We each begin life as a single cell harboring a single genome, which— over the course of development— gives rise to the trillions of cells comprising the body. From skin cells, to heart cells, to neurons of the brain, each bears a copy of the original cell’s genome. But as anyone who has used a copy machine or played the childhood game of “telephone” knows, copies are never perfect. Every cell in an individual actually has a unique genome; an imperfect copy of its cellular ancestor differentiated by inevitable somatic mutations arising from errors in DNA replication and other mutagenic forces (1). Somatic mutation is the fundamental process leading to all genetic diseases including cancer. Every inherited genetic disease also has its origins in mutation events that occurred in an ancestor’s germline cells. Yet how many and what kinds of somatic mutations accumulate in our cells as we develop and age has long been unknown and a blindspot in our understanding of the origins of genetic disease.
While in the laboratory of Christopher Walsh at Boston Children’s Hospital and Harvard Medical School, I became intrigued by reports of neurologic diseases caused by somatic mutations, including rare cases of epilepsy, neurodegeneration, intellectual disability, brain malformations, and autism spectrum disorder (2–8). There were also long-standing hypotheses in the field that somatic genetic diversity may be prevalent in the human brain (9). Still, the common view was that the brain operates from a unitary genome.
I wondered, how much of a genomic patchwork is our brain? What kinds and how many somatic mutations are present, and do they affect brain function? Could somatic mutations underlie some of the neuropsychiatric diseases whose causes remain unknown?
Answering these questions, we recognized, would require development of a technology to sequence the vanishingly small amount of DNA (6 picograms) present in single brain cells. Any individual somatic mutation may be present in only a very small fraction of cells or even just one cell, making it undetectable by standard DNA sequencing, which mixes together DNA from thousands or millions of cells. Together with colleagues, I developed methods to sequence the genomes of single brain cells, allowing detection of even the rarest somatic mutations. Along with novel bioinformatic approaches developed with Eunjung Alice Lee in the lab of Peter Park, this provided the first systematic, genome-wide measurements of somatic mutation in the brain (10,11). Together with single-cell studies of cancer and sperm (12–14), this heralded the emergence of the field of single-cell genome sequencing, spurred by the confluence of whole genome amplification technologies (15) and decreasing DNA sequencing costs.
Our single-neuron genomics studies have identified remarkably diverse somatic mutations that reveal a wide gamut of mutation processes impacting the brain, from small point mutations and microsatellite polymorphisms to larger retrotransposon insertions, copy number variants, and aneuploidy (10,11,16,17) (Figure 1). Importantly, we are finding that each type of mutation occurs at distinct rates and patterns (10,11,16–18). These and single-neuron studies by others (19) provide a proof of principle for the systematic quantification of somatic mutations in any human tissue.
Figure 1. Single-cell genomics detects diverse somatic mutations and enables tracing of cell lineages in the human brain.
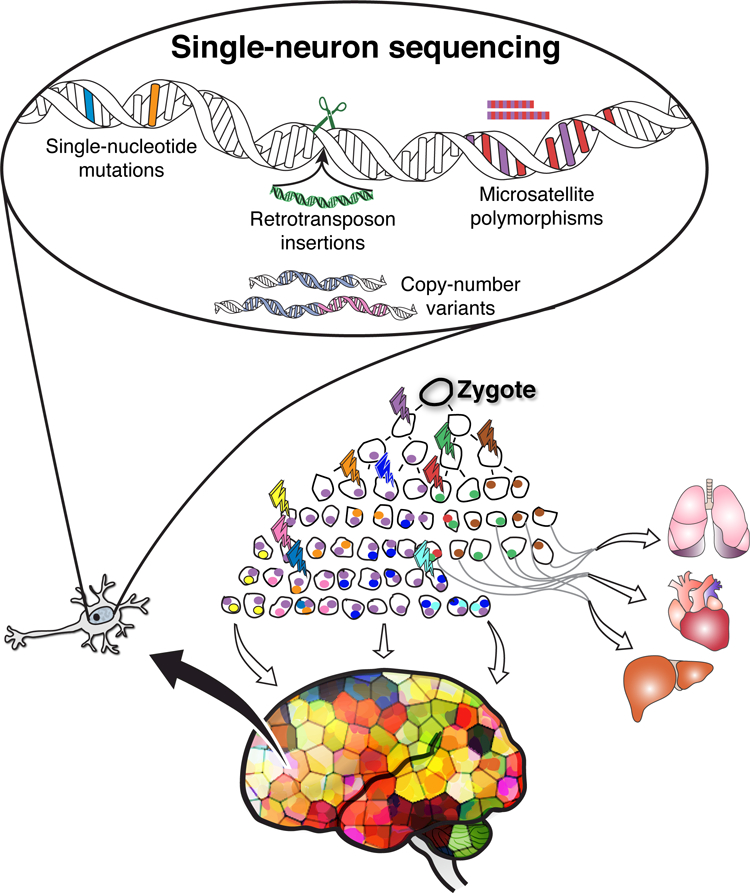
Somatic mutations occur during every cell division from the zygote throughout normal body and brain development. Single-cell genomics enables systematic measurement of somatic mutation rates. Each somatic mutation event (lightning bolt) is inherited by all offspring of the cell in which it occurred. Somatic mutations can therefore also be used as spontaneously occurring, endogenous markers for lineage tracing in human tissues, enabling reconstruction of patterns of progenitor proliferation and migration in the brain.
During my doctoral work, I was part of a team led by Dr. Ann Poduri that identified the first brain-specific somatic mutations causing neurologic disease (20). The disease in question was hemimegalencephaly, a rare congenital brain malformation in which one brain hemisphere is severely dysplastic and grows too large, leading to intractable epilepsy. Using single-cell sequencing, we found that ~20–30% of both glial and neuronal cells carry the disease-causing mutations, allowing us to pinpoint neuroglial progenitors of the cortex as the cell type where the mutations occurred (8,10,16). This finding provides insight into the source of hemimegalencephaly and related focal neurologic diseases and may potentially be relevant to other epilepsies of unknown origin. While hemimegalencephaly is visible by imaging, our findings suggest that radiographically-invisible somatic mutations— for example in ion channels or synaptic proteins— may be an occult cause of some neurologic diseases. As a result of these studies, numerous groups have begun systematic investigations of somatic mutations in neuropsychiatric disease.
A key tool in developmental biology is a technique known as lineage tracing, in which the fate of all the offspring of a particular cell (or group of cells) are tracked as the body forms. In animal models, this is achieved by tagging cells with fluorescent proteins or other invasive markers which cannot be used in humans. Somatic mutations, however, occur naturally and, as we were able to demonstrate, can serve as non-invasive lineage markers in humans (1,11,17).
Using somatic mutations identified by our single-neuron sequencing, we have been able to trace cell lineages in the human brain and reconstruct their proliferation and migration during development (11,17). Clear spatial patterns were revealed, including cell lineages distributed over an entire hemisphere at surprisingly low mosaicism indicating spatial mixing among early brain progenitors, as well as somatic mutations marking focally distributed lineages present in only one small (<1 cm2) area of the frontal cortex (11,17). This latter pattern would seem to indicate that every brain is, in fact, a mosaic patchwork of focal somatic mutations. It is therefore possible that rare, as-yet unrecognized brain disorders may exist in which a focal somatic mutation affects only one small region responsible for a particular cognitive function, while sparing the rest of the brain.
Our studies have generated a number of captivating new questions. Might the brain be particularly susceptible to harmful somatic mutations because of the unique interconnectedness of its cells? Would neurogenetic diseases, for example genetic cases of autism, manifest differently if the inciting mutation were present in only one hemisphere, one lobe, or just one gyrus of the brain? Single-neuron sequencing may also be useful for measuring the extent to which neurons, whose genomes must function for decades, accumulate mutations with age and determining whether these mutations eventually impair function. We believe that single-cell genomics combined with single-cell epigenomics, transcriptomics, and proteomics will ultimately revolutionize our understanding of brain development and function.
ACKNOWLEDGEMENTS
I am grateful to Christopher A. Walsh for his mentorship, Peter J. Park and Eunjung Alice Lee for rewarding collaborations, and Xuyu Cai and all members of the Walsh lab.
This work was supported by NIH MSTP grant T32GM007753, the Louis Lange III Scholarship in Translational Research, and the Howard Hughes Medical Institute.
REFERENCES
- 1.Shapiro E, Biezuner T, Linnarsson S, Nat. Rev. Genet 14, 618–630 (2013). [DOI] [PubMed] [Google Scholar]
- 2.Gleeson JG, et al. , Am. J. Hum. Genet 67, 574–581 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Depienne C, et al. , J. Med. Genet 47, 404–410 (2010). [DOI] [PubMed] [Google Scholar]
- 4.Topcu M, et al. , Eur. J. Hum. Genet 10, 77–81 (2002). [DOI] [PubMed] [Google Scholar]
- 5.Alzualde A, et al. , Am. J. Med. Genet. B Neuropsychiatr. Genet 153B, 1283–1291 (2010). [DOI] [PubMed] [Google Scholar]
- 6.Castronovo P, et al. Clin. Genet 78, 560–564 (2010). [DOI] [PubMed] [Google Scholar]
- 7.Beck JA, et al. Hum. Mol. Genet 13, 1219–1224 (2004). [DOI] [PubMed] [Google Scholar]
- 8.Poduri A, Evrony GD, Cai X, Walsh CA, Science 341, 1237758 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Muotri AR, Gage FH, Nature 441, 1087–1093 (2006). [DOI] [PubMed] [Google Scholar]
- 10.Evrony GD, et al. , Cell 151, 483–496 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Evrony GD, et al. , Neuron 85, 49–59 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Navin N, et al. , Nature 472, 90–94 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hou Y, et al. , Cell 148, 873–885 (2012). [DOI] [PubMed] [Google Scholar]
- 14.Wang J, Fan HC, Behr B, Quake SR, Cell 150, 402–412 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dean FB, et al. , Proc Natl Acad Sci USA 99, 5261–5266 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cai X, et al. , Cell reports 8, 1280–1289 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lodato MA, et al. , Science 350, 94–98 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Evrony GD, Lee E, Park PJ, Walsh CA, Elife 5, e12966 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McConnell MJ, et al. , Science 342, 632–637 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Poduri A, et al. , Neuron 74, 41–48 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]


