Abstract
Research on early warning indicators has generally focused on assessing temporal transitions with limited application of these methods to detecting spatial regimes. Traditional spatial boundary detection procedures that result in ecoregion maps are typically based on ecological potential (i.e. potential vegetation), and often fail to account for ongoing changes due to stressors such as land use change and climate change and their effects on plant and animal communities. We use Fisher information, an information theory based method, on both terrestrial and aquatic animal data (U.S. Breeding Bird Survey and marine zooplankton) to identify ecological boundaries, and compare our results to traditional early warning indicators, conventional ecoregion maps, and multivariate analyses such as nMDS and cluster analysis. We successfully detected spatial regimes and transitions in both terrestrial and aquatic systems using Fisher information. Furthermore, Fisher information provided explicit spatial information about community change that is absent from other multivariate approaches. Our results suggest that defining spatial regimes based on animal communities may better reflect ecological reality than do traditional ecoregion maps, especially in our current era of rapid and unpredictable ecological change.
Keywords: spatial regimes, spatial resilience, regime shifts, Fisher information, boundary detection, community change
Introduction
The possibility of multiple regimes for ecosystems is now well documented, and methods to detect temporal regime shifts have received a great deal of attention (Scheffer & Carpenter 2003; Dakos et al. 2008; Guttal & Jayaprakash 2008). Less well developed is the application of these tools to the identification of spatial regimes that reflect the boundary between two types of ecosystems (though see Kéfi and others 2014). Spatial data has unique challenges in that while it is not necessary for data points to be equally spaced (Dai et al. 2013; Cline et al. 2014), sufficient spatial sampling resolution is needed to distinguish one spatial regime from another. The identification of spatial regimes is increasingly important due to habitat fragmentation, which increases the proportion of boundaries in landscapes (Kent et al. 2006), and anthropogenic climate change, which is expected to shift ecological boundaries. Studies have already shown rapid altitudinal shifts in montane ecological boundaries in response to climate change (Allen & Breshears 1998; Beckage et al. 2008). Similarly, climate-driven boundary shifts are being detected in marine systems as both spatial shifts in primary production and in individual species ranges, as well as in phenological shifts and changes in community composition (Beaugrand et al. 2002; Edwards & Richardson 2004; Grebmeier et al. 2006). Because ecological boundaries in terrestrial systems typically demarcate the distribution of vegetation and ecosystem type, they provide critical information about the extent and rate of the biological processes shaping the boundary and driving the maintenance of the regime within the boundary (Yarrow & Salthe 2008). This has implications for both environmental management and biological conservation (Kent et al. 2006).
Boundary identification has been an active area of research in terrestrial ecology and biogeography, and is generally both data intensive and statistically challenging, particularly when it involves vegetation sampling (Kent et al. 2006). The use of remotely-sensed data is less laborious than field work, but the method is poor at distinguishing between physically similar but floristically different vegetation; hence, it may require labor-intensive ground-truthing to verify ecological transitions in plant assemblages (Kent et al. 2006). Boundary detection is further complicated by the multiplicity of scales at which different processes and physical patterns are expressed (Fagan et al. 2003; Strayer et al. 2003), and that the relationship between abiotic variables such as climate, and biotic variables such as vegetation, is often non-linear across boundaries (Danz et al. 2012). Typically, terrestrial ecological boundaries defined for ecoregion maps such as those used by U.S. federal agencies are based on potential plant communities, which in turn reflect differences in bedrock, soil, altitude, temperature, and moisture (Bailey 1983; Omernik 1987). Terrestrial plant communities may not respond as rapidly as animal communities to direct anthropogenic change and climate change (Pearson 2006; Pearman et al. 2008), therefore defining the boundaries between animal communities may better represent current biotic and abiotic conditions. Variation in animal population dynamics provides information on the stability of ecosystem mechanisms, processes, and linkages, and may serve as an early warning signal of shifting regimes (Cline et al. 2014).
Pelagic marine ecological boundaries are typically defined by primary production characteristics (Longhurst 1998) which reflect aquatic properties such as currents, temperature, salinity, nutrients, and bathymetry, but are complicated by the ephemeral nature of features such as oceanographic fronts. Landforms, such as straights, may create another form of boundary between biological communities. Advection across fronts or through physical constrictions between water masses can serve as a driver of both physical and ecological homogeneity, though the degree of connectivity can vary rapidly in space and time (Wassmann et al. 2015). There is much current discussion of appropriate variables by which to track marine ecological change (Rice & Rochet 2005; Samhouri et al. 2009; Rombouts et al. 2013). A priori, it is difficult to know which individual taxa or processes represent a spatial regime and thus ecological boundaries. Because of the central role played by zooplankton as a prey item and a grazer, zooplankton data have commonly been used (Hooff & Peterson 2006; Pace et al. 2013), although Scheffer et al. (2003) warn that zooplankton community composition and abundance may be too chaotic to be useful for regime shift prediction except at very high level aggregate states.
Ideally, a monitoring program should be able to forecast far-reaching change such as a regime shift. However, too often monitoring focuses on particular species of interest, effectively barring community-level or ecosystem-level analyses. We use spatially explicit avian and zooplankton community species composition data to test for the identification and location of spatial regimes using Fisher information, an information-theory method with no strict data requirements that is a powerful tool for understanding system-level change within a location, or over space.
Regime shifts and Fisher information
There is widespread acceptance in the scientific community that some ecosystems exhibit multiple regimes, and that the transition between regimes can be abrupt and discontinuous (though see Fukami & Nakajima, 2011; Hastings & Wysham, 2010). Statistical indicators of regime shifts that can act as an early warning signal are thought to represent generic properties that behave in similar and predictable ways across system types (Dakos et al. 2011), and are proposed to have the added advantage that detailed mechanistic knowledge is not necessary for their use. The indicators include critical slowing down, which can manifest as slower recovery rates from perturbation, increased autocorrelation, and increased variance (Scheffer et al. 2009); changing skewness (Guttal & Jayaprakash 2008); conditional heteroscedasticity (Seekell et al. 2011), and the variance index (Brock & Carpenter 2006).
These indicators have transformed our ability to identify variables that change in response to exogenous or endogenous drivers and signal an impending regime shift. However, much remains uncertain. For example, although the various indicators have been tested on model systems and historical data sets with known temporal regime shifts (Lindegren et al. 2012), their performance is not consistent (Seekell et al. 2011; Perretti & Munch 2012; Batt et al. 2013; Dakos et al. 2013) and their ability to predict future regime shifts is unknown (Boulton et al. 2014). Some methods, such as conditional heteroscedasticity, require large, high resolution samples (Seekell et al. 2011) and their applicability to complex systems with multivariate data is questionable because most studies have been conducted using either simulated data or very simple systems (Scheffer et al. 2009; Drake & Griffen 2010; Dai et al. 2012; Dakos et al. 2012). When models have incorporated realistic levels of ecological noise, the indicators tend to perform poorly (Perretti & Munch 2012). A difficulty in developing early warning indicators is that the critical variables driving system transitions are typically unknown. Brock and Carpenter (2012) cite this lack of knowledge as a “fundamental problem” in leading indicators research.
Researchers have urged that multiple ecosystem variables should be evaluated when interpreting indictors for real systems (Carpenter et al. 2009; Lindegren et al. 2012). For example, Litzow et al. (2013) found that when analysing rising variance in catch data from fisheries, trends in individual fisheries largely failed to be statistically significant, while pooling multiple populations increased their ability to detect a collapse. The variance index (VI) was developed to capture dominant variance trends in multivariate systems (Brock & Carpenter 2006). VI should spike prior to a transition, but results from this index are sometimes unclear (Eason et al. 2014).
Fisher information may address some of the issues listed above. Fisher information is an information theory approach (Fisher 1922) that captures patterns in system dynamics as evidenced by the trends in variables that characterize the system’s condition. The approach collapses the behavior of multiple variables into an index that can be used to track changes in dynamic order, including regimes and regime shifts. Historical applications of information theory-based approaches include assessing ecosystem functioning, stability, complexity, and diversity (Anand & Orloci 2000; Svirezhev 2000; Fath & Cabezas 2004; Patricio et al. 2004). More recently, Fisher information has been employed for sustainable environmental management at various spatial scales (Karunanithi et al. 2011; Eason & Garmestani 2012) and to examine temporal patterns in both terrestrial (Mayer et al., 2007; Eason and Cabezas, 2012;) and aquatic systems (Mantua 2004; Spanbauer et al. 2014; Eason et al. 2016).
While other methods like time series analysis requires a sufficient resolution of data to separate noise from a genuine signal of an impending regime shift, the data requirements for Fisher information are more lenient. A strength of Fisher information is that it can readily incorporate a wide variety of data types and variables and has been used to identify regime changes in various types of systems with data resolutions from relatively small and moderate (Eason & Cabezas 2012) to quite large (Spanbauer et al. 2014). Furthermore, there is no minimum or maximum number of variables needed to compute the index. When assessing a complex system characterized by multiple variables, methods like Spearman rank order correlation have been used in conjunction with Fisher information to determine which variables or groups of variables are critical for shaping the Fisher information signal (Eason & Cabezas 2012). Accordingly, one of the key limitations of traditional statistical indicators is avoided because there is no need to make assumptions about which variables best act as indicators of an impending regime shift, particularly when much is uncertain and our knowledge is limited.
Purpose
Our goal is to identify spatial regimes in avian and zooplankton community data using Fisher information, and compare the extent to which Fisher-identified regime boundaries are coincident with our a priori understanding of where these ecological boundaries exist, as per classification systems such as Bailey’s (1983) and Omernik’s (1987) for terrestrial systems, and marine domain descriptions found in Carmack et al. (2010) and Archambault et al. (2010). The terrestrial ecoregion maps rely heavily on potential natural vegetation based on underlying geological and climatic variables, so significant discrepancies between actual land use, actual vegetative cover, and potential vegetation can exist, and should be reflected in the composition of the animal community. Boundaries in marine systems are not as spatially constrained as in terrestrial systems and the key habitat determinants of species’ distributions and community structure are not as easily defined. It is important to note that we are not trying to identify regime shifts that represent a critical transition (e.g. Scheffer 2009), but rather the geospatial point or region at which one ecosystem type transitions into another.
Although Fisher information is suited to multivariate data encompassing a wide range of biotic and abiotic data that characterize any given regime, we used a single taxon dataset from each system (birds and zooplankton). Limiting the data in this way had the benefit of making this a conservative test of the performance of Fisher information that reflects the data readily available to others working on similar problems. We compared the Fisher information results with a range of early warning indicators (critical slowing down, captured by the lag-1 autocorrelation coefficient; variance; kurtosis; skewness; and the variance index), and multivariate methods commonly employed by community ecologists (nMDS (Oksanen 2013), and cluster analysis).
Methods
Terrestrial data
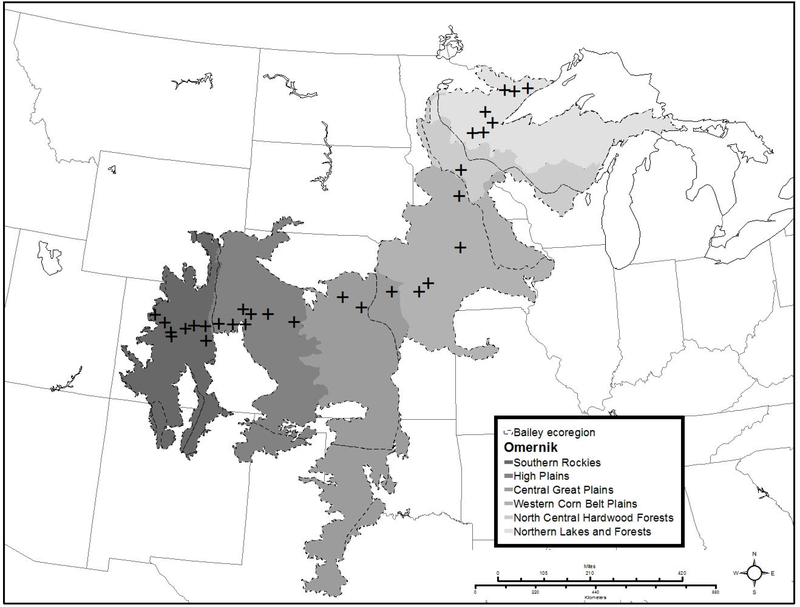
We used USGS Breeding Bird Survey data (BBS) from 30 survey routes along a ~ 1900 km transect. Each BBS route is 41 km long and has 50 stop points located at 800 m intervals; at each stop point, a 3-minute point count of sighted and heard birds is recorded, and data from each stop point are totalled for the route (Sauer et al. 2014). The routes begin in the Rocky Mountains, move due east through the central prairie region, and then veer north into Minnesota, terminating at the western border of Lake Superior (Figure 1A). The species abundance data are a snapshot of the 2007 bird community at each route location. The routes are located in 5 Omernik Level III ecoregions (Omernik 1987), but were selected such that there were roughly an equal number of routes in four gross ecosystem types: 8 routes from the Southern Rockies (montane forest), 7 from the High Plains (grassland), 3 from the Central Great Plains and 4 from the Western Cornbelt Plains (total of 7 routes from grassland-agriculture matrix), and 8 from the Northern Lakes and Forest ecoregion (northern forest-wetland matrix). The unequal number of routes among ecosystems was due to data availability; not all routes are covered in all years, as route coverage relies on volunteers. Although we used the Omernik ecoregions as an underlying map layer when selecting routes, there are multiple ecoregion maps used by U.S. land agencies, with sometimes substantial differences between them. None are ‘right’ per se, but all are best approximations of potential vegetation based on areas with similar geology, physiography, vegetation, climate, soils, land use, wildlife, water quality, and hydrology (United States Department of the Interior). We downloaded the complete species abundance list for each route (Sauer et al. 2014) and used it to create a route-species abundance matrix, where abundance is the number of individual birds for each species at each route, with values ranging from 0 – 293.
Figure 1A.
The USGS Breeding Bird Survey route locations in the central and northern United States. The Omernik Level III ecoregion boundaries are colored in grayscale , while the Bailey Level III ecoregion boundaries are shown using dotted lines.
Sampling biases are an issue with BBS data, resulting primarily from under detection of wary, rare, and aquatic species, as well as differences between observers. However, those biases are present across all routes and should not impact the very coarse pattern extracted from the absence/abundance data. Remotely-sensed data for land cover type is also available for a 400 m buffer around each route (Sauer et al. 2014). The land cover data provides a sense of the heterogeneity of the habitat type for each ecoregion. We averaged the percent of each land cover type across all routes for each of the five Omernik ecoregions.
Marine data
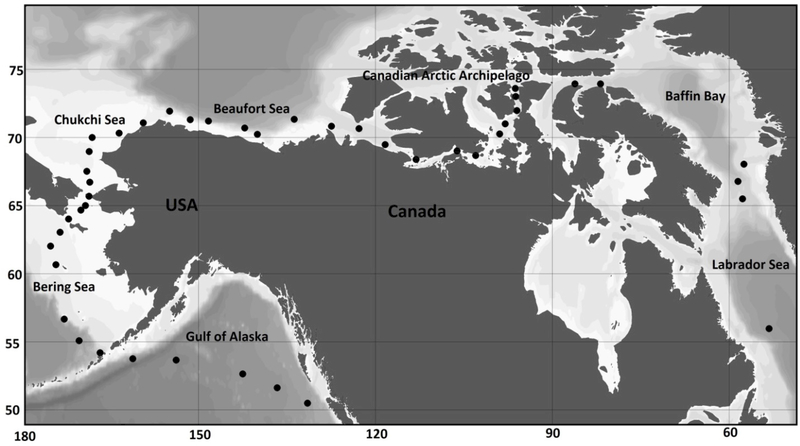
Zooplankton community surveys were conducted in 2008, and samples analysed under the auspices of the International Polar Year program, Canada’s Three Oceans project (Carmack et al. 2008). The survey traverses 12,000 km from coastal British Columbia just north of Vancouver Island to the Labrador Sea on the eastern side of Canada, crossing through 6 oceanic domains: the Gulf of Alaska, the Bering Sea, the Chukchi Sea, the Beaufort Sea Shelf, the Canadian Arctic Archipelago, and terminates in the Davis Strait/Labrador Sea (Figure 1B). Although these oceanic domains share some zooplankton species, they are known to be distinct from each other to varying degrees (Archambault et al. 2010; Pomerleau et al. 2011, 2014). There were 44 sampling locations irregularly spaced along the transect.
Mixed zooplankton samples were collected from August to September by vertical net hauls with a 236 micron net (typically to 100 m or 7 metres above the bottom), and were preserved in 95% ethanol and 10% buffered formalin. The zooplankton samples were keyed out to the lowest possible taxonomic unit and enumerated and 4th root transformed, as is standard for marine zooplankton data. When possible, the developmental stages of each taxa was counted separately. A site-taxa abundance matrix was created. Sites were ordered from western-most to eastern-most station.
Statistical Methods
Fisher information was developed by Fisher (1922) as a measure of the amount of information about a particular parameter (or system characteristic) that can be obtained by observation. The form of Fisher information used in this work is based on the probability of observing various conditions (p(s)) of the system (Fath et al. 2003; Mayer et al. 2007).
| (1) |
This is appropriate for our study because we are interested in determining patterns of change in the condition (or state: s) of a system. From this equation, note that Fisher information is proportional to the change in the probability of observing a system state (dp(s)) over the change in state ds (i.e. ). The significance of this proportionality may be examined using two cases. The first example is a system in which the overall condition does not change from one observation to the next. While such a system may fluctuate within a basin of attraction, it is considered stable because the overall conditions are predictable and the patterns are evident; accordingly, the probability of observing a particular state of the system is high and Fisher information tends toward infinity. The exact opposite is true of a system that is constantly changing. In this case, the system displays no bias toward a particular condition and there are no distinct patterns useful for characterizing the way the system behaves; hence, there is equal probability of the system functioning in any state and Fisher information is zero (Pawlowski & Cabezas 2008).
Karunanithi et al (2008) adapted Equation 1 to handle empirical data from real systems. Through a series of derivation steps, Fisher information (henceforth denoted as FI) is numerically estimated as:
| (2) |
where p(s) is replaced by its amplitude (q2(s) ≡ p(s)) to reduce calculation errors from very small p(s). Further details on the derivation and calculation may be found in (Mayer et al. 2007; Karunanithi et al. 2008; Cabezas & Eason 2010).
Fisher information has traditionally been used to explore temporal patterns, however, the method can be applied to examine spatial dynamics. The core of the FI approach is to assess patterns in data based on tracking systematic changes in line with some ordering principle such that trends are evaluated over a series of points (e.g., point a, point b, etc.). This sequence may be defined temporally or spatially. The key distinction is that rather than using time as the basis for assessing changes, spatial location is the ordering principle. The basic algorithm for computing FI is as follows: (1) select variables (e.g. , i = 1: n variables) that characterize the condition of the system (in this case various animal species) and gather data (i.e., species abundance) from each sampling location (lj) across the route: (, j = 1 : m sampling locations), such that the abundance of each species at each site defines one point (e.g.. ; (2) assemble the data into a m × n matrix and divide it into a sequence of overlapping windows that advances one route location per iteration; (3) determine the measurement uncertainty for each variable () and use this to define a boundary (tolerance) around each system state. If the measurement uncertainty is unknown then the variation in a stable portion of data may be used as a proxy. This boundary (size of states) defines how much a measurement can vary within a particular state; (4) Use the size of states to determine which points are similar (dimensions stay within the boundary defining a minimum range of variation) and group (bin) similar points together into discrete states; (5) Compute p(s) by counting the number of points binned in each state and dividing this value by the total number of points in the window; (6) compute q(s) and calculate FI using Equation 2. This process is repeated for each window. Based on empirical assessments, a hwin ≥ 8 was suggested (Cabezas & Eason 2010), however, it is generally set based on the amount of data available. Increasing the hwin tends to decrease the magnitude of the FI result and number of FI points, but the basic trends remain intact (Cabezas & Eason 2010).
Different system regimes are controlled by fundamentally distinct processes and exhibit unique patterns. Tracking FI affords the ability to assess changes in these patterns. Regimes are identified as periods over time or across space in which FI is non-zero and the values are relatively stable (i.e., dFI/dl ≈ 0). While steadily increasing FI indicates rising dynamic order, less change and possible movement to more consistent patterns, declining FI signifies unstable dynamics, loss of resilience and may provide warning of an impending shift (Eason et al. 2014). Although FI typically declines prior to a regime shift (Mayer et al. 2007; Eason & Cabezas 2012; Eason et al. 2014), researchers examined model dynamics to study the behavior of FI in the neighbourhood of a tipping point and found that the behavior of FI depends heavily on the trends in the variables as the system approaches a shift (Eason et al. 2014; Gonzalez-Mejia et al. 2015). It is therefore possible for FI to increase as a system transitions from one regime to another. Such a result is in line with Seekell et al. (2011, 2012), who found both increasing and decreasing trends in early warning indicators prior to a shift.
Once a shift has been identified, the underlying variables can be explored to determine (or compare) the condition of the system in its new state (Eason & Garmestani 2012). Although higher FI values are generally associated with a greater degree of dynamic order, the level of dynamic order is not as important as the ability of the system to remain stable within a desirable regime. When interpreting FI, a regime is denoted by a relatively stable FI trend (i.e., dFI/dl ≈ 0) with a high mean (↑μFI) and low standard deviation in FI (↓σFI) or low coefficient of variation in FI (↓ ) (Gonzalez-Mejia 2011; Eason & Garmestani 2012). Transitions are identified as periods outside of stable regimes characterized by relatively high σFI and cvFI.
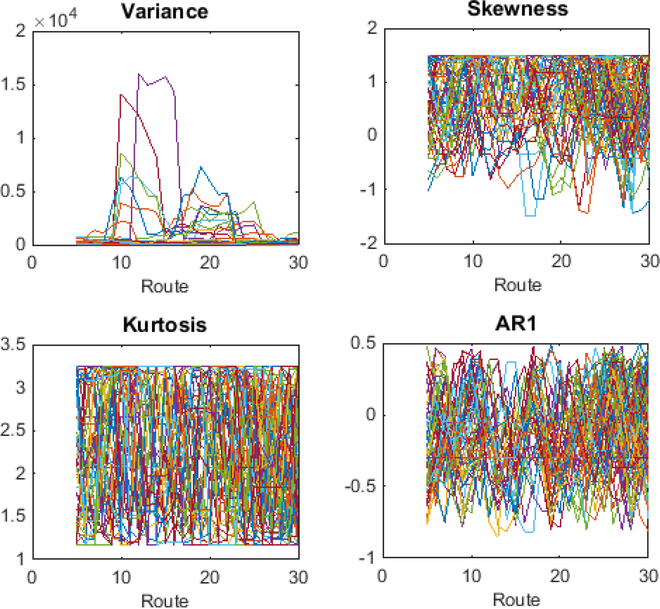
The traditional temporal early warning indicators (variance, skewness, and kurtosis) were computed using standard functions. The spatial variants (Moran’s I spatial autocorrelation and spatial variance and skewness) were not used because the sequential one-dimensional ordering of the sampling stations lent itself to a space-for-time substitution. Since critical slowing down can be understood as increases in short-term autocorrelation, the lag-1 autocorrelation coefficient was used as an estimate (Dakos et al. 2008). The VI was computed as the maximum eigenvalue of the covariance matrix from the dataset (Brock & Carpenter 2006). Note that the VI and traditional indicators are expected to spike or increase prior to a regime shift, while FI tends to decline (Eason et al. 2014). Fisher information and the traditional indicators were computed in MATLAB (v. 2014b) using a 5 station moving window that advanced one station at a time, where a station was either a BBS route or a plankton sampling site. A window size of 5 ensured that there were FI results for each ecoregion for both studies; using smaller or larger windows resulted in similar trends in the FI results, similar to other studies (Cabezas & Eason 2010). Multivariate analyses were conducted using metaMDS and ordicluster from package ‘vegan’ (R Development Core Team 2013). The distance matrices for the nMDS were created using Bray-Curtis, and multiple dimensions were plotted in a scree diagram to find the lowest dimensionality with an adequate ordination fit as expressed by a stress value (<0.2, (Clarke 1993)). The mean, standard deviation, and the coefficient of variation (CV) in FI were calculated for each regime to explore regime stability.
Results
Terrestrial data
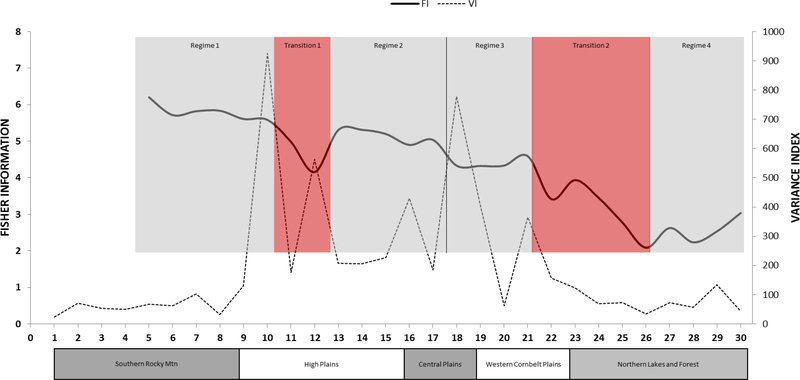
Fisher information detected four regimes and two transition zones which are roughly congruent with a priori expectations based on ecoregion maps, but diverge in significant ways (Figure 2). The total drop in FI between the high point in regime 1 and the low point in transition 1 is greater than that between regime 2 and regime 3 (∆FI of 2.05 and 0.98, respectively), suggesting that the difference in FI between the Southern Rocky Mountains and the 3 Plains ecoregions is greater than the difference among the Plains regions, which is to be expected. Likewise, the total drop in FI between regime 3 (all Plains routes) and regime 4 (Northern Lakes and Forest) is the largest of all (∆FI of 2.51), indicating that the greatest variation in bird community structure exists between these two regimes.
Figure 1B.
Zooplankton data collection locations.
The FI results suggest that avian community structure loses order from west to east, and this aligns with the reality of increasing intensive agricultural land use. FI classified the community structure in the first High Plains route as being similar enough to the eastern Southern Rocky Mountains to include it in the first regime. There followed a steady loss of order, as reflected in the FI value, across the western High Plains. When FI did stabilize, indicating a new regime, that regime captured routes from both the eastern High Plains and western Central Plains ecoregions, indicating a blurring of the distinction between the two Plains ecoregions in terms of vegetative cover and avian community structure. Similarly, the third regime incorporates routes from the eastern Central Plains and most of the Western Cornbelt Plains ecoregions, indicating that avian community structure did not significantly differ between the two Plains ecoregions. This is not an unexpected result, given that those two ecoregions are, in reality, a grassland-agriculture matrix.
The traditional indicators did not provide clear results and yielded graphs with no interpretable pattern (Figure 3), however, VI provided results that were complementary to FI (Figure 2). The VI peaks in several places which are congruent with regime shifts identified by FI (routes 10, 18, and 21). In general, the VI provides complementary information that supports the trend captured by FI, but is significantly more difficult to interpret when evaluated alone because it is not possible to ascertain whether a peak marks the beginning or end of a stable regime or of a transition zone.
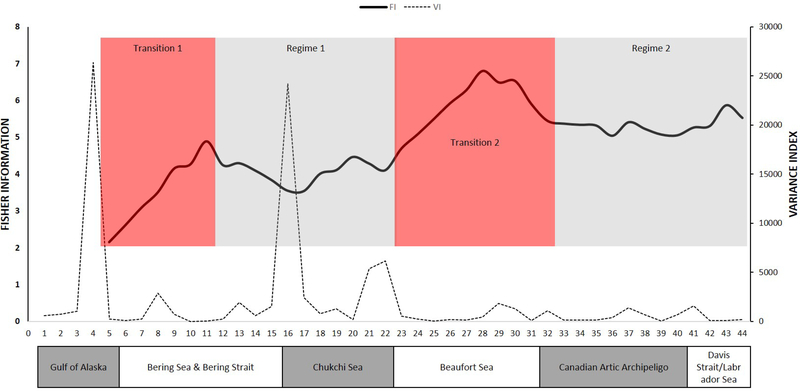
Figure 2.
Fisher information ((FI; bold solid line)) and Variance Index (VI; faint dotted line) for Breeding Bird Survey community data from 30 routes (numbered from 1 to 30 on the x-axis, reflecting the west to east ordering of the routes in geographic space). Regimes identified by FI are shown as shaded boxes around the plotted line. The Omernik ecoregion domains under the x-axis allow comparison as to how well the regimes align with the ecoregions, which represent potential rather than actual vegetation. Because one FI value is produced per window, the first FI value is at route 5.
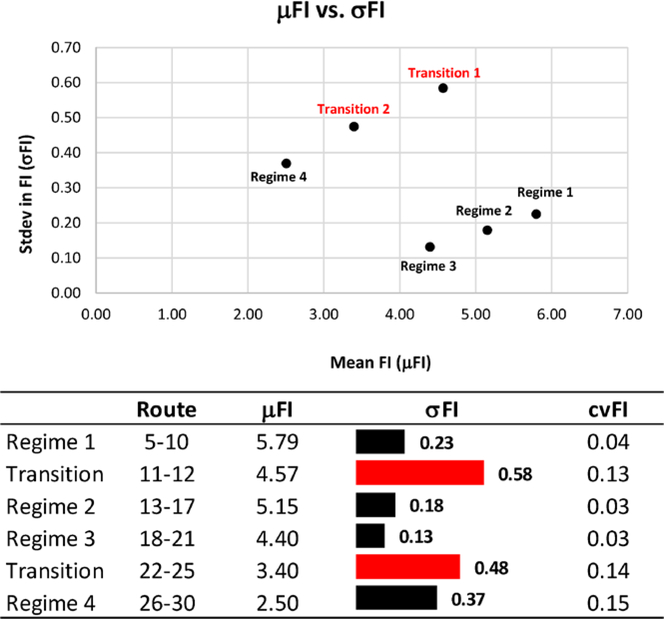
While all three descriptive statistics (mean (μFI), standard deviation (σFI), and coefficient of variation (cvFI) in FI) indicate relative stability in each of the first three regimes, the fourth regime, wholly comprised of routes from the Northern Lakes and Forest region, has a lower mean, higher standard deviation, and higher coefficient of variation in FI than the other regions, indicating that there is greater variation in community structure within this ecoregion (Figure 4). Furthermore, the two transition zones have a higher CV than the regimes (except the 4th regime), indicating zones of high variability as community structure transitions from one regime to another.
Figure 3.
Results for traditional regime shift parameters applied to the BBS avian community data: variance, skewness, kurtosis, and ARI (critical slowing down). The graphs are largely uninterpretable when used on multivariate data such as this.
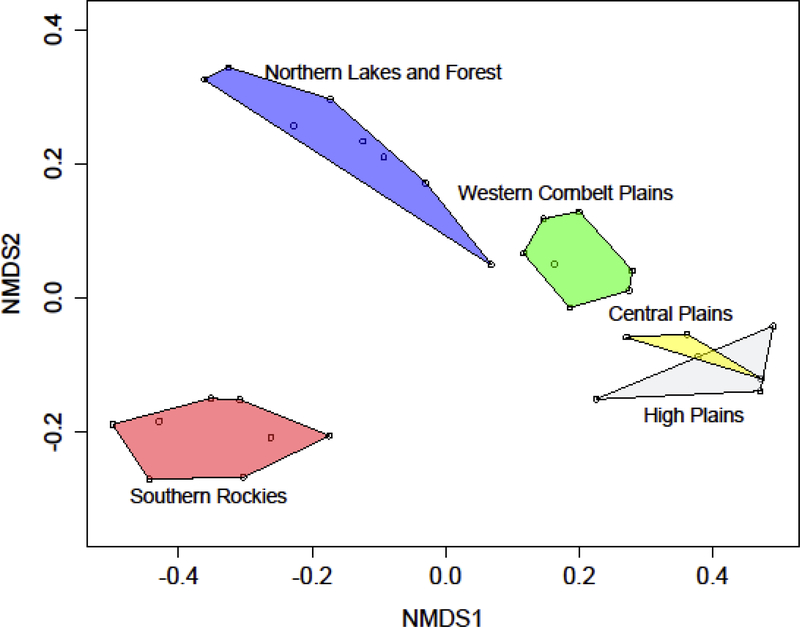
The results of the multivariate analyses suggest that while the nMDS (stress value of 0.080 for 2 dimensions) and cluster analysis (not shown on Figure 5 because results are identical to the nMDS) identifies distinct communities that align with the a priori expectations of the Omernick ecoregions, they do not distinguish between the High Plains and Central Plains communities. The nMDS (Figure 5) shows the dissimilarity in community structure in terms of the relative position of each route to every other in ordination space, as well as how those routes align with ecoregion expectations by drawing polygons that connect the routes belonging to each Omernik-defined ecoregion. The routes from the three Plains ecoregions are closer to each other in ordination space than either the Southern Rockies or Northern Lakes and Forest routes, indicating that they are more similar in community structure. The first route of the Northern Lakes and Forest region, indicated by FI as part of a long transition zone between regimes, is also very proximate in ordination space to the Cornbelt Plains routes, reflecting their closeness in geographic space. However, the High Plains and Central Plains overlap each, indicating that the nMDS does not perceive them as dissimilar.
Figure 4.
The stability of each terrestrial regime over space, as defined by the mean (μFI), standard deviation (σFI), and coefficient of variation (cvFI) of FI. While regimes 1–3 are clustered together and relatively stable with high μFI, low σFI and cvFI, Regime 4 was highly variable (low μFI, high σFI and cvFI). The transition periods exhibited the least amount of stability.
Marine data
Fisher information detected two regimes and two transition zones, which partially align with the a priori expectations for the locations of the oceanic domains (Figure 6). FI is low and rises steadily throughout two-thirds of the Bering Sea domain. Since FI never stabilizes in this domain, much of the Bering Sea is classified as a transition zone. The first regime extends from the northern Bering Sea through the Chukchi Sea. As the transect enters the Beaufort Sea, FI climbs steeply without stabilizing, indicating increasing dynamic order in community structure and classifying the Beaufort Sea as a second transition zone. The second regime extends from the more geographically closed-in waters of the Canadian Arctic Archipelago through the sixth oceanic domain, the Davis Strait/Labrador Sea. The entire distance from the western edge of the Archipelago to the Labrador Sea is represented by only 12 stations, so it is relatively under-represented compared to the western half of the survey.
Figure 5.
Ordination plot for the BBS avian community data (k = 2, stress = 0.080). The BBS routes are shown with open circles, while the polygons contain all the routes that fall into the ecoregions (Omernik 1987). The overlap between the High Plains and the Central Plains suggests that these two ecoregions do not substantially differ in avian community structure.
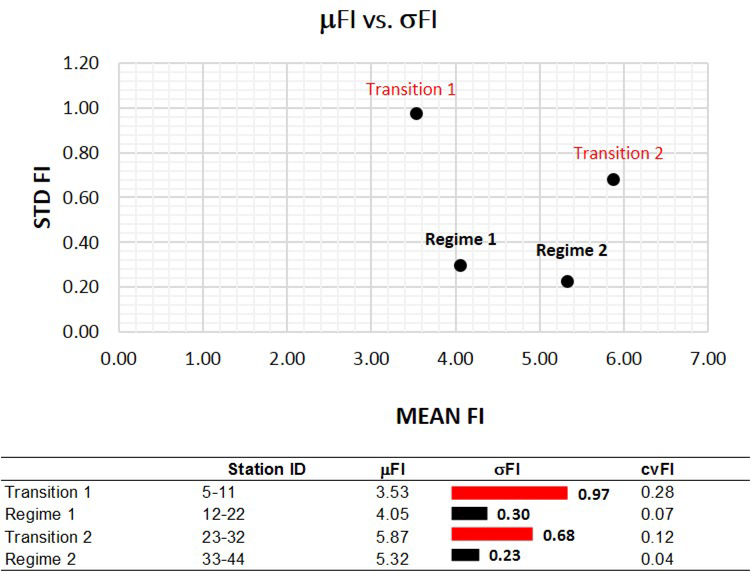
Like the terrestrial case study, when the FI trends are compared to the traditional regime shift indicators, only the VI was able to provide sensible results (Figure 6). The Variance Index peaks at the boundary of the Bering Sea, the Chukchi Sea, and to a lesser extent the Beaufort Sea Shelf. However, it does not distinguish whether the increased variance denotes the beginning of a stable regime, or signals a transition zone. The descriptive statistics support an overall picture of change in community structure which reflects successive patterns of an ecoregion with high variability (i.e. high σFI and cvFI) transitioning into a more stable regime (high μFI, and low σFI and cvFI) (Figure 7).
Figure 6.
Fisher information (FI; bold solid line) and Variance Index (VI; faint dotted line) for zooplankton community data collected from 44 stations along a transect that begins in the Pacific ocean, traverses the Arctic, and ends in the Labrador Sea (numbered from 1 to 44 and ordered from west to east along the x-axis). Because one FI value is produced per window, the first FI value is at route 5. The regimes and transition zones identified by FI are shown as boxes drawn around the FI plotted line. The a priori-defined oceanic domains are under the x-axis, to see how well the location of the regimes identified by FI align with the oceanic domains identified in the literature.
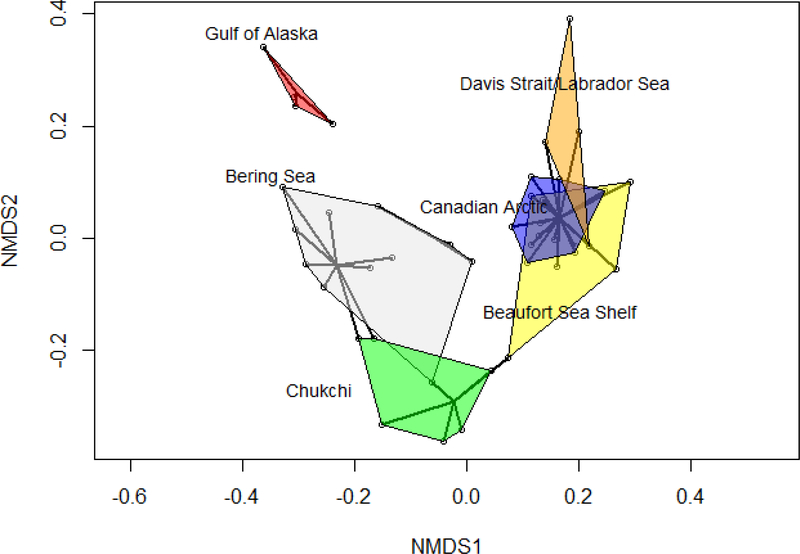
The multivariate analyses support the FI results, and suggest that the boundaries between the a priori defined ecological domains are soft, particularly between the Bering Sea and Chukchi Sea. When viewed in ordination space, the nMDS places the stations so they more or less flow from west to east along the arc, but there is also strong overlap in community structure at sampling locations near the edges of the domains (Figure 8; (stress value of 0.121 for 3 dimensions)). The cluster analysis (Figure 8; pruned to 6 clusters) divides the stations of the Bering Sea into two clusters, and places two of the Bering Sea stations in the Chukchi cluster, as well as fails to distinguish between the Canadian Arctic and the Davis Strait/Labrador Sea. The overall result is that the zooplankton communities do not have crisp boundaries which fully align with the a priori defined domains described in the methods, but have softer boundaries with considerable overlap in community structure between domains. Furthermore, FI communicates a richer story of community structure transitioning across space than either the nMDS or cluster analysis. However, unlike the BBS case study, the transition zones were marked by a rise in FI, as opposed to a drop, which may suggest a possible slowing down of changes in community structure before the patterns destabilized and the system organized into a new regime. Further work on the underlying system dynamics would be instructive.
Figure 7.
The stability of each marine regime over space, as defined by the mean (μFI), standard deviation (σFI) and coefficient of variation of FI (cvFI). While the two regimes are relatively stable with high μFI, low σFI and low cvFI, the transition periods exhibited the least stability. Note: Regimes reflect the domains identified by the trend in FI, not the regimes a priori identified using Carmack et al. (2010)) and Archambault et al. (2010).
Discussion
Detecting spatial regimes with Fisher information
Given animal community data, we found that Fisher information was able to detect spatial regimes and transitions between spatial regimes in both terrestrial and aquatic ecosystems, across regional scales (1900 and 12,000 kilometres respectively). These studies were an important step towards determining the utility of FI in detecting spatial regimes in both aquatic and terrestrial systems, even given data limitations. In contrast, the traditional indictors we examined, such as variance, skewness, kurtosis, and critical slowing down, were unable to detect spatial regimes, though this was unsurprising as they are not suited for multivariate data. The VI helped to confirm general trends, but it does not reveal details about the regime dynamics that are useful for assessing the behavior of the system, e.g., whether there is a stable regime between two peaks, or whether changes in the VI are capturing a transition. Our results suggest that Fisher information can be a powerful, easy-to-use tool to assess regime shifts in animal (or other) community data, providing a biological link between anthropogenic disturbances such as land use and climate change and spatial shifts in ecological communities.
The ecological reality of community regimes
Our analyses demonstrated that the bird community boundaries only roughly coincided with the expectations of ecoregion maps. There are substantial differences between the potential vegetation underpinning the ecoregion classifications, and the actual spatial locations of stable avian communities. If FI were to fully coincide with the ecoregion maps, then we would expect to see a stable FI value through the center of each ecoregion, with evidence of increasing variability at the borders, indicated by declining FI. Instead, the High Plains had high variability in community structure throughout the core of the ecoregion. And rather than FI identifying three distinct Plains regimes, as per the ecoregion expectation, it identified two regimes, each of which straddled routes from the Central Plains. In other words, the avian community structure was simplified relative to ecological expectations, with a blurring of the boundaries between what are considered distinct ecoregion types by US land agencies. Indeed, the difference in FI between regime 2 and regime 3 is such that the argument could be made that the entire Great Plains is one regime, with a slow but steady loss of order as one moves from west to east, corresponding with an increasing intensity of agriculture. The transitions to and from the Plains are both much steeper than that between the two Plains regimes, as would be expected.
The land cover summary (Table 1) supports the findings of FI as it demonstrates that the three prairie landscapes exist on a gradient of actual vegetative cover. As we move east from the High Plains to the Cornbelt Plains, the percent grassland cover drops dramatically from 60% to 5%, and the percent of row crop land cover rises 14% to 74% (Table 1). The most significant changes occur between the High Plains and the Central Great Plains. These patterns are in contradiction to ecoregion maps (Omernik 1987; Bailey 2015), which hold the difference between the Central Great Plains and the Western Cornbelt Plains as much more fundamental (a Level I division) than that between the High Plains and the Central Great Plains (a Level III division). To the extent that the land use cover in each 400 m route buffer around the ~40 km route reflects on a gross level the land cover of each ecoregion, it seems likely that the heterogeneity within the Plains landscapes due to agriculture and grazing has been reduced.
Table 1.
Land cover classification for a 400 m buffer around each 41 km BBS route. The dominant land cover type for each ecoregion is in bold. Note that Northern Lakes and Forest is roughly evenly split between Deciduous Forest and Woody Wetlands, evidence for the hetereogeneity of the region.
| Landcover Type | Southern Rockies |
High Plains |
Central Plains |
Western Cornbelt |
Northern Lakes and Forest |
|---|---|---|---|---|---|
| Open Water | 0.01 | 0.01 | 0.01 | 0.04 | |
| Low Intensity Residential | 0.02 | ||||
| Deciduous Forest | 0.14 | 0.02 | 0.03 | 0.25 | |
| Evergreen Forest | 0.47 | 0.12 | |||
| Mixed Forest | 0.01 | 0.11 | |||
| Shrubland | 0.15 | ||||
| Grassland/Herbaceous | 0.18 | 0.61 | 0.20 | 0.05 | |
| Pasture/Hay | 0.02 | 0.04 | 0.08 | 0.12 | 0.10 |
| Row Crops | 0.14 | 0.66 | 0.74 | 0.03 | |
| Small Grains | 0.13 | 0.02 | 0.01 | ||
| Fallow | 0.07 | ||||
| Woody Wetlands | 0.28 | ||||
| Emergent Herb Wetland | 0.01 | 0.04 | |||
*Only showing those categories for which at least one ecoregion has > 1%
The length of each transition zone is suggestive of soft, rather than the hard boundaries depicted on ecoregion maps (Bailey 1983; Omernik 1987). The long transition from the Cornbelt Plains to the Northern Lakes and Forest, which covered more than 400 kilometres, may be impacted by two factors: First, the final two routes in the Cornbelt Plains occur on the upward sweep of the transect and so are substantially more northern than the other Cornbelt Plains routes. Latitude is known to affect animal communities (Clergeau et al. 2006). Second, the first route in the Northern Lakes ecoregion technically falls into a narrow band of the North Central Hardwood Forest. This rapid shifting across three ecoregions is captured by FI as a long transition before the fourth regime begins. Finally, the higher cvFI and thus relative variability of FI in the fourth regime, which falls wholly within the Northern Lakes and Forest ecoregion, is possibly explained by the heterogeneity of the land cover, though it is also possible that further data points would reveal the fourth regime as another transition. Simply put, community structure in this ecoregion is likely more variable than in the other regimes because the landscape itself is more variable, as it is a patchy mosaic of water features and forest (Table 1).
The zooplankton data tell a similar story to the avian data. Although there is correspondence between zooplankton community structure, large scale oceanic structure, and regime transitions as detected by FI, some boundaries are less defined than a priori expectations. Domains thought to contain distinct communities, such as the Bering Sea or Beaufort Sea Shelf (Springer et al. 1989; Hopcroft et al. 2010; Pomerleau et al. 2014), appear to be transition zones between stable communities. The failure of both FI and the nMDS to distinguish between the Canadian Arctic Archipelago and Davis Strait/Labrador Sea may be a function of inconsistent sample coverage. Further work examining how the frequency of sampling affects the power and sensitivity of FI is warranted.
The inability of FI to crisply distinguish between the Bering Sea and the Chukchi Sea is consistent with our understanding of the region as a mixing zone where Bering Shelf water mixes with water from the Anadyr current, which enters from the west, and Alaska coastal water, which enters the Bering Strait from the east (Coachman et al. 1975). These three water masses are believed to harbour unique zooplankton communities (Springer et al. 1989), and as the water masses do not mix until they pass through the Bering Strait into the Chukchi Sea, the zooplankton community contains a mixture of communities that differ from the southern Bering Sea and have high patchiness (Eisner et al. 2014; Pomerleau et al. 2014). As the transect enters the Beaufort Sea, there is a decline in both Pacific taxa and zooplankton community patchiness associated with the mixing of the three Pacific water masses and Arctic water, corresponding to greater similarity among samples and increasing dynamic order in FI. The expectation was that the Chukchi, understood to be a mixing zone of watermasses, would be identified by FI as a transition zone, while the Beaufort Sea Shelf would be a stable regime. Instead, the northern part of the Bering Sea and the Chukchi had a stable FI value denoting it as a regime, while the Beaufort Sea Shelf underwent a long and significant increase in dynamic order that never flattened sufficiently to qualify as a regime. This means that the variability in zooplankton community structure as the transect traverses the Beaufort Sea was much higher than that of the northern Bering/Chukchi Sea, despite the latter region consisting of a mixing zone of multiple water masses. The FI results suggest that studies on dominant zooplankton species within each domain (Nelson et al. 2009; Walkusz et al. 2010; Pomerleau et al. 2014) may not strictly correlate to bigger picture studies which assess variability in community structure over space, or that zooplankton species compositional data or the way in which they are collected are not a good proxy for spatial regimes.
What Fisher information captures that multivariate analysis does not
The nMDS analysis largely aligned with the a priori ecoregion and oceanographic domain expectations, but was not always able to distinguish between ecoregions (the High Plains and Central Plains) or domains (Canadian Arctic and Davis Strait/Labrador Sea), though in the case of the zooplankton data, may be a function of insufficient sampling stations in those domains. Perhaps most importantly, the multivariate analyses are largely visual; ordination methods create their own space, and thus do not tell us about spatial shifts in the location of a community. Routes that were geographically farther away from each other tended to be more dissimilar than routes that were close together. However, this rather crude depiction of community structure does not tell us where the boundaries between communities occur, whether they are hard or soft, or if the soft boundaries are themselves ecotones with stable community structure. Furthermore, the approach does not provide any insight on the spatial extent of the transitions. The ability to assess whether or not a particular community is gaining or losing order over time could allow land use managers to anticipate a potential regime shift within a location, or document if community locations shift in space over time. That said, our ability to detect change using FI may be improved by employing post-hoc tests to assess trends in the index. Researchers have explored approaches such as cut-offs, Mann-Kendall tests, and Bayesian methods to help reduce interpretive uncertainty (Heberling & Hopton 2010; Vance et al. 2015; González-Mejía et al. 2016), but these methods are still under development.
Idio- or non-idiosyncratic changes in animal community regimes?
To what extent can we expect changes in plant and animal communities to occur in a fashion detectable by monitoring and analytical methods like the one presented here? Our contention is that it will depend on whether or not species’ response to anthropogenic change is idiosyncratic within and across taxa. If species’ responses are fully idiosyncratic, then the patterns at the community level will become chaotic as a function of independent species’ responses as anthropogenic impacts accumulate and intensify. Accordingly, tracking spatial regimes and the location of the transition zones between them would not be a useful activity for managers or scientists. There are, however, constraints on individual response such that pattern identification will remain useful and feasible on shorter timescales, though the possibility of no-analog communities seems highly likely for multi-decadal or longer time scales (Williams & Jackson 2007). In general, we expect to see changes in animal abundances in the short term as a response to climate change and anthropogenic influence, as opposed to changes in presence/absence. Changes may result from range shifts, as there is substantial evidence documenting vagile species recently shifting their ranges to track their climatic niche (Parmesan, 2006; Parmesan & Yohe, 2003; Tingley et al., 2009), but the rate of climate change is such that migration capabilities are unlikely to keep up with the rate of thermal change (Thuiller et al. 2008), and the ability to shift ranges is further impeded by habitat fragmentation, which has been shown to reduce range shift (Iverson et al. 2004; Thuiller et al. 2008). As a result, range contraction due to a lack of suitable habitat and reduced survivorship within their original range is also expected (Davis & Shaw 2001; Parmesan 2006).
These issues confound the identification of ecological boundaries and our ability to track changes in boundaries over time. Fisher information can assist researchers and managers in tracking changes in the patterns of community structure associated with habitat types or biogeographical distribution areas, as well as the temporal dissolution of community structure as no-analog communities assemble over time. A substantial benefit to Fisher information is that it circumvents many of the difficulties currently present in defining ecological boundaries, such as problems of non-linear responses across ecotones, landscape fragmentation, and land use change in terrestrial systems, or the ephemeral nature of some oceanographic boundaries, as well as the vast spatial scales involved, all of which can be difficult to capture without exhaustive data collection (Strayer et al. 2003; Kent et al. 2006; Danz et al. 2012). Other researchers have discussed the challenges of tracking boundary region shifts as a way to monitor climate change, when, for example, little to no native vegetation remains (less than 5% of the original prairie in the United States due to land conversion), and critical structuring processes have been repressed or altered (natural fire regimes supressed) (Danz et al. 2012). Fisher information allows for the simultaneous analysis of multiple, disparate variables and provides a synoptic approach that may allow for detection of ecological change and boundary shift without pre-supposing key taxa as bell-weather species of change. However, future studies wishing to estimate more precisely the location of boundaries and how they may shift over time may also need to account for phonological/seasonal detection differences in the taxon under question.
We also propose that monitoring animal populations is more likely to reflect currently changing conditions and is easier than detecting variation in plant communities or oceanographic properties. Remotely-sensed data remain challenged to identify physically similar but floristically different species, and ground-truthing large ecological regions is unfeasible. Animal species’ responses are likely to occur more rapidly than plants, as there can be a large mismatch between vegetation and climate change, with changes in vegetation lagging substantially behind changes in climate (Beckage et al. 2008). Long-lived species such as trees can exhibit ecosystem responses to land use and climate change at century-scales because of the spatial and temporal processes structuring forests (Starfield & Chapin 1996), while terrestrial animal species are more vagile and can act as a leading indicator of vegetation change, or of a change in climatic variables such as temperature. Furthermore, as we demonstrated, there can be significant differences between ecoregion mapping, which is based on potential vegetation as a function of geomorphology and soils, and the location of spatial regimes actually present after decades of land use changes. All of these issues make it critical to identify reliable spatially-explicit tools for mapping the effects of climate and land use change on biodiversity (Mokany & Ferrier 2011), and our research suggests that Fisher information can be one of those tools.
Conclusion
Our analyses confirmed that when using multivariate data, traditional early warning indicators are very difficult to interpret, and integrated indicators such as FI and VI more consistently detect regime shifts. We found that Fisher information provided the clearest, most detailed, and interpretable signal of spatial regime shifts. Although the Variance Index did not provide clear signals as a stand-alone indicator, some congruent trends are found when the results are presented in conjunction with FI. Fisher information has the further benefit of being highly flexible in terms of the choice of variable selection and data input, and is able to detect a clear signal without the need for difficult-to-acquire high resolution data.
This research had the further benefit of highlighting the incongruence between terrestrial ecoregion maps, which are focused on ecological potential, and the ecological reality of community regimes given land use and climate change. The method presented would allow researchers to track both the shifting spatial locations of communities over time, as well as the change over time within a location, both of which are critical as the consequences of anthropogenic change manifests in community structure and dynamics over time and space.
We appreciate that for both systems analysed, a different taxa could show spatial regimes in different locations. Reptile or mammal community regime location may or may not overlap bird regime location, and the transitions between ecoregions may be more or less steep given the taxa under consideration. Neither mammals nor reptiles tend to be as vagile as birds, and their ability to disperse in response to climate or land use change is accordingly more limited. Further research evaluating the spatial regimes of other taxa and the extent to which they overlap bird and zooplankton species would be useful.
Finally, further studies that looked more deeply into community structure within a spatial regime could inform managers as to which subgroups of species are most dominant within each regime, while correlation analysis could identify the subgroups of species responsible for driving the value of Fisher information within each regime, both of which would allow managers to objectively select subgroups of species to monitor as the primary indicators of ecological stability within a community.
Figure 8.
Ordination plot for the zooplankton community data (k = 3; stress = 0.121. The sampling stations are shown with open circles. The results of a cluster analysis (pruned to 6 clusters) are shown with black spiders, while the oceanic domains a priori identified from the literature are represented by the colored polygons. Both the nMDS and the cluster analysis fail to assign some sampling stations to the ‘correct’ oceanic domain for all domains except the Gulf of Alaska.
Acknowledgements
This research arose from a workshop series, “Understanding and managing for resilience in the face of global change”, which was funded by the USGS John Powell Center for Synthesis and Analysis, and the USGS National Climate Change and Wildlife Center. Many thanks to Powell for supporting collaborative and interdisciplinary research efforts. Thanks to JC Nelson at the USGS Upper Midwest Environmental Sciences Center for creating Figure 1A. The Nebraska Cooperative Fish and Wildlife Research Unit is jointly supported by a cooperative agreement between the United States Geological Survey, the Nebraska Game and Parks Commission, the University of Nebraska−Lincoln, the United States Fish and Wildlife Service, and the Wildlife Management Institute. The views expressed in this article are those of the authors and do not necessarily represent the views or policies of the U.S. Environmental Protection Agency.
Footnotes
Data accessibility: Should the manuscript be accepted, the zooplankton data will be archived at http://www.arcodiv.org/Database/Plankton_datasets.html and the data DOI will be included at the end of the article. The Breeding Bird Survey data is in the public domain.
Statement of authorship: CRA designed the study with input from all authors, SMS and JN managed the data, SMS and TE did the analyses, SMS, TE and JN wrote the first draft, and all authors contributed substantially to revisions.
References
- 1.Allen CD & Breshears DD (1998). Drought-induced shift of a forest-woodland ecotone : Rapid landscape response to climate variation. Proc. Natl. Acad. Sci. U. S. A, 95, 14839–14842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anand M & Orloci L (2000). On partitioning of an ecological complexity function. Ecol. Modell, 132, 51–61 [Google Scholar]
- 3.Archambault P, Snelgrove P, Fisher J, Gagnon J-M & Garbary D (2010). From sea to sea: Canada’s Three Oceans of Biodiversity. PLoS One, 5, e12182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bailey RG (1983). Delineation of ecosystem regions. Environ. Manage, 7, 365–373 [Google Scholar]
- 5.Bailey RG (2015). Ecoregions of the United States. Available at: http://www.fs.fed.us/rm/ecoregions/products/map-ecoregions-united-states/. Last accessed 20 May 2009
- 6.Batt RD, Brock WA, Carpenter SR, Cole JJ, Pace ML & Seekell DA (2013). Asymmetric response of early warning indicators of phytoplankton transition to and from cycles. Theor. Ecol, 6, 285–293 [Google Scholar]
- 7.Beaugrand G, Reid P, Ibanez F, Lindley J & Edwards M (2002). Reorganization of North Atlantic marine copepod biodiversity and climate. Science (80-. )., 96, 1692–1694 [DOI] [PubMed] [Google Scholar]
- 8.Beckage B, Osborne B, Gavin DG, Pucko C, Siccama T & Perkins T (2008). A rapid upward shift of a forest ecotone during 40 years of warming in the Green Mountains of Vermont. Proc. Natl. Acad. Sci. U. S. A, 105, 4197–4202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boulton CA, Allison LC & Lenton TM (2014). Early warning signals of Atlantic Meridional Overturning Circulation collapse in a fully coupled climate model. Nat. Commun, 5, 5752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brock WA & Carpenter SR (2006). Variance as a leading indicator of regime shift in ecosystem services. Ecol. Soc, 11 [Google Scholar]
- 11.Brock WA & Carpenter SR (2012). Early warnings of regime shift when the ecosystem structure is unknown. PLoS One, 7, e45586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burrows MT, Schoeman DS, Buckley LB, Moore P, Poloczanska ES, Brander KM, et al. (2011). The pace of shifting climate in marine and terrestrial ecosystems. Science (80-. )., 334, 652–655 [DOI] [PubMed] [Google Scholar]
- 13.Cabezas H & Eason T (2010). Fisher information and order. In: San Luis basin sustainability metrics project: A methodology for assessing regional sustainability (eds. Heberling MT & Hopton ME). EPA Report, pp. 163–222 [Google Scholar]
- 14.Carmack E, McLaughlin F, Vagle S & Melling H (2008). Canada’s Three Oceans (C3O): A Canadian contribution to the International Polar Year. PICES Press, 16, 22–25 [Google Scholar]
- 15.Carmack EC, McLaughlin FA, Vagle S, Melling H & Williams WJ (2010). Structures and property distributions in the three oceans surrounding Canada in 2007: A basis for a long-term ocean climate monitoring strategy. Atmosphere-Ocean, 48, 211–224 [Google Scholar]
- 16.Carpenter SR, Brock WA, Cole JJ & Pace ML (2009). Leading indicators of phytoplankton transitions caused by resource competition. Theor. Ecol, 2, 139–148 [Google Scholar]
- 17.Clarke K (1993). Non-parametric multivariate analyses of changes in community structure. Aust. J. Ecol, 18, 117–143 [Google Scholar]
- 18.Clergeau P, Croci S, Jokimäki J, Kaisanlahti-Jokimäki ML & Dinetti M (2006). Avifauna homogenisation by urbanisation: Analysis at different European latitudes. Biol. Conserv, 127, 336–344 [Google Scholar]
- 19.Cline TJ, Seekell D, Carpenter S, Pace M, Hodgson J, Kitchell J, et al. (2014). Early warnings of regime shifts: evaluation of spatial indicators from a whole-ecosystem experiment. Ecosphere, 5, Art 102 [Google Scholar]
- 20.Coachman L, Aagaard K & Tripp R (1975). Bering Strait: The regional and physical oceanography. University of Washington Press, Seattle, Washington [Google Scholar]
- 21.Dai L, Korolev KS & Gore J (2013). Slower recovery in space before collapse of connected populations. Nature, 496, 355–359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dai L, Vorselen D, Korolev KS & Gore J (2012). Generic indicators for loss of resilience before a tipping point leading to population collapse. Science (80-. )., 336, 1175–1177 [DOI] [PubMed] [Google Scholar]
- 23.Dakos V, Carpenter SR, Brock WS, Ellison AM, Guttal V, Ives AR, et al. (2012). Methods for detecting early warnings of critical transitions in time series illustrated using simulated ecological data. PLoS One, 7, e41010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dakos V, Kéfi S, Rietkerk M, van Nes EH & Scheffer M (2011). Slowing down in spatially patterned ecosystems at the brink of collapse. Am. Nat, 177, E153–66 [DOI] [PubMed] [Google Scholar]
- 25.Dakos V, van Nes EH & Scheffer M (2013). Flickering as an early warning signal. Theor. Ecol, 6, 309–317 [Google Scholar]
- 26.Dakos V, Scheffer M, van Nes EH, Brovkin V, Petoukhov V & Held H (2008). Slowing down as an early warning signal for abrupt climate change. Proc. Natl. Acad. Sci, 105, 14308–14312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Danz NP, Frelich LE, Reich PB & Niemi GJ (2012). Do vegetation boundaries display smooth or abrupt spatial transitions along environmental gradients? Evidence from the prairie-forest biome boundary of historic Minnesota, USA. J. Veg. Sci, 24, 1129–1140 [Google Scholar]
- 28.Davis MB & Shaw RG (2001). Range shifts and adaptive responses to quaternary climate change. Science (80-. )., 292, 673–680 [DOI] [PubMed] [Google Scholar]
- 29.Drake JM & Griffen BD (2010). Early warning signals of extinction in deteriorating environments. Nature, 467, 456–459 [DOI] [PubMed] [Google Scholar]
- 30.Eason T & Cabezas H (2012). Evaluating the sustainability of a regional system using Fisher information in the San Luis Basin, Colorado. J. Environ. Manage, 94, 41–9 [DOI] [PubMed] [Google Scholar]
- 31.Eason T & Garmestani AS (2012). Cross-scale dynamics of a regional urban system through time. Reg. Dev, 36, 55–76 [Google Scholar]
- 32.Eason T, Garmestani AS & Cabezas H (2014). Managing for resilience: early detection of regime shifts in complex systems. Clean Technol. Environ. Policy, 16, 773–783 [Google Scholar]
- 33.Eason T, Garmestani AS, Stow CA, Rojo C, Alvarez-Cobelas M & Cabezas H (2016). Managing for resilience: an information theory-based approach to assessing ecosystems. J. Appl. Ecol, n/a-n/a [Google Scholar]
- 34.Edwards M & Richardson AJ (2004). Impact of climate change on marine pelagic phenology and trophic mismatch. Nature, 430, 881–884 [DOI] [PubMed] [Google Scholar]
- 35.Eisner L, Napp J, Mier K, Pinchuk A & Andrews III A (2014). Climate-mediated changes in zooplankton community structure for the eastern Bering Sea. Deep Sea Res. Part II Trop. Stud. Oceanogr, 109, 157–171 [Google Scholar]
- 36.Fagan WF, Fortin M-J & Soykan C (2003). Integrating edge detection and dynamic modeling in quantitative analyses of ecological boundaries. Bios, 53, 730–738 [Google Scholar]
- 37.Fath BD & Cabezas H (2004). Exergy and Fisher Information as ecological indices. Ecol. Modell, 174, 25–35 [Google Scholar]
- 38.Fath BD, Cabezas H & Pawlowski CW (2003). Regime changes in ecological systems: An information theory approach. J. Theor. Biol, 222, 517–530 [DOI] [PubMed] [Google Scholar]
- 39.Fisher RA (1922). On the mathematical foundations of theoretical statistics. Philos. Trans. R. Soc. A, 222, 309–368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fukami T & Nakajima M (2011). Community assembly: alternative stable states or alternative transient states? Ecol. Lett, 14, 973–984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gonzalez-Mejia AM (2011). Fisher information: Sustainability analysis of several US metropolitan statistical areas. University of Cincinnati [Google Scholar]
- 42.González-Mejía AM, Eason TN & Cabezas H (2016). System learning approach to assess sustainability and forecast trends in regional dynamics: The San Luis Basin study, Colorado, U.S.A. Environ. Model. Softw, 81, 1–11 [Google Scholar]
- 43.Gonzalez-Mejia AM, Vance L, Eason T & Cabezas H (2015). Recent development in the application of Fisher information to sustainable environmental management In: Assessing and measuring environmental impact and sustainabililty (ed. Klemes J). Elsevier, New York [Google Scholar]
- 44.Grebmeier J, Overland J, Moore S, Farley E, Carmack E, LW C, et al. (2006). Major ecosystem shift in the Northern Bering Sea. Science (80-. ), 311, 1461–1464 [DOI] [PubMed] [Google Scholar]
- 45.Guttal V & Jayaprakash C (2008). Changing skewness: an early warning signal of regime shifts in ecosystems. Ecol. Lett, 11, 450–60 [DOI] [PubMed] [Google Scholar]
- 46.Hastings A & Wysham DB (2010). Regime shifts in ecological systems can occur with no warning. Ecol. Lett, 13, 464–72 [DOI] [PubMed] [Google Scholar]
- 47.Heberling MT & Hopton ME (2010). San Luis Basin Sustainability Metrics Project: A methodology for evaluating regional sustainability [DOI] [PubMed]
- 48.Hooff RC & Peterson WT (2006). Copepod biodiversity as an indicator of changes in ocean and climate conditions of the northern California current ecosystem. Limnol. Oceanogr, 51, 2607–2620 [Google Scholar]
- 49.Hopcroft R, Kosobokova K & Pinchuk A (2010). Zooplankton community patterns in the Chukchi Sea during summer 2004. Deep Sea Res. Part II Trop. Stud. Oceanogr, 57, 27–39 [Google Scholar]
- 50.Iverson LR, Schwartz MW & Prasad AM (2004). How fast and far might tree species migrate in the eastern United States due to climate change? Glob. Ecol. Biogeogr, 13, 209–219 [Google Scholar]
- 51.Karunanithi AT, Cabezas H, Frieden BR & Pawlowski CW (2008). Detection and assessment of ecosystem regime shifts from Fisher Information. Ecol. Soc, 13 [Google Scholar]
- 52.Karunanithi AT, Garmestani AS, Eason T & Cabezas H (2011). The characterization of socio-political instability, development and sustainability with Fisher information. Glob. Environ. Chang, 21, 77–84 [Google Scholar]
- 53.Kéfi S, Guttal V, Brock WA, Carpenter SR, Ellison AM, Livina VN, et al. (2014). Early warning signals of ecological transitions: Methods for spatial patterns. PLoS One, 9, e92097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kent M, Moyeed RA, Reid CL, Pakeman R & Weaver R (2006). Geostatistics, spatial rate of change analysis and boundary detection in plant ecology and biogeography. Prog. Phys. Geogr, 30, 201–231 [Google Scholar]
- 55.Lindegren M, Dakos V, Gröger JP, Gårdmark A, Kornilovs G, Otto SA, et al. (2012). Early detection of ecosystem regime shifts: a multiple method evaluation for management application. PLoS One, 7, e38410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Litzow MA, Mueter FJ & Urban JD (2013). Rising catch variability preceded historical fisheries collapses in Alaska. Ecol. Appl, 23, 1475–1487 [DOI] [PubMed] [Google Scholar]
- 57.Longhurst A (1998). Ecological Geography of the Seas. Academic Press, San Diego [Google Scholar]
- 58.Mantua N (2004). Methods for detecting regime shifts in large marine ecosystems: A review with approaches applied to North Pacific data. Prog. Oceanogr, 60, 165–182 [Google Scholar]
- 59.Mayer AL, Pawlowski CW, Fath BD & Cabezas H (2007). Applications of Fisher information to the management of sustainable environmental systems In: Exploratory Data Analysis Using Fisher Information (eds. Frieden BR & Gatenby RA). Springer-Verlag, London, pp. 217–243 [Google Scholar]
- 60.Mokany K & Ferrier S (2011). Predicting impacts of climate change on biodiversity: a role for semi-mechanistic community-level modelling. Divers. Distrib, 17, 374–380 [Google Scholar]
- 61.Nelson R, Carmack E & McLaughlin F (2009). Penetration of Pacific zooplankton into the western Arctic Ocean tracked with molecular population genetics. Mar. Ecol. Prog. Ser, 381, 129–138 [Google Scholar]
- 62.Oksanen J (2013). Multivariate analysis of ecological communities in R: vegan tutorial
- 63.Omernik JM (1987). Ecoregions of the conterminous United States. Ann. Assoc. Am. Geogr, 77, 118–125 [Google Scholar]
- 64.Pace ML, Carpenter SR, Johnson RA & Kurtzweil JT (2013). Zooplankton provide early warnings of a regime shift in a whole lake manipulation. Limnol. Oceanogr, 58, 525–532 [Google Scholar]
- 65.Parmesan C (2006). Ecological and evolutionary responses to recent climate change. Annu. Rev. Ecol. Evol. Syst, 37, 637–669 [Google Scholar]
- 66.Parmesan C & Yohe G (2003). A globally coherent fingerprint of climate change impacts across natural systems. Nature, 421, 37–42 [DOI] [PubMed] [Google Scholar]
- 67.Patricio J, Ulanowicz RE, Pardal MA & Marques JC (2004). Ascendency as an ecological indicator: A case study of estuarine pulse eutrophication. Estuar. Coast. Shelf Sci, 60, 23–35 [Google Scholar]
- 68.Pawlowski CW & Cabezas H (2008). Identification of regime shifts in time series using neighborhood statistics. Ecol. Complex, 5, 30–36 [Google Scholar]
- 69.Pearman PB, Randin CF, Broennimann O, Vittoz P, van der Knaap WO, Engler R, et al. (2008). Prediction of plant species distributions across six millennia. Ecol. Lett, 11, 357–369 [DOI] [PubMed] [Google Scholar]
- 70.Pearson RG (2006). Climate change and the migration capacity of species. Trends Ecol. Evol, 21, 111–3 [DOI] [PubMed] [Google Scholar]
- 71.Perretti CT & Munch SB (2012). Regime shift indicators fail under noise levels commonly observed in ecological systems. Ecol. Appl, 22, 1772–1779 [DOI] [PubMed] [Google Scholar]
- 72.Pomerleau C, Nelson RJ, Hunt BPV, Sastri AR & Williams WJ (2014). Spatial patterns in zooplankton communities and stable isotope ratios in relation to oceanographic conditions in the sub-Arctic Pacific and western Arctic regions during the summer of 2008. J. Plankton Res, 36, 757–775 [Google Scholar]
- 73.Pomerleau C, Winkler G, Sastri AR, Nelson RJ, Vagle S, Lesage V, et al. (2011). Spatial patterns in zooplankton communities across the eastern Canadian sub-Arctic and Arctic waters: insights from stable carbon and nitrogen isotope ratios. J. Plankton Res, 33, 1779–1792 [Google Scholar]
- 74.R Development Core Team. (2013). R: A language and environment for statistical computing. Available at: http://www.r-project.org. Last accessed
- 75.Rice JC & Rochet MJ (2005). A framework for selecting a suite of indicators for fisheries management. ICES J. Mar. Sci, 62, 516–527 [Google Scholar]
- 76.Rombouts I, Beaugrand G, Artigas LF, Dauvin J-C, Gevaert F, Goberville E, et al. (2013). Evaluating marine ecosystem health: Case studies of indicators using direct observations and modelling methods. Ecol. Indic, 24, 353–365 [Google Scholar]
- 77.Samhouri JF, Levin PS & Harvey CJ (2009). Quantitative evaluation of marine ecosystem indicator performance using food web models. Ecosystems, 12, 1283–1298 [Google Scholar]
- 78.Sauer JR, Hines J & Fallon JE (2014). USGS Patuxent Wildlife Research Center. North Am. Breed. Bird Surv. 1966–2012. Available at: http://www.pwrc.usgs.gov/bbs. Last accessed 1 January 2011
- 79.Scheffer M (2009). Critical transitions in nature and society. Princeton University Press, Princeton, New Jersey [Google Scholar]
- 80.Scheffer M, Bascompte J, Brock WA, Brovkin V, Carpenter SR, Dakos V, et al. (2009). Early-warning signals for critical transitions. Nature, 461, 53–59 [DOI] [PubMed] [Google Scholar]
- 81.Scheffer M & Carpenter SR (2003). Catastrophic regime shifts in ecosystems: linking theory to observation. Trends Ecol. Evol, 18, 648–656 [Google Scholar]
- 82.Scheffer M, Rinaldi S, Huisman J & Weissing FJ (2003). Why plankton communities have no equilibrium: Solutions to the paradox. Hydrobiologia, 491, 9–18 [Google Scholar]
- 83.Seekell DA, Carpenter SR, Cline TJ & Pace ML (2012). Conditional heteroskedasticity forecasts regime shift in a whole-ecosystem experiment. Ecosystems, 15, 741–747 [Google Scholar]
- 84.Seekell DA, Carpenter SR & Pace ML (2011). Conditional heteroscedasticity as a leading indicator of ecological regime shifts. Am. Nat, 178, 442–51 [DOI] [PubMed] [Google Scholar]
- 85.Spanbauer TL, Allen CR, Angeler DG, Eason T, Fritz SC, Garmestani AS, et al. (2014). Prolonged instability prior to a regime shift. PLoS One, 9, e108936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Springer AM, McRoy CP & Turco KR (1989). The paradox of pelagic food webs in the northern Bering Sea II. Zooplankton communities. Cont. Shelf Res, 9, 359–386 [Google Scholar]
- 87.Starfield AM & Chapin FS (1996). Model of transient changes in arctic and boreal vegetation in response to climate and land use change. Ecol. Appl, 6, 842–864 [Google Scholar]
- 88.Strayer DL, Power ME, Fagan WF, Pickett STA & Belnap J (2003). A classification of ecological boundaries. Bioscience, 53, 723–729 [Google Scholar]
- 89.Svirezhev YM (2000). Thermodynamics and ecology. Ecol. Modell, 132, 11–22 [Google Scholar]
- 90.Thuiller W, Albert C, Araújo MB, Berry PM, Cabeza M, Guisan A, et al. (2008). Predicting global change impacts on plant species’ distributions: Future challenges. Perspect. Plant Ecol. Evol. Syst, 9, 137–152 [Google Scholar]
- 91.Tingley MW, Monahan WB, Beissinger SR & Moritz C (2009). Birds track their Grinnellian niche through a century of climate change. Proc. Natl. Acad. Sci, 106 Suppl, 19637–19643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.United States Department of the Interior. (n.d.). Omernik’s Level III Ecoregions of the Continental United States. Map Layer Info. Available at: http://nationalatlas.gov/mld/ecoomrp.html.Last accessed [Google Scholar]
- 93.Vance L, Eason T & Cabezas H (2015). An information-based approach to assessing the sustainability and stability of an island system. Int. J. Sustain. Dev. World Ecol, 22, 64–75 [Google Scholar]
- 94.Walkusz W, Paulic J, Kwasniewski S, Williams W, Wong S & Papst M (2010). Distribution, diversity and biomass of summer zooplankton from the coastal Canadian Beaufort Sea. Polar Biol, 33, 321–335 [Google Scholar]
- 95.Wassmann P, Kosobokova K, Slagstad D, Drinkwater K, Hopcroft R, Moore S, et al. (2015). The contiguous domains of Arctic Ocean advection: Trails of life and death. Prog. Oceanogr. [Google Scholar]
- 96.Williams JW & Jackson ST (2007). Novel climates, no-analog communities, and ecological surprises. Front. Ecol. Environ, 5: 9, 475–482 [Google Scholar]
- 97.Yarrow MM & Salthe SN (2008). Ecological boundaries in the context of hierarchy theory. Biosystems, 92, 233–244 [DOI] [PubMed] [Google Scholar]