Abstract
Popularity of electronic cigarettes (ECIGs) has increased tremendously among young people, in part due to flavoring additives in ECIG liquids. Pyrazines are an important class of these additives, and their presence in tobacco cigarettes has been correlated with increased acceptability of smoking among smokers and bystanders. Pyrazine use by the tobacco industry is therefore thought to encourage smoking. However, the extent of transfer of pyrazines present in the liquid to aerosols upon vaping remains unclear. We present a simple analytical method to quantify six pyrazine derivatives in liquids and aerosols of ECIGs that allows the isolation of pyrazines from interfering compounds, like nicotine. Standard pyrazine solutions and commercial ECIG samples of different brands and flavors were tested for their pyrazine content in the liquids and in the generated aerosols from these solutions. Testing on ECIG commercial liquids revealed a heterogeneous distribution in the levels and types of pyrazines, with acetyl and alkyl pyrazines present in more than 70% of the samples. This method confirmed that pyrazine additives are common in ECIG and that labels do not usually reflect the type and quantity of pyrazines in the liquid. Pyrazines were not correlated with the nicotine content or the brand of the liquid. The aerosols showed similar pyrazine profiles to their corresponding liquids. The efficiency of transfer of pyrazines into the particle phase was approximately 46%. Therefore, addition of pyrazines to ECIGs should be regulated, because they act synergistically with nicotine to increase product appeal, ease smoking initiation, and discourage cessation.
Keywords: Electronic cigarette, pyrazine, aerosol, nicotine, thermogravimetric analysis
Introduction
Electronic cigarette (ECIG) use is gaining popularity around the globe (Brown et al. 2014, McMillen et al. 2015, Breland et al. 2016, Filippidis et al. 2016, Syamlal G et al. 2016). The high prevalence of ECIG use among young people is usually attributed to the perception that it is a safer alternative to tobacco cigarettes and to its availability in a wide array of flavors that appeal to younger age groups (ASH 2016, Dai and Hao 2016). Recently, the Food and Drug Administration (FDA) reported that almost 82% of current youth ECIG users link their attraction to ECIGs to the availability of appealing flavors (Bach 2016); more than 7700 unique flavors of ECIGs are currently available in the market (Zhu et al. 2014).
Pyrazines, a family of N-heterocyclic compounds, are among the most frequently used additives in aroma and flavoring of tobacco cigarettes (Leffingwell JC et al. 1972, Alpert et al. 2016). Pyrazines can also arise from biosynthesis and chemical transformations during the curing process of tobacco leaves (Schmeltz and Hoffmann 1977), as well as during smoking due to the Maillard reaction of dicarbonyls with nitrogen sources like amines and/or amino acids. These dicarbonyls are usually produced by the degradation of carbohydrates present in the tobacco leaves (Leffingwell 1976, Ishiguro and Sugawara 1977, Weeks et al. 1995). Interestingly, similar transformations are usually detected in roasted foods, such as coffee and cocoa products, beans, baked potatoes, popcorn, and others (Heinzler and Eichner 1992, Huang and Barringer 2011, Treesuwan et al. 2012, Pickard et al. 2013, Yang et al. 2013).
The addition of pyrazines to cigarettes favors the acceptability of smoking to both smokers and bystanders, since it reduces the side-stream odor and masks the noxious irritating effects of tobacco smoke (Connolly et al. 2000). Pyrazines, due to their chemosensory effects, reduce the harshness of nicotine aerosol in the airway so that the smoker experiences a smoother smoke (Alpert et al. 2016). Despite their harmless presence in roasted food, pyrazines are considered “unsafe” in cigarette smoke because they act synergistically with nicotine to make smoking more appealing, thereby discouraging cessation (Alpert et al. 2016).
Analytical methods have been developed to determine the pyrazine contents of roasted foods and wine; most of these methods have relied on head space solid phase micro extraction (HS-SPME) coupled with gas chromatography (GC) (Sala et al. 2002). In tobacco leaves, pyrazine concentrations have been determined by solvent extraction (Peng et al. 2004), whereas their content in tobacco smoke has been determined following collection on Tenax tubes or filter pads and subsequent thermal desorption or extraction with organic solvents, respectively (Lu et al. 2004, Marcilla et al. 2012, Marco and Grimalt 2015).
Few studies have addressed the quantification of pyrazines in ECIG liquids and aerosols (Tierney et al. 2016). In 2012, four alkyl pyrazines were measured in liquid and aerosol of one Italian ECIG brand, but nothing was mentioned regarding the flavoring in that brand (Pellegrino et al. 2012). Trimethylpyrazine (TMP) and acetylpyrazine (AcP) were identified in the general chemical profile of 28 ECIG liquids and aerosols following GC analysis (Hutzler et al. 2014, Herrington and Myers 2015) and more recently in refill liquids and ECIG pre-fillable cartridges by HS-SPME GC-MS analysis (Lim and Shin 2017).
The synergistic role of pyrazine with nicotine in increasing the appeal of cigarettes, easing smoking initiation, and discouraging cessation (Riveles et al. 2004) may also apply to ECIG vaping and could therefore lead to similar calls for restrictions on their use as additives in ECIGs. Therefore, this study focuses on the development of a simple liquid-liquid extraction method for the isolation of pyrazines from the ECIG matrix [including nicotine, propylene glycol (PG), vegetable glycerin (VG) and other flavor interferences], followed by GC-MS quantification. The developed method is then used to quantify six pyrazine derivatives (Scheme S1) in standard solutions, in a sample set of ECIG commercial liquids, and in aerosols generated by ECIGs upon activation. The study was also designed to assess the efficiency of pyrazine transfer into the aerosols and the stability of pyrazines during vaping.
Materials and Methods
Materials
Six pyrazine derivatives [ethylpyrazine (EP), 2,5-dimethylpyrazine (DMP), 2,3-diethylpyarzine (≥97%) (DEP), 2,3,5-trimethylpyrazine (TMP), 2,3,5,6-tetramethylpyrazine (≥98%) (TeMP) and acetylpyrazine (97%) (AcP)], (hexadecane (≥99%) (used as an internal standard in pyrazine quantification), formic acid, propylene glycol (PG; 99.5%), vegetable glycerin (VG; 99–101%), β-citronellol (used in PG/VG quantification), and High Performance Liquid Chromatography (HPLC) grade ethyl acetate and deionized water (DI) were procured from Sigma-Aldrich. Toluene (HPLC grade) was purchased from VWR and quartz filters (ADVENTEC, QR-100,47mm) were procured from Whatman International. Eight different commonly used ECIG liquid brands, with 14 different flavors, were purchased from internet vendors.
Study Design
A sample set of eight commercial brands with 14 different flavors and three standard liquid solutions were tested for pyrazines, PG, VG, and nicotine content in the liquids and in generated aerosols upon activation of ECIG. STD1 was formed by mixing commercial pure PG and VG stock solutions in a 70/30 PG/VG ratio. STD2 was prepared by adding nicotine to a mixture of PG/VG (70/30) to form a solution of 18 mg/mL nicotine concentration and STD3 consisted of a mixture of PG/VG (70/30) with 18 mg/mL of nicotine and six pyrazines of ~80 µg/mL concentration each.
ECIG Liquid Chemical Characterization
Pyrazine extraction and quantification
The liquid-liquid extraction step (toluene-acidified water extraction) allows the isolation of pyrazines from interfering compounds, such as nicotine, PG, VG, and other flavors in the matrix. Nicotine is considered a major interferent because both pyrazine and nicotine have nitrogen atoms in their ring structures, hence isolating nicotine, which is present in larger amounts compared to pyrazines, is of paramount importance in order to ensure clean and high extraction efficiency. The extraction method consisted of acidifying the liquid ECIG solution by first preparing a solution of the ECIG liquid to contain a nicotine concentration of ~500 µg/mL by dissolving a volume (X mL; X = 70 to 105 µL) based on the labeled nicotine concentration of the commercial sample in 3 mL of deionized water, followed by addition of 0.1 μL of pure formic acid (pKa = 3.77). For STD1 and the commercial sample with zero nicotine, an aliquot of 100 µL was dissolved in 3 mL of de-ionized water. The added acid protonated the pyrrolidine, but not the pyridine, ring of nicotine and increased its solubility in water. A 3 mL volume of toluene was then added and the whole mixture was shaken for 30 min. The mixture was then left to stand until the two immiscible phases separated. The aqueous phase contained PG, VG, and the protonated nicotine, whereas the toluene phase contained the dissolved pyrazines. This extraction step was repeated twice. An aliquot from each extraction step (S1 and S2) was injected into the GC-MS system for analysis.
The GC-MS analysis was performed on a Thermo Trace GC-2000 POLARIS QMS instrument equipped with a thermostatically controlled AI 3000 auto-sampler and a TG-5MS Thermo Scientific fused silica capillary GC column (30 m × 0.25 mm × 0.25 µm). The mobile phase was helium delivered at a 1 mL/min flow rate. The injector temperature was 250 °C, with a split-less injection mode of 1µL. The oven temperature program was: 60 °C, 5 °C/min ramp to 120 °C, and another ramp of 12 °C/min to 200 °C. The total run time was 18.67 min and the solvent delay time was 4.45 min. The quantification was performed using a selected target ion m/z = 107, 108, 121, 122, 136, and 94 for ethylpyrazine, 2,5-dimethylpyrazine, 2,3-diethylpyrazine, 2,3,5-trimethylpyrazine, 2,3,5,6-tetramethylpyrazine and acetylpyrazine, respectively. The signal at m/z =41 was for hexadecane, the internal standard.
Quality control and quality assurance
For quality control and quality assurance, a standard liquid solution was prepared from a mixture of six standard pyrazine derivatives (~80 µg/mL) in PG/VG (70/30) solvent (Table 1). The extraction recoveries of the standard pyrazine liquid samples were calculated by comparing the GC-MS peak areas of the extracted samples to the pyrazine standard solutions directly injected onto the GC-MS. Table 1 includes a list of the recoveries that were computed in triplicates for three different standard liquid concentrations (n=9) in the absence of nicotine. For commercial liquids, the presence of nicotine is assumed not to affect the recoveries because adding formic acid to the extraction medium will protonate nicotine which becomes highly dissolved in the aqueous layer. Quantitative analysis was done according to an extracted calibration curve with a linear range (0.5–15 µg/mL). The limit of detection (LOD) and limit of quantification (LOQ) of the tested pyrazines ranged between 0.02–0.05 and 0.08–0.16 µg/mL, respectively. The recoveries ranged from 66–103%, with 7–12% relative standard deviation from the mean.
Table 1.
Quality control and quality assurance measurements for pyrazine quantification
| Pyrazine | CAS Number |
Molecular Weight (g/mol) |
LOD (µg/mL) |
LOQ (µg/mL) |
Repeatability (%RSD of n = 6) |
%Recovery from liquids (n = 9)* |
%Recovery from filters (n =9)* |
|---|---|---|---|---|---|---|---|
| Ethylpyrazine (EP) | 13925–00-3 | 108.14 | 0.03 | 0.10 | 11.8 | 89 – 100 | 85–90 |
| 2,5-dimethylpyrazine (DMP) | 123–32-0 | 108.14 | 0.04 | 0.12 | 7.4 | 76 – 98 | 85–92 |
| 2,3-diethylpyrazine (DEP) | 15707–24-1 | 136.19 | 0.05 | 0.16 | 6.8 | 88 – 102 | 80–100 |
| 2,3,5-trimethylpyrazine (TMP) | 14667–55-1 | 122.17 | 0.04 | 0.12 | 8.8 | 81 – 99 | 64–82 |
| 2,3,5,6-tetramethylpyrazine (TeMP) | 1124–11-4 | 136.19 | 0.04 | 0.13 | 7.3 | 81 – 103 | 76–104 |
| Acetylpyrazine (AcP) | 22047–25-2 | 122.12 | 0.02 | 0.08 | 6.8 | 66 – 86 | 67–88 |
Recoveries were estimated on three different concentrations, each repeated in triplicates.
PG/VG quantification
PG and VG were quantified using a previously reported method (Talih et al. 2017). In brief, 2 µL of liquid was dissolved in 1 mL of ethyl acetate and sonicated for 30 min. A 10-fold diluted solution was then spiked with the internal standard, β-citronellol (50 µg/mL), and separated by gas chromatography with flame ionization detection (GC-FID). The quantification was done against a calibration curve of 20–315 µg/mL of PG or VG in ethyl acetate solutions. The recoveries of standard solutions prepared in the lab were > 90%.
Thermal degradation of pyrazines
A known mass of AcP was weighed into an Al2O crucible and placed in a NETZSCH thermobalance coupled to a Bruker Fourier-Transform-Infrared (FTIR) spectrophotometer to conduct the thermogravimetric analysis (TGA). AcP was used as an example since it was identified in most of the tested samples. TGA follows changes in the mass of the sample as a function of temperature. Gases released directly from the sample were directed to the FTIR spectrophotometer for identification. AcP, in its pure solid form, was heated under O2 at 5 °C/min to assess its degradation within the temperature range of 30 to 500 °C normally expected in ECIGs.
Aerosol Characterization
Aerosol generation and sampling
The AUB Aerosol Lab Vaping Instrument (ALVIN; Talih et. al., 2015) was used to generate ECIG aerosols. Puff duration, inter-puff interval, and flow rate were selected to represent an experienced ECIG user (4 sec puff duration, 10 sec inter-puff interval, 1 L/min flow rate,(Talih et al. 2015). All liquids were vaped in a VaporFi platinum tank equipped with a 2.2 Ω coil and operated at a 3.3 V (power = 5 W). For each test condition, a 47 mm quartz filter was installed at the mouth end of the ECIG to collect the total particulate matter (TPM) generated from 45 puffs. Quartz filters are neutral and do not affect the distribution of analytes between the acidified water and toluene. Each test condition was repeated in triplicate and the results were reported as the mean of the three measurements. The amount of TPM was determined gravimetrically by weighing the filter pad and its holder before and after each sampling session.
Pyrazine quantification
Five commercial liquids (selected from Table 2) and three standard solutions vaped under normal puffing conditions and 5 W power output were used to quantify pyrazines in aerosols. The samples were selected to represent different brands, flavors within the same brand, and PG/VG ratios. The quartz filters holding the particles were immersed in 3 mL of deionized water, 0.1 µL of formic acid (pKa = 3.77) was added, followed by 3 mL of toluene, and the whole mixture was shaken for 30 min. This extraction step was repeated twice. An aliquot from each extraction step (S1 and S2) was injected into the GC-MS system for analysis. Recoveries of pyrazines on filters were assessed by spiking the prepared standard pyrazine mixture on quartz filters followed by filter extraction. Table 1 shows the extraction recoveries from spiked filters determined in triplicates for three different concentrations (n=3×3).
Table 2.
Pyrazine concentration in e-liquid (µg/mL) of commercial ECIG brands with nicotine label listed in µg/mL for comparison
| Sample | Brand | Flavor | Nicotine label | EP | DMP | DEP | TMP | TeMP | AcP | Total |
|---|---|---|---|---|---|---|---|---|---|---|
| I | wow | Hazelnut coffee | 18000 | 5.3 | - | - | 48.6 | - | 48.0 | 101.9 |
| II | wow | Peanut butter | 18000 | - | 13.6 | - | 37.3 | - | 19.3 | 70.2 |
| III | EC blend | Kettle corn | 18000 | - | - | - | 8.8 | - | 1702.4 | 1711.2 |
| IV | EC blend | Buttered keoke rum coffee | 18000 | - | - | - | - | 11.4 | 26.3 | 37.7 |
| V | EC blend | Dragon’s milk | 0 | - | - | - | - | - | 20.6 | 20.6 |
| VI | Apollo | Blubery kona coffee | 12000 | - | - | - | 8.6 | - | - | 8.6 |
| VII | Apollo | Tobacco | 12000 | - | - | - | 100.9 | 508.4 | 62.3 | 671.6 |
| VIII | Halo | Tribeca | 18000 | - | - | - | 9.3 | 13.5 | 75.4 | 98.2 |
| IX | Halo | Malibu | 18000 | - | - | - | - | - | - | - |
| X | Retro | Cheese cake walts | 3000 | - | - | - | - | - | 138.4 | 138.4 |
| XI | Retro | Roots | 12000 | - | - | - | - | - | - | - |
| XII | Vaporzone | Red tobacco | 18000 | - | - | - | - | - | - | - |
| XIII | South beach | Red tobacco | 18000 | - | - | - | - | - | - | - |
| XIV | Medusa | Charon liquorice | 18000 | - | - | - | 25.3 | 35.2 | - | 60.5 |
|
XV |
STD3 |
18000 |
81.6 |
81.6 |
76.1 |
81.1 |
90.8 |
78.8 |
490 |
|
EP: ethyl pyrazine, DMP: 2,5-dimethyl pyrazine, DEP: 2,3-diethyl pyrazine, TMP: 2,3,5-trimethyl pyrazine, TeMP: 2,3,5,6-tetramethyl pyrazine, AcP: acetyl pyrazine. *: The measured nicotine content for seven samples agreed with the label within a 5% error (data not shown).
Results
Even though pyrazine was not listed on the label as one of the ECIG additives, it was detected in most of the analyzed samples, as shown in Table 2. The total pyrazine concentration ranged between 0 in Retro Roots, Vaporzone Red Tobacco, South Beach Red Tobacco, and Halo Malibu to 1711µg/mL in ECBlend Kettle Corn. Only two of the 14 tested samples had only one type of pyrazine compound: Apollo Blueberry Kona Coffee contained 8.6 µg/mL of TMP, and Retro-Cheesecake Waltz contained 138.4 µg/mL of AcP. Samples that had a “nutty” or “coffee” flavor showed more than one derivative of pyrazine (e.g., Wow Hazelnut Coffee, Wow Peanut Butter, ECBlend Kettle Corn, and ECBlend Buttered Keoke Rum Coffee). High concentrations and three different pyrazine derivatives were detected in tobacco and liquorice flavors (e.g., Apollo Tobacco, Halo Tribeca, and Medusa Charon Liquorice).
Table 3 shows the quantified PG/VG ratio and the concentrations of pyrazine derivatives in the aerosols. The concentration of pyrazines is reported as mass of pyrazine per unit volume of the collected aerosol particles. The volume of the particles was estimated based on the density of the ECIG liquid and the mass of the collected TPM. The pyrazine concentrations were lower in the aerosols than in the parent liquids; less than half of the original concentration of pyrazines in the commercial and standard (STD3) liquids was recovered in the particle phase trapped on the filter pads.
Table 3.
Pyrazine concentration (µg/mL) in aerosols (A) for several commercial e-cigarette (ECIG) brands and standard solutions (STD) Measured PG/VG ratio (v/v) in the parent liquid is also shown. Values below the limit of detection are indicated by a dash
| Sample | PG/VG | EP | DMP | DEP | TMP | TeMP | AcP | Total |
|---|---|---|---|---|---|---|---|---|
|
Wow, Hazelnut |
71.9/28.1 | - | - | - | 15.8 ± 0.4 | - | 19.8± 0.2 | 35.6± 0.3 |
|
Wow, Peanut Butter |
84.1/15.9 | - | 2.6± 0.47 | - | 10.4 ± 2.4 | - | 12.8± 3.1 | 25.8± 1.4 |
|
ECBlend, Kettle Corn |
70.2/29.8 | - | - | - | - | - | 714.3 ± 23.6 | 714.3 ± 23.6 |
|
Halo, Tribeca |
72.0/28.0 | - | - | - | 0.2± 0.1 | 0.1± 0.0 | 27.8± 1.3 | 28.1± 1.4 |
|
Retro, Cheese Cake Waltz |
52.2/47.8 | - | - | - | - | - | 61.2± 1.9 | 61.3± 1.9 |
| STD1 | 71.0/29.0 | - | - | - | - | - | - | - |
| STD2 | 71.0/29.0 | - | - | - | - | - | - | - |
| STD3 | 71.0/29.0 | 26.5± 2.9 | 32.6± 3.8 | 29.0 ± 2.7 | 36.3 ± 3.8 | 40.1 ± 3.3 | 37.8± 2.0 | 202.3 ± 18.4 |
EP: ethyl pyrazine, DMP: 2,5-dimethyl pyrazine, DEP: 2,3-diethyl pyrazine, TMP: 2,3,5-trimethyl pyrazine, TeMP: 2,3,5,6-tetramethyl pyrazine, AcP: acetyl pyrazine, L: liquid phase; A: aerosol phase.
TGA of AcP heated under O2 showed that AcP evaporation into the gas phase was initiated at 80 °C and was completed by 130 °C (Figure S1). As such, AcP evaporated at temperatures that are assumed to be lower than the normal vaping conditions of ECIGs. No decomposition products were detected in this temperature range, as confirmed by the IR spectra in Figure 1. All identified IR peaks were characteristic of AcP.
Figure 1.
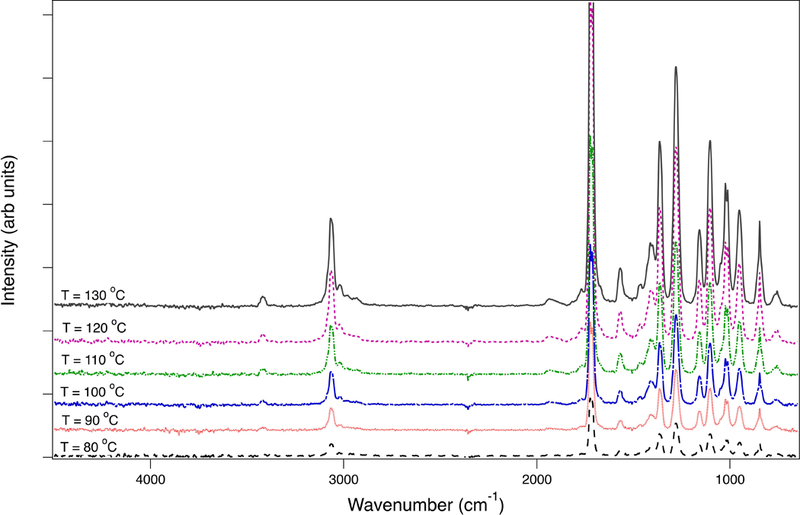
Fourier-Transform-Infrared (FTIR) plots of evaporated acetylpyrazine (AcP) during thermogravimetric analysis (TGA) between 80 and 130 °C. The plots show no new peaks indicative of possible degradation products and confirm that AcP evaporation was completed by 130 °C.
Discussion
The method presented here for toluene-acidified water extraction coupled with GC-MS is a simple, reproducible, and accurate method for qualitative and quantitative identification of pyrazines in ECIG liquids and aerosols. In the considered sample set, the range of the detected pyrazines was 0–0.15% (w/w in the liquid), with the AcP concentration in the liquid of the ECBlend Kettle Corn approaching 10% of the measured nicotine content. The presence of pyrazines in ECIG liquids may induce a preference among ECIG users, as suggested by Alpert et al. (2016). Some tobacco-flavored ECIG brands contained different derivatives and elevated levels of pyrazines [e.g., Apollo Tobacco (672 µg/mL) and Halo Tribeca (98.2 µg/mL)]. Noted that there is no correlation between nicotine level and total pyrazine concentration and the pyrazine profile is flavor-specific and independent of the brand. This raises a question about the source of the pyrazines in ECIG, as it could be either added to the liquid or originated from the natural extraction of tobacco (Farsalinos et al. 2015).
Aerosols showed pyrazine profiles similar to the ones determined in liquids, but with lower concentrations. The concentrations of EP in aerosols from Wow Hazelnut Coffee and TMP in ECBlend Kettle Corn were below the LOQ of the analytical method. A scatter plot of the different pyrazine concentrations in aerosols versus those in liquids (Figure 2) indicated a slope of 46% (R2=0.95). The relative depletion of the particle phase pyrazines compared to the parent liquid may indicate that pyrazine compounds are thermally converted to other products during aerosolization, or that they are partitioned between the gas and particle phase of the aerosol. Interestingly, a fate study of tobacco ingredients showed that only 46% of 13C-labelled TeMP was recovered intact after smoking (Purkis et al. 2011). Incomplete combustion products, like 13CO and 13CO2 resulting from the degradation of the labelled pyrazine, were detected. In our case, however, no decomposition products were detected by FTIR when the samples were heated to 510 °C on TGA. AcP represented well the behavior of the pyrazines in the commercial samples since it was the most abundant in the tested liquids. We observed complete evaporation of AcP from the sample holder at 130 °C, which was considered a lower temperature than the reported temperatures in the vicinity of the ECIG heating element (Geiss et al. 2016, Talih et al. 2017).
Figure 2.
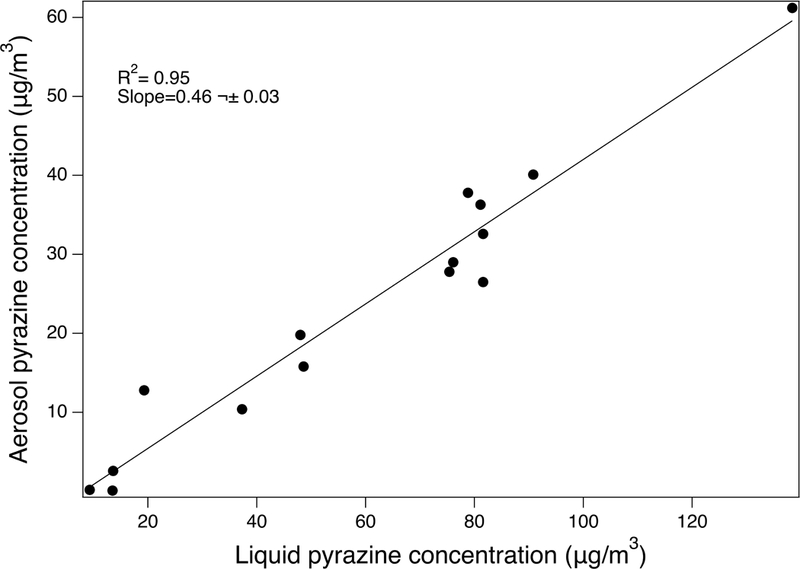
Plot of the measured pyrazine concentration in aerosol particulate matter versus the concentration measured in the parent e-cigarette liquids
The fraction of the pyrazines found in the particle phase of the aerosol can be estimated using the equilibrium partitioning theory (Pankow 1994, Pankow 2001). The calculation is based on the AcP evaporation temperature of 80 °C that was determined by TGA analysis. This temperature represents the lower limit of the partitioning of pyrazines to the particle phase since condensation is expected to take place at lower temperatures. Following Pankow’s method (Pankow 2017) and based on the vapor pressure of DMP and TMP at 353 K (80 °C), we calculated particle fractions of 37% for DMP and 43% for TMP (the calculations are shown in the Supplementary Information) (Sakoguchi et al. 1995, Chickos and Acree 2003). These values indicate that, when aerosolized, roughly half the pyrazines in the parent liquid will be transferred to the vapor phase of the resulting aerosol, thereby possibly explaining the relatively lower concentrations measured in the particle phase trapped on the quartz filters in this study. The relative partitioning of pyrazines between the particle and gas phase of the aerosol may have implications for the delivery of these compounds to the olfactory receptors, in a way similar to the effect of nicotine partitioning and its delivery process as suggested by Caldwell et al. (Caldwell et al. 2012).
Previous studies that reported synergistic effects of pyrazines on nicotine addiction and their role in “normalizing” smoking among youth (Connolly et al. 2000, Alpert et al. 2016) suggested that pyrazine emissions in ECIG aerosols may contribute to initiation and adherence of ECIG use. Therefore, pyrazine content should be taken into consideration in any future effort for regulating this category of tobacco products. Moreover, the reproductive toxicity of pyrazine derivatives was recently questioned and other studies showed that their presence in tobacco smoke significantly inhibited oviductal functioning in animals (Adams et al. 2002, Melkonian et al. 2003, Talbot 2008). This raises questions about their safety when inhaled by smokers (Riveles et al. 2004) and, similarly, by vapers.
Conclusion
This study introduced a simple, reproducible, and accurate method for pyrazine quantification in ECIG liquids and aerosols. Pyrazines were detected in the liquids of diverse brands and flavor combinations. Previous reports have shown that ECIG liquids can contain pyrazines, but this study specifies for the first time the transfer efficiency and content of pyrazines in the ECIG aerosols using machine smoking according to a human-derived puffing regimen.
Supplementary Material
Acknowledgments
Funding Sources
This work was supported by the National Institute on Drug Abuse of the National Institutes of Health (grant number P50DA036105) and the Center for Tobacco Products of the U.S. Food and Drug Administration. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the Food and Drug Administration.
Footnotes
Conflict of Interest Disclosure
The authors have no conflict of interest to declare.
References
- Adams TB, Doull J, Feron VJ, Goodman JI, Marnett LJ, Munro IC, Newberne PM, Portoghese PS, Smith RL, Waddell WJ and Wagner BM (2002). The FEMA GRAS assessment of pyrazine derivatives used as flavor ingredients. Food Chem. Toxicol 40(4): 429–451. [DOI] [PubMed] [Google Scholar]
- Alpert HR, Agaku IT and Connolly GN (2016). A study of pyrazines in cigarettes and how additives might be used to enhance tobacco addiction. Tob. Control 25: 444–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ASH. (2016). “Use of electronic cigarettes (vapourisers) among adults in Great Britain.” from http://www.ash.org.uk/files/documents/ASH_891.pdf.
- Bach L (2016). “Electronic Cigarettes and Youth.” Retrieved December 2016, from http://www.tobaccofreekids.org/research/factsheets/pdf/0382.pdf.
- Breland A, Soule E, Lopez A, Ramôa C, El-Hellani A and Eissenberg T (2016). Electronic cigarettes: what are they and what do they do? Ann. N. Y. Acad. Sci 1394: 5–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J, West R, Beard E, Michie S, Shahab L and McNeill A (2014). Prevalence and characteristics of e-cigarette users in Great Britain: Findings from a general population survey of smokers. Addict. Behav 39(6): 1120–1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell B, Sumner W and Crane J (2012). A Systematic Review of Nicotine by Inhalation: Is There a Role for the Inhaled Route? Nicotine Tob. Res 14(10): 1127–1139. [DOI] [PubMed] [Google Scholar]
- Chickos JS and Acree WEJ (2003). “Enthalpies of Vaporization of Organic and Organometallic Compounds, 1880–2002.” from http://nist.gov/data/PDFfiles/jpcrd628.pdf.
- Connolly GN, Wayne GD, Lymperis D and Doherty MC (2000). How cigarette additives are used to mask environmental tobacco smoke. Tob. Control 9(3): 283–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai H and Hao J (2016). Flavored Electronic Cigarette Use and Smoking Among Youth. Pediatrics 138. [DOI] [PubMed] [Google Scholar]
- Farsalinos K, Gillman I, Melvin M, Paolantonio A, Gardow W, Humphries K, Brown S, Poulas K and Voudris V (2015). Nicotine Levels and Presence of Selected Tobacco-Derived Toxins in Tobacco Flavoured Electronic Cigarette Refill Liquids. Int. J. Environ. Res. Public Health 12(4): 3439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filippidis FT, Laverty AA, Gerovasili V and Vardavas CI (2016). Two-year trends and predictors of e-cigarette use in 27 European Union member states. Tob. Control 26: 98–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiss O, Bianchi I and Barrero-Moreno J (2016). Correlation of volatile carbonyl yields emitted by e-cigarettes with the temperature of the heating coil and the perceived sensorial quality of the generated vapours. Int. J. Hyg. Environ. Health 219(3): 268–277. [DOI] [PubMed] [Google Scholar]
- Heinzler M and Eichner K (1992). The role of Amadori compounds during cocoa processing-formation of aroma compounds under roasting conditions. Z Lebensm Unters Forsch 21: 445–450. [Google Scholar]
- Herrington JS and Myers C (2015). Electronic cigarette solutions and resultant aerosol profiles. J. Chromatogr. A 1418: 192–199. [DOI] [PubMed] [Google Scholar]
- Huang Y and Barringer SA (2011). Monitoring of Cocoa Volatiles Produced during Roasting by Selected Ion Flow Tube-Mass Spectrometry (SIFT-MS). J. Food Sci 76(2): C279–C286. [DOI] [PubMed] [Google Scholar]
- Hutzler C, Paschke M, Kruschinski S, Henkler F, Hahn J and Luch A (2014). Chemical hazards present in liquids and vapors of electronic cigarettes. Arch. Toxicol 88(7): 1295–1308. [DOI] [PubMed] [Google Scholar]
- Ishiguro S and Sugawara S (1977). Comparisons of Volatile N-Containing Compounds in the Smoke of Lamina and Midrib of Flue-cured Tobacco Leaves. Agric. Biol. Chem 41(2): 377–382. [Google Scholar]
- Leffingwell JC (1976). Nitrogen components of leaf and their relationship to smoking quality and aroma. Recent Adv. Tob. Sci 2(1–31). [Google Scholar]
- Leffingwell JC, Young HJ and EB (1972). Tobacco flavoring for smoking products Winston-Salem: R.J. Reynolds Tobacco Company; http://www.leffingwell.com/download/TobaccoFlavorBook.pdf. [Google Scholar]
- Lim H-H and Shin H-S (2017). Determination of volatile organic compounds including alcohols in refill fluids and cartridges of electronic cigarettes by headspace solid-phase micro extraction and gas chromatography–mass spectrometry. Anal. Bioanal. Chem 409(5): 1247–1256. [DOI] [PubMed] [Google Scholar]
- Lu X, Zhao M, Kong H, Cai J, Wu J, Wu M, Hua R, Liu J and Xu G (2004). Characterization of complex hydrocarbons in cigarette smoke condensate by gas chromatography–mass spectrometry and comprehensive two-dimensional gas chromatography–time-of-flight mass spectrometry. J. Chromatogr. A 1043(2): 265–273. [DOI] [PubMed] [Google Scholar]
- Marcilla A, Martínez I, Berenguer D, Gómez-Siurana A and Beltrán MI (2012). Comparative study of the main characteristics and composition of the mainstream smoke of ten cigarette brands sold in Spain. Food Chem. Toxicol 50(5): 1317–1333. [DOI] [PubMed] [Google Scholar]
- Marco E and Grimalt JO (2015). A rapid method for the chromatographic analysis of volatile organic compounds in exhaled breath of tobacco cigarette and electronic cigarette smokers. J. Chromatogr. A 1410: 51–59. [DOI] [PubMed] [Google Scholar]
- McMillen RC, Gottlieb MA, Shaefer RMW, Winickoff JP and Klein JD (2015). Trends in Electronic Cigarette Use Among U.S. Adults: Use is Increasing in Both Smokers and Nonsmokers. Nic. Tob. Res 17(10): 1195–1202. [DOI] [PubMed] [Google Scholar]
- Melkonian G, Eckelhoefer H, Wu M, Wang Y, Tong C, Riveles K and Talbot P (2003). Growth and Angiogenesis Are Inhibited in Vivo in Developing Tissues by Pyrazine and Its Derivatives. Toxicol. Sci 75(2): 393–401. [DOI] [PubMed] [Google Scholar]
- Pankow JF (1994). An absorption model of gas/particle partitioning of organic compounds in the atmosphere. Atmos. Environ 28(2): 185–188. [Google Scholar]
- Pankow JF (2001). A Consideration of the Role of Gas/Particle Partitioning in the Deposition of Nicotine and Other Tobacco Smoke Compounds in the Respiratory Tract. Chem. Res. Toxicol 14(11): 1465–1481. [DOI] [PubMed] [Google Scholar]
- Pankow JF (2017). Calculating compound dependent gas-droplet distributions in aerosols of propylene glycol and glycerol from electronic cigarettes. Journal of Aerosol Science 107: 9–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellegrino RM, Tinghino B, Mangiaracina G, Marani A, Vitali M, Protano C, Osborn JF and Cattaruzza MS (2012). Electronic cigarettes: an evaluation of exposure to chemicals and fine particulate matter (PM). Ann. Ig 24: 279–288. [PubMed] [Google Scholar]
- Peng F, Sheng L, Liu B, Tong H and Liu S (2004). Comparison of different extraction methods: steam distillation, simultaneous distillation and extraction and headspace co-distillation, used for the analysis of the volatile components in aged flue-cured tobacco leaves. J. Chromatogr. A 1040(1): 1–17. [DOI] [PubMed] [Google Scholar]
- Pickard S, Becker I, Merz K-H and Richling E (2013). Determination of the Alkylpyrazine Composition of Coffee Using Stable Isotope Dilution–Gas Chromatography–Mass Spectrometry (SIDA-GC-MS). J. Agric. Food Chem 61(26): 6274–6281. [DOI] [PubMed] [Google Scholar]
- Purkis SW, Mueller C and Intorp M (2011). The fate of ingredients in and impact on cigarette smoke. Food Chem. Toxicol 49(12): 3238–3248. [DOI] [PubMed] [Google Scholar]
- Riveles K, Roza R, Arey J and Talbot P (2004). Pyrazine derivatives in cigarette smoke inhibit hamster oviductal functioning. Reprod. Biol. Endocrinol 2(1): 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakoguchi A, Ueoka R, Kato Y and Arai Y (1995). Vapor Pressures of Alkylpyridines and Alkylpyrazines. KAGAKU KOGAKU RONBUN 21(1): 219–223. [Google Scholar]
- Sala C, Mestres M, Martí MP, Busto O. and Guasch J. (2002). Headspace solid-phase microextraction analysis of 3-alkyl-2-methoxypyrazines in wines. J. Chromatogr. A 953(1–2): 1–6. [DOI] [PubMed] [Google Scholar]
- Schmeltz I and Hoffmann D (1977). Nitrogen-containing compounds in tobacco and tobacco smoke. Chem. Rev 77: 295–311. [Google Scholar]
- Syamlal G, Jamal A, King BA and JM, M. (2016). Electronic Cigarette Use Among Working Adults — United States, 2014. MMWR Morb Mortal Wkly Rep 65: 557–561. [DOI] [PubMed] [Google Scholar]
- Talbot P (2008). In vitro assessment of reproductive toxicity of tobacco smoke and its constituents. Birth Defects Research (Part C) 84(1): 61–72. [DOI] [PubMed] [Google Scholar]
- Talih S, Balhas Z, Eissenberg T, Salman R, Karaoghlanian N, El Hellani A, Baalbaki R, Saliba N and Shihadeh A (2015). Effects of User Puff Topography, Device Voltage, and Liquid Nicotine Concentration on Electronic Cigarette Nicotine Yield: Measurements and Model Predictions. Nicotine Tob. Res 17(2): 150–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talih S, Balhas Z, Salman R, El-Hage R, Karaoghlanian N, El-Hellani A, Baassiri M, Jaroudi E, Eissenberg T, Saliba N and Shihadeh A (2017). Transport phenomena governing nicotine emissions from electronic cigarettes: Model formulation and experimental investigation. Aerosol Sci. Technol 51(1): 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tierney PA, Karpinski CD, Brown JE, Luo W and Pankow JF (2016). Flavour chemicals in electronic cigarette fluids. Tob. Control 25(e1): e10–e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treesuwan W, Hirao H, Morokuma K and Hannongbua S (2012). Characteristic vibration patterns of odor compounds from bread-baking volatiles upon protein binding: density functional and ONIOM study and principal component analysis. J. Mol. Model 18(5): 2227–2240. [DOI] [PubMed] [Google Scholar]
- Weeks WW, Campos MP and Moldoveanu S (1995). Pyrolysis of Cherry Red Tobacco and 1-Deoxy-1-[(S)-2-(3-pyridyl)-1-pyrrolidinyl]-.beta.-D-fructose (Pyranose and Furanose Isomers) Amadori Products of Cherry Red Tobacco. J. Agric. Food Chem 43(8): 2247–2253. [Google Scholar]
- Yang J, Pan Z, Takeoka G, Mackey B, Bingol G, Brandl MT, Garcin K, McHugh TH and Wang H (2013). Shelf-life of infrared dry-roasted almonds. Food Chem 138(1): 671–678. [DOI] [PubMed] [Google Scholar]
- Zhu S-H, Sun JY, Bonnevie E, Cummins SE, Gamst A, Yin L and Lee M (2014). Four hundred and sixty brands of e-cigarettes and counting: implications for product regulation. Tob. Control 23(suppl 3): iii3–iii9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


