two haplotypes (alleles at SNPs 2, 3, 4, 5, 6, 8, 9 and 11) (Table 6): AGCTCGTC occurs only in controls (frequency 0.060), while haplotype GGCGTGCC occurs only in cases (frequency of 0.0714). The most common haplotype in cases, GGCGCACC, is the same in each of the three ethnic groups. This is also the most common haplotype in controls for each ethnic group with a frequency consistently lower than in cases (Table 7).
Table 6.
Frequencies of the haplotypes in OPRK1 in Hispanic group
| Frequencies | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| IVS1113G>A | IVS1133G>A | IVS1339C>T | 36G>T | 459C>T | 843A>G | 846C>T | 948C>T | Controls | Cases |
| Haplotypes | |||||||||
| A | G | C | T | C | G | T | C | 0.060 | 0 |
| G | G | C | G | C | A | C | C | 0.589 | 0.729 |
| G | G | C | G | C | G | C | C | 0.190 | 0.143 |
| G | G | C | G | T | G | C | C | 0 | 0.071 |
| Sum | 1 | 1 | |||||||
Table 7.
Frequencies of the most common haplotype GGCGACC in cases and controls in the three ethnic groups
| Ethnicity | Controls | Opiate-dependent |
|---|---|---|
| Caucasians | 0.6571 | 0.8119 |
| Hispanics | 0.5890 | 0.7286 |
| African-Americans | 0.2416 | 0.3779 |
Linkage disequilibrium in controls
Results of pairwise linkage disequilibrium analysis for consecutive SNPs in the hKOR gene region in three different control populations (African-American, Caucasian and Hispanic) are presented in Table 8. We considered only the eight markers that showed minor-allele frequencies above 0.01. In Table 8, we present the sample sizes used (n), the value of the chi-square statistic that tests whether D’ is non-zero (i.e. evidence for linkage disequilibrium), the P-value corresponding to the chi-square statistic (P-value), and estimates of two measures of linkage disequilibrium, Δ2and D’. Those chi-square values with corresponding P-values that are less than 0.05 are indicated in bold.
Table 8.
Linkage disequilibrium measures for consecutive markers in hKOR region in control subjects
| Population | First marker | Second marker | Distancea | nb | Chi-square | P | Δ2 | D′c |
|---|---|---|---|---|---|---|---|---|
| African-American | 113G>A | 133G>A | 20 | 34 | 0.76 | 0.382 | 0.006 | 1 |
| 133G>A | 339C>T | 226 | 34 | 0.16 | 0.691 | 0.006 | 0.095 | |
| 339C>T | 36G>T | 107 | 34 | 1.29 | 0.255 | 0.023 | 1 | |
| 36G>T | 459C>T | 16 092 | 34 | 2.04 | 0.153 | 0.056 | 1 | |
| 459C>T | 843A>G | 5313 | 34 | 7.29 | 0.007 | 0.198 | 1 | |
| 843A>G | 846C>T | 3 | 34 | 5.29 | 0.021 | 0.144 | 1 | |
| 846C>T | 948C>T | 102 | 34 | 12.81 | < 0.001 | 0.362 | 1 | |
| Caucasian | 113G>A | 133G>A | 20 | 64 | 0 | 1 | 0 | 0 |
| 133G>A | 339C>T | 226 | 64 | 0 | 1 | 0 | 0 | |
| 339C>T | 36G>T | 107 | 64 | 0.34 | 0.559 | 0.001 | 1 | |
| 36G>T | 459C>T | 16 092 | 64 | 1.74 | 0.188 | 0.008 | 1 | |
| 459C>T | 843A>G | 5313 | 64 | 12.24 | < 0.001 | 0.207 | 1 | |
| 843A>G | 846C>T | 3 | 64 | 19.24 | < 0.001 | 0.295 | 1 | |
| 846C>T | 948C>T | 102 | 64 | 4.57 | 0.033 | 0.136 | 1 | |
| Hispanic | 113G>A | 133G>A | 20 | 25 | 0.25 | 0.615 | 0.003 | 1 |
| 133G>A | 339C>T | 226 | 25 | 0.17 | 0.683 | 0.002 | 1 | |
| 339C>T | 36G>T | 107 | 25 | 0.43 | 0.512 | 0.005 | 1 | |
| 36G>T | 459C>T | 16 092 | 25 | 0.65 | 0.419 | 0.007 | 1 | |
| 459C>T | 843A>G | 5313 | 25 | 5.87 | 0.015 | 0.136 | 1 | |
| 843A>G | 846C>T | 3 | 25 | 3.1 | 0.079 | 0.136 | 1 | |
| 846C>T | 948C>T | 102 | 25 | 0 | 1 | 0 | 0 |
Distance is given in nucleotides from the indicated single nucleotide polymorphism (SNP) to the next SNP according to the map of chromosome 8.
n, Number of individuals.
D′ values of 0.0 indicate that one of the two SNPs was not polymorphic in the particular population.
The marker pairs that show the strongest linkage disequilibrium across multiple populations are: (459C>T, 843A>G), (843A>G, 846C>T) and (846C>T, 948C>T). All show significant linkage dis-equilibrium at the P < 0.05 level for at least two populations.
Table 9 presents the results for those marker pairs that show the most linkage disequilibrium in each population. The chi-square value for every marker pair in Table 9 has a corresponding P-value less than 0.05. In all three populations, the marker pairs with the strongest linkage disequilibrium are: IVS1113G>A-846C>T and IVS1113G>A-36G>T.
Table 9.
All possible marker pairs in hKOR region that show most significant evidence for linkage disequilibrium in control subjects
| Population | First marker | Second marker | Distancea | nb | Chi-square | P | Δ2 | D′c |
|---|---|---|---|---|---|---|---|---|
| African-American | 113G>A | 36G>T | 333 | 34 | 17.1 | < 0.001 | 0.409 | 1 |
| 113G>A | 846C>T | 21 741 | 34 | 20.2 | < 0.001 | 0.561 | 1 | |
| 133G>A | 843A>G | 21 728 | 34 | 5.2 | 0.023 | 0.075 | 1 | |
| 36G>T | 846C>T | 21 408 | 34 | 6.58 | 0.01 | 0.161 | 0.47 | |
| 459C>T | 843A>G | 5313 | 34 | 7.29 | 0.007 | 0.198 | 1 | |
| 843A>G | 846C>T | 3 | 34 | 5.29 | 0.021 | 0.144 | 1 | |
| 846C>T | 948C>T | 102 | 34 | 12.81 | < 0.001 | 0.362 | 1 | |
| Caucasian | 113G>A | 36G>T | 333 | 64 | 21.23 | 0 | 0.266 | 1 |
| 113G>A | 843A>G | 21 738 | 64 | 16.38 | < 0.001 | 0.251 | 1 | |
| 113G>A | 846C>T | 21 741 | 64 | 34.38 | 0 | 0.85 | 1 | |
| 36G>T | 846C>T | 21 408 | 64 | 15.82 | < 0.001 | 0.212 | 0.82 | |
| 459C>T | 843A>G | 5313 | 64 | 12.24 | < 0.001 | 0.207 | 1 | |
| 843A>G | 846C>T | 3 | 64 | 19.24 | < 0.001 | 0.295 | 1 | |
| 846C>T | 948C>T | 102 | 64 | 4.57 | 0.033 | 0.136 | 1 | |
| Hispanic | 113G>A | 846C>T | 21 741 | 25 | 18.54 | < 0.001 | 1 | 1 |
| 113G>A | 36G>T | 333 | 25 | 13.19 | < 0.001 | 0.574 | 1 | |
| 36G>T | 846C>T | 21 408 | 25 | 13.19 | < 0.001 | 0.574 | 1 | |
| 459C>T | 843A>G | 5313 | 25 | 5.87 | 0.015 | 0.136 | 1 |
Distance is given in nucleotides from the indicated single nucleotide polymorphism (SNP) to the next SNP according to the map of chromosome 8 on 29 April 2004.
n, Number of individuals.
D′ values of 0.0 indicate that one of the two SNPs was not polymorphic in the particular population.
Discussion
In the present study, we have cloned cDNA-RACE fragments containing the 5’- and 3’-termini of the hOPRK1 gene and studied the exon/intron structure of hOPRK1 gene upstream of the previously reported exon containing the translation initiation codon. The results of these studies demonstrate that the hOPRK1 gene has at least four major exons and three introns, similar to rodent OPRK1 genes. Furthermore, we have shown that the hOPRK1 gene has at least three transcription initiation sites indicating that the 5’-UTR of hOPRK1 mRNA is between 215 and 299 nucleotides in length. The mRNA with 238 nucleotides of the 5’-UTR was found in 14 of 20 sequenced clones and, hence, was the predominant form. Although there is a substantial difference in the sequences of exon 1 between the human and mouse OPRK1 genes, the exon/intron organization of the gene (i.e. having four major exons and three introns) is conserved among these species. During the course of this study, a splice variant of the hOPRK1 (KOR1A) mRNA sequence, containing most of exon 1 (reported herein) was submitted to GenBank, accession AY168006 [40]. This splice variant contained 236 nucleotides of 5’-UTR and a 42-nucleotide deletion in exon 3 (formerly exon 2).
The identified 3’-UTR of the hOPRK1 mRNA of 3096 nucleotides in length contains the rare noncanonical polyadenylation signal, AATAAT, located 27 nucleotides upstream from the 3’ polyadenylation site. However, the 3’-UTR, as well as genomic sequences of human OPRK1, downstream of the identified 3’-terminus, contain additional potential polyadenylation sites that may produce mRNA of different sizes in a tissue-specific manner. Utilization of alternative polyadenylation sites has been demonstrated for the mouse OPRK1 mRNA in P19 cells [38].
This information on the organization and structure of the hOPRK1 gene should facilitate functional studies on the transcriptional regulation of this gene. Regulatory elements in promoter regions and, particularly, in intron 1 of the mouse OPRK1 gene have been studied. The presence of the proximal promoter [41] and the functional transcription inhibitory control elements, Ik-1 (Ikaros) and E-box (mediated by retinoic acid and nitric oxide, respectively), in the intron 1 have been characterized [42,43]. In addition, sequence variations in the 3’-UTR in the hOPRK1 mRNA and utilization of alternative polyadenylation sites may influence mRNA post-transcriptional processing, mRNA stability and mRNA localization (e.g. axonal or dendritic localization).
The identification of novel SNPs in exon 1 and intron 1, −88C>G, IVS1113G>A, IVS1133G>A, IVS1339C>T, and three rare SNPs, 508C>T in exon III, 852C>T, 948C>T and 1008C>T in exon 4, increases the number of known common SNPs in the hOPRK1 gene: 36G>T in exon 2, 459C>T in exon 3, 843A>G and 846C>T in exon 4 [4]. The novel 508C>T SNP was found in one subject only. This polymorphism occurs in the second intracellular loop and alters the amino acid sequence of the receptor protein, substituting a cysteine residue for arginine at amino acid position 169. Other SNPs in the OPRK1 coding regions altered the third position of its respective codon but did not alter the predicted amino acid sequence of the receptor.
In Hispanics, the haplotypes defined herein are significantly associated with opiate addiction. Specifically, within this ethnic group, the AGCTCGTC haplotype is found only in control subjects, while the GGCGTGCC is found only in subjects with opiate addiction. These haplotypes may be markers for unknown, unidentified polymorphisms with functional impact. The markers that define these haplotypes span most of three exons and three introns, in a region spanning more than 20 kb. A functional undetected polymorphism, one that may alter the gene regulation or processing, may exist in either intron 2 or 3 and therefore may influence the vulnerability to develop opiate addiction.
Several of the SNPs (including IVS1113G>A, IVS 1846C>T and 36G>T) studied in this work showed strong inter-marker linkage disequilibrium across populations (Table 7). This information is useful in determining the feasibility of mapping complex trait genes using this gene as a candidate. Background linkage disequilibrium in controls is often used as a surrogate for marker-trait linkage disequilibrium [44], which in turn is used to compute power and sample size calculations for association analyses [45,46].
The novel IVS1113G>A, IVS1133G>A and IVS1339 C>T SNPs are located in intron 1, and their functional significance remains to be elucidated. However, it has been shown that the mouse OPRK1 intron 1 contains the functionally active proximal promoter which regulates tissue-specific expression of splice variants of the receptor [41,47]. In a recent study of sequence variations in the OPRK1 gene in mouse strains differing in alcohol consumption, there were no differences in the coding region of the OPRK1 gene in the alcohol-preferring C57BL/6ByJ (B6) and alcohol-avoiding BALB/cJ mice. However, variants in the promoter region of the OPRK1 gene that may be related to the difference in OPRK1 expression levels were found between these two strains [48]. For example, in the cortex, OPRK1 mRNA levels were significantly higher in the B6 than in the BALB/cJ mice.
Although synonymous SNPs in the coding region of OPRK1 do not alter protein sequence, it does not mean that they are without functional significance. Synonymous SNPs, particularly in GC-ending codons, may affect gene expression or translational selection [49]. A recent cell culture study of functional effects of six naturally occurring synonymous variants in the human dopamine receptor D2 gene showed that a synonymous transition of C>T at nucleotide 957 altered the predicted mRNA folding, and led to a decrease in mRNA stability and translation, thereby dramatically reducing dopamine-induced up-regulation of DRD2 expression [50]. Another synonymous transition of G>A at nucleotide 1101 in DRD2 was not functional by itself, but compensated for the effects of the 957T variant, demonstrating that combinations of polymorphisms that do not affect protein structure can have functional consequences.
Chronic morphine administration has been reported to increase preprodynorphin mRNA levels in the rat striatum [23]. The promoter region of the preprodynorphin gene (PDYN) has a variable repeat element that contains a putative AP-1 transcription factor binding site. In cellular studies, increased phorbal ester-stimulated activation was observed in reporter constructs containing three or four, but not one or two, copies of this repeat [51]. In our earlier human genetic study, we found that allelic variants of the PDYN gene with three or four repeats were associated with decreased vulnerability to develop cocaine abuse/dependence [26]. Different transcriptional activity of the PDYN gene allelic variants may therefore lead to differential stimulation of the KOR with possible impact on the development of cocaine addiction.
In conclusion, identification of the 5’ and 3’ ends of the hOPRK1 mRNA and elucidation of the complete hOPRK1 genomic structure will contribute to our further understanding of the regulation of the hOPRK1 expression in tissue- and cell-specific manners. The redefinition of the exon/intron organization of the hOPRK1 gene will facilitate the localization of promoter regions of the OPRK1 gene, a region not yet explored for functional polymorphisms. Significance of differences in haplotype frequencies between opioid-dependent subjects and controls in Hispanic subjects will require replication. Further studies are also needed determine whether any functional differences are introduced by the hOPRK1 SNPs.
Acknowledgements
We thank Kathy Bell, RN, Elizabeth Ducat, RN, Dorothy Melia, RN, Gavin Bart, MD, Pauline McHugh, MD, and Scott Kellogg, PhD, for recruiting, screening and assessment of study subjects. Jonathan Ball assisted in the reading of electropherograms. We thank Eduardo Butelman for a critical reading of the manuscript.
Sponsorship: This study was supported by the National Institutes of Health (NIH) National Institute on Drug Abuse grants K05-DA00049 (M.J.K.), DA12848 (M.J.K.); NCRR General Clinical Research Center grant M01-RR00102; K01-HG00055 (D.G.); and MH44292 (J.O.).
References
- 1.Kreek MJ. Opiates, opioids and addiction. Mol Psychiatry 1996; 1:232–254. [PubMed] [Google Scholar]
- 2.Kreek MJ. Opioid receptors: some perspectives from early studies of their role in normal physiology, stress responsivity and in specific addictive diseases. J Neurochem Res 1996; 21:1469–1488. [DOI] [PubMed] [Google Scholar]
- 3.LaForge KS, Yuferov V, Kreek MJ. Opioid receptor and peptide gene polymorphisms: potential implications for addictions. Eur J Pharmacol 2000; 410:249–268. [DOI] [PubMed] [Google Scholar]
- 4.Mayer P, Höllt V. Allelic and somatic variations in the endogenous opioid system in humans. Pharmacol Ther 2001; 91:167–177. [DOI] [PubMed] [Google Scholar]
- 5.Kreek MJ. Opioid and cocaine addictions: challenge for pharmacothera-pies. Pharm Biochem Behav 1997; 57:551–569. [DOI] [PubMed] [Google Scholar]
- 6.Kreek MJ, Schluger J, Borg L, Gunduz M, Ho A. Dynorphin A1–13 causes elevation of serum levels of prolactin through an opioid receptor mechanism in humans: gender differences and implications for modulation of dopaminergic tone in the treatment of addictions. J Pharmacol Exp Ther 1999; 288:260–269. [PubMed] [Google Scholar]
- 7.Bart G, Borg L, Schluger JH, Green M, Ho A, Kreek MJ. Suppressed prolactin response to dynorphin A1–13 in methadone-maintained versus control subjects. J Pharmacol Exp Ther 2003; 306:581–587. [DOI] [PubMed] [Google Scholar]
- 8.Specker S, Wananukul W, Hatsukami D, Nolin K, Hooke L, Kreek MJ et al. Effects of dynorphin A(1–13) on opioid withdrawal in humans. Psychopharmacology (Berl) 1998; 137:326–332. [DOI] [PubMed] [Google Scholar]
- 9.Portenoy PK, Caraceny A, Chemy NI, Goldblum R, Ingham J, Inturrisi JH, et al. Dynorphin A(1–13) analgesia in opioid-treated patients with chronic pain. A control pain study. Clin Drug Invest 1999; 17:33–42. [Google Scholar]
- 10.Butelman ER, Harris TJ, Perez A, Kreek MJ. Effects of systemically administered dynorphin A(1–17) in rhesus monkeys. J Pharmacol Exp Ther 1999; 290:678–686. [PubMed] [Google Scholar]
- 11.Spanagel R, Herz A, Shippenberg TS. The effects of opioid peptides on dopamine release in the nucleus accumbens: an in vivo microdialysis study. J Neurochem 1990; 55:1734–1740. [DOI] [PubMed] [Google Scholar]
- 12.Claye LH, Maisonneuve IM, Yu J, Ho A, Kreek MJ. Local perfusion of dynorphin A(1–17) reduces extracellular dopamine levels in the nucleus accumbens. NIDA Res Monogr 1997; 174:113. [Google Scholar]
- 13.Zhang Y, Butelman ER, Schlussman SD, Ho A, Kreek MJ. Effect of the endogenous kappa opioid agonist dynorphin A(1–17) on cocaine-evoked increases in striatal dopamine levels and cocaine-induced place preference in C57BL/6J mice. Psychopharmacology (Berl) 2004; 172: 422–429. [DOI] [PubMed] [Google Scholar]
- 14.Zhang Y, Butelman ER, Schlussman SD, Ho A, Kreek MJ. Effect of the kappa opioid agonist R-84760 on cocaine-induced increases in striatal dopamine levels and cocaine-induced place preference in C57BL/6J mice. Psychopharmacology (Berl) 2004; 173:146–152. [DOI] [PubMed] [Google Scholar]
- 15.Glick SD, Maisonneuve IM, Raucci J, Archer S. Kappa opioid inhibition of morphine and cocaine self-administration in rats. Brain Res 1995; 681:147–152. [DOI] [PubMed] [Google Scholar]
- 16.Spealman RD, Bergman J. Opioid modulation of the discriminative stimulus effects of cocaine: comparison of micro, kappa and delta agonists in squirrel monkeys discriminating low doses of cocaine. Behav Pharmacol 1994; 5:21–31. [DOI] [PubMed] [Google Scholar]
- 17.Heidbreder CA, Shippenberg TS. U-69593 prevents cocaine sensitization by normalizing basal accumbens dopamine. Neuroreport 1994; 5:1797–1800. [DOI] [PubMed] [Google Scholar]
- 18.Hurd YL, Herkenham M. Influence of a single injection of cocaine, amphetamine or GBR 12909 on mRNA expression of striatal neuropep-tides. Mol Brain Res 1992; 16:97–104. [DOI] [PubMed] [Google Scholar]
- 19.Steiner H, Gerfen CR. Cocaine-induced c-fos messenger RNA is inversely related to dynorphin expression in striatum. J Neurosci 1993; 13:5066–5081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Daunais JB, McGinty JF. Cocaine binges differentially alter striatal preprodynorphin and zif/268 mRNAs. Mol Brain Res 1995; 29:201–210. [DOI] [PubMed] [Google Scholar]
- 21.Spangler R, Unterwald EM, Kreek MJ. ‘Binge’ cocaine administration induces a sustained increase of prodynorphin mRNA in rat caudateputamen. Mol Brain Res 1993; 19:323–327. [DOI] [PubMed] [Google Scholar]
- 22.Hurd YL, Brown EE, Finley JM, Fibiger HC, Gerfen CR. Cocaine self-administration differentially alters mRNA expression of striatal peptides. Mol Brain Res 1992; 13:165–170. [DOI] [PubMed] [Google Scholar]
- 23.Przewlocka B, Turchan J, Lason W, Przewlocki R. The effect of single and repeated morphine administration on the prodynorphin system activity in the nucleus accumbens and striatum of the rat. Neuroscience 1996; 70:7449–7454. [DOI] [PubMed] [Google Scholar]
- 24.Maisonneuve IM, Ho A, Kreek MJ. Chronic administration of a cocaine ‘binge’ alters basal extracellular levels in male rats: an in vivo microdialysis study. J Pharmacol Exp Ther 1995; 272:652–657. [PubMed] [Google Scholar]
- 25.Hurd YL, Herkenham M. Molecular alterations in the neostriatum of human cocaine addicts. Synapse 1993; 13:357–369. [DOI] [PubMed] [Google Scholar]
- 26.Chen ACH, LaForge KS, Ho A, McHugh PF, Kellogg S, Bell K, et al. Potentially functional polymorphism in the promoter region of prodynorphin gene may be associated with protection against cocaine dependence or abuse. Am J Med Gen 2002; 114:429–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yasuda K, Espinosa R III, Takeda J, Le Beau MM, Bell GI. Localization of the kappa opioid receptor gene to human chromosome band 8q11.2. Genomics 1994; 19:596–597. [DOI] [PubMed] [Google Scholar]
- 28.Wei L-N, Loh HH. Regulation of opioid receptor expression. Curr Opin Pharmacol 2002; 2:69–75. [DOI] [PubMed] [Google Scholar]
- 29.Mansson E, Bare L, Yang D. Isolation of a human kappa opioid receptor cDNA from placenta. Biochem Biophys Res Commun 1994; 202: 1431–1437. [DOI] [PubMed] [Google Scholar]
- 30.Simonin F, Gaveriaux-Ruff C, Befort K, Matthes H, Lannes B, Micheletti G, et al. Kappa-opioid receptor in humans: cDNA and genomic cloning, chromosomal assignment, functional expression, pharmacology, and expression pattern in the central nervous system. Proc Natl Acad Sci USA 1995; 92:7006–7010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhu J, Chen C, Xue J-C, Kunapuli S, DeRiel K, Liu-Chen L-Y. Cloning of a human kappa opioid receptor from the brain. Life Sci 1995; 56: PL201–PL207. [DOI] [PubMed] [Google Scholar]
- 32.Liu HC, Lu S, Augustin LB, Felsheim RF, Chen HC, Loh HH, et al. Cloning and promoter mapping of mouse kappa opioid receptor gene. Biochem Biophys Res Commun 1995; 209:639–647. [DOI] [PubMed] [Google Scholar]
- 33.Yakovlev AG, Krueger KE, Faden AI. Structure and expression of a rat kappa opioid receptor gene. J Biol Chem 1995; 270:6421–6424. [DOI] [PubMed] [Google Scholar]
- 34.Chiano MN, Clayton DG. Fine genetic mapping using haplotype analysis and the missing data problem. Ann Hum Genet 1998; 62:55–60. [DOI] [PubMed] [Google Scholar]
- 35.Westfall PH, Young SS. Resampling-Based Multiple Testing: Examples and Methods for P-Value Adjustment. New York, NY: John Wiley & Sons, Inc; 1993. [Google Scholar]
- 36.Abecasis GR, Cookson WO. GOLD-graphical overview of linkage disequilibrium. Bioinformatics 2000; 16:182–183. [DOI] [PubMed] [Google Scholar]
- 37.Wingender E, Chen X, Hehl R, Karas H, Liebich I, Matys V, et al. TRANSFAC: an integrated system for gene expression regulation. Nucleic Acids Res 2000; 28:316–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hu X, Bi J, Loh HH, Wei LN. Regulation of mouse kappa opioid receptor gene expression by different 3’-untranslated regions and the effect of retinoic acid. Mol Pharmacol 2002; 62:881–887. [DOI] [PubMed] [Google Scholar]
- 39.Fisher RA. Statistical Methods for Research Workers, 14th edn New York, NY: Hafner/MacMillan; 1970. [Google Scholar]
- 40.Lu LD, Mansson E. Homo sapiens DRG kappa 1 splice variant KOR 1A mRNA, complete cds. GenBank 25 December 2002; Accession AY168006. [Google Scholar]
- 41.Lu S, Loh HH, Wei LN. Studies of dual promoters of mouse kappa-opioid receptor gene. Mol Pharmacol 1997; 52:415–420. [DOI] [PubMed] [Google Scholar]
- 42.Hu X, Bi J, Loh HH, Wei LN. An intronic Ikaros-binding element mediates retinoic acid suppression of the kappa opioid receptor gene, accompanied by histone deacetylation on the promoters. J Biol Chem 2001; 276:4597–4603. [DOI] [PubMed] [Google Scholar]
- 43.Park SW, Li J, Loh HH, Wei LN. A novel signaling pathway of nitric oxide on transcriptional regulation of mouse kappa opioid receptor gene.J Neurosci 2002; 22:7941–7947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gordon D, Simonic I, Ott J. Significant evidence for linkage disequilibrium over a 5-cM region among Afrikaners. Genomics 2000; 66:87–92. [DOI] [PubMed] [Google Scholar]
- 45.Gordon D, Finch SJ, Nothnagel M, Ott J. Power and sample size calculations for case-control genetic association tests when errors are present: application to single nucleotide polymorphisms. Hum Hered 2002; 54:22–33. [DOI] [PubMed] [Google Scholar]
- 46.Purcell S, Cherny SS, Sham PC. Genetic power calculator: design of linkage and association genetic mapping studies of complex traits. Bioinformatics 2003; 19:149–150. [DOI] [PubMed] [Google Scholar]
- 47.Wei LN, Hu X, Bi J, Loh HH. Post-transcriptional regulation of mouse k-opioid receptor expression. Mol Pharmacol 2000; 57:401–408. [PubMed] [Google Scholar]
- 48.Saito M, Ehringer MA, Toth R, Oros M, Szakall I, Sikela JM, et al. Variants of kappa-opioid receptor gene and mRNA in alcohol-preferring and alcohol-avoiding mice. Alcohol 2003; 29:39–49. [DOI] [PubMed] [Google Scholar]
- 49.Iida K, Akashi H. A test of translational selection at ‘silent’ sites in the human genome: base composition comparisons in alternatively spliced genes. Gene 2000; 261:93–105. [DOI] [PubMed] [Google Scholar]
- 50.Duan J, Wainwright MS, Comeron JM, Saitou N, Sanders AR, Gelernter J, et al. Synonymous mutations in the human dopamine receptor D2 (DRD2) affect mRNA stability and synthesis of the receptor. Hum Mol Genet 2003; 12:205–216. [DOI] [PubMed] [Google Scholar]
- 51.Zimprich A, Kraus J, Woltje M, Mayer P, Rauch E, Höllt V. An allelic variation in the human prodynorphin gene promoter alters stimulus-induced expression. J Neurochem 2000; 74:472–477. [DOI] [PubMed] [Google Scholar]
- 52.Schwartz S, Kent WJ, Smit A, Zhang Z, Baertsch R, Hardison RC, et al. Human-mouse alignments with BLASTZ. Genome Res 2003; 13: 103–107. [DOI] [PMC free article] [PubMed] [Google Scholar]