Abstract
Purpose:
To examine the ultrastructure of lipofuscin bodies and melanosomes in retinal epithelium of elderly rhesus monkeys and determines changes in their number and morphology as a function of retinal eccentricity.
Methods:
Electron microscopy was used to describe and quantify two major organelles in elderly monkey retinal epithelium, lipofuscin bodies and melanosomes, at different retinal loci extending from the macula to the peri-macula, equator, periphery and ora serrata. Osmium tetroxide was used to distinguish lipofuscin bodies from melanosomes.
Results:
Lipofuscin bodies and melanosomes diminished in number with advanced age but there was an inverse relationship between these two organelles. Lipofuscin bodies were more numerous in the macula and melanosomes more numerous in the peripheral retina. Three types of lipofuscin bodies were identified: 1) smaller and tending to locate in the middle third of the epithelial cell, 2) larger, less common, and located more basally, and 3) extremely rare, melano-lipofuscin, containing a melanosome. When osmicated, all lipofuscin bodies contained electron dense materials. When osmium tetroxide was not used for fixation, the first two types of lipofuscin bodies lost their electron densities while the third type retained its electron density due to the melanosome it contained.
Conclusion:
As previously reported for human retina, lipofuscin is most abundant in the macular and peri-macular epithelium and least abundant in the periphery, whereas melanosomes show the opposite relationship. This distribution pattern could contribute to the macula’s greater vulnerability to photo-toxicity. Three types of lipofuscin bodies are found in aging monkey retinal epithelium. All types contain electron dense material, but the most prominent two types lose their densities in the absence of osmium tetroxide during fixation. Most of the electron densities in lipofuscin bodies must contain a material that binds strongly to osmium tetroxide such as polyunsaturated fatty acids.
Keywords: Retina, epithelium, electron microscopy, monkey, lipofuscin, aging
Introduction
Rhesus monkeys develop a drusenoid maculopathy resembling age-related macular degeneration (AMD) in humans and having similar genetic and environmental risk factors.1,2 However, monkeys rarely, if ever, develop the advanced forms of AMD that occur in humans, such as geographic atrophy or choroidal neovascularization, even though monkeys develop the maculopathy earlier in their relative lifespan.1 As in humans, the macular epithelium of the aging rhesus monkey accumulates large amounts of lipofuscin and loses melanosomes.3 We have examined these age-related changes at different retinal locations extending from the macula to the retinal periphery, complementing earlier in vivo and ex vivo optical imaging and ultrastructural work in humans4–8 and most recently in vivo lipofuscin autofluorescence measurements in rhesus monkeys.9
Most previous studies of aging human retinal epithelium used fluorescence to estimate lipofuscin content but did not count the lipofuscin bodies that contain the fluorescent material. However, Feeney-Burns et al.5 measured the number and distribution of lipofuscin granules, melanosomes and melanolipofuscin by electron microscopy (EM). They found that lipofuscin bodies and melanosomes showed inverse relationships with retinal eccentricity, as lipofuscin bodies decreased while melanosomes increased with retinal eccentricity. Weiter et al.6 found a similar pattern in lipofuscin and melanin concentration as estimated by autofluorescence and optical density, respectively. Our results demonstrate that this pattern is even more striking in aging monkey retinal epithelium. A new classification of lipofuscin bodies is introduced based on their morphology, cellular location, and response to osmium tetroxide fixation.
Methods
All procedures were approved by the Institutional Animal Care and Use Committees of the Oregon Health and Science University, Portland, OR, or the National Institute on Aging Intramural Research Program, Baltimore, MD, and conformed to the Guide for the Care and Use of Laboratory Animals (8th edition, 2011).
Eyes were obtained from two rhesus monkeys (Macaca mulatta), a 24-year old female and a 44-year old male. Rhesus monkeys in this age range are considered old, which is based on a rhesus:human age ratio of approximately 3:1. The previously reported median survival for rhesus in captivity was 26 years, and maximal survival was approximately 40 years.10
Immediately after euthanasia, the eyes were removed and fixed by 4% paraformaldehyde and 0.45% glutaraldehyde. Diffusion of fixative was facilitated by piercing the eye with an 18-gauge needle at the limbus and injecting 0.5–1 ml of fixative into the vitreous. After a minimum of one week in fixative, the eyes were washed with a balanced salt solution and dissected with the aid of a surgical microscope. Progressing temporally along the horizontal meridian rectangular segments, about 4 mm in length and 2 mm in width, were cut out of the retina and choroid at the macula, perimacula, equator, periphery, and ora serrata. These segments were respectively 4, 5–8, 9–13, 14–18 and 19–23 mm from the fovea. Each segment was cut in half and one half was postfixed with 2% osmium tetroxide while the other was not. This comparison tested whether electron dense material in lipofuscin bodies was due to melanin because melanosomes are unaffected by osmium tetroxide fixation. In contrast, almost all lipofuscin bodies, except the few containing melanosomes, lose their electron densities if osmium fixation was not performed, as previously described.11–13
Each segment was dehydrated, embedded in epon and sectioned. All sections were stained with lead citrate and uranyl acetate and examined by EM. Several sections, approximately 0.8 mm in length, were mounted on each grid, each containing the profiles of 20–40 epithelial cells. Three to five cells, lacking a large nuclear profile or a grid bar, were selected for measurements. Digital photographs were viewed on a large screen monitor and examined using Photoshop (Adobe, Mountain View, CA), which allowed adjustments of brightness, contrast, and magnification to facilitate distinguishing organelles. Hundreds of lipofuscin bodies and melanosomes were counted and their length measured in the selected cells from each segment. The area in which the counts were made was measured and the data expressed as organelles/μm2.
Results
Retinal epithelial cells from the macula (A), peri-macula (B), equator (C) and periphery (D) are illustrated for the 24-year old (Figure 1) and the 44-year old (Figure 2) monkeys. The macula cells in both monkeys contain predominantly lipofuscin bodies. Three different types of lipofuscin bodies can be distinguished in Figure 1(a): small ones (black arrowhead), large ones (black arrow), and a melano-lipofuscin (white arrow). A melanosome is seen at the left (white arrowhead). The small lipofuscin bodies, which tend to be located more centrally in the cell, are the most common. They are often slightly darker, tend to be quasi-circular, and are often pleomorphic with a more irregular perimeter than the large ones. Additionally, they can have strong electron densities that may be particulate rather than homogeneous. The large ones are usually located more basally in the cell, have a smooth perimeter, and have a dark central homogenous area surrounded by a lighter peripheral ring. Melanosomes are rare in these macular cells. A few ellipsoidal melanosomes can be seen at the upper left of the 24-year-old macula section (Figure 1(a)) and at the upper right of the peri-macular section (Figure 1(b). No melanosomes are seen in either the macula (Figure 2(a)) or peri-macula (Figure 2(b)) sections of the 44-year old monkey. The perimacular sections of both monkeys resemble the macula sections. The large type of lipofuscin bodies appear larger in the 44-year-old compared to the 24-year old monkey and larger in the peri-macula than at the macula. For both monkeys, the equatorial and peripheral sections resemble each other more than they resemble the macula and peri-macula sections. The larger type of lipofuscin bodies are not seen in these loci. There are also fewer of the smaller lipofuscin bodies, which appear lighter than they are at the macula. More melanosomes, both elliptical and circular, are seen in the equatorial and peripheral sections than in macular or peri-macular regions; the circular melanosomes seem to increase in number at a faster rate with eccentricity. At the ora serrata (not illustrated) only circular melanosomes are seen.
Figure 1.
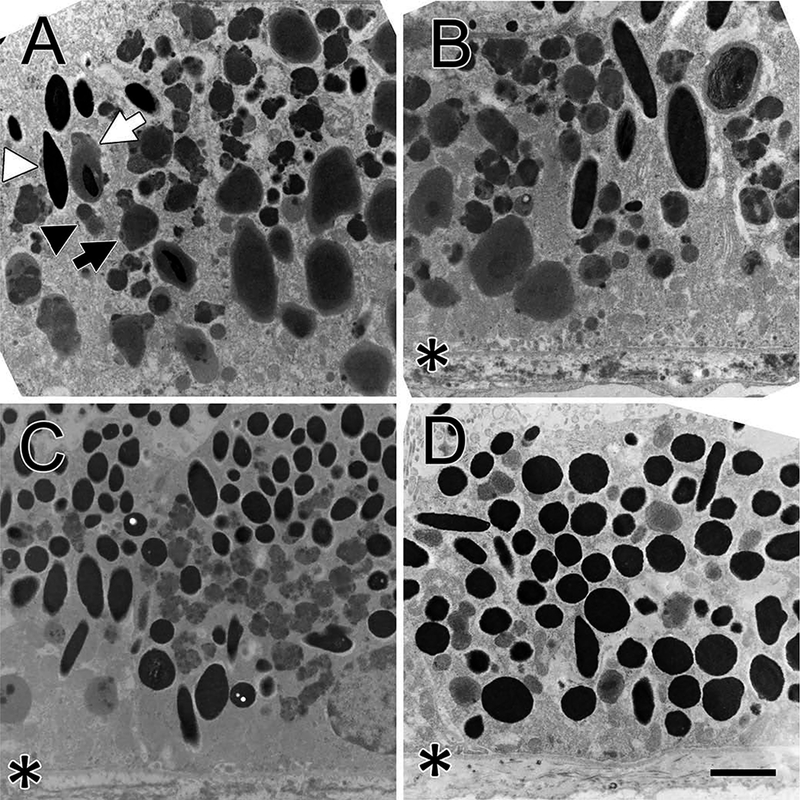
The figure shows osmicated retinal epithelial cells of the 24-year old monkey. The basal third of the cell is at the bottom of all images. An asterisk indicates the basal membrane where it is visible. Panel A shows a macular epithelial cell. Three types of lipofuscin bodies are seen. The first type are small (black arrowhead) and tend to locate at the middle of the cell; the second type are larger (black arrow) and tend to be located at the basal region of the cell. A few melanosomes (white arrowhead) are seen upper left. Several melano-lipofuscin bodies are present, one marked with a white arrow. Panel B shows the peri-macular area, which contains fewer lipofuscin bodies and more melanosomes. Three of the larger type of lipofuscin bodies are present near the basal plasma membrane; most of the lipofuscin bodies are the smaller variety. Panel C shows a cell at the retinal equator that contains more melanosomes and fewer lipofuscin bodies. Only the smaller variety of lipofuscin bodies are seen and they have fewer electron densities than in the macula. Panel D shows a cell in the peripheral retina with no lipofuscin bodies but many round melanosomes filling the cytoplasm without any stratification. A few elliptical melanosomes are also seen. Bar, 2 μm.
Figure 2.

The figure shows osmicated retinal epithelial cells of the 44-year old monkey. The basal side of the cell is at the bottom of each image. An asterisk indicates the basal membrane where it is visible. Panel A shows a macular epithelial cell with no melanosomes but many lipofuscin bodies in its cytoplasm. Both large and small lipofuscin bodies are present with the large bodies clustering toward the basal side and the smaller bodies toward the middle of the cell. A peri-macular cell (Panel B) shows numerous lipofuscin bodies and no melanosomes. The basal oriented lipofuscin bodies are larger than they are in the macula. A cell from the equatorial region (Panel C) contains only the smaller type of lipofuscin bodies interspersed among numerous melanosomes. A peripheral cell (Panel D) contains predominantly melanosomes, both round and elliptical. Bar, 2 μm.
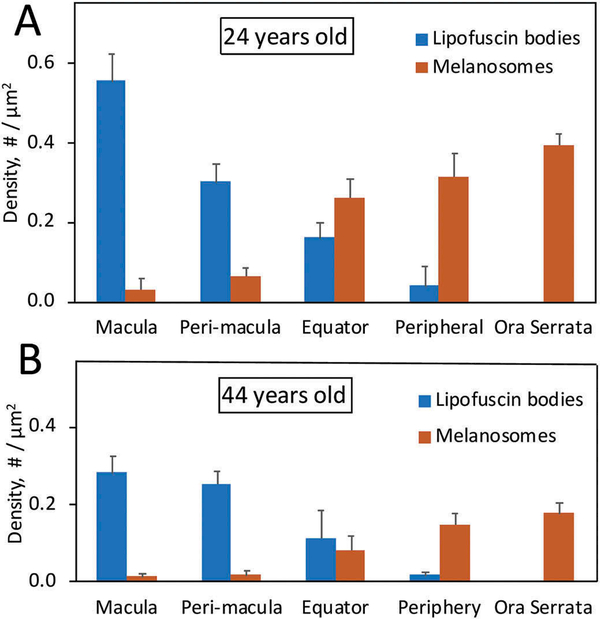
The lipofuscin bodies and melanosomes were quantified in both the 24-year-old (Figure 3(a)) and the 44-year-old (Figure 3(b)) monkeys by measuring the number and length (not shown) of these organelles in 3–5 selected cells from each of the different retinal areas. Density data are reported as the mean organelles/μm2 with standard deviation. The density of lipofuscin bodies is maximal at the macula and decreases with retinal eccentricity toward the ora serrata, whereas the density of melanosomes follows the opposite pattern. Absolute values for lipofuscin bodies are higher in the 24-year-old monkey, but the proportion at the peri-macula is higher in the 44-year-old (38%) than in the 24-year-old (29%). There was a tendency for the length of the lipofuscin bodies to decrease with retinal eccentricity while the length of melanosomes appeared to be similar across the retina (not shown), but these observations need to be evaluated statistically before considering physiological significance.
Figure 3.
The figure shows the organelles/μm2 of the lipofuscin bodies (blue) and melanosomes (red) at different eccentricities in the 24-year-old monkey (A) and 44-year-old monkey (B). Values are reported as the mean of 3–5 cells per region for each monkey, with standard deviations indicated by vertical lines. In both animals, lipofuscin bodies are most numerous at the macula and perimacula region with decreasing density toward the periphery, whereas melanosomes show the opposite distribution.
In the current study, distinguishing lipofuscin bodies from melanosomes was essential and based on several characteristics. Lipofuscin bodies are diverse in morphology as noted above, but generally are pleomorphic with an irregular perimeter (Figure 4(a)), and can have strong electron densities that may be particulate rather than homogeneous. Melanosomes have geometric shapes, either ellipsoidal or circular, with a linear perimeter; they also have a very strong and homogeneous electron density. But the most striking and unambiguous difference between lipofuscin bodies and melanosomes is their response to osmium tetroxide fixation. Most lipofuscin bodies lose all of their electron density in the absence of osmium tetroxide fixation. This occurs for both the small (Figure 4(a,b)) and the large (Figure 4(c–f) lipofuscin bodies. The only lipofuscin bodies that retain an electron density in the absence of osmium fixation are those that have incorporated a melanosome (center of Figure 4(f)). Such melanosome-containing lipofuscin bodies are rare in monkey retinal epithelium.
Figure 4.
The figure compares osmicated (A, C, E) with non-osmicated (B, D, F) organelles in the macula of a 24 year old monkey. A and B compare predominantly the small variety of lipofuscin bodies, and C and F compare predominantly the large variety of lipofuscin bodies. Melanosomes are not changed by osmication. One melanosome is seen in D and in F two are seen inside lipofuscin bodies. Bar, 1 μm.
Discussion
The results indicate that lipofuscin bodies and melanosomes decrease in number with age. They also show that a relative reduction in the number of lipofuscin bodies and a relative increase in the number of melanosomes with retinal eccentricity in aging monkey retinal epithelial cells. This difference in the spatial distribution of two major organelles in retinal epithelium has also been found in aging human retinal epithelium, and this represents one of many parallels between retinal aging in the two species.6 Lower melanin content may make the macula more vulnerable to photo-oxidation and consequently aging. The overall melanin content of the human retinal epithelium decreases more than 4-fold with age but is always lowest in the macula.14 Melanosomes are difficult to break down, and lysosomes appear unable to dissolve melanin.15 Melanosomes may be lost by extrusion through the basal membrane of the epithelium into Bruch’s membrane, where they might be dissolved by macrophages. Melanosomes are also subject to photo-toxicity16 which can be modulated by lipofuscin.17
For the current study, we used the osmium tetroxide test to determine if the electron density in a lipofuscin body was due to a melanosome. We discovered that most of the lipofuscin bodies in aging monkey retinal epithelium are electron dense but lose this density in the absence of osmication, and there-fore did not contain melanosomes but contained another material that has a strong affinity for osmium tetroxide, such as polyunsaturated fatty acids.11–13 This test for lipofuscin bodies eliminated the potential for inaccurately identifying melano-lipofuscin bodies.3
The large number of lipofuscin bodies in the central retina reflects the well-known finding that fluorescence is maximal in and around the macular and peri-macular region. A difference between the EM and fluorescence measurements is that the latter can detect small decreases in fluorescence in the central macula, whereas EM detection would require serial sectioning through the entire macula. It is interesting that the peri-macula seems to have more of the larger type of lipofuscin bodies than the macula, a difference which could account for the typical central dip in fluorescence; however, this dip may also be due, in part, to the filtering effect of macular pigment.9
EM reveals three types of lipofuscin bodies in aging monkey retinal epithelium that differ in morphology, location, and density. The most common tends to be smaller with an irregular perimeter, and often contains an interior with particulate electron densities. These small lipofuscin bodies tend to stratify at the middle of the epithelial cell. The second type of lipofuscin body tends to be larger, less numerous, with a smooth perimeter and a homogeneous electron density that is often encircled by a lighter rim. The larger type tends to stratify at the basal side of the epithelial cell. The third type, and least common lipofuscin body, is a lysosome that has incorporated a melanosome (melano-lipofuscin).
The current finding is consistent with earlier studies that reported only small amounts of protein in purified lipofuscin bodies, but substantial amounts of lipid including fatty acids.18,19 Whether the fatty acids contribute to the fluorescence, as retinoids do20, is unknown. It is intriguing that multiple lysosomal pathways seem to exist for lipofuscin bodies. These lysosomal path-ways play a role in the degradation and recycling of two important classes of molecules in this epithelium, the retinoid compounds and the fatty acids; the former are involved in photo-transduction and the latter in membrane structure, with a uniquely high proportion of very highly unsaturated omega-3 fatty acids in the photoreceptor outer segment membranes that are shed and phagocytized by the retinal epithelium on a daily basis.
A key question in the pathogenesis of aging and AMD is understanding why the macula accumulates far more lipofuscin than the peripheral retina. If the buildup of lipofuscin reflects a cumulative or age-related inability of the retinal epithelium to digest the outer segment material it phagocytoses daily, then the lipofuscin accumulation should be related to the density of photoreceptors and the resulting quantity of outer segments to be processed. Indeed, the spatial distribution of lipofuscin, as measured by fundus autofluorescence in human eyes, seems to be correlated with the density of rod photoreceptors.7,8 Furthermore, we previously found an absence of tissue autofluorescence and lipofuscin bodies at the ora serrata, where photoreceptors are absent.21 Our observation that the number of lipofuscin bodies seems to decrease with age is apparently at odds with the idea that lipofuscin accumulation triggers cell damage in an aging eye. Future studies should clarify the issue perhaps by focusing on the mechanism of lipofuscinmediated cell death.
Another possible contributor to epithelial cell degeneration is oxidative damage to highly vulnerable polyunsaturated fatty acids derived from shed photoreceptor membranes in these lipofuscin bodies. Other factors may also contribute to the macula’s unique vulnerability to age-related degeneration, such as greater oxidative stress and light exposure, and a paucity of melanosomes. For example, the effective pupil area decreases with eccentricity following a cosine function and light intensity decreases with eccentricity following this function. A cell culture study has shown that RPE cell death triggered by light stress and lipofuscin may be modulated by melanosomes.17 The accumulation of potentially toxic retinoid compounds also contributes to autofluorescence, but this cannot explain the unique vulnerability of the macula. Reports that bisretinoids are more concentrated in the peripheral retina than at the macula, in both human and monkey eyes, cast doubt on the involvement of retinoid compounds in macula fluorescence.22,23
Although patterns of age-related changes in lipofuscin and melanin and their regional distribution appear largely similar between human and macaque, our results suggest several notable differences. For example, the relative distribution of ultrastructural lipofuscin subtypes appears different based on our small macaque sample size. In aging human eyes, the melano-lipofuscin bodies account for more than 40% of the total lipofuscin bodies5 compared to a very small percentage identified in the aging monkey retinal epithelium. In addition, the human studies do not report two morphologically different types of non-melanosome containing lipofuscin bodies. The relationship of lipofuscin morphology and melano-lipofuscin prominence to macular disease is unknown. However, it is tempting to speculate that these ultrastructural differences may have some relationship to the aging monkey’s resistance to the more serious, advanced forms of AMD that occur in humans.
Acknowledgments
Funding
This work was funded, in part, by the Intramural Research Program of the National Institute on Aging, NIH, and NIH grants P51OD011092 (MN), R01EY015293 (PG), 2P30EY019007 (PG, TN, Core Facilities for Vision Research), and grants from Research to Prevent Blindness (PG, TN, MN) and Eye Surgery Fund (TN).
References
- 1.Gouras P, Ivert L, Landauer N, Mattison JA, Ingram DK, Neuringer M. Drusenoid maculopathy in rhesus monkeys (Macaca mulatta): effects of age and gender. Graefes Arch Clin Exp Ophthalmol. 2008;246(10):1395–402. doi: 10.1007/s00417-008-0910-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Francis PJ, Appukuttan B, Simmons E, Landauer N, Stoddard J,Hamon S, Ott J, Ferguson B, Klein M, Stout JT, et al. Rhesus monkeys and humans share common susceptibility genes for agerelated macular disease. Hum Mol Genet. 2008;17(17):2673–80. doi: 10.1093/hmg/ddn167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gouras P, Brown K, Ivert L, Neuringer M. A novel melanolysosome in the retinal epithelium of rhesus monkeys. Exp Eye Res. 2011;93(6):937–46. doi: 10.1016/j.exer.2011.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wing GL, Blanchard GC, Weiter JJ. The topography and age relationship of lipofuscin concentration in the retinal pigment epithelium. Invest Ophthalmol Vis Sci. 1978;17(7):601–07. [PubMed] [Google Scholar]
- 5.Feeney-Burns L, Hilderbrand ES, Eldridge S. Aging human RPE: morphometric analysis of macular, equatorial, and peripheral cells. Invest Ophthalmol Vis Sci. 1984;25(2):195–200. [PubMed] [Google Scholar]
- 6.Weiter JJ, Delori FC, Wing GL, Fitch KA. Retinal-pigment epithelial lipofuscin and melanin and choroidal melanin in human eyes. Invest Ophthalmol Vis Sci. 1986;27(2):145–52. [PubMed] [Google Scholar]
- 7.Delori FC, Goger DG, Dorey CK. Age-related accumulation and spatial distribution of lipofuscin in RPE of normal subjects. Invest Ophthalmol Vis Sci. 2001;42(8):1855–66. [PubMed] [Google Scholar]
- 8.Ach T, Huisingh C, McGwin G, Messinger JD, Zhang TJ, Bentley MJ, Gutierrez DB, Ablonczy Z, Smith RT, Sloan KR, et al. Quantitative autofluorescence and cell density maps of the human retinal pigment epithelium. Invest Ophthalmol Vis Sci. 2014;55(8):4832–41. doi: 10.1167/iovs.14-14802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McGill TJ, Renner LM, Neuringer M. Elevated fundus autofluorescence in monkeys deficient in lutein, zeaxanthin, and omega-3 fatty acids. Invest Ophthalmol Vis Sci. 2016;57(3):1361–69. doi: 10.1167/iovs.15-18596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Colman RJ, Anderson RM. Nonhuman primate calorie restriction.Antioxid Redox Signal. 2011;14(2):229–39. doi: 10.1089/ars.2010.3224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Riemersma JC. Osmium tetroxide fixation of lipids for electron microscopy. A possible reaction mechanism. Biochim Biophys Acta. 1968;152(4):718–27. doi: 10.1016/0005-2760(68)90118-5. [DOI] [PubMed] [Google Scholar]
- 12.Feeney L Lipofuscin and melanin of human retinal pigment epithelium. Fluorescence, enzyme cytochemical, and ultrastructural studies. Invest Ophthalmol Vis Sci. 1978;17(7):583–600. [PubMed] [Google Scholar]
- 13.Belazi D, Sole-Domenech S, Johansson B, Schalling M, Sjovall P.Chemical analysis of osmium tetroxide staining in adipose tissue using imaging ToF-SIMS. Histochem Cell Biol. 2009;132(1):105–15. doi: 10.1007/s00418-009-0587-z. [DOI] [PubMed] [Google Scholar]
- 14.Schmidt SY, Peisch RD. Melanin concentration in normal human retinal pigment epithelium. Regional variation and age-related reduction. Invest Ophthalmol Vis Sci. 1986;27(7):1063–67. [PubMed] [Google Scholar]
- 15.Otaki N, Seiji M. Degradation of melanosomes by lysosomes. J Invest Dermatol. 1971;57(1):1–5. doi: 10.1111/1523-1747.ep12292038. [DOI] [PubMed] [Google Scholar]
- 16.Ito S, Pilat A, Gerwat W, Skumatz CM, Ito M, Kiyono A, Zadlo A,Nakanishi Y, Kolbe L, Burke JM, et al. Photoaging of human retinal pigment epithelium is accompanied by oxidative modifications of its eumelanin. Pigment Cell Melanoma Res. 2013;26 (3):357–66. doi: 10.1111/pcmr.12078. [DOI] [PubMed] [Google Scholar]
- 17.Zareba M, Skumatz CM, Sarna TJ, Burke JM. Photic injury to cultured RPE varies among individual cells in proportion to their endogenous lipofuscin content as modulated by their melanosome content. Invest Ophthalmol Vis Sci. 2014;55(8):4982–90. doi: 10.1167/iovs.14-14310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ng KP, Gugiu B, Renganathan K, Davies MW, Gu X, Crabb JS,Kim SR, Rozanowska MB, Bonilha VL, Rayborn ME, et al. Retinal pigment epithelium lipofuscin proteomics. Mol Cell Proteomics. 2008;7(7):1397–405. doi: 10.1074/mcp.M700525-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bazan HE, Bazan NG, Feeney-Burns L, Berman ER. Lipids in human lipofuscin-enriched subcellular fractions of two age populations. Comparison with rod outer segments and neural retina. Invest Ophthalmol Vis Sci. 1990;31(8):1433–43. [PubMed] [Google Scholar]
- 20.Sparrow JR, Gregory-Roberts E, Yamamoto K, Blonska A, Ghosh SK, Ueda K, Zhou J. The bisretinoids of retinal pigment epithelium. Prog Retin Eye Res. 2012;31(2):121–35. doi: 10.1016/j.preteyeres.2011.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gouras P, Ivert L, Neuringer M, Mattison JA. Topographic and age-related changes of the retinal epithelium and Bruch’s membrane of rhesus monkeys. Graefes Arch Clin Exp Ophthalmol. 2010;248(7):973–84. doi: 10.1007/s00417-010-1325-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ablonczy Z, Higbee D, Anderson DM, Dahrouj M, Grey AC, Gutierrez D, Koutalos Y, Schey KL, Hanneken A, Crouch RK. Lack of correlation between the spatial distribution of A2E and lipofuscin fluorescence in the human retinal pigment epithelium. Invest Ophthalmol Vis Sci. 2013;54(8):5535–42. doi: 10.1167/iovs.13-12250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pallitto P, Ablonczy Z, Jones EE, Drake RR, Koutalos Y, Crouch RK, Donello J, Herrmann J. A2E and lipofuscin distributions in macaque retinal pigment epithelium are similar to human. Photochem Photobiol Sci. 2015;14(10):1888–95. doi: 10.1039/C5PP00170F. [DOI] [PMC free article] [PubMed] [Google Scholar]