SUMMARY
Profound cardiovascular and/or respiratory dysfunction is part of the terminal cascade in sudden unexpected death in epilepsy (SUDEP). Central control of ventilation is mediated by brainstem rhythm generators, that are influenced by a variety of inputs, many of which use the modulatory neurotransmitter serotonin to mediate important inputs for breathing. The aim of this study was to investigate epileptic seizure induced changes in serum serotonin levels and if there are potential implications for SUDEP. Forty-one epileptic patients were pooled into 2 groups based on seizure type, as a) generalized tonic-clonic seizures (GTCS) of genetic generalized epilepsy and focal to bilateral tonic-clonic seizures (FBTCS) (n=19), and b) focal seizures (n=26) based on clinical signs using surface video electroencephalography (VEEG). Post-ictal serotonin levels were statistically significantly higher after GTCS and FBTCS, compared to interictal levels (p=0.002) but not focal seizures (p=0.941). The change in serotonin (post-ictal - interictal) was inversely associated with a shorter duration of tonic phase of generalized seizures. The interictal serotonin level was inversely associated with a shorter period of post-ictal generalized EEG suppression (PGES). This data suggests that peripheral serum serotonin levels may play a role in seizure features and earlier post-seizure recovery; these findings merit further study.
Keywords: generalized, focal, SUDEP, tonic, PGES
Introduction
Sudden Unexpected Death in Epilepsy (SUDEP) is the second leading neurological cause of total years of potential life lost after stroke in the United States.1 The occurrence of SUDEP is usually seizure-related, and SUDEP risk factors include frequent generalized seizures and long-standing epilepsy.2 The precise pathophysiological mechanisms of death are unclear, but ictal, post-ictal or interictal cardiorespiratory dysfunction, with arousal failure, are thought to account for most deaths that have been monitored.3
Serotonin (5-HT, 5-hydroxytryptamine) is a major neurotransmitter that is produced by serotonergic raphe neurons in the brainstem, which project throughout the central nervous system.4 These serotonergic neurons play an important role in cardiovascular control, breathing, arousal mechanisms, and a “serotonin axis” may comprise the common link between these mechanisms, seizures and SUDEP.5 The majority of serotonin (>95%) in the body is found outside the nervous system.6 Peripheral serotonin is synthesized by enterochromaffin cells in the gut, which release some of it to be taken up by platelets that express serotonin transporter (SERT), but not serotonin synthesizing proteins. The pool of serotonin in the periphery is largely separate from that in the brain, because serotonin does not easily cross the blood brain barrier (BBB).7 However, during intense seizure activity, such as status epilepticus, the permeability of the BBB increases,8 potentially allowing exchange of 5-HT between peripheral circulation and the central nervous system. We set out to examine if seizures induced changes in peripheral serotonin levels, which have hitherto not been systematically studied in humans.
Methods
We studied patients with intractable epilepsy who were admitted to the Epilepsy Monitoring Unit and consented to participate in a multi-institution, IRB approved, prospective, multicenter SUDEP study as part of the NINDS Center for SUDEP Research’s Autonomic and Imaging Biomarkers project. Seizures of 41 epileptic patients were monitored by standard surface video-EEG methods using the Nihon-Kohden system (Tokyo, Japan). Peripheral capillary oxygen saturation (SpO2) was measured using pulse oximetry (Nellcor OxiMax N-600x Convidien). Epilepsy phenotypic and electroclinical data were collected, including age, gender, body mass index, epilepsy syndrome, seizure types, etiology, seizure frequency, seizure duration, seizure phase (tonic, clonic, jittery), duration of PGES,9 and medications. Post-ictal and interictal venous blood samples were collected in serum separator tubes, spun down, and serum was frozen and sent to a reference lab (LabCorp, Burlington, NC) for measurement of serotonin levels using high-pressure liquid chromatography (HPLC) with electrochemical detection. Since seizure occurrence during admission was not guaranteed, interictal sampling was only carried out if post-ictal samples were successfully obtained. Interictal samples were obtained at rest and always at least 12 hours after the last recorded clinical seizure. The normal lab reference values for serum serotonin are 21–321 ng/mL. Seizures were classified according to the ILAE 2017 seizure classification. Statistical analysis was done using SPSS software version 24.0 (IBM Corp, NY). A bivariate Spearman correlation coefficient was used for comparing interictal serum serotonin levels and PGES or duration of the tonic phase. P<0.05 was considered to be statistically significant.
Results
Patient characteristics are shown in Table 1. A total of 41 patients were enrolled in the study (18 males and 23 females) with an average age of 40.6±14 (range 20–77) years and mean body mass index (BMI) of 29.3±7.6 (range 19–53). Patients were pooled into two groups based on the type of seizures recorded, with 19 seizures in the group with generalized seizures (GTCS of genetic generalized epilepsy and FBTCS) and 26 seizures in the group with focal seizures without secondary generalization. The distribution of gender and race was similar in both groups. No significant differences were seen in age, BMI or number of anti-epileptic drugs (AEDs) used (Table 1). Patients with generalized seizures had a history of epilepsy for a mean duration of 20.5±13.3 years, whereas patients with focal seizures had a mean duration of epilepsy of 16.2±16.3 years (p=0.33). Pre-existing health issues associated with cardiac, pulmonary, sleep or psychiatric disorder was similar in both groups. The psychiatric disorders were mainly anxiety, depression or bipolar disorder. However, none of the patients were on selective serotonin re-uptake inhibitors (SSRIs). The epileptogenic zone was mainly temporal for focal seizures. Seizure semiologies for both groups are shown in Table 1. No differences were seen for EEG seizure duration and clinical seizure duration (Table 1). SpO2 nadir (%) significantly differed (p<0.001) between the two seizure groups, with a more pronounced decline in oxygen levels in the generalized seizure group when compared to the focal seizure group. Of the 19 generalized seizure patients, the seizure during which the serotonin samples were taken was the first during the admission in 14/19 (74%), second seizure in 4/19 (21%), and third seizure in 1/19 (5%).
Table 1.
Clinical characteristics of the patients.
| Generalized Convulsive Seizures1 (n=19) |
Focal Seizures (n=26) |
P value | |
|---|---|---|---|
| Demographics: | |||
| Gender – Male Female |
10 (58%) 9 (42%) |
8 (36%) 14 (64%) |
0.68 |
| Age, y | 41.6 ± 15.5 | 39.9 ± 13.0 | 0.70 |
| Body mass index, BMI | 30.1 ± 7.5 | 28.6 ± 7.8 | 0.53 |
| History: | |||
| Anti-epileptic medications, n | 2.7 ± 1.1 | 2.5 ± 1.2 | 0.49 |
| Epilepsy duration, y | 20.5 ± 13.3 | 16.2 ± 16.3 | 0.33 |
| Cardiac disorder | 5 (26.3%) | 8 (30.8%) | |
| Pulmonary disorder | 1 (5.3%) | 4 (15.4%) | |
| Sleep disorder | - | 2 (7.7%) | |
| Psychiatric disorder2 | 6 (31.6%) | 6 (23.1%) | |
|
Type of Epilepsy: Generalized Focal |
4 (21.0%) 15 (79.0%) |
- 26 (100.0%) |
|
|
Epileptogenic zone: Generalized Temporal Frontal Parietal Unknown |
4 (21.0%) 3 (15.8%) 4 (21.0%) 1 (5.3%) 7 (33.3%) |
- 15 (57.7%) 4 (15.3%) 3 (11.5%) 4 (15.3%) |
|
| Seizure semiology3: | |||
| Generalized onset motor tonic-clonic | 4 (21.1%) | - | |
| FOIA clonic | - | 2 (7.7%) | |
| FOIA non-motor onset | - | 8 (30.8%) | |
| FOA motor onset automatisms | - | 3 (11.5%) | |
| FOIA motor onset hyperkinetic | - | 13 (50%) | |
| Focal to bilateral tonic-clonic | 15 (78.9%) | - | |
| Seizure parameters: | |||
| EEG Sz. duration (sec) | 94.8 ± 52.2 | 166.6 ± 451.6 | 0.43 |
| Clinical Sz. duration (sec) | 87.0 ± 51.9 | 167.0 ± 471.0 (n=24) | 0.42 |
| No. of Sz. with Apnea | 5 (26.3 %) | - | |
| No. of Sz. with Hypoxemia | 5 (26.3%) | - | |
| SpO2 Nadir (%) | 63.6 ± 16.3 (n=14) | 92.8 ± 3.6 (n=18) | <0.001 |
| Tonic phase duration (sec) | 6.0 ± 2.7 (n=12) | - | |
| Jittery phase duration (sec) | 12.9 ± 10.6 (n=13) | - | |
| Clonic phase duration (sec) | 27.3 ± 15.5 (n=18) | - | |
| PGES duration (sec) | 28.0 ± 19.2 (n=11) | - | |
| Post-ictal EEG Burst suppression (sec) | 99.8 ± 142.8 (n=4) | - | |
| Post-ictal EEG Continuous slow (sec) | 723.3 ± 519.9 (n=8) | - | |
| EEG Return to Baseline duration (sec) | 1363.3 ± 1374.5 (n=7) | - | |
| EEG Seizure End to Blood draw (min) | 11.2 ± 12.1 | 7.2 ± 7.6 | 0.21 |
Generalized tonic-clonic seizures and focal to bilateral tonic-clonic seizures
None of the patients were on SSRIs
ILAE classification of seizures; FOIA-focal onset impaired awareness; FOA-focal onset aware
Post-ictal serotonin levels in serum were increased after generalized seizures compared to interictal levels but not after focal seizures. The average time elapsed between post-ictal blood draw and end of seizure for generalized seizures was 11.2±12.1 minutes and 7.2±7.6 minutes for focal seizures; there was no significant difference between these two groups (Table 1). Interictal blood samples were drawn after a 12h seizure-free period. Mean post-ictal serotonin levels after generalized seizure, were 173.1±91.8 ng/ml (range 30–386), and in the interictal state were 119.4±63.3 ng/ml (range 26–227) (Figure 1a). For focal seizures, mean post-ictal and interictal serotonin levels were similar, at 130.9±95.9 ng/ml (range 25–353), and 132.2±79.6 ng/ml (range 31–416) (Figure 1a). Increase in post-ictal serotonin levels compared to interictal levels using a paired sample T-test was statistically significant for the generalized seizure group (p=0.002), but not for the focal seizure group (p=0.941, Figure 1a). The change in serum serotonin levels (post-ictal - interictal) was also statistically significant (p=0.027) between the generalized and focal seizure groups. The difference in serotonin level (post-ictal to interictal) was associated with reduced duration of tonic phase during generalized seizures (p=0.03, Figure 1b). Higher levels of interictal serotonin were significantly associated with shorter duration of PGES (p=0.04, Figure 1c). In the generalized seizure group, comparison of patients who had PGES (n=11) with patients who did not have PGES (n=8), revealed no significant difference in post-ictal serotonin levels between the two groups. No other associations were observed between serotonin levels and other clinical features of the seizure.
Figure 1.
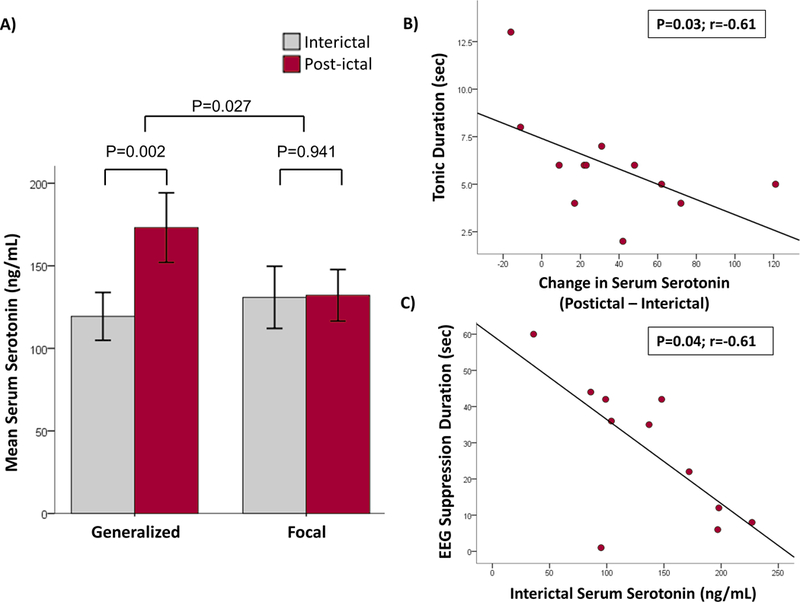
A) Serum serotonin levels increase after seizures. The mean serum post-ictal serotonin levels in ng/mL are shown in red bars and interictal serotonin levels are shown in grey bars for the two seizure groups; Generalized (n=19) and Focal (n=26). Elevated levels of post-ictal serum serotonin after generalized seizures were statistically significant when compared to interictal levels (p=0.002), but not after focal seizures (p=0.941, paired sample T-test). Also, the change in serum serotonin level (post-ictal - interictal) was statistically significant (p=0.027, independent 2 sample T-test) between the generalized and focal seizure groups.
B) Association of tonic duration with serum serotonin. The difference between post-ictal and interictal serum serotonin levels were plotted against the tonic duration of the seizure. Increased levels of serotonin was significantly associated with reduced duration of tonic phase during generalized seizures (n=12).
C) Association of PGES duration with serum serotonin. The interictal serum serotonin levels were plotted against the post-ictal EEG suppression (PGES) duration of the seizure. Higher interictal serotonin was significantly associated with shorter period of EEG suppression during generalized seizures (n=11).
Discussion
Serotonin is synthesized through the actions of two different tryptophan hydroxylase isoforms encoded by different genes, TPH1 and TPH2, which are expressed in enterochromaffin cells of the intestine and serotonergic neurons in the brainstem, respectively. The source of almost all serotonin in the brain originates from a small group of neurons in the midbrain and medullary raphe nuclei.10 Raphe serotonin neurons project throughout the neuraxis and are important for many brain functions, including mood, sleep/arousal, motor output, thermoregulation, autonomic control and breathing.5,10 However, the BBB, which consists of a single layer of endothelial cells throughout the cerebral vasculature connected via tight junctions, covered by a basement membrane and surrounded by astrocytic endfeet acts as a barrier that prevents many substances from exchanging between the brain and blood.7 Serotonin is relatively impermeable across the BBB, so the brain is unlikely to be the source of serotonin that leads to the increase in serum 5-HT seen in our study.
It has been reported that during intensive acute exercise, serum serotonin levels increase when compared to controls.11 Moreover, moderate exercise in horses (a 450m run) causes both free plasma 5-HT and whole blood 5-HT to increase by over 3 fold.12 The authors concluded that the source of 5-HT was enterochromaffin cells that are thought to release 5-HT in response to exercise. Thus, the exertion associated with generalized seizures may lead to alterations similar to other forms of exercise. 5-HT release induced by muscular activity during a seizure would be consistent with our observation that 5-HT rose with generalized seizures, but not focal seizures. This finding further suggests that seizure activity within the brain during seizures causes indirect rather than a direct rise in 5-HT.
Our data does not exclude an alternative possibility that seizures cause release of 5-HT from platelets, since we measured serum 5-HT and not whole blood 5-HT. In the periphery, platelet SERT is required for serotonin uptake from the plasma, which does not synthesize 5-HT; platelets regulate serotonin levels to prevent vasoconstriction and thereby keep blood flow stable.13 A reduction in density of platelet membrane SERT in patients having epileptic seizures compared to normal controls or patients with non-epileptic seizures has been reported.14 This suggests that serotonin may be released from platelets during seizures and reuptake reduced by decreased expression of SERT on platelet membranes.
An inverse correlation between interictal serum 5-HT levels and the duration of PGES observation raises the question of what relationship potentially exists between interictal serum serotonin levels and brain serotonin. A correlation between human platelet serotonin transport and brain synaptosomal transport has been demonstrated in human brain in patients undergoing epilepsy surgery,15 so that differences observed in peripheral blood 5-HT may be paralleled by similar differences in brain 5-HT. It is also possible that differences in 5-HT synthesis or metabolism could lead to higher peripheral 5-HT levels, and that parallel differences occur in the brain. Also, mice lacking 5-HT neurons in the brain have been shown to display a lower threshold for seizures, and are more likely to have PGES and death after seizures.16
Alternatively, high peripheral 5-HT levels may possibly lead to increased brain 5-HT if seizures caused breakdown of the BBB, as has been demonstrated under some conditions,17 allowing transfer of serotonin into the central nervous system. BBB disruption occurs within minutes after induction of bicuculline-induced seizures in rats. Increased micropinocytosis in cerebral capillaries has been reported during seizures, and epilepsy per se, may compromise the BBB.18
Serotonin within the brain enhances respiration in response to hypercapnia, and also contributes to arousal.16 It is also well known that seizures in animal models cause release of serotonin from brainstem raphe neurons.19 Such increases in synaptically-released serotonin coupled with the peripherally-generated serotonin that may pass into the brain due to seizure-induced disruption of the BBB7 may add to stimulatory effects of serotonin on respiration and arousal. How higher peripheral serum serotonin levels pertain to observed shorter PGES duration (correlated with post-ictal immobility duration and oxygen desaturation) and tonic phase durations is unclear; both are markers of seizure severity, and thus potentially of SUDEP.20 It is possible that serotonin plays a role in early recovery by reducing seizure severity, although we have no definitive evidence of this. DBA/1 and DBA/2 mice have reduced serotonin availability and are susceptible to post-seizure death if unresuscitated. Pretreatment with serotonin-reuptake inhibitors (SSRIs) prevents death. In this small sample, our data suggest that peripheral serum serotonin levels in epileptic patients are inversely correlated with potential biomarkers of SUDEP and merit further study.
Acknowledgements
Carl Faingold would like to thank the Epilepsy Foundation for their support.
Footnotes
Disclosure:
Samden Lhatoo is funded by the Center for SUDEP Research: NIH/NINDS U01-NS090405 and NIH/NINDS U01-NS090407. Bilal Zonjy, M R Sandhya Rani, Stephan Schuele, Rup Sainju, Maromi Nei, Ronald Harper, Beate Diehl, and Lisa Bateman are funded by NIH/NINDs U01-NS090407. Orrin Devinsky is funded by Center for SUDEP Research NIH/NINDS UO1-NS090415 and NIH/NINDS U01-NS090407. George B. Richerson is funded by Center for SUDEP Research NIH/NINDS UO1-NS090414. Stephan Schuele is on the speaker’s bureau for Sunovion and Eisai. Arun Murugesan, Johnson Hampson, Ronald Harper and Nuria Lacuey report no disclosures.
We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that his report is consistent with those guidelines.
References
- 1.Devinsky O, Hesdorffer DC, Thurman DJ, et al. Sudden unexpected death in epilepsy: epidemiology, mechanisms, and prevention. Lancet Neurol 2016;15:1075–1088. [DOI] [PubMed] [Google Scholar]
- 2.Lhatoo SD, Nashef L, Tomson T, et al. Association of prone position with sudden unexpected death in epilepsy. Neurology 2015;85:1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ryvlin P, Nashef L, Lhatoo SD, et al. Incidence and mechanisms of cardiorespiratory arrests in epilepsy monitoring units (MORTEMUS): a retrospective study. Lancet Neurol 2013;12:966–977. [DOI] [PubMed] [Google Scholar]
- 4.Buchanan GF, Richerson GB. Central serotonin neurons are required for arousal to CO2. Proc Natl Acad Sci U S A 2010;107:16354–16359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Richerson GB, Buchanan GF. The serotonin axis: Shared mechanisms in seizures, depression, and SUDEP. Epilepsia 2011;52 Suppl 1:28–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tyce GM. Origin and metabolism of serotonin. J Cardiovasc Pharmacol 1990;16 Suppl 3:S1–7. [PubMed] [Google Scholar]
- 7.Daneman R, Prat A. The blood-brain barrier. Cold Spring Harb Perspect Biol 2015;7:a020412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van Vliet EA, Aronica E, Gorter JA. Blood-brain barrier dysfunction, seizures and epilepsy. Semin Cell Dev Biol 2015;38:26–34. [DOI] [PubMed] [Google Scholar]
- 9.Lhatoo SD, Faulkner HJ, Dembny K, et al. An electroclinical case-control study of sudden unexpected death in epilepsy. Ann Neurol 2010;68:787–796. [DOI] [PubMed] [Google Scholar]
- 10.Pilowsky PM. Peptides, serotonin, and breathing: the role of the raphe in the control of respiration. Prog Brain Res 2014;209:169–189. [DOI] [PubMed] [Google Scholar]
- 11.Zimmer P, Stritt C, Bloch W, et al. The effects of different aerobic exercise intensities on serum serotonin concentrations and their association with Stroop task performance: a randomized controlled trial. Eur J Appl Physiol 2016;116:2025–2034. [DOI] [PubMed] [Google Scholar]
- 12.Alberghina D, Giannetto C, Piccione G. Peripheral serotoninergic response to physical exercise in athletic horses. J Vet Sci 2010;11:285–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mercado CP, Kilic F. Molecular mechanisms of SERT in platelets: regulation of plasma serotonin levels. Mol Interv 2010;10:231–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cupello A, Audenino D, Scarrone S, et al. Epileptic seizures but not pseudoseizures are associated with decreased density of the serotonin transporter in blood platelet membranes. Neurochem Res 2008;33:2263–2268. [DOI] [PubMed] [Google Scholar]
- 15.Rausch JL, Johnson ME, Li J, et al. Serotonin transport kinetics correlated between human platelets and brain synaptosomes. Psychopharmacology (Berl) 2005;180:391–398. [DOI] [PubMed] [Google Scholar]
- 16.Buchanan GF, Murray NM, Hajek MA, et al. Serotonin neurones have anti-convulsant effects and reduce seizure-induced mortality. J Physiol 2014;592:4395–4410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Janigro D Blood-brain barrier, ion homeostatis and epilepsy: possible implications towards the understanding of ketogenic diet mechanisms. Epilepsy Res 1999;37:223–232. [DOI] [PubMed] [Google Scholar]
- 18.van Vliet EA, Aronica E, Gorter JA. Role of blood-brain barrier in temporal lobe epilepsy and pharmacoresistance. Neuroscience 2014;277:455–473. [DOI] [PubMed] [Google Scholar]
- 19.Lin WH, Huang HP, Lin MX, et al. Seizure-induced 5-HT release and chronic impairment of serotonergic function in rats. Neurosci Lett 2013;534:1–6. [DOI] [PubMed] [Google Scholar]
- 20. Tao JX, Yung I, Lee A, et al. Tonic phase of a generalized convulsive seizure is an independent predictor of postictal generalized EEG suppression. Epilepsia 2013;54:858–865. [DOI] [PubMed] [Google Scholar]


