To The Editor,
Clostridium difficile is emerging as a major enteric and nosocomial pathogen worldwide. Antibiotic usage is an important risk factor for C.difficile infection (CDI). The incidence of both nosocomial- and community-acquired CDI has been steadily increasing in the last 15 years. The increase in CDI rates has been primarily attributed to C.difficile PCR ribotype 027. Infection with C.difficile ribotype 027 has been associated with increased morbidity and mortality. Therefore, C.difficile ribotype 027 has been referred to as “hypervirulent”. The hypervirulence in ribotype 027 has been linked to a) increased transmissibility b) increased relapse rates and c) poor clinical outcomes as compared to typical endemic strains. In 2008, CDI with C.difficile PCR ribotype 078 was reported to cause severe disease. In addition, ribotypes 027 and 078 share virulence factors including tcdA-, tcdB- and binary toxin-genes [1]. Thus ribotypes 027 and 078 are both referred to as hypervirulent ribotypes of C.difficile. Although other ribotypes have been associated with epidemics and increased toxin production [2] there is no conclusive evidence of increased morbidity and mortality; therefore, the use of the term “hypervirulent” has thus far been limited to C.difficile ribotypes 027 and 078. In addition, differences in enzymes, expression of flagella, capsule production and enhanced adhesion have been attributed to hypervirulence in C.difficile. While a plethora of factors have been linked to ribotypes 027 and 078, it is accepted that hypervirulence is a complex process that is are not necessarily linked to toxin production, sporulation or resistance to antibiotics [3]. Despite extensive research, the specific genomic attributes of hypervirulence in C.difficile remain poorly understood.
Recent studies suggest that depletion of CpGs in virus genomes is linked to enhanced virus replication [4] and poor prognosis [5]. In addition, CpG dinucleotides content of virus genomes has been associated with virus pathogenesis [6], host methylation capabilities [7] and differences in evolutionary pressures [8]. Another recent report highlights how innate immune responses target non-self RNA in a CpG-dependent manner [9]. It is not known if the CpG content of bacterial genomes is associated with pathogenesis or virulence. Differences in CpG content of C.difficile strains has not been investigated as a potential factor in hypervirulence. We hypothesized that the CpG dinucleotide content in C.difficile is associated with hypervirulence.
A total of 21 whole genome sequences of C.difficile were retrieved from NCBI Genome database (https://www.ncbi.nlm.nih.gov/genome/genomes/535?) and analyzed. This includes all available full-length sequences from the hypervirulent ribotypes 027 and 078 (n = 15) and all available full-length sequences from the non-hypervirulent ribotype 012 (n = 6). Differences in transmission rates, ability to cause epidemics, toxin production, sporulation and antimicrobial resistance has been documented across C.difficile ribotypes [10,11]; nonetheless, only ribotypes 027 and 078 have been designated as “hypervirulent” based on several factors including increased morbidity and mortality associated with CDI by these ribotypes [1,12,13]. In addition, most studies investigating the mechanisms underlying hypervirulence use ribotype 012 as the reference, representing non-hypervirulent or “typical” strains. Therefore, for this study we considered ribotypes 027 and 078 as hypervirulent ribotypes. We used the historic ribotype 012 strain 630 [2] and all full-length sequences of ribotype 012 as “typical” or non-hypervirulent strains of C.difficile. The accession numbers of all the full-length C.difficile sequences analyzed are provided in Supplementary Table 1. The computation of dinucleotide ratio for double stranded C.difficile genome was carried out using the following formula [14]:
Where f(X), f(Y) and f(XpY) denote the mononucleotide frequencies and dinucleotide frequency respectively in one strand; f(X’), f(Y’) and f(Y’pX’) denote the frequency of complementary mononucleotides and reverse complement of the dinucleotide respectively in the same strand. G denotes the total length of genome.
Dinucleotide frequencies were calculated for all the whole genome sequences (n = 21). In addition, the dinucleotide frequencies in the coding DNA sequences (CDS) were calculated from the annotated Genbank files downloaded from NCBI (https://www.ncbi.nlm.nih.gov/sites/batchentrez). The calculation of dinucleotide frequencies for the coding regions was carried out for all annotated sequences [n = 12; this includes 8 sequences from hypervirulent ribotypes (027 and 078) and 4 sequences from ribotype 012]. Statistical analysis was carried out using the Mann Whitney U test, Student t test and Wilcoxon signed-rank test as appropriate. The bar graphs and box plots were made using the software Origin. The obtained results were considered significant at a p value <0.05.
We found that CpG dinucleotides were the most depleted dinucleotides among the C.difficile strains analyzed (i.e. both hyper-virulent and non-hypervirulent ribotypes). The relative abundance of CpG dinucleotides in C.difficile strains analyzed is 0.307 (Figure 1(a); i.e. only about 30% of the expected number of CpGs are present). Interestingly, CpG dinucleotides were the most variable dinucleotides (about 9% for CpG vs about 1% for other dinucleotides) between the hypervirulent and the non-hypervirulent ribotypes of C.difficile (Figure 1(b)). Importantly, the hypervirulent ribotypes of C.difficile had significantly lower CpG content as compared to the non-hypervirulent ribotype (Figure 1(c); p value = 0.03). These findings suggest that CpG is a rapidly evolving dinucleotide in C.difficile genomes and also highlight the potential link between depletion of CpG content and hypervirulence in C.difficile. Given that higher CpG content in bacterial genomes has been linked with enhanced activation of the toll-like receptor 9 (TLR-9) [15], our finding of higher CpG content in non-hypervirulent ribotypes of C.difficile as compared to hypervirulent ribotypes is particularly interestingly. Furthermore, C.difficile encoded toxins have been shown to bind to C.difficile genomic DNA with high affinity leading to activation of TLR-9 responses [16]. Therefore, our finding of CpG depletion from hypervirulent C.difficile ribotypes may be potentially associated with weak TLR-9 responses.
Figure 1.
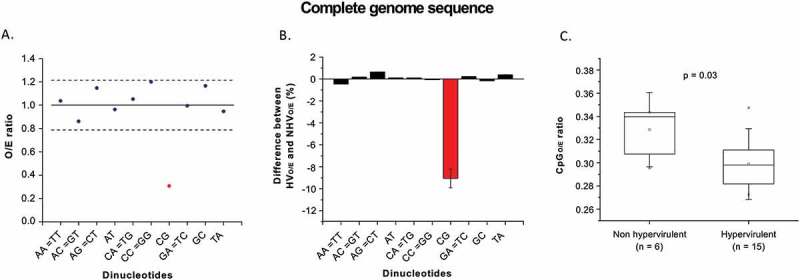
(a) CpG dinucleotides are depleted in C.difficile: Graph illustrating mean ± SD (the continuous black line indicates the mean and the broken black lines indicate standard deviation) of dinucleotide O/E ratio for C.difficile (both hypervirulent and non hypervirulent) (1 ± 0.213). All dinucleotides except the CpG dinucleotides (red) lie within the confidence interval of (0.787–1.213). (b) CpG dinucleotides are the most variable dinucleotides between hypervirulent and non-hypervirulent C.difficile strains: The difference in dinucleotide O/E ratios between hypervirulent (HV) and non-hypervirulent (NHV) C.difficile strains in percentage is shown in the Y-axis (A positive value for a given dinucleotide implies higher O/E ratios for the hypervirulent strains as compared to the non-hypervirulent strains). The complete genome sequences were analyzed. The differences between the hypervirulent and the non-hypervirulent strains were less than 1% except for the CpG dinucleotide for which the difference is about 9% (red bar). (c) Hypervirulent C.difficile strains are more CpG depleted than the non-hypervirulent strains: Box-plot diagram showing the CpG (O/E) in the whole genome sequence in non-hypervirulent and hypervirulent strains of C.difficile. The relative abundance of CpG dinucleotides was significantly higher in non-hypervirulent strains (Median = 0.341) as compared to the hypervirulent strains (Median = 0.298) [p < 0.05].
Further analysis of the coding regions suggests that the differences between hypervirulent and non-hypervirulent ribotypes for all other dinucleotides (except CpG dinucleotides) were marginal (<1% for all other dinucleotides as compared to 11.5% for CpG dinucleotides; Figure 2(a)). Importantly, the CpG dinucleotide depletion in the hypervirulent ribotypes is more pronounced in the coding DNA sequences (CDS) compared to the whole genome (0.263 in the CDS vs 0.298 in the whole genome; Figures 2(b) and 1(c); p value = 0.008). The differences in the CpG content (both at the whole genome level and within the CDS) between hypervirulent and non-hypervirulent ribotypes is more pronounced as compared to that of other dinucleotides. Our results suggest a potential role for CpG depletion in the hypervirulence of C.difficile although we do not identify specific underlying mechanisms. To the best of our knowledge, this is the first report, linking CpG depletion in bacterial genomes to virulence.
Figure 2.
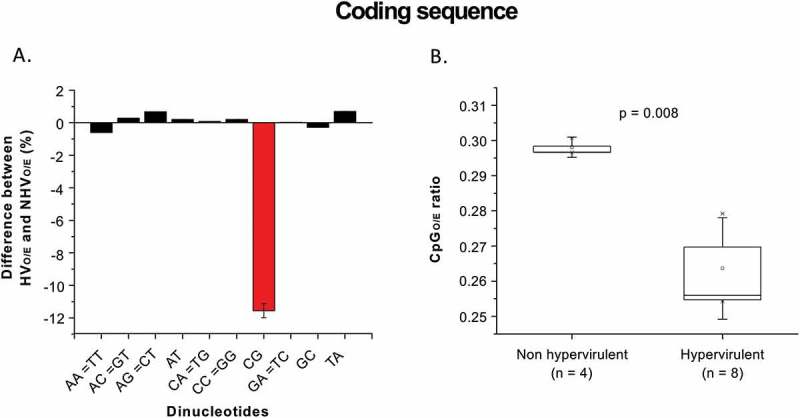
(a) Within the coding regions CpG dinucleotides are the most variable dinucleotides between hypervirulent and non-hypervirulent C.difficile strains: The difference in dinucleotide O/E ratios between hypervirulent (HV) and non-hypervirulent (NHV) C.difficile strains within the coding region (in percentage) is shown in the Y-axis (A positive value for a given dinucleotide implies higher O/E ratios for the hypervirulent strains as compared to the non-hypervirulent strains). The differences between the hypervirulent and the non-hypervirulent strains were less than 1% except for the CpG dinucleotide for which the difference is about 11.5% (red bar). (b) Within the coding region CpG depletion is more pronounced in the hypervirulent C.difficile strains as compared to the non-hypervirulent strains: Box-plot diagram showing the CpG (O/E) in the coding region in non-hypervirulent and hypervirulent strains of C.difficile. The relative abundance of CpG dinucleotides was significantly lower in the hypervirulent strains (Median = 0.263) as compared to the non-hypervirulent strains (Median = 0.298) [p < 0.05].
As with eukaryotic genomes, methylation of CpGs in bacterial genomes has been linked to the depletion of CpGs [17]. If the depletion of CpG dinucleotides from the hypervirulent ribotypes of C.difficile is due to mutational pressure, we would expect that the loss of CpGs from the whole genome is more pronounced than that from the coding. The increased depletion of CpG dinucleotides from the coding regions of the hypervirulent ribotypes suggests a major role for translational selection (i.e. codon usage bias); nonetheless we cannot rule out a role for mutational pressure. A recent report has documented CpG depletion in bacterial genomes [17]; this report also suggests a potential link between CpG-specific DNA methyltransferases encoded by bacteria and the loss of CpG dinucleotides. In addition, cytosine methylation in bacterial genomes has been associated with modulation of gene expression, motility, adhesion and virulence [18]. Although CpG methylation is well-documented in bacteria, the role of CpG depletion in bacterial pathogenesis has not been investigated.
Hypervirulence in C.difficile has been linked to adaptation to the host [19] and differences in gene expression [20]. In our study, we found that transcripts encoded by hypervirulent C.difficile ribotypes contain significantly lower number of CpGs than those encoded by the non-hypervirulent ribotypes. The reduced CpG content in transcripts of hypervirulent C.difficile ribotypes may be associated with increased translation efficiencies or escape from innate immune responses such as ZAP that targets CpG-containing mRNA.
In sum, our results suggest that (a) C.difficile genomes are CpG depleted (b) CpG dinucleotides are the most rapidly evolving dinucleotides in C.difficile and (c) Hypervirulent C.difficile ribotypes are significantly CpG depleted (both at the whole genome level and within coding regions) as compared to the non-hypervirulent strains. This work highlights previously unknown differences between hypervirulent and non-hypervirulent ribotypes of C.difficile. Importantly, these results provide a novel perspective on hypervirulence in C.difficile and lay the groundwork for studies investigating systematic CpG depletion in bacterial pathogenesis.
Disclosure statement
No potential conflict of interest was reported by the authors.
Supplementary material
Supplementary data can be accessed here
References
- [1].Goorhuis A, Bakker D, Corver J, et al. Emergence of Clostridium difficile infection due to a new hypervirulent strain, polymerase chain reaction ribotype 078. Clin Infect Dis. 2008;47:1162–1170. [DOI] [PubMed] [Google Scholar]
- [2].Vohra P, Poxton IR.. Comparison of toxin and spore production in clinically relevant strains of Clostridium difficile. Microbiology. 2011;157:1343–1353. [DOI] [PubMed] [Google Scholar]
- [3].Smits WK. Hype or hypervirulence: a reflection on problematic C. difficile strains. Virulence. 2013;4:592–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Simmonds P, Tulloch F, Evans DJ, et al. Attenuation of dengue (and other RNA viruses) with codon pair recoding can be explained by increased CpG/UpA dinucleotide frequencies. Proc Natl Acad Sci. 2015;112:E3633–E3634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Wasson MK, Borkakoti J, Kumar A, et al. The CpG dinucleotide content of the HIV-1 envelope gene may predict disease progression. Sci Rep. 2017;7:8162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Sankar S, Borkakoti J, Ramamurthy M, et al. Identification of tell-tale patterns in the 3ʹ non-coding region of hantaviruses that distinguish HCPS-causing hantaviruses from HFRS-causing hantaviruses. Emerg Microbes Infect. 2018;7:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Upadhyay M, Samal J, Kandpal M, et al. CpG dinucleotide frequencies reveal the role of host methylation capabilities in parvovirus evolution. J Virol. 2013;87:JVI–02515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Upadhyay M, Vivekanandan P. Depletion of CpG Dinucleotides in papillomaviruses and polyomaviruses: a role for divergent evolutionary pressures. PloS One. 2015;10:e0142368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Takata MA, Gonçalves-Carneiro D, Zang TM, et al. CG dinucleotide suppression enables antiviral defence targeting non-self RNA. Nature. 2017;550:124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Vedantam G, Clark A, Chu M, et al. Clostridium difficile infection: toxins and non-toxin virulence factors, and their contributions to disease establishment and host response. Gut Microbes. 2012;3:121–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Marsh JW, Arora R, Schlackman JL, et al. Association of relapse of Clostridium difficile disease with BI/NAP1/027. J Clin Microbiol. 2012;50:4078–4082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Quesada-Gómez C, López-Ureña D, Acuña-Amador L, et al. Emergence of an outbreak-associated Clostridium difficile variant with increased virulence. J Clin Microbiol. 2015;53:1216–1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Stabler RA, Gerding DN, Songer JG, et al. Comparative phylogenomics of Clostridium difficile reveals clade specificity and microevolution of hypervirulent strains. J Bacteriol. 2006;188:7297–7305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Burge C, Campbell AM, Karlin S. Over-and under-representation of short oligonucleotides in DNA sequences. Proc Natl Acad Sci. 1992;89:1358–1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Dalpke A, Frank J, Peter M, et al. Activation of toll-like receptor 9 by DNA from different bacterial species. Infect Immun. 2006;74:940–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Yang X, Li D, Xu H, et al. P-258 YI clostridium difficile toxin A-associated DNA augments the host inflammatory response. Inflamm Bowel Dis. 2012;18:S113–S113. [Google Scholar]
- [17].Wojciechowski M, Czapinska H, Bochtler M. CpG underrepresentation and the bacterial CpG-specific DNA methyltransferase M. MpeI Proc Natl Acad Sci. 2013;110:105–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Sánchez-Romero MA, Cota I, Casadesús J. DNA methylation in bacteria: from the methyl group to the methylome. Curr Opin Microbiol. 2015;25:9–16. [DOI] [PubMed] [Google Scholar]
- [19].Kansau I, Barketi-Klai A, Monot M, et al. Deciphering adaptation strategies of the epidemic Clostridium difficile 027 strain during infection through in vivo transcriptional analysis. PLoS One. 2016;11:e0158204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Borriello SP. Pathogenesis of Clostridium difficile infection. J Antimicrob Chemother. 1998;41:13–19. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


