Abstract
Progressive pseudorheumatoid dysplasia is a skeletal genetic disorder affecting primarily the articular carti lage, causing joint stiffness and leading to a crippling status. More than two-thirds of the reported patients belong to Arab and Mediterranean populations. The disease locus has been mapped to chromosome 6q22 in a region of 12.9 cM using a Jordanian family. We examined two additional families, one Jordanian and one Palestinian, to test for homogeneity of the disorder and the presence of a common haplotype, to fine map the disorder, and to use all the information to derive a tool for hétérozygote identification. The two families showed linkage to the same previously reported locus, thus suggesting homogeneity, but they did not share a common haplotype. They also provided information that refined the genetic region for the disease locus to 2.1 cM with three microsatellite markers. The absence of a common haplotype indicates that no common ancestor mutations were inherited by our patients. Genotyping for the three-marker haplotype showed that it can be used as a heterozygote identification tool.
INTRODUCTION
PROGRESSIVE PSEUDORHEUMATOID DYSPLASIA (PPD) (MIM# 208230) is a member of the osteochondrodysplasias, showing an autosomal recessive mode of inheritance (El-Shanti et al, 1997). It is characterized by a picture, clinically but not radiographically, resembling rheumatoid arthritis with radiographie changes in the spine similar to spondyloepiphyseal dysplasia tarda (Wynne-Davies et al, 1982; Spranger et al, 1983a,b). The disorder is manifested by progressive swelling and stiffness of all joints, eventually leading to severe joint contractures. Although rare, it has a relatively high prevalence amongst Arabs and other Mediterranean populations; about two-thirds of the reported patients belong to this group (El-Shanti et al, 1997). The literature contains reports of several families and sporadic cases that outline the clinical picture, and emphasize the autosomal recessive mode of inheritance (Wynne-Davies et al, 1982; Kaibara et al, 1983; Spranger et al, 1983a,b; Al-Awadi et al, 1984; Kozlowski et al, 1986; Teebi and Al-Awadi 1986; Robinson et al, 1989; Sood et al, 1991; Lewkonia and Bech-Hansen 1992; Legius et al, 1993; Razai-Delui et al, 1994; El-Shanti et al, 1997; Fischer et al, 1998). The disease locus was mapped to a 12.9-cM region on chromosome 6ql6–q22 in a large inbred family from Jordan (El-Shanti et al, 1998).
The region of linkage was further identified as 3 cM by Fischer et al (1998). Here we report on two additional families from Jordan with 8 affected individuals. Linkage is shown to the previously mapped locus, thus, supporting linkage homogeneity for this disorder. We also narrow the region of linkage to a 2.1 -cM segment flanked by two microsatellite markers and containing a third marker. We use the three markers for the identification of carriers in the families.
PATIENTS, MATERIALS, AND METHODS
Patient evaluation
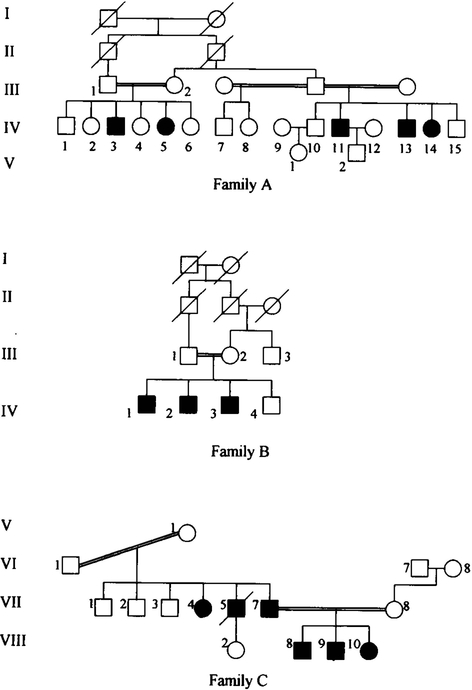
We identified two additional families with 8 affected individuals (families A and B) who are unrelated to the family C for which linkage was previously reported by our group (El-Shanti et al, 1998) (Fig. 1). The two families were ascertained through the clinical genetics services provided by the National Center for Diabetes, Endocrinology, and Genetics (Amman, Jordan) and the Princess Rahma Children’s Hospital (Irbid, Jordan). The two families were invited to participate in the study and an informed consent was obtained from family members or their legal guardians. All individuals in the same sibship were examined thoroughly and the necessary radiographie and laboratory investigations were performed.
FIG. 1.
Pedigrees of the three unrelated families. Blackened symbols denote affected individuals.
Genotyping
DNA was extracted from leukocytes in venous blood by a simple standard procedure of salting out (Grimberg et al, 1989; Miller et al, 1988). Only one sibship in family A and their parents agreed to participate in the study (III. 1, III.2, IV. 1, IV.2, IV.3, IV.4, and IV.5). All members of family B participated in the study. Six microsatellite DNA markers (D6S1056, GCT5E07, D6S1021, ATA56D06, D6S1023, and D6S1040) known to be linked to the PPD locus were used to test for linkage in families A and B. Amplification of these markers was performed with 40 ng of template DNA (2 μL) in an 8.4 μl PCR reaction mixture containing 1.25 μl of PCR buffer (100 mM Tris-HCl, pH 8.8, 500 mM KC1, 15 mM MgCl2, 0.01% wt/v of gelatin); 200 μM each dATP, dCTP, dGTP, and dTTP; 2.5 pmol of each forward and reverse primers (0.25 μl); and 0.25 U of Taq polymerase. The reaction mixture was subjected to 35 cycles at 94°C for 30 sec, 55°C for 30 sec, and 72°C for 30 sec. Products were analyzed on 6% denaturing polyacry lamide gels (7.7M urea). The gels were then silver-stained (Bassam et al, 1991).
Statistical analysis
Two-point LOD scores were calculated using MLINK of the LINKAGE 5.1 computer program package (Lathrop et al., 1984). A LOD score of 3 was used as a criterion for significance. The analysis was carried out assuming an autosomal recessive mode of inheritance, where the disease phenotype is fully penetrant with no phenocopies. The recombination fraction was assumed to be equal for males and females. The disease frequency was set at 0.001. Because the actual disease frequency is unknown, linkage analysis was also carried out varying the disease frequency between 0.01 and 0.00001, to evaluate the effect of changing the alíele frequencies on the LOD score. The alíele frequencies for the markers were assumed to be equal, because the true alíele frequency for this population is unknown. To avoid obtaining a false-positive result due to using too low an alíele frequency for a common alíele, the linkage analysis was also carried out with the allele frequency doubled for the alíele that segregates with the disease alíele. We used the Marshfield map (Center for Medical Genetics, Marshfield Medical Research Foundation) and the Whitehead RH map (Whitehead Institute for Biomédical Research/MIT Center for Genome Research) to arrange the markers, but the reference genetic map used for the analysis was generated by the Cooperative Human Linkage Center (CHLC) from combined CEPH data (Murray et al, 1994)
Haplotype analysis
Genotypes for five DNA markers were determined for the members of families A and B. Recombination events are recognized on the basis of haplotypes passed from parents to children. Alleles for the five markers were grouped in the most likely haplotype, with the paternal haplotype on the left and the maternal haplotype on the right.
Hétérozygote identification
Individuals identified from the pedigrees as possible heterozygotes were invited for participation. The shortest identified region of linkage was used as a haplotype and it was made of three microsatellite markers. In family A, individual IV.6 did not want to know her carrier status. In family B, the maternal uncle III.3 agreed to participate. In family C, all of the offspring of individuals VII. 1 and VII.3 (14 individuals) agreed to participate
RESULTS
Clinical picture
Individuals IV.3 and IV.5 of family A are currently 21 and 18 years old, respectively. Both presented at the age of about 3 with bowing of the lower limbs, which was corrected surgically. They both acquired progressive limitation in movement of their jints, as well as swelling, starting around the age of 8 years. They are both currently short in stature, with scoliosis and lumbar lordosis. Radiographie findings demonstrate the platyspondyly and the spinal curvature abnormalities. The X-rays also show enlarged epiphyseal ends of bones, loss of joint space, and osteoporosis. Individuals IV. 11, IV. 13, and IV. 14 were not examined by us, but their clinical presentations were similar to their cousins
Individuals IV. 1, IV.2, and IV.3 of family B are currently 13, 11, and 8 years of age. They all presented with bowing of the lower limbs around the age of 3 years. The two oldest had swellings and progressive limitation of almost all joints. The youngest started showing the joint involvement just last year, mostly in the fingerjoints. The radiographie findings in all three are platyspondyly and enlarged epiphyses of all bones.
Linkage results
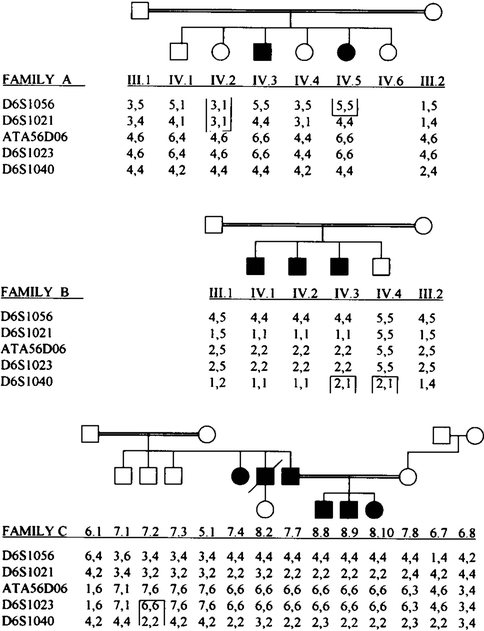
Five microsatellite markers were used in the analysis (Fig. 2). These markers spanned a region of 26 cM on the long arm of chromosome 6 encompassing the PPD gene locus. Significant LOD scores were obtained for all but one marker (D6S1040) in family B (Table 1). When the alíele frequency was doubled for the marker alíele that segregated with the disease alíele, the LOD scores remained significant for markers D6S1056, D6S1021, ATA56D06, and D6S1023. The results also remained significant for these four marker loci when the disease alíele frequency was varied between 0.01 and 0.0001. A maximum LOD score of 7.06 at θ =0 was obtained for marker ATA56D06. Significant LOD scores for adjacent markers (D6S1021 and D6S1056) confirm linkage of the disease locus to 6q.
FIG. 2.
Partial pedigrees of the affected families including all genotyped individuals. The genotyping data for the five listed markers are shown as a haplotype. Open boxes denote centromeric and telomeric boundaries of the region of linkage.
Table 1.
Two LOD Scores for Each of the Three PPD Families with Five Chromosome 6 Markers
| Recombination fraction | ||||||
|---|---|---|---|---|---|---|
| Locus | Family | 0.00 | 0.01 | 0.05 | 0.10 | 0.20 |
| D6S1056 | A | 1.65 | 1.61 | 1.44 | 1.24 | 0.85 |
| B | 1.86 | 1.83 | 1.67 | 1.46 | 1.04 | |
| C | 2.39 | 2.34 | 2.10 | 1.81 | 1.23 | |
| 5.90 | 5.78 | 5.21 | 4.51 | 3.12 | ||
| D6S1021 | A | 1.65 | 1.61 | 1.46 | 1.28 | 0.90 |
| B | 1.86 | 1.83 | 1.67 | 1.46 | 1.04 | |
| C | 3.30 | 3.22 | 2.91 | 2.51 | 1.74 | |
| 6.81 | 6.66 | 6.04 | 5.25 | 3.68 | ||
| ATA56D06 | A | 1.58 | 1.54 | 1.37 | 1.17 | 0.76 |
| B | 1.90 | 1.86 | 1.70 | 1.50 | 1.06 | |
| C | 3.58 | 3.50 | 3.20 | 2.80 | 2.01 | |
| 7.06 | 6.90 | 6.27 | 5.47 | 3.83 | ||
| D6S1023 | A | 1.58 | 1.54 | 1.37 | 1.17 | 0.76 |
| B | 1.90 | 1.86 | 1.70 | 1.50 | 1.06 | |
| C | −∞ | 1.92 | 2.30 | 2.22 | 1.72 | |
| −∞ | 5.32 | 5.37 | 4.89 | 3.54 | ||
| D6S1040 | A | 0.88 | 0.86 | 0.77 | 0.66 | 0.45 |
| B | −∞ | −0.11 | 0.44 | 0.54 | 0.47 | |
| C | −∞ | −0.59 | 0.54 | 0.82 | 0.76 | |
| −∞ | 0.16 | 1.75 | 2.02 | 1.68 | ||
Haplotypes
The linkage phases could not be absolutely determined due to the lack of a third generation. The change in the arrangement of the haplotypes in individual IV.2 of family A could not have happened unless a recombination in one of the parent’s méioses took place. This suggests that D6S1021 is the centromeric boundary for the disease locus.
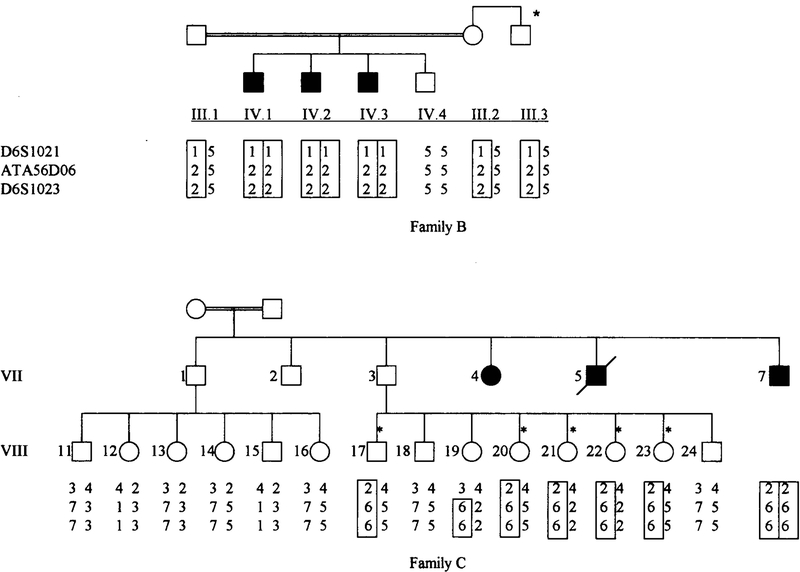
Heterozygote identification
For carrier identification, we used only the three markers that encompass the gene in a 2.1-cM interval. Figure 3 shows the disease haplotype for families B and C. Affected individuals are homozygous for the disease haplotype and obligate carriers are heterozygous for the disease haplotype. In family B, the maternal uncle (III.3), had one copy of the disease haplotype denoting that he is a carrier of the disease gene. Figure 3 shows that the sibship of VII. 11 -VIII-16 are not carriers, because their father is not a carrier and their mother is not related to their father. Five out of eight individuals in the sibship of VII.17-VIII-24 are carriers. A crossover having two alíeles of the disease haplotype was present in one individual VIII. 19, giving her a probability of 98% of being a carrier. Two individuals did not have the disease haplotype and were, thus, non-carriers.
FIG. 3.
Pedigrees of families B and C showing the three marker haplotypes for the maternal uncle from family B and the off-spring of individuals VII. 1 and VII.3 from family C. Individuals marked with an asterisk are carriers.
DISCUSSION
The analysis of these two Jordanian families clearly supports linkage of the disease gene to the previously assigned locus (El-Shanti et al, 1998). Three other families from different geographical locations were reported to have the same locus (Fischer et al, 1998). Thus far, a total of 6 families show linkageto the same locus without suggestion of locus heterogeneity. Linkage data to date support that PPD is a homogeneous disorder, although locus heterogeneity cannot be conclusively excluded.
Crossing over defined the margins of the locus interval; this was reflected as a change in the arrangement of the haplotype in one offspring due to recombination in one ofthe parent’s meioses. The identity of this parent could not be ascertained due to lack of grandparental genotypes. Nevertheless, a change in the haplotype arrangement is an evidence of crossing over, which places marker D6S1021 as the centromeric boundary of the region of linkage. The telomeric boundary of the region is D6S1023, which has been reported previously (El-Shanti et al, 1998). This places the responsible gene between marker D6S1021 and marker D6S1023. This region is estimated to be 2.1 cM on the basis of CHLC reference maps obtained from combined CEPH data.
The disease-causing alíele is not in linkage disequilibrium with a certain allelic constitution of the three markers— D6S1021, ATA56D06, and D6S1023—in that order from centromere to telomere in the three families studied and in the three chromosomes that carry the disease-causing gene. A common haplotype was not present amongst chromosomes of families from Jordan. In fact there were three distinct haplotypes in these families. The absence of a common haplotype, as evidenced by the absence of linkage disequilibrium between the disease-causing gene and specific alíeles of the linked markers makes it unlikely that there is an original mutation that is responsible forall PPD chromosomes.
The telomeric boundary for the region of linkage was defined previously by marker D6S1023 (El-Shanti et al, 1998). In this study, the centromeric boundary was defined using haplotype analysis as marker D6S1021, thus narrowing down the region of linkage. COL10A1 gene and prolyl endopeptidase, a procollagen-processing enzyme, are potential candidate genes that map to 6q22 (Fischere et al., 1998). However, no mutations were identified within the coding sequence of COL10A1 gene (El-Shanti et al, 1998). In addition, analysis of radiation hybrids for a 3-cM interval did not contain the two proposed genes (Fischer et al, 1998). Accordingly, we did not pursue these candidate genes further. The five markers used spanned a region of 26 cM, whereas the three middle markers spanned a region of 2.1 cM, on the basis of CHLC reference maps obtained from combined CEPH data (Murray et al, 1994). A distance of 2.1 cM indicates a probability of 2.1% recombination. A recombination event, evidenced by a change in the three marker haplotypes, leaving two alíeles unchanged will give us a probability of about 98% that the individual is a carrier. When there is no recombination, the probability of prediction of carrier status approaches 100%. The region is too small for two crossover events.
In family C, the offspring of individual VII. 1 were not carriers, and this was expected because this individual does not have the disease gene and his wife is not related to him.
Carrier detection is a useful way to prevent the expression of the disorder in these large inbred families. Consanguineous marriage in Jordan and in the Arab countries in general is a widespread custom, and this practice leads to the expression of very rare disorders such as PPD. Our simple and inexpensive test provides an efficient tool for testing couples from within the family with the intention of marriage by giving them the exact probability of having affected children. This will lessen the problem of having more affected individuals in the family. In addition, any monogenic disorder that is autosomal recessive with a known locus could be handled with the same approach.
ACKNOWLEDGMENTS
The authors wish to thank the families who participated in this study. The primer pairs were a kind donation from the laboratory of Dr. Jeffrey C. Murray of the University of Iowa, Iowa City, Iowa.
REFERENCES
- AL-AWADI S, FARAG T, NAGUIB K, EL-KHALIFA MY, CUSCHIERI A, HOSNY G, ZAHRAN M, and AL-ANSARI AG (1984). Spondyloepiphyseal dysplasia tarda with progressive arthropathy. J. Med. Genet. 21, 193–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BASSAM BJ, CAETANO-ANOLLES G, and GRESSHOFF PM (1991). Fast and sensitive silver staining of DNA in polyacrylamide gels. Anal. Biochem. 196, 80–83. [DOI] [PubMed] [Google Scholar]
- CENTER FOR MEDICAL GENETICS, MARSHFIELD MEDICAL RESEARCH FOUNDATION, http://www.marshmed.org/genetics.
- EL-SHANTI HE, OMARI HZ, and QUBAIN HI (1997). Progressive pseudorheumatoid dysplasia: report of a family and review. J. Med. Genet. 34, 559–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EL-SHANTI H, MURRAY J, SEMINA E, BEUTOW K, SCHERPBIER T, and AL-ALAMI J (1998). The assignment of the gene responsible for progressive pseudorheumatoid dysplasia to the long arm of chromosome six and examination of COL10A1 as a candidate gene. Eur. J. Hum. Genet. 6, 251–256. [DOI] [PubMed] [Google Scholar]
- FISCHER J, URTIZBEREA JA, PAVEC S, VANDIEDONCK C, BRULS T, SAKER S, ALKATIP Y, PRUD’HOMME JF, and WEISSENBACH J (1998). Genetic linkage of progressive pseudorheumatoid dysplasia to a 3 cM interval of chromosome 6q22. Hum. Genet. 103, 60–64. [DOI] [PubMed] [Google Scholar]
- GRIMBERG J, NAWOSCHIK S, BELLUSCIO L, McKEE R, TURK A, and EISENBERG A (1989). A simple and efficient non-organic procedure for the isolation of genomic DNA from blood. Nucleic Acids Res. 17, 8390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAIBARA N, TAKAGISHI K, KATSUKI I, EGUCHI M, MASUMI S, and NISHIO A (1983). Spondyloepiphyseal dysplasia tarda with progressive arthropathy. Skeletal Radiol. 10, 13–16. [DOI] [PubMed] [Google Scholar]
- KOZLOWSKI K, KENNEDY J, LEWIS IC (1986). Radiographiec features of progressive pseudorheumatoid arthritis. Australas. Radiol. 30, 244–250. [DOI] [PubMed] [Google Scholar]
- LATHROP GM, LALONEL JM, JULIER C, and OTT J (1984). Strategies for multilocus linkage analysis in humans. Proc. Nati. Acad. Sei. USA 81, 3443–3446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEGIUS E, MULIER M, VAN DAMME B, and FRYNS JP (1993). Progressive pseudorheumatoid arthritis of childhood (PPAC) and normal adult height. Clin. Genet. 44, 152–155. [DOI] [PubMed] [Google Scholar]
- LEWKONIA RM, and BECH-HANSEN NT (1992). Spondyloepiphyseal dysplasia tarda simulating juvenile arthritis: clinical and molecular genetic observations. Clin. Exp. Rheumatol. 10, 411–414. [PubMed] [Google Scholar]
- MILLER SA, DYKES DD, and POLESKY HF (1988). A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res. 16, 1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MURRAY JC, BEUTEW KH, WEBER JL, et al. (1994). A comprehensive human linkage map with centimorgan density. Science 265, 2049–2054. [DOI] [PubMed] [Google Scholar]
- RAZAI-DELUI H, MAMOORI G, SADRI-MAHVELATI E, and NOORI NM (1994). Progressive pseudorheumatoid chondrodysplasia: a report of nine cases in three families. Skeletal Radiol. 23, 411–119. [DOI] [PubMed] [Google Scholar]
- ROBINSON D, TIELDER M, HALPERIN N, and COPE-LIOVITCH L (1989). Spondyloepiphyseal dysplasia associated with progressive arthropathy: an unusual disorder mimicking juve nile rheumatoid arthritis. Arch. Orthop. Trauma Surg. 108, 397–399. [DOI] [PubMed] [Google Scholar]
- SOOD S, GUPTA AK, and BERRY M (1991). Spondyloepiphy- seal dysplasia tarda with progressive arthropathy. Indian J. Pedatr. 28,671–673. [PubMed] [Google Scholar]
- SPRANGER J, ALBERT C, SHILLING F, and BARTSOCAS C (1983a). Progressive pseudorheumatoid arthropathy of childhood (PPAC) a hereditary disorder simulating rheumatoid arthritis (letter). Am. J. Med. Genet. 14, 399–101. [DOI] [PubMed] [Google Scholar]
- SPRANGER J, ALBERT C, SHILLING F, BARTSOCAS C, and STOSS H (1983b). Progressive pseudorheumatoid arthritis of childhood (PPAC) a hereditary disorder simulating rheumatoid arthritis. Eur. J. Pediatr. 140, 34–40. [DOI] [PubMed] [Google Scholar]
- TEEBI AS, and AL-AWADI SA (1986). Spondyloepiphyseal dysplasia tarda with progressive arthropathy: a rare disorder frequently diagnosed among Arabs (letter). J. Med. Genet. 23, 189–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHITEHEAD INSTITUTE FOR BIOMEDICAL RESEARCH/MIT CENTER FOR GENOME RESEARCH, http://www-genome.wi.mit.edu.
- WYNNE-DAVIES R, HALL B, and ANSELL MB (1982). Spondyloepiphyseal dysplasia tarda with progressive arthropathy— a “new” disorder. J. Bone Joint Surg. Br. 64, 442–445. [DOI] [PubMed] [Google Scholar]