Summary
A new multi-national study of the non-commercial MODS assay shows excellent performance in detecting M/XDR-tuberculosis
INTRODUCTION
The World Health Organization’s (WHO) End Tuberculosis Strategy calls for the early diagnosis of tuberculosis (TB) and universal drug susceptibility testing (DST) in order to start patients on the most effective treatment regimen as early as possible. DSTs using solid or liquid media and rapid molecular-based DST to detect drug-resistant TB are available and endorsed by WHO; however, in 2015, WHO has reported that among new cases of bacteriologically confirmed TB only 12% were subjected to DST.[1] Most National Tuberculosis Programs do not offer universal DST,[2] and in 2015 twelve countries reported no capacity to perform phenotypic DST[1] while 28 countries had neither in-country capacity nor a linkage with a partner laboratory for second-line DSTs.
Molecular-based DSTs are both costly and can be technically demanding to administer, thus are not widely implemented in many low-income countries.[2] Non-commercial DST methods such as the microscopic observation drug susceptibility (MODS) assay have the potential to help close this gap in diagnostic capacity because they can be implemented in resource-poor settings at low cost and with little training.[3, 4] MODS has been endorsed by the WHO as a DST for isoniazid (INH) and rifampicin (RIF),[5] and there is enormous potential for MODS to be used as a DST for second-line drugs.[6] The objective of this study was evaluate the performance of MODS for the simultaneous detection of resistance to seven different anti-TB drugs and evaluate cut-points for capreomycin (CAP).
MATERIALS AND METHODS
Specimens were collected as part of a observational cohort study (ClinicalTrials registration number NCT02170441) conducted by the Global Consortium for Drug-resistant TB Diagnostics (GCDD), descriptions of protocols have been published previously.[7]
MGIT960 DST was selected as the phenotypic reference standard using the critical drug concentrations recommended by the WHO at the time of the study: INH 0.1, RIF 1.0, moxifloxacin (MOX) 0.25, ofloxacin (OFX) 2.0, amikacin (AMK) 1.0, kanamycin (KAN) 2.5, and CAP 2.5 (μg/ml).[8, 9]
The MODS assay[6] was performed with drug concentrations of INH 0.4, RIF 1.0, MOX 0.5, OFX 1.0, AMK 2.0, and KAN 5.0 (μg/ml).[6, 10] and multiple concentrations of CAP 1.25, 2.5, 5.0, and 10.0 (μg/ml). The pyrosequencing platform[11, 12] scanned regions associated with mutations in rrs (positions 1397 to 1406) and eis promoter regions (positions −5 to −47) to investigate resistance to injectable drugs (KAN, AMK, and CAP).
The isolates used in Trollip et al. (6) were not the same samples collected in this prospective study however, as the methods and staff employed were exactly the same, it was deemed appropriate to pool those results with the current results.
RESULTS
Of 1,128 study participants enrolled between April 2012 and June 2013, 213 (18.9%) were culture negative, 1 (0.1%) was culture contaminated and 914 (81.0%) were culture positive (demographic data have previously been published[7]). A total of 826 (73%) samples were smear positive while 302 (27%) were smear negative.
Of the 1,128 MODS tests, 731 (64.8%) were positive, 288 (25.5%) were negative, 55 (4.9%) were contaminated, and 43 (3.8%) were indeterminate. Eleven (1.1%) had some discordance between the two drug-free control wells. Overall sensitivity for MODS to detect Mtb compared with MGIT was 84.9% while specificity was 98.8%. For smear positive samples, overall sensitivity was 91.8% and specificity was 97.4% while for smear negative samples sensitivity was 46.6% and specificity was 99.2%.
MODS and MGIT DST results were available for 729 samples. Sensitivity overall was high: INH 97.0%, RIF 99.6%, AMK 90.0%, KAN 61.9%, MOX 97.8%, OFX 98.2%. Overall specificity was excellent: INH 98.7%, RIF 97.8%, AMK 99.5%, KAN 99.8%, MOX 97.1%, OFX 98.0%. Of the 18 non-CAP site/drug combinations only six had sensitivity lower than 95% (India-KAN; Moldova-AMK, KAN, MOX, OFX; South Africa-INH) and only four had specificity lower than 98% (India-INH, MOX, OFX; South Africa-RIF).
Kanamycin
All MODS discordant KAN results in Moldova (n=40) were false susceptible of which 26 (65%) had mutations in the eis promoter region scanned. Of the six discordant results in India, two (33%) had had mutations in the eis promoter region. There were no discordant results for KAN in South Africa and no South African samples had mutations in the eis promoter region.
Capreomycin
Trollip et al. (6) reported provisional cut-points for CAP and our results showed moving from 2.5 to a 1.25 μg/ml breakpoint yielded an increase of 6% in sensitivity but a concomitantly decrease of 2.1% in specificity.
Analysis of Receiver Operating Characteristic curves (ROC) (Figure 1) shows agreement between this study and two previously published studies on breakpoints using MODS to test for Mtb resistance to capreomycin.[6, 13] Trollip et al. (6) reported CAP results for four concentrations (n=55, 29 were CAP resistant) and Fitzwater et al. (2013) reported CAP results for six concentrations (n= 94, 51 were CAP resistant).
FIGURE 1.
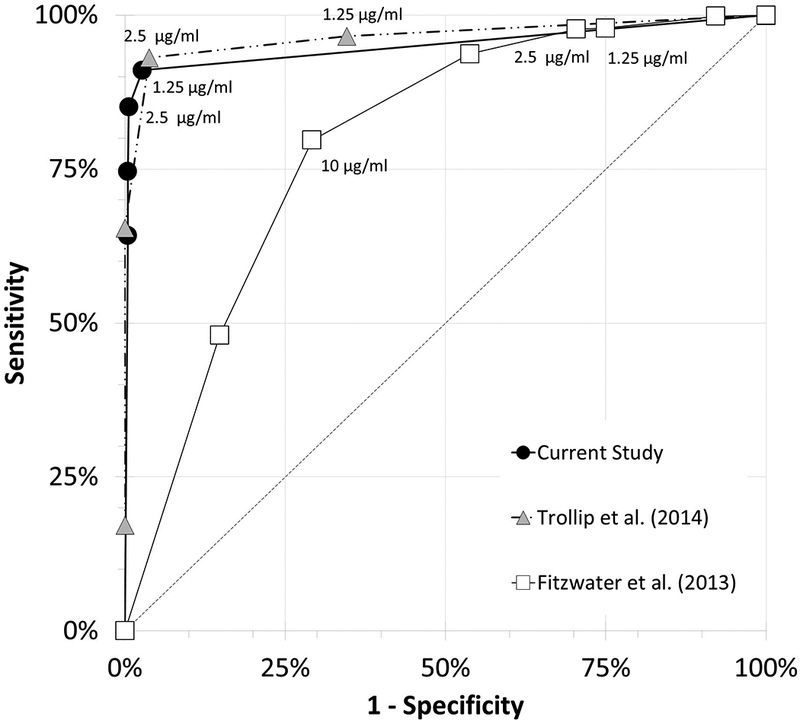
Receiver operating characteristic curves for capreomycin. In the current study and that of TROLLIP et al. [6], the reference drug susceptibility testing (DST) method was MGIT960 with a critical concentration of 2.5 μg⋅mL−l (n=729 and n = 55, respectively); in the study of FITZWATER et al. [13], the reference DST method was a proportional method with an unreported critical concentration (n=94).
ROC curve analysis supports the lowering of the critical concentration CAP for MODS to 1.25 μg/ml (Figure 1). Based on pooling this study results with Trollip et al. (6), if breakpoint concentration for CAP is lowered to 1.25 μg/ml a combined sensitivity for CAP of 92.7% (95% confidence interval 86–97) and specificity of 96.1% (95% confidence interval 94–97) would be achieved.
DISCUSSION
This is the first multi-national study to prospectively assess the clinical performance of the MODS assay for the detection of M/XDR-TB. The findings from our large study (n=1,128) significantly bolster the current WHO Policy Guidance which endorses MODS to screen patients of having MDR-TB[5] and adds to the growing body of literature with regards to using MODS to detect resistance to second-line anti-TB drugs.
The MODS test demonstrated a clear dose-response relationship between smear grade/culture and the MODS test produced a positive, negative or indeterminate result (not shown). Lower sensitivity of MODS could be due to our use of presumptive identification of Mtb with the MODS assay while employing a confirmatory identification of Mtb for our reference standard. It is likely that combining the MODS test with a test to confirm Mtb (e.g. para-nitrobenzoic acid (PNB) or MPT64) would decrease the discordance between MODS and MGIT culture.
In the present study, the 95% Confidence Intervals surrounding MODS sensitivity and specificity as a DST for INH, RIF, AMK, MOX and OFX[7] will meet and/or exceed the WHO Target Product Profiles (TPP) for a tuberculosis diagnostics.[14] We found higher sensitivity and specificities than the only other prospective study of MODS (n=540 in one country) for INH, RIF, OFX, and CAP; though our estimates were lower for KAN.[15] Our low sensitivity for AMK was driven by a low number of AMK resistance samples (n=10). Clear performance increases were observed when lowering the CAP breakpoint to 1.25 μg/ml.
Investigation of genetic mutations known to confer resistance to KAN revealed that mutation status of samples can have a dramatic effect on accuracy, especially sensitivity, due to the presence of mutations in rrs and eis promoter region which confer high-level resistance (rrs) or low-level resistance (eis promoter) to KAN.
The MODS assay yielded comparable results to MGIT first-line and second-line DST and results are obtained much faster than MGIT (but not as fast as the MTBDRsl,[7]) - thus we believe that MODS can be deployed in resource poor laboratory settings. Given its non-commercial nature, MODS is a low cost alternative to other WHO endorsed DST methods and operation research would be helpful to determine the cost/benefits of implementing MODS in individual resource poor laboratory settings.
ACKNOWLEDGEMENTS
The authors would like to acknowledge the site investigators of Valeriu Crudu (Phthysiopneumology Institute), Tommie Victor (Stellenbosch University), and Camilla Rodriques (P.D. Hinduja National Hospital & Medical Research Centre) as well as their clinical and laboratory staff for their assistance in acquiring and testing patient specimens.
FINANCIAL SUPPORT
Data and funding for this project were provided by the NIAID funded GCDD (Grant #U01-AI082229; PI: A. Catanzaro). T.C.R. was partly funded under NIH grant number P30 AI036214–20 and receives salary support from FIND, a nonprofit organization. The terms of this arrangement have been reviewed and approved by the UCSD.
REFERENCES
- 1.World Health Organization (WHO). Global Tuberculosis Report 2015, Geneva, Switzerland, 2015. [Google Scholar]
- 2.(WHO) WHO. Tuberculosis Diagnostics Technology and Market Landscape. 3rd Edition UNITAID Secretariat, World Health Organization, Geneva, Switzerland, 2014. [Google Scholar]
- 3.Caviedes L, Lee TS, Gilman RH, Sheen P, Spellman E, Lee EH, Berg DE, Montenegro-James S. Rapid, efficient detection and drug susceptibility testing of Mycobacterium tuberculosis in sputum by microscopic observation of broth cultures. The Tuberculosis Working Group in Peru. Journal of clinical microbiology 2000: 38(3): 1203–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moore DA, Evans CA, Gilman RH, Caviedes L, Coronel J, Vivar A, Sanchez E, Pinedo Y, Saravia JC, Salazar C, Oberhelman R, Hollm-Delgado MG, LaChira D, Escombe AR, Friedland JS. Microscopic-observation drug-susceptibility assay for the diagnosis of TB. N Engl J Med 2006: 355(15): 1539–1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.World Health Organization (WHO). WHO policy statement: Noncommercial culture and drug-susceptibility testing methods for screening patients at risk for multidrug-resistant tuberculosis. Geneva, Switzerland, 2011. [PubMed] [Google Scholar]
- 6.Trollip AP, Moore D, Coronel J, Caviedes L, Klages S, Victor T, Romancenco E, Crudu V, Ajbani K, Vineet VP, Rodrigues C, Jackson RL, Eisenach K, Garfein RS, Rodwell TC, Desmond E, Groessl EJ, Ganiats TG, Catanzaro A. Second-line drug susceptibility breakpoints for Mycobacterium tuberculosis using the MODS assay. The international journal of tuberculosis and lung disease : the official journal of the International Union against Tuberculosis and Lung Disease 2014: 18(2): 227–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Catanzaro A, Rodwell TC, Catanzaro DG, Garfein RS, Jackson RL, Seifert M, Georghiou SB, Trollip A, Groessl E, Hillery N, Crudu V, Victor TC, Rodrigues C, Lin GS, Valafar F, Desmond E, Eisenach K. Performance Comparison of Three Rapid Tests for the Diagnosis of Drug-Resistant Tuberculosis. PLoS One 2015: 10(8): e0136861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rodrigues C, Jani J, Shenai S, Thakkar P, Siddiqi S, Mehta A. Drug susceptibility testing of Mycobacterium tuberculosis against second-line drugs using the Bactec MGIT 960 System. The international journal of tuberculosis and lung disease : the official journal of the International Union against Tuberculosis and Lung Disease 2008: 12(12): 1449–1455. [PubMed] [Google Scholar]
- 9.WHO. Policy guidance on drug-susceptibility testing (DST) of second-line antituberculosis drugs. Geneva, Switzerland: World Health Organization; 2008. [PubMed] [Google Scholar]
- 10.Park WG, Bishai WR, Chaisson RE, Dorman SE. Performance of the microscopic observation drug susceptibility assay in drug susceptibility testing for Mycobacterium tuberculosis. Journal of clinical microbiology 2002: 40(12): 4750–4752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lin SY, Rodwell TC, Victor TC, Rider EC, Pham L, Catanzaro A, Desmond EP. Pyrosequencing for rapid detection of extensively drug-resistant Mycobacterium tuberculosis in clinical isolates and clinical specimens. Journal of clinical microbiology 2014: 52(2): 475–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Georghiou SB, Seifert M, Catanzaro D, Garfein RS, Valafar F, Crudu V, Rodrigues C, Victor TC, Catanzaro A, Rodwell TC. Frequency and Distribution of Tuberculosis Resistance-Associated Mutations between Mumbai, Moldova, and Eastern Cape. Antimicrobial agents and chemotherapy 2016: 60(7): 3994–4004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fitzwater SP, Sechler GA, Jave O, Coronel J, Mendoza A, Gilman RH, Friedland JS, Moore DA. Second-line anti-tuberculosis drug concentrations for susceptibility testing in the MODS assay. Eur Respir J 2013: 41(5): 1163–1171. [DOI] [PubMed] [Google Scholar]
- 14.World Health Organization (WHO). High-priority target product profiles for new tuberculosis diagnostics: report of a consensus meeting. WHO, Geneva, 2014; p. 98. [Google Scholar]
- 15.Huang Z, Qin C, Du J, Luo Q, Wang Y, Zhang W, Zhang X, Xiong G, Chen J, Xu X, Li W, Li J. Evaluation of the microscopic observation drug susceptibility assay for the rapid detection of MDR-TB and XDR-TB in China: a prospective multicentre study. The Journal of antimicrobial chemotherapy 2015: 70(2): 456–462. [DOI] [PubMed] [Google Scholar]


