Abstract
An in vitro method based on 15N-labelled forage nitrogen (N) was developed to study ruminal N metabolism of soluble N (SN), insoluble N (ISN) and neutral detergent insoluble N (NDIN) fractions of timothy forage. Timothy grass was grown on replicated experimental plots with one plot receiving 15N-labelled and the other unlabelled N fertilizer. Harvested grass was preserved as dried grass or as formic acid treated or untreated silage. The intact forages and their corresponding N fractions were incubated in buffered rumen fluid in vitro to determine degradation parameters based on the 15N fluxes between labelled feed N and ammonia N pools. A high percentage (25–38%) of 15N-labelled ammonia disappeared from ammonia N pool during the first 15 min of incubation. Microbial uptake of dried grass SN fraction was higher than of silage SN fractions. Fractional degradation rates of SN from formic acid treated silage, untreated silage and dried grass during the first 6 hours of incubation were 0.145, 0.125 and 0.115 /h, respectively. By the end of the incubation period (28 h), 69, 66 and 43%, of the SN fraction of formic acid treated silage, untreated silage and dried grass, respectively were recovered as ammonia. The percentage of ISN fractions degraded to ammonia N were 9, 34 and 27%, respectively. Based on the changes in 15N-labelled ammonia N pool in blank incubation and appearance of 15N to ammonia N pool from 15N-labelled NDIN fractions, it was estimated that a significant portion of microbial lysis occurred when incubations were carried out for longer than 20 hours. With dried grass the contribution of ammonia N for microbial N synthesis was greater than with silages. Use of 15N-labelled forages together with this in vitro method is a promising technique for determining soluble N degradation parameters, but it requires further development to be used for determining degradation parameters of insoluble N fractions and work with whole feeds.
Introduction
Grass forage, and especially ensiled grass is the major component of dairy cow diet in Northern Europe, often consisting >50% of diet DM. Therefore even small improvements in forage protein utilisation could reduce the need for supplementary protein [1]. However, the processes during silage fermentation change the composition of forage, particularly the content of readily fermentable components such as soluble proteins and carbohydrates [2]. Similarly to ruminal fermentation, microbes in silage use water-soluble carbohydrates during silage fermentation to produce energy for growth. This leads to losses of energy and changes in the composition of the fermentable substrate available for ruminal organisms [3,4], and consequently reduced ruminal microbial efficiency, especially in poorly preserved forages [3]. In studies with growing [5] and lactating cattle [6], ruminal protein degradability was lower with hay-based diets compared with silage-based diets. Jaakkola and Huhtanen [5] reported greater microbial nitrogen (N) flow from the rumen with silage-based than with hay based diets, but the differences in total non-ammonia N flow were not significant. These conflicting results indicate a need to improve understanding of the effects of forage preservation method on ruminal protein metabolism in dairy cows as total rumen protein degradability alone seems insufficient for evaluating forage protein value. Restricted silage fermentation has been shown to increase production of milk fat and protein, mainly due to increased dry matter intake [7].
The models employed to optimise nutrient efficiency within existing feeding evaluation systems such as CNCPS [8], NRC [9] and Volden [10] use different levels of N fractionation. Furthermore, changes in the fractional composition of plant N due to preservation method (ensiling, drying, etc.) can affect ruminal N metabolism and the supply of metabolisable protein to the small intestine [11]. Most current feed evaluation systems rely on protein degradation parameters estimated by the in situ method [12]. However, the inherent shortcomings of the in situ method, in which feedstuff is incubated inside porous nylon bags inside cows rumen to measure disappearance (degradation) of substrate over period of time, make it difficult to accurately evaluate all N fractions [13]. The escape of soluble N from nylon bags, restricted microbial access to feed in the bags or microbial contamination of feed residues are likely, but not only causes of the low reproducibility of the in situ method [14]. Therefore various in vitro systems have been developed to estimate ruminal protein degradation characteristics [15,16]. Techniques involving 15N-labelled forage have made it possible to investigate N kinetics in the rumen [17] and the nitrogen isotope 15N has been successfully used as a microbial N marker in in vitro studies [18,19]. When used together with nitrogen isotope 15N to measure degradation of crude protein (CP) and/or microbial N synthesis, this allows ruminal protein metabolism to be better predicted [18]. The objectives of the present study were therefore to: 1) develop an in vitro method using 15N-labelled forages to estimate the in vitro ruminal digestion kinetics of plant soluble N (SN), insoluble N (ISN) and neutral-detergent insoluble N (NDIN) fractions of dried and ensiled timothy grass, and 2) compare the effects of preservation methods on the composition of N fractions and digestion kinetics of timothy grass.
Material and methods
Production of 15N-labelled forages
Timothy (Phleum pratense, cv. Grindstad) was grown in two replicate plots of 2 m2 each in the experimental field at Röbäcksdalen research station, Umeå, Sweden (63°35´N, 20°45´E). Both plots received 10 g N/m2 as NH4NO3 fertiliser (applied in water solution) and 1.8 g/m2 of phosphorus (P) and 9.5 g/m2 of potassium (K) as commercial compound fertiliser on 8 May 2013, when approximately 5 cm shoots were visible. To produce 15N labelled forages with 1–2 atom% excess of 15N, the timothy grass was fertilised with labelled 15NH415NO3 (Larodan Fine Chemicals AB, Malmö, Sweden) containing 2% 15N/N. Control timothy grass received unlabelled commercial NH4NO3.
Forages were harvested on 9 June 2013 when grass was between boot and early flowering stage. One-third of the forage from each plot was dried (dried grass) in a forced air oven for 48 h at 50°C to avoid protein denaturation. The aim was to compare traditional hay against silages, and therefore oven drying was used to avoid quality changes due to bad weather conditions during the harvest period. The rest of the forage was chopped using handheld gardening shears and thereafter wilted for 2 h on a paper sheet outdoors. The wilted forage was conserved in 1-L glass jars without additives (untreated silage) or with formic-acid based additive (formic acid treated silage) (ProMyr XR801, Perstorp AB, Perstorp, Sweden) applied at a rate of 4 mL/kg wilted forage. After 100 days, the silages were removed from the jars, packed into polythene grip-seal bags and kept frozen at -20°C until freeze-dried or analysed. Dry matter concentration of preserved forages and N fractions was determined by drying at 105°C for 16 h and ash concentration by incinerating at 500°C for 4 h. All forages and ISN and NDIN fractions were analyzed for CP [20] using a 2020 Digestor and a 2400 Kjeltec Analyzer Unit (Foss Analytical A/S, Hilleröd, Denmark). Metabolizable energy concentration in silages and dried grass was calculated following the procedure of Lindgren [21]. The frozen silage samples were thawed and pressed, and the pH in the liquid was measured with a pH meter (Metrohm, Herisau, Switzerland) and further analyzed for VFA and lactic acid according to Ericson and André [22]. Analysis for silage ammonium-N was done by direct distillation after adding MgO with Kjeltec 2100 Distillation Unit (Foss Analytical A/S). Organic matter digestibility was determined for all forages after 96 hr gas in vitro incubations, as described by Hetta et al., [23]
Preparation of forage N fractions
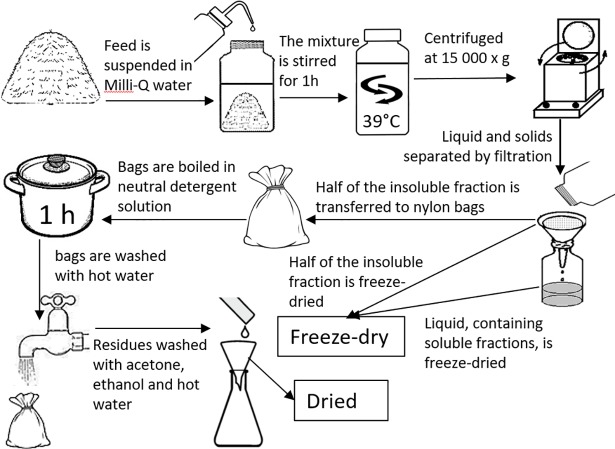
Freeze-dried silage and oven-dried grass samples were milled through a 1 mm screen with a Cyclotec 1093 sample mill (Foss Tecator, Högnäs, Sweden) and then separated into the three N fractions: SN, ISN and NDIN. One-quarter of the milled sample material was retained as a whole forage. Fig 1 depicts the procedures of N fractionation. Portions of 20 g were suspended in 400 mL of ultrapure water (Milli-Q, Merck Millipore Corporation, Darmstadt, Germany) and stirred for 1 h at 39°C. The suspension was centrifuged at 15 000 x g for 15 min (Avanti J26S XP, Beckman Coulter, Inc. Brea, CA, USA) and filtered through Whatman no. 1 filter paper. The filtrate consisting of soluble fraction was frozen at -20°C and freeze-dried. The N content in soluble fraction was determined simultaneously with determination of 15N enrichment with Flash EA 2000 elemental analyser (Thermo Fisher Scientific, Bremen, Germany) as detailed under section “Determination of 15N abundance”. The insoluble residues were rinsed twice with milli-Q water to remove all remaining soluble N and half were frozen at -20°C and later freeze-dried (ISN fraction). The remaining half was allocated into nylon bags and then boiled in neutral detergent solution (without sodium sulphite) for 1 h to prepare the NDIN fraction. After extraction, the bags were thoroughly rinsed in hot running water and dried at 60°C overnight. The dried residues were washed several times with boiling water, ethanol and acetone to remove all neutral detergent residues.
Fig 1. Procedures used for extracting forage N fractions from silages and dried grass.
(Images acquired from http://getdrawings.com/ and procedures notes DOC316.53.01186, Hack company/Hach Lange GmbH).
Neutral detergent fibre (NDF) was extracted from a good quality timothy grass silage using the same procedure as for NDIN fraction, but with added Na2SO3 (0.25 g per 1 g sample) to remove NDF-bound N.
Experimental design
The three N fractions were incubated in rumen fluid in vitro as mixed substrates. These substrates were prepared from the respective N fraction, isolated NDF, soybean meal and a carbohydrate mixture containing pectin, starch and maltose (2:1:1 on DM basis) to achieve similar concentrations of N and NDF as in the whole forages (Table 1). Isolated NDF was mixed with the SN fraction but ISN and NDIN fractions were supplemented with soybean meal and carbohydrate mixture dissolved in buffer solution [24]. The experimental design included two treatments (non-labelled and 15N-labelled), three forages (formic acid treated and untreated silage and dried grass) and four fractions (whole forages, SN, ISN and NDIN). A complete randomised design was used with the rule that each substrate had to be represented in each run at least once. Each forage and the substrates containing the N fractions were incubated in three in vitro runs with the incubation bottle being an experimental unit. In each in vitro run one sample of each substrate was represented and one-third of the substrates was replicated, resulting in four replicate observations per sample. All samples within a run were randomly distributed spatially within and between water baths. Within each run, a blank (buffered rumen fluid without a sample) and a blank with 15N-labelled ammonium in the buffer were incubated in duplicate.
Table 1. In vitro substrate formulation (mg/g DM) and chemical composition.
| Substrates1 | |||||
|---|---|---|---|---|---|
| Whole forage | SN | ISN | NDIN | ||
| Forage | 1000 | 0 | 0 | 0 | |
| SN | 0 | 330–5622 | 0 | 0 | |
| ISN | 0 | 0 | 670–684 | 0 | |
| NDIN | 0 | 0 | 0 | 507–536 | |
| Soybean meal | 0 | 0–198 | 109–212 | 163–262 | |
| NDF | 0 | 438–480 | 0 | 0 | |
| CHO3 | 0 | 0 | 111–205 | 196–300 | |
| Chemical composition | |||||
| DM, g/kg | 860–940 | 936–950 | 940–950 | 940–950 | |
| CP, g/kg DM | 132–166 | 142–168 | 147–159 | 143–155 | |
| NDF, g/kg DM | 500–550 | 480–546 | 500–547 | 500–547 | |
1 Substrates containing: SN–soluble N fraction; ISN–insoluble N fraction; NDIN–neutral detergent-insoluble N fraction.
2 The substrates were prepared to have similar CP and NDF concentrations. Since concentrations of CP and/or NDF in the same fraction differed between forages, the ratios of substrate components varied.
3 CHO–carbohydrate mixture containing pectin, starch and maltose (2:1:1 on DM basis).
In vitro procedures
All animals used for in vitro procedures were treated and kept with permission acquired beforehand from the Swedish Ethical Committee on Animal Research represented by the Court of Appeal for Northern Norrland in Umeå, Sweden and with permission from ethical committee of Umeå Djurförsöksetiska nämnd (Document nr. A97-07). Rumen fluid was collected between the morning milking and morning feeding from two rumen-cannulated lactating Nordic Red cows fed a diet containing grass silage and commercial concentrate (0.60:0.40 on DM basis) twice a day. A total of 800 mL of rumen fluid was strained through four layers of cheesecloth into Erlenmeyer flasks: Rumen fluid together with 8 g of a mixture of carbohydrates (maltose, starch, xylose, pectin; 2:1:1:1) and 2.8 g of NaHCO3) was pre-incubated for 3 hours in a water bath at 39°C. The purpose of pre-incubation [25] was to reduce the amount of digestible substrate originating from the rumen, by mechanically removing larger particles and giving time for microbes to degrade the rest. All procedures with rumen fluid were performed under a constant flow of CO2 to maintain anaerobic conditions. The rumen fluid mixture was stirred for 5 min and then the stirrer was turned off. After 25 min, the buoyant layer on rumen fluid was removed by vacuum aspiration. Thereafter the stirrer was turned on again and the rumen fluid mixture was continuously stirred for 2.5 h. The pre-incubated rumen fluid was mixed into buffered mineral solution [24] with a 1:4 volume ratio of rumen fluid to buffer. Substrates (forages and N fractions) of 500 mg were incubated in 60 mL of buffered rumen fluid in 250 mL serum bottles (Schott, Mainz, Germany). The freeze-dried forage SN fraction was solubilised in 4 mL of buffer solution (same as used for rumen fluid) before being added to the incubation bottles. Same amount of buffer solution was added to the other incubation bottles. In bottles containing unlabelled substrates, the ammonia in the inoculum was labelled with 15N by adding 1 mg of enriched (15NH4)2SO4 with 99% 15N/N (Larodan Fine Chemicals AB, Malmö, Sweden) dissolved in 0.2 mL of milliQ water. Bottles containing 15N labelled substrates received the same amount of non-enriched (NH4)2SO4. All bottles were kept in a water bath at 39°C for 44 h and continuously agitated.
The in vitro procedures were performed with a fully automated system [26] recording gas production every 12 min as described in detail by Hetta et al. [23]. Simultaneously with gas recordings, 0.6 mL of fluid from rumen samples was extracted at 0.25, 0.5, 1, 2, 3, 4, 6, 9, 20 and 28 h for determination of ammonia N and soluble non-ammonia N (SNAN) concentration in the liquid phase. The system for allowing collection of fluid samples during incubation is described in detail by Karlsson et al. [27]. The fluid samples were transferred to Eppendorf tubes containing 0.024 mL of 18 M H2SO4 for preservation and then frozen (-20°C). Prior to analysis, the samples were thawed and centrifuged at 12500 × g for 10 min. Thereafter 0.1 mL aliquots of supernatant were transferred to test-tubes and diluted to 1:20 with milli-Q water. The concentration of NH3-N was analysed using a continuous flow analyser (AutoAnalyzer 3 HR, SEAL Analytical Ltd) following the instructions provided by the manufacturer (Method No. G-102-93 Rev 7 (multitest MT7)).
Determination of 15N abundance
To determine the 15N-atom% in forages and N fractions, the milled samples were further homogenised in a ball mill and a sample containing 20–100 μg N was weighed and closed within a tin capsule. The 15N-atom% was determined using a Flash EA 2000 elemental analyser (Thermo Fisher Scientific, Bremen, Germany) connected to a DeltaV isotope ratio mass spectrometer (Thermo Fisher Scientific, Bremen, Germany).
To determine the 15N-atom% for ammonia N, the frozen samples were thawed at room temperature and centrifuged at 12 000 x g for 5 min. Then 0.3 mL of supernatant was pipetted into a 2 mL micro centrifuge tube and 0.3 mL of 10 N NaOH was added. A polyethylene mesh was rolled up and placed within the mouth of the micro centrifuge tube. A fibreglass filter (⌀ 5.5 mm, Whatman GD/C) disc impregnated with 1 μL of 5 M H2SO4 was placed on top of the mesh and the cap was closed tightly. The tubes were kept for 4 days at room temperature under constant slow agitation on a rotary shaker. Thereafter, the filter discs were removed from the tubes and placed in a 96-well microtiter plate which was placed inside a desiccator together with a container of 18 M H2SO4 and dried overnight. The dry filter discs were sealed in tin capsules and sent for analysis. In addition, some untreated and acid-impregnated filter papers were prepared and added as controls to check for contamination.
To determine the N concentration and 15N-atom% in SNAN, the samples were thawed and centrifuged in the same way as the ammonia N samples. Each fibreglass filter disc was placed inside a tin capsule in a 96-well microtiter plate and 80 μL of supernatant were pipetted onto the filter paper. The plate was placed in a desiccator together with a container of 18 M H2SO4, and dried at 60°C overnight. On the following day, the samples were left to cool to room temperature within the desiccator, after which the tin capsules were sealed and placed back in the microtiter plate. The capsules were analysed for 15N-atom% the same way as the forages described before. The size of the SNAN pool in digesta was calculated by subtracting the ammonia N pool from the total SN pool.
Calculations
The pool sizes of ammonia N and SNAN in the incubation bottles were calculated using the N concentrations determined in the respective samples and the volume of the total incubation medium. The change in volume of the incubation medium during the sampling period was taken into account.
The 15N-atom% values in unlabelled forages were very similar, and therefore an average of 0.372% [SD = 0.0014) was used to calculate 15N-atom% excess in labelled feeds. The 15N-atom% value measured in ammonia N and SN samples of unlabelled blanks were used as background 15N concentrations to calculate 15N atom% excess in the respective pools.
The size of the 15N excess pool (15NEP, μg) of the ammonia N pool (μg) was calculated as:
| (1) |
The fractional degradation rate of SN was calculated based on 15N disappearance from the SN pool, estimated from the slope of the linear regression line using natural logarithmic values of the 15NEP size.
Gas recordings were used to only check the normality of in vitro degradation processes. A mean asymptotic cumulative gas production (mL) was calculated per sample OM (g).
Statistical analysis
The effects of preservation method on in vitro protein degradation parameters such as ammonia N concentrations and microbial uptake and fractional degradation rate of SN fractions was analysed separately for each feed using the mixed procedure in SAS (SAS Inst. 2008. Version 9.4 Inc., Cary, NC) and the following model:
| (2) |
where μ is the overall mean, Ti is the treatment method (formic acid treated silage, untreated silage and dried grass), rj is the random effect of run and eij is the error. Within 15N labelled and unlabelled forages and ISN and SN fractions the treatments were analysed for treatment × time interactions using following model:
| (3) |
where μ is the overall mean, Ti is the treatment method (formic acid treated silage, untreated silage and dried grass), Pj is time, TPij is the treatment × time interaction, rk is the random effect of run and eijk is the error. Time was set as repeated measurement. Treatment effects and interactions were considered significant at P ≤ 0.05.
Model development
The kinetic parameters describing degradation of forage N fractions were estimated using observed values of 15NEP. As the aim was to develop models to describe protein degradation in vitro, the estimations were made using only mean data values of 4 replicates, for each time point, and therefore it was not possible to perform further statistical evaluation of model predictions between treatments. The model describing SN degradation consisted of three compartments: ammonia N, SNAN and microbial N, where SNAN can be degraded to ammonia N or incorporated directly to microbial N. The model describing microbial N synthesis from ammonia N for SN and ISN had two compartments: ammonia N, and microbial N together with SNAN, where ammonia N can only be incorporated to microbial N. Estimated parameters in the models were defined to be significantly different from zero (P < 0.05), i.e. the fractional standard deviation (FSD = SD / mean) was below 0.5 for all parameters in all datasets. Only data from labelled ammonia N with unlabelled ISN fractions were used for the ISN model, because the appearance of 15N in ammonia and SNAN pools from labelled ISN fractions was too low to estimate the parameters satisfactorily. The models were developed and evaluated using WinSAAM version 3.0.7 software (www.winsaam.com; New Bolton Center, Biostatistics Unit, University of Pennsylvania) [28].
Results
Descriptive data on timothy silage and dried grass and their N fractions
All silages were of good quality, with similar DM concentration, in vitro organic matter digestibility and average metabolisable energy content of 10.5 (SD = 0.21) MJ/kg DM (Table 2). The pH and ammonia N concentration were lower for the formic acid treated silages than for untreated. The 15N-labelled silages and dried grass had higher CP and also higher SN concentration than the unlabelled forages (Table 3). On average, dried grass had a lower SN concentration than the silages. The CP concentration was higher in the silage SN fraction than in SN from dried grass, whereas the CP concentration was lower in the silage ISN and NDIN fractions than in the corresponding fractions in dried grass. The mean asymptotic gas production for dried grass and silages was 275 (SE = 6.5) and 281 (SE = 6.9) mL/g organic matter (OM), respectively. There were no significant differences between comparative substrates.
Table 2. Fermentation characteristics of silages (values expressed as g/kg DM, unless otherwise stated).
| FAS151 | UTS15 | FAS | UTS | |
|---|---|---|---|---|
| DM, g/kg | 266 | 260 | 262 | 256 |
| ME2 | 10.6 | 10.3 | 10.8 | 10.5 |
| OMD3 | 725 | 708 | 735 | 720 |
| pH | 3.88 | 4.88 | 3.89 | 4.13 |
| AN4, g/kg N | 38 | 99 | 28 | 60 |
| Lactic acid | 86.4 | 49.1 | 84.3 | 73.7 |
| Acetic acid | 16.0 | 20.3 | 16.9 | 19.5 |
| Propionic acid | 3.6 | 11.4 | 5.1 | 2.9 |
| Butyric acid | 0.3 | 1.4 | 0.3 | 0.3 |
| Ethanol | 11.9 | 24.8 | 11.6 | 17.8 |
1 FAS15 and FAS = Formic acid-treated 15N-labelled and unlabelled grass silage, UTS15 and UTS = untreated 15N-labelled and unlabelled grass silage.
2 ME = metabolisable energy (MJ/kg DM).
3 OMD = in vitro OM digestibility.
4 AN = ammonia N concentration of silages and soluble N fractions.
Table 3. Chemical composition of forages and N fractions used in vitro.
| DM2, g/kg | g/kg DM | g/kg N |
15N A% / N4 |
||||
|---|---|---|---|---|---|---|---|
| Item1 | Ash | CP | NDF | Soluble N | AN3 | ||
| FAS15 | 860 | 66 | 155 | 521 | 772 | 1.642 | |
| FAS15-SN | 974 | NA | 270 | NA | 90.0 | 1.645 | |
| FAS15-ISN | 907 | 21 | 63 | 744 | 1.646 | ||
| FAS15-NDIN | 904 | 10 | 29 | 971 | 1.596 | ||
| UTS15 | 883 | 71 | 166 | 550 | 785 | 1.563 | |
| UTS15-SN | 971 | NA | 290 | NA | 123 | 1.593 | |
| UTS15-ISN | 904 | 20 | 65 | 785 | 1.476 | ||
| UTS15-NDIN | 905 | 10 | 22 | 978 | 1.432 | ||
| FAS | 862 | 66 | 149 | 518 | 692 | 0.374 | |
| FAS-SN | 973 | NA | 299 | NA | 95.9 | 0.371 | |
| FAS-ISN | 908 | 21 | 70 | 740 | 0.371 | ||
| FAS-NDIN | 901 | 9 | 32 | 975 | 0.372 | ||
| UTS | 873 | 65 | 142 | 543 | 637 | 0.373 | |
| UTS-SN | 969 | NA | 321 | NA | 115 | 0.374 | |
| UTS-ISN | 905 | 18 | 72 | 776 | 0.374 | ||
| UTS-NDIN | 905 | 9 | 25 | 979 | 0.373 | ||
| DG15 | 940 | 64 | 144 | 502 | 297 | 1.609 | |
| DG15-SN | 954 | NA | 157 | NA | 14.3 | 1.691 | |
| DG15-ISN | 906 | 28 | 139 | 714 | 1.607 | ||
| DG15-NDIN | 950 | 11 | 107 | 902 | 1.568 | ||
| DG | 938 | 59 | 132 | 500 | 237 | 0.369 | |
| DG-SN | 954 | NA | 137 | NA | 15.4 | 0.372 | |
| DG-ISN | 907 | 26 | 131 | 714 | 0.372 | ||
| DG-NDIN | 905 | 11 | 109 | 902 | 0.372 | ||
1 FAS15 and FAS = Formic acid-treated 15N-labelled and unlabelled grass silage, UTS15 and UTS = untreated 15N-labelled and unlabelled grass silage, DG15 and DG = 15N-labelled and unlabelled dried grass; SN = soluble N fraction; ISN = insoluble N fraction; NDIN = neutral detergent-insoluble N fraction.
2 DM content of feeds when used in vitro.
3 AN = ammonia N concentration of soluble N fractions.
4 15N A%/N = 15N-atom% of total N.
The N15 atom% excess of labelled forages was 1.27, 1.19 and 1.24 for the N15 labelled formic acid treated and untreated silage and dried grass, respectively. The N15 atom% excess in the SN fraction was slightly higher than that in whole forages, while it was about 3.5% lower in the NDIN fraction than in whole forages.
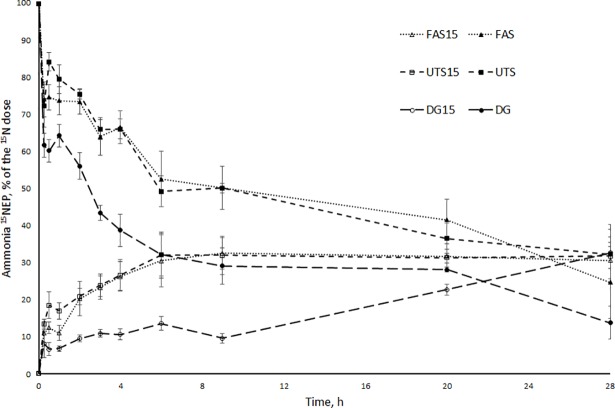
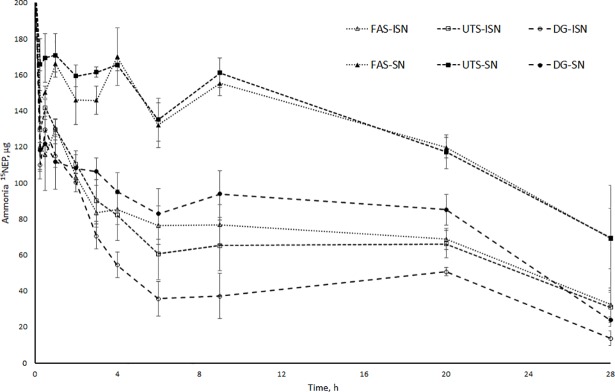
The appearance of 15N in the ammonia 15NEP from the N15 labelled untreated silage during the first 30 min was almost two-fold of that from N15 labelled formic acid treated silage and dried grass (Fig 2). After the first hour, the appearance rate of 15N from silages to ammonia 15NEP was almost identical until the end of the observation period (treatment × time interaction P = 0.019).
Fig 2. Changes in ammonia 15NEP sizes with forages.
Ammonia 15N excess pool (15NEP) size (% of the initial dose) from 15N-labelled forages (open symbols) and 15N-labelled ammonia with unlabelled forages (filled symbols). Formic acid-treated silages (FAS15, FAS; dotted line), untreated silages (UTS15, UTS; dashed line), dried grasses (DG15, DG; long dashed line). Time × treatment interaction for 15N labelled forages P = 0.019 and unlabelled forages P = 0.002.
The initial ammonia 15NEP was calculated based on the ammonia N concentration and the extra N administered as (15NH4)2SO4 into incubation vessels. The disappearance of labelled ammonia 15N was rapid for all forages, with 25–38% of the initial 15N dose (212 μg) disappearing within 15 min (Fig 2). However the initial disappearance was followed by an increase in ammonia 15NEP. This pattern of rapid initial disappearance of excess ammonia 15N followed by an increase in ammonia 15NEP size between 15 and 60 min of incubation was observed for all fractions and also with the N15 labelled blank. During the incubation, the decrease of ammonia 15NEP was faster and reached a higher extent with dried grass than with silages (treatment × time interaction P = 0.002). After the initial changes the ammonia 15NEP in N15 labelled blanks, did not change significantly despite an increase in the total ammonia N pool size from 2.3 to 4.4 mg between 4 and 20 h.
Soluble N
The SN in silages and dried grass contained 9.0–12.4% and 1.4–1.5% of ammonia N, respectively (Table 3). The excess of 15N in the labelled SN of dried grass substrate was only 51 μg, compared with 147 and 140 μg in the SN of formic acid treated and untreated silage substrates, respectively. Fig 3 presents the observed values and the model estimates for in vitro metabolism of only the SN fraction of labelled formic acid treated silage as an example, since the figures for the labelled untreated silage and dried grass SN fractions were similar. The first observation at 15 min indicated that 8, 18 and 11% of labelled N from the SN fraction in formic acid treated and untreated silage and dried grass, respectively, appeared in the ammonia N pool. By the end of the incubation period, about 67, 62 and 19%, respectively, of 15N in the initial SN fraction was recovered as ammonia N with minimal excess 15N remaining in the SNAN pool. The immediate microbial uptake of SN was 20% of the 15N dose in the SN fraction of labelled dried grass, but for the silages the predicted initial microbial uptake of SN was only 0.6–1.2%. The fractional degradation rate of the SN fractions of N15 labelled dried grass during the first 9 h were significantly (P = 0.016) faster (0.198 /h) than of the silages and lowest for the untreated silage (0.145 and 0.110 /h respectively).
Fig 3. Predicted and observed 15NEP sizes with 15N-labelled SN fraction of FAS15-SN.
Predicted (solid line) and observed (●) pool size of soluble non-ammonia 15N pool. Predicted (dotted line) and observed (▲) pool size of ammonia 15N pool. Predicted (dashed line) and observed (■) pool size of microbial 15N pool.
The 15N enrichment of ammonia N pool increased linearly (R2 = 0.93 and 0.98) during the first 6 hours, with a rate of 0.148 and 0.235 /h for the 15N-labelled silage and dried grass SN substrates, respectively. However, apart from the initial decrease in ammonia 15NEP during 0–15 min, there were no significant changes during the first 9 hours when 15N was administered as ammonia N to unlabelled silage-SN fraction of unlabelled silage. For the SN of dried grass, the ammonia 15NEP decreased linearly (R2 = 0.96) from 121 to 94 μg (from 0.58 to 0.44 of initial dose) between 15 min and 9 hours during the incubation, but remained unchanged for the rest of the incubation period.
The rate of microbial uptake of SNAN estimated by the SN model was slightly faster for the SN fraction of labelled dried grass than for the SN fractions of silages (Table 4). The rate of SNAN degradation to ammonia N was fastest for the SN of labelled formic acid silage and slowest for the SN of labelled dried grass. However, the rate of ammonia N uptake was 4- to 9-fold higher with SN of dried grass than with SN of silages. The proportion of SN estimated by the SN model to be recovered immediately in the ammonia N pool was similar to the proportion of ammonia N in the SN fraction (Tables 3 and 4). The immediate uptake of SNAN and ammonia N by microbes was similar for both silages, but higher for the SN fraction of labelled dried grass. No recycling of microbial N to ammonia N was detected, based on the modelling of appearance of 15N in ammonia 15NEP.
Table 4. Parameter estimates for the soluble nitrogen (SN) model.
| Parameter | FAS15-SN1 | UTS15-SN | DG15-SN | |||||
|---|---|---|---|---|---|---|---|---|
| Estimate | FSD2 | Estimate | FSD | Estimate | FSD | |||
| kd_SNAN-AN3, /h | 0.079 | 0.052 | 0.064 | 0.106 | 0.055 | 0.155 | ||
| ks_SNAN-MN4, /h | 0.050 | 0.057 | 0.047 | 0.080 | 0.057 | 0.161 | ||
| P15 | 0.065 | 0.102 | 0.172 | 0.073 | 0.137 | 0.066 | ||
| P26 | 0.007 | 0.291 | 0.013 | 0.249 | 0.198 | 0.064 | ||
| FAS-SN | UTS-SN | DG-SN | ||||||
| ks_AN-MN7, /h | 0.013 | 1.200 | 0.029 | 0.417 | 0.114 | 0.168 | ||
| P38 | 0.247 | 0.055 | 0.220 | 0.065 | 0.416 | 0.031 | ||
1 FAS15-SN = soluble N fraction (SN) of formic acid treated 15N labelled grass silage, UTS15-SN = SN of untreated 15N labelled grass silage, DG15-SN = SN of 15N labelled dried grass, FAS-SN = SN of unlabelled formic acid treated silage, UTS-SN = SN of unlabelled untreated grass silage, DG-SN = SN of unlabelled dried grass.
2 FSD = fractional standard deviation (SD / mean)
3 kd_SNAN-AN = rate of soluble non-ammonia N (SNAN degradation to ammonia N by microbes
4 ks_SNAN-MN = rate of direct microbial N synthesis from SNAN
5 P1 = proportion of SN immediately found in ammonia N pool
6 P2 = proportion of SN immediately found in microbial N pool
7 ks_AN-MN = rate of microbial N synthesis from ammonia N
8 P3 = proportion of ammonia N immediately taken up by microbes.
Insoluble N
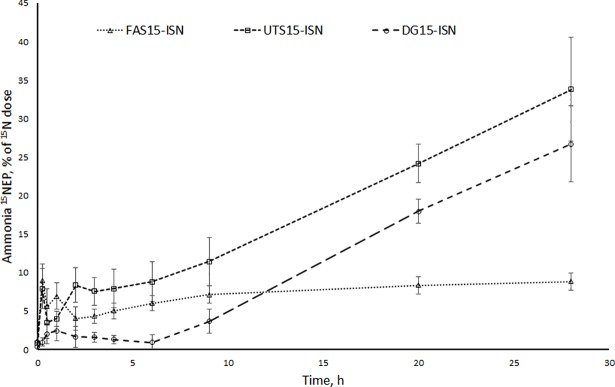
The first sampling at 15 min showed that 7.9 and 8.9% of the initial dose of 15N from the ISN fraction of labelled formic acid treated and untreated silage was recovered in ammonia 15NEP (Fig 4). With the ISN fraction of labelled dried grass, only 2.0% of the initial dose was recovered in ammonia 15NEP after 15 min of incubation, which remained virtually unchanged for the first 9 h. The percentage of initial 15N from labelled ISN fraction found in ammonia 15NEP after 28 hours was lower for the labelled formic acid treated silage than for the untreated silage and dried grass (14, 30 and 24%, respectively; P < 0.001).
Fig 4. Changes in ammonia 15NEP sizes with 15N-labelled grass forage ISN fractions.
Ammonia 15N excess pool (15NEP) size (% of the initial dose) Formic acid-treated silages (FAS15-ISN; dotted line), untreated silages (UTS15-ISN; dashed line), and dried grass (DG15-ISN; long dashed line). Time × treatment interaction is P < 0.001.
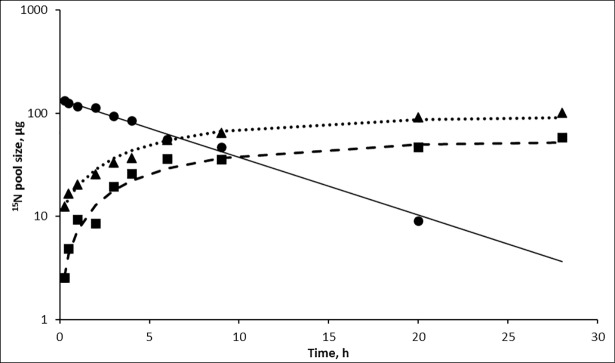
The rapid disappearance of 15N dose from the ammonia N pool (40–47% within 15 min) was not possible to model and therefore the initial condition of ammonia 15NEP was used as an adjustable parameter in the model. The ammonia 15NEP decreased linearly until 6 hours, after which there were no significant changes (Fig 5). The rate of microbial N synthesis from ammonia N was 1.6- and 2.3-fold higher for the ISN of untreated silage and dried grass compared with ISN of formic acid treated silage (Table 5). However, the rate of microbial N recycling to ammonia N was similar for all three forages.
Fig 5. Changes in ammonia 15NEP size when 15N was administered into ammonia N.
Ammonia 15N excess pool (15NEP) size (μg) with unlabelled soluble N (SN, filled symbols) and insoluble N (ISN, open symbols) fractions. Formic acid-treated silages (FAS; dotted line), untreated silages (UTS; dashed line), dried grass (DG15; long dashed line). Time × treatment interaction for ISN fraction is P = 0.14 and for SN fraction P < 0.001.
Table 5. Parameter estimates for the insoluble nitrogen (ISN) model.
| Parameter | FAS-ISN1 | UTS-ISN | DG-ISN | |||
|---|---|---|---|---|---|---|
| Estimate | FSD2 | Estimate | FSD | Estimate | FSD | |
| ks_AN-MN3, /h | 0.152 | 0.126 | 0.241 | 0.153 | 0.350 | 0.123 |
| kr_MN-AN4, /h | 0.072 | 0.165 | 0.084 | 0.215 | 0.072 | 0.182 |
| P15 | 0.355 | 0.041 | 0.295 | 0.093 | 0.314 | 0.098 |
1 FAS-SN = insoluble N fraction (ISN) of formic acid-treated unlabelled grass silage, UTS-ISN = ISN of untreated unlabelled grass silage, DG-ISN = SN of unlabelled dried grass.
2 FSD = fractional standard deviation (SD / mean).
3 ks_AN-MN = rate of microbial N synthesis from ammonia N.
4 kr_MN-AN = rate of microbial N recycling to ammonia N.
5 P1 = proportion of ammonia N immediately taken up by microbes.
The 15N disappearance from labelled ammonia 15NEP was faster and reached a higher extent with ISN fractions than with SN fractions (Fig 5). The decrease in ammonia 15NEP size was higher with the SN and ISN fractions of dried grass than with the corresponding silage N fractions. The decrease in ammonia 15NEP was greatest with the ISN fraction of dried grass, with 76% of the ammonia 15N disappearing in 9 hours, compared with 60% for the SN fraction of dried grass.
Neutral detergent insoluble N
None of the 15N from the labelled silage NDIN fraction appeared in ammonia 15NEP and only 12% of 15N in the NDIN fraction of labelled dried grass was recovered as ammonia 15N by the end of the incubation.
The disappearance of 15N from the labelled ammonia 15NEP followed the same pattern as for ISN, but the differences between forages were smaller. The ammonia 15NEP decreased linearly (R2 = 0.94–0.98) until 6 hours, after which there were no significant changes during the rest of the incubation period.
Discussion
Forage quality and N fractions
The first objective of this study was to grow 15N-labelled and unlabelled timothy grass in as similar a way as possible, in order to achieve similar N fractionation in the forages for comparison of the effects of preservation method. The similar DM concentration in all silages (261 g/kg DM, SD = 0.4) and similar fermentation quality for corresponding 15N-labelled and unlabelled silages (Table 2) show that this objective was generally achieved. The effect of the acid-based additive in reducing silage pH and ammonia N concentration was as expected [29]. The higher CP concentration in the NDIN fraction of formic acid-treated silages than that of untreated silages may indicate reduced hydrolysis of fibre-bound N in treated silages [30]. The higher CP concentration in the dried grass NDIN fraction supports this assumption, as less breakdown/hydrolysis occur during drying than during ensiling [9]. Nsereko and Rooke [31] reported a higher proportion of peptides in soluble N in formic acid-treated silage than in untreated silage.
There were no significant differences in 15N enrichment between dried grass and different silages, but the lower enrichment of NDIN fraction and slightly higher enrichment of SN fraction compared with total N in the whole forages agree with the findings by Hristov et al. [17]. They reported much lower 15N enrichment in ADF-bound N than in other N fractions.
Pre-incubation of ruminal fluid proved effective in normalising the conditions between the runs. The average ammonia concentration between runs at the start of the incubation was 44.5 mg/L (SD = 2.1). Based on authors earlier experience [32] and with current data it is recommended that in vitro medium should always be pre-incubated when evaluating protein digestion. The extent and rate of gas production determined in this study suggests that the in vitro fermentation proceeded normally [33].
The rapid initial disappearance of 15N from labelled ammonia 15NEP observed for all samples remains unexplained. The proportional changes in the total ammonia N pool between the start and 15 min of incubation were not large enough to explain the decrease in ammonia 15NEP size. It can be speculated that this disappearance was due to N uptake into microbial cells, because the rumen fluid was deprived of ammonia during the pre-incubation. Blake et al. [34] observed an almost 50% decrease in ammonia 15N atom% excess in just 30 min after infusion of 15NH4Cl into the rumen of steers. In addition, they observed a significant increase in 15N enrichment of intracellular ammonia in bacteria after only 2 min, demonstrating the ability of microbes for rapid uptake of ammonia N. In such a short time, ammonia N cannot be completely used for microbial protein synthesis, which suggests that some of ammonia is taken into intracellular pools [35]. A substantial proportion of the 15N had reappeared in the ammonia 15NEP after 30 or 60 min in the present study, suggesting release of some of the absorbed 15N from microbes. This is supported by the slower 15N enrichment of bacterial AA than of intracellular ammonia reported by Blake et al. [34]. Previous tests conducted without pre-incubation of rumen fluid in our laboratory [unpublished data) indicated that microbial uptake and release of ammonia can occur in a short time and exceed the extent observed in the present study.
The increase in total ammonia production in the blanks observed after 20 hours may be due to microbial lysis [36]. However, since there was no increase in ammonia 15NEP, the increase in the ammonia N pool size mostly likely resulted from the lysis of protozoal or inoculum bacteria. Protozoa grow slowly and survive only in small numbers in this type of batch culture in vitro system [37], and therefore incorporation of 15N into protozoal cells was likely to be negligible.
Soluble N
As expected, the ammonia N concentration in silage SN was higher than that in dried grass SN, since the ammonia concentration in hay is normally very low compared with that in silage [17,38]. The SNAN model fitted both the dried grass and silage data well, although random variation was greater for the dried grass data, likely due to lower N concentration in SN fractions of dried grasses compared with silage.
The greater proportion of the 15N in SN fraction of labelled untreated silage appeared to ammonia N pool during the first 15 min of incubation compared with the SN fraction of formic acid treated silage and dried grass (0.18, 0.08 and 0.11, respectively) may partly be because of the higher ammonia N concentration in SN fraction of untreated silage than in formic acid treated silage (Table 3). However, this does not explain the relatively high value with the SN fraction of labelled dried grass since the ammonia concentration in SN fraction of dried grass was only 10–15% of that in silage SN. These differences in degradation could be explained by differences in the rate of hydrolysis of different soluble proteins and to more favourable composition of SN in restrictedly fermented silage and dried grass [38,39,40]. Wallace [41] found that the protein adsorption rate to bacteria corresponded closely to their initial rate of hydrolysis. Soluble N in untreated grass silage has a significantly lower peptide concentration but greater free amino acids (AA) concentration compared with formic acid-treated grass silage [33,42]. Microbes may prefer peptides and AA from restrictedly fermented silage and use these components directly as N source for microbial synthesis [43,44]. This is also supported by the lower initial microbial uptake of ammonia 15N from labelled rumen fluid observed with whole silage and silage SN fractions than with the dried grass and SN of dried grass. The overall slower appearance of 15N into ammonia 15NEP and higher disappearance of 15N from labelled ammonia 15NEP with dried grass than silages suggest slower degradation [45, 46] and/or higher microbial N synthesis from direct incorporation of SN with dried grass [41].
The fractional degradation rates of SN fractions observed here were about two-fold lower than previously reported by Peltekova and Broderick [47]. In dairy cows, given a single dose of 15N-labelled grass silage SN fraction, the SNAN disappearance rate was 1.24 /h, with the rate of SNAN degradation to ammonia N being about five-fold faster than direct bacterial uptake of SNAN [48]. In our study the degradation rate of silage SN fractions to ammonia N was only about 50% faster than the rate of microbial uptake of SN, and with SN from dried grass the rates of uptake and degradation were similar. However, part of the difference can be due to microbes using some of the ammonia 15N produced from SN degradation for microbial N synthesis. This is supported by the fact that estimated rate of microbial N synthesis from ammonia N was slowest for the SN faction of formic acid treated silage (0.013 /h) and fastest for the SN fraction of dried grass (0.114 /h).
Insoluble N
The 15N from silage ISN fractions recovered in ammonia 15NEP during the early stage of incubation suggests better availability of silage ISN for microbial synthesis than dried grass ISN [47]. This also may explain the lower ammonia 15NEP with the ISN fraction of the labelled formic acid treated silage than with the ISN fraction of the untreated silage at the end of incubation, indicating better utilisation of ISN from restrictively fermented silage.
Lower appearance of 15N in ammonia 15NEP from the ISN fraction of the labelled formic acid treated silage and the corresponding lower rate of microbial uptake of labelled ammonia 15N with the ISN of the unlabelled formic acid treated silage compared with untreated silage and dried grass suggests better utilisation of the ISN fraction of the formic acid-treated silage. In extensively fermented silage, the remaining N may be less available for microbial synthesis [4]. On the other hand, hay usually has lower degradability than silage when determined by the in situ method [10,49].
The greater extent of 15N uptake from labelled ammonia 15NEP with ISN fractions compared with SN fractions suggest that ammonia played a more important role as N source for microbial protein synthesis with the ISN fraction. These results are in agreement with observations by Peltekova and Broderick [47], who used 15N-labelled ammonia N to measure in vitro ruminal degradation of soluble and insoluble fractions of alfalfa silage and hay and observed lower bacterial 15N enrichment (0.474 N15 atom% excess) with soluble N fraction compared with the insoluble fraction (0.664 N15 atom% excess).
Neutral detergent insoluble N
None of the dose of 15N in NDIN appeared in ammonia 15NEP. If any of the NDIN was degraded to ammonia N, this must have occurred intracellularly and the ammonia N must have been directly incorporated into microbial protein. This agrees with the suggestion that fibre-bound bacteria use protein directly without degrading it to the external ammonia N pool [50,51]. Cellulolytic bacteria have a preference for ammonia as N source [52], although they can also utilise other N sources [51,53]. Because of low CP concentration in NDF, particle-associated bacteria utilise degradation products directly for cell synthesis rather than excreting them into extracellular pools. In addition, the in vivo ruminal degradability of the NDIN fraction is rather low [54].
With both the unlabelled NDIN and ISN fractions, the ammonia 15NEP decreased only for the first 6–9 hours, after which it remained mostly unchanged or even increased. This observation supports the idea that rate of microbial lysis or recycling and ammonia uptake reached an equilibrium after 6 hours, as speculated previously concerning ammonia 15NEP changes in N15 labelled blanks. Wells and Russell [36] discussed bacterial lysis and presented similar data showing two-phasic ammonia 15N dilution kinetics for intraruminal enrichment of N following a single injection of 15N. Using measured data reported by Nolan and Leng [55], Wells and Russell [36] developed hypothetical values showing the dilution rate of 15N-labelled ammonia to be 14-fold greater in the first 5–6 hours than at later times. Thus it was concluded that about 35% or more ammonia N was turned over, prolonging the dilution of 15N-labelled ammonia.
Implications
Using 15N labelled feed N fractions allows to study ruminal N metabolism in more detail both in vitro and in vivo [17]. The results have indicated that the degradation rate of SNAN fraction to ammonia N is not infinite and that quantitatively an important fraction escapes ruminal degradation. This is contrast to the assumptions of most feed protein evaluation systems in calculating RUP and MP. The evidence also suggest that SNAN is a better N source for rumen microbes than ammonia N. Therefore, feed protein evaluation systems could be improved by taking into account escape of the SNAN and the nature of N on microbial protein synthesis. Although rather labour-intensive, kinetic studies using 15N labelled feed N fractions could be used to determine parameter values required in both mechanistic and static feed evaluation models [48].
Conclusions
Timothy grass forages labelled with 15N and preserved as formic acid-treated or untreated silage or as dried grass were used to estimate the ruminal degradation kinetics of different forage protein fractions in vitro. Formic acid treatment decreased ammonia N concentration in silage and reduced degradation and improved microbial N synthesis from ammonia N, but there were no significant differences in overall degradation of formic acid treated silages compared with untreated silage. Differences in degradation of SN fractions between forages indicated significantly higher true protein concentration in dried grass than in silages. The degradation rates of SN fractions were lower than normally observed in vivo. The microbial uptake of rumen ammonia was significantly greater with insoluble N fractions than with soluble N fractions, indicating higher importance of soluble N, compared to ammonia N for direct microbial synthesis. More work is needed to relate the results of in vitro predictions to in vivo conditions. The method of using 15N-labelled plants to study ruminal degradation kinetics is a promising approach for future in vitro and in vivo studies.
Supporting information
(XLSX)
Acknowledgments
The Swedish research council Formas is gratefully acknowledged for financial support for this work. The authors would like to express their gratitude to Prof. Kerstin Huss-Danell and Dr. Mårten Hetta for their help in planning and preparing for this study and to Prof. Glen Broderick for assistance with 15N analysis. The authors also wish to thank Mrs. Ann-Sofi Hahlin, Ms. Evelina Viklund and Ms. Marie Koukolová for their assistance with sample preparation and laboratory procedures.
Data Availability
All data underlying the study are within the paper and its Supporting Information files.
Funding Statement
This work was supported by Formas, (www.formas.se/en/), Grant number 2011-1334. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Huhtanen P, Broderick GA. Improving utilisation of forage protein in ruminant production by crop and feed management. In: Höglind M, Bakken AK, Hovstad KA, Kallioniemi E, Riley H, Steinshamn H, et al. The multiple roles of grassland in the European bioeconomy: Proceedings of the 26th EGF General Meeting. 2016 Sept 4–8; Trondheim, Norway: Wageningen; 2016. pp. 340–349.
- 2.McDonald P. The Biochemistry of Silage. Chichester: Wiley; 1981 [Google Scholar]
- 3.Chamberlain DG. Silage fermentation in relation to the utilization of nutrients in the rumen. Process Biochem. 1987; 22: 60–63. [Google Scholar]
- 4.Van Soest PJ. Nutritional Ecology of the Ruminant. 2nd ed. Ithaca: Cornell University Press; 1994. [Google Scholar]
- 5.Jaakkola S, Huhtanen P. The effects of forage preservation method and proportion of concentrate on nitrogen digestion and rumen fermentation in cattle. Grass Forage Sci. 1993; 48: 146–154. 10.1111/j.1365-2494.1993.tb01847.x [DOI] [Google Scholar]
- 6.Halmemies-Beauchet-Filleau A, Kairenius P, Ahvenjärvi S, Crosley LK, Muetzel S, Huhtanen P, Vanhatalo A, Toivonen V, Wallace RJ, Shingfield KJ. Effect of forage conservation method on ruminal lipid metabolism and microbial ecology in lactating cows fed diets containing a 60:40 forage-to-concentrate ratio. J. Dairy Sci. 2013; 96: 2428–2447. 10.3168/jds.2012-6043 [DOI] [PubMed] [Google Scholar]
- 7.Huhtanen P, Nousiainen JI, Khalili H, Jaakkola S, Heikkila T. Relationships between silage fermentation characteristics and milk production parameters: analyses of literature data. Livest Prod Sci. 2003; 81: 57–73. 10.1016/S0301-6226(02)00195-1 [DOI] [Google Scholar]
- 8.Fox DG, Sniffen CJ, OConnor JD, Russell JB, Van Soest P. A net carbohydrate and protein system for evaluating cattle diets: III. Cattle requirements and diet adequacy. J. Animal Sci. 1992; 70: 3578–3596. 10.2527/1992.70113578x [DOI] [PubMed] [Google Scholar]
- 9.NRC. Nutrient Requirements of Dairy Cattle. 7th rev. ed. Washington: National Academy Press; 2001. doi: 10.17226/9825 [Google Scholar]
- 10.Volden H. NorFor—The Nordic feed evaluation system. Wageningen: Wageningen Academic Publishers; 2011. 10.3920/978-90-8686-718-9 [DOI] [Google Scholar]
- 11.Broderick GA. Manipulation of the Dietary N-Fractions to Improve Ruminal Microbial Synthesis and Yield. In: Proceedings of 22nd Florida Ruminant Nutrition Symposium; 2011 Feb 1–2; Gainesville, USA; 2011. pp. 81–88
- 12.Ørskov ER, McDonald I. The estimation of protein degradability in the rumen from incubation measurements weighted according to rate of passage. J Agric Sci. 1979; 92: 499–503. 10.1017/S0021859600063048 [DOI] [Google Scholar]
- 13.Broderick GA, Cochran RC. In vitro and in situ methods for estimating digestibility In: Theodorou MK, France J, editors. Feeding Systems and Feed Evaluation Models. Wallingford: CABI Publishing; 2000. pp. 53–85. [Google Scholar]
- 14.Hvelplund T, Weisbjerg MR. In situ techniques for the estimation of protein degradability and postrumen availability In: Givens DI, Owen E, Axford RFE and Omed HM, editors. Forage Evaluation in Ruminant Nutrition. Wallingford: CABI publishing; 2000. pp. 233–258. 10.1079/9780851993447.0233 [DOI] [Google Scholar]
- 15.Broderick GA. In Vitro Procedures for Estimating Rates of Ruminal Protein Degradation and Proportions of Protein Escaping the Rumen Undegraded. J. Nutr. 1978; 108: 181–190. 10.1093/jn/108.2.181 [DOI] [PubMed] [Google Scholar]
- 16.Raab L, Cafantaris B, Jilg T, Menke KH. Rumen protein degradation and biosynthesis. 1. A new method for determination of protein degradation in rumen fluid in vitro. Br. J. Nutr. 1983; 50: 569–582. 10.1079/BJN19830128 [DOI] [PubMed] [Google Scholar]
- 17.Hristov AN, Huhtanen P, Rode LM, Acharya SN, McAllister TA. Comparison of the ruminal metabolism of nitrogen from 15N-labeled alfalfa preserved as hay or as silage. J. Dairy Sci. 2001; 84: 2738–2750. 10.3168/jds.S0022-0302(01)74728-5 [DOI] [PubMed] [Google Scholar]
- 18.Hristov AN. Fractionation of ammonia nitrogen isotopes by ruminal bacteria in vitro. Anim. Feed Sci. Technol. 2002; 100: 71–77. 10.1016/S0377-8401(02)00078-0 [DOI] [Google Scholar]
- 19.Ahvenjärvi S, Stefanski T, Huhtanen P. In vitro method for determining the ruminal degradation rate of rapeseed meal protein using 15N isotope labelled ammonia nitrogen. Anim. Feed Sci. Technol. 2009; 153: 88–100. 10.1016/j.anifeedsci.2009.06.006 [DOI] [Google Scholar]
- 20.Nordic Committee on Food Analysis. Nitrogen. Determination in food and feed according to Kjeldahl. Report no. 6. 1979; Uppsala, Sweden.
- 21.Lindgren E. The nutritional value of roughages determined in vivo and by laboratory methods (In Swedish). Report no. 45. 1979; The Swedish University of Agricultural Sciences, Uppsala, Sweden.
- 22.Ericson B, André J. HPLC—Applications for agricultural and animal science. Proceedings of 1st Nordic Feed Science Conference; 2010 June 22–23; 2010. Uppsala, Sweden; pp. 23–26.
- 23.Hetta M, Cone JW, Gustavsson A-M, Martinsson K. The effect of additives in silages of pure timothy and timothy mixed with red clover on chemical composition and in vitro rumen fermentation characteristics. Grass Forage Sci. 2003; 58: 249–257. 10.1046/j.1365-2494.2003.00376.x [DOI] [Google Scholar]
- 24.Menke KH, Steingaß H. Estimation of the energetic feed value obtained from chemical analysis and in vitro gas production using rumen fluid. Anim. Res. Dev. 1988; 28: 7–55. [Google Scholar]
- 25.Hristov AN, Broderick GA. In vitro determination of ruminal protein degradability using [15N] ammonia to correct for microbial nitrogen uptake. J. Animal Sci. 1994; 72: 1344–1354. [DOI] [PubMed] [Google Scholar]
- 26.Cone JW, van Gelder AH, Visscher GJW, Oudshoorn L. Influence of rumen fluid and substrate concentration on fermentation kinetics measured with a fully automated time related gas production apparatus. Anim. Feed Sci. Technol. 1996; 61: 113–128. 10.1016/0377-8401(96)00950-9 [DOI] [Google Scholar]
- 27.Karlsson L, Hetta M, Udén P, Martinsson K. New methodology for estimating rumen protein degradation using the in vitro gas production technique. Anim. Feed Sci. Technol. 2009; 153: 193–202. 10.1016/j.anifeedsci.2009.06.010 [DOI] [Google Scholar]
- 28.Wastney ME, Patterson BH, Linares OA, Greif PC, Boston RC. Investigating Biological Systems Using Modeling. San Diego: Academic Press; 1998. [Google Scholar]
- 29.McDonald P, Henderson AR, Heron SJE. The Biochemistry of Silage. 2nd ed. Kingston: Chalcombe Publications; 1991 [Google Scholar]
- 30.Barry TN. Effect of treatment with formaldehyde, formic acid, and formaldehyde-acid mixtures on the chemical composition and nutritive value of silage. New Zeal. J. Agr. Res. 1975:18; 285–294. 10.1080/00288233.1975.10423646 [DOI] [Google Scholar]
- 31.Nsereko VL, Rooke JA. Effects of peptidase inhibitors and other additives on fermentation and nitrogen distribution in perennial ryegrass silage. The J. Sci. Food Agric. 1999; 79: 679–686. [DOI] [Google Scholar]
- 32.Vaga M, Huss-Danell K, Hetta M. Huhtanen P. Investigation of protein digestion kinetics in vitro using 15N labelled timothy and red clover. J. Dairy Sci. 2015; 98: Suppl. 2 pp. 482. [Google Scholar]
- 33.Ramin M, Huhtanen P. Development of an in vitro method for determination of methane production kinetics using a fully automated in vitro gas system—A modelling approach. Anim. Feed Sci. Technol. 2012; 174: 190–200. 10.1016/j.anifeedsci.2012.03.008 [DOI] [Google Scholar]
- 34.Blake JS, Salter DN, Smith RH. Incorporation of nitrogen into rumen bacterial fractions of steers given protein- and urea-containing diets. Ammonia assimilation into intracellular bacterial amino acids. Br. J. Nutr. 1983; 50: 769–782. 10.1079/BJN19830148 [DOI] [PubMed] [Google Scholar]
- 35.Russell JB and Strobel HJ. Concentration of ammonia across cell membranes of mixed rumen bacteria. J. Dairy Sci. 1987; 70: 970–976. 10.3168/jds.S0022-0302(87)80101-7 [DOI] [PubMed] [Google Scholar]
- 36.Wells JE, Russell JB. Why do many ruminal bacteria die and lyse so quickly? J. Dairy Sci. 1996; 79: 1487–1495. 10.3168/jds.S0022-0302(96)76508-6 [DOI] [PubMed] [Google Scholar]
- 37.Bonhomme A. Rumen ciliates: their metabolism and relationships with bacteria and their hosts. Anim. Feed Sci. Technol. 1990; 30: 203–266. 10.1016/0377-8401(90)90016-2 [DOI] [Google Scholar]
- 38.Hedqvist H, Udén P. Measurement of soluble protein degradation in the rumen. Anim. Feed Sci. Technol. 2006; 126: 1–21. 10.1016/j.anifeedsci.2005.05.011 [DOI] [Google Scholar]
- 39.Mangan JL. Quantitative studies on nitrogen metabolism in the bovine rumen: The rate of proteolysis of casein and ovalbumin and the release and metabolism of free amino acids. Br. J. Nutr. 1972; 27: 261–283. 10.1079/BJN19720092 [DOI] [PubMed] [Google Scholar]
- 40.Wallace RJ. Hydrolysis of 14C-labelled proteins by rumen micro-organisms and by proteolytic enzymes prepared from rumen bacteria. Br. J. Nutr. 1983; 50: 345–355. 10.1079/BJN19830102 [DOI] [PubMed] [Google Scholar]
- 41.Wallace RJ. Adsorption of soluble proteins to rumen bacteria and the role of adsorption in proteolysis. Br. J. Nutr. 1985; 53: 399–408. 10.1079/BJN19850047 [DOI] [PubMed] [Google Scholar]
- 42.Nsereko VL, Rooke JA. Characterisation of peptides in silages made from perennial ryegrass with different silage additives. J. Sci. Food Agric. 2000; 80: 725–731. [DOI] [PubMed] [Google Scholar]
- 43.Russell JB, Sniffen CJ, Van Soest PJ. Effect of carbohydrate limitation on degradation and utilization of casein by mixed rumen bacteria. J. Dairy Sci. 1983; 66: 763–775. 10.3168/jds.S0022-0302(83)81856-6 [DOI] [PubMed] [Google Scholar]
- 44.Walker ND, Newbold CJ, Wallace RJ. Nitrogen metabolism in the rumen In: Pfeffer E and Hristov AN, editors. Nitrogen and Phosphorus nutrition of cattle: reducing the environmental impact of cattle operations. Wallingford: CABI Publishing; 2005. pp. 71–115 [Google Scholar]
- 45.Varvikko T, Vanhatalo A. Use of a combined synthetic fibre bag method to estimate the true total tract digestion of organic matter and nitrogen of hay and grass silage in cows. Arch Tierernahr. 1993; 43: 53–61. 10.1080/17450399309386023 [DOI] [PubMed] [Google Scholar]
- 46.Martineau R, Lapierre H, Ouellet DR, Pellerin D, Berthiaume R. In situ degradation of timothy conserved as restrictively or extensively fermented silage or as hay. Can. J. Anim. Sci. 2006; 86: 299–306. 10.4141/A05-046 [DOI] [Google Scholar]
- 47.Peltekova VD, Broderick GA. In vitro ruminal degradation and synthesis of protein on fractions extracted from alfalfa hay and silage. J. Dairy Sci. 1996; 79; 612–619. 10.3168/jds.S0022-0302(96)76406-8 [DOI] [PubMed] [Google Scholar]
- 48.Ahvenjärvi S, Vaga M, Vanhatalo A, Huhtanen P. Ruminal metabolism of grass silage soluble N fractions. J. Dairy Sci. 2018; 101: 279–294. 10.3168/jds.2016-12316 [DOI] [PubMed] [Google Scholar]
- 49.Verbič J, Ørskov ER, Žgajnar J, Chen XB, Vida Žnidaršič-Pongrac. The effect of method of forage preservation on the protein degradability and microbial protein synthesis in the rumen. Anim. Feed Sci. Technol. 1999; 82: 195–212. 10.1016/S0377-8401(99)00102-9 [DOI] [Google Scholar]
- 50.Wallace RJ, Atasoglu C, Newbold JC. Role of peptides in rumen microbial metabolism–Review. Asian-Australas J Anim Sci. 1999; 12: 139–147. 10.5713/ajas.1999.139 [DOI] [Google Scholar]
- 51.Carro MD, Miller EL. Effect of supplementing a fibre basal diet with different nitrogen forms on ruminal fermentation and microbial growth in an in vitro semi-continuous culture system (RUSITEC). Br. J. Nutr. 1999; 82: 149–157. 10.1017/S0007114599001300 [DOI] [PubMed] [Google Scholar]
- 52.Russell JB, O'Connor JD, Fox DG, Van Soest PJ, Sniffen CJ. A net carbohydrate and protein system for evaluating cattle diets: I. Ruminal fermentation. J Anim Sci. 1992; 70: 3551–3561. 10.2527/1992.70113551x [DOI] [PubMed] [Google Scholar]
- 53.Atasoglu C, Newbold CJ, Wallace RJ. Incorporation of [(15)N] ammonia by the cellulolytic ruminal bacteria Fibrobacter succinogenes BL2, Ruminococcus albus SY3, and Ruminococcus flavefaciens 17. Appl. Environ. Microbiol. 2001; 67; 2819–2822. 10.1128/AEM.67.6.2819-2822.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ahvenjärvi S, Vanhatalo A, Huhtanen P Varvikko T. Effects of supplementation of a grass silage and barley diet with urea, rapeseed meal and heat-moisture-treated rapeseed cake on omasal digesta flow and milk production in lactating dairy cows. Acta Agric. Scand. A. 1999; 49: 179–189. 10.1080/090647099424097 [DOI] [Google Scholar]
- 55.Nolan JV, Leng RA. Dynamic aspects of ammonia and urea metabolism in sheep. Br. J. Nutr.1972; 27: 177–194. 10.1079/BJN19720081 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLSX)
Data Availability Statement
All data underlying the study are within the paper and its Supporting Information files.