Abstract
Introduction
Mycoplasma pneumoniae is a major cause of atypical community-acquired pneumonia (CAP) with a prevalence range of 15-20% and up to 40% in adults and children, respectively. In Iran, the recorded frequency ranges between 1-6.15%. We aimed to investigate the frequency of M. pneumoniae among patients with atypical pneumonia acquired from the community.
Methods
Over a period of 5 months between January and June 2017, 520 patients with suspected CAP, who had been to the hospital outpatient clinics of Tehran University, were enrolled in this study. Throat swab specimens were obtained from 110 outpatients who presented with symptoms of atypical pneumonia. M. pneumoniae was identified via culture and biochemical tests, such as fermentation of glucose and arginine, hemolysis, and hemadsorption. For confirmation, PCR was performed to amplify the gene fragment coding for p1 adhesin.
Results
The major and minor clinical signs of the patients were dyspnea (67.3%) and nausea (15.5%), respectively. Out of 110 specimens, 25 (22.7%) and 29 (26.4%) isolates were identified to be M. pneumoniae via culture and molecular assay, respectively. Comparing the results of the two methods, the PCR showed better sensitivity and rapidity for the detection of M. pneumoniae. There was a high congruence between culture and the PCR assay; kappa level was ‘almost perfect’ (κ=0.90).
Conclusion
This is the first report of high frequency of M. pneumoniae in our region. This finding can serve as baseline information for further investigation and confirmation of the potential epidemics of M. pneumoniae pneumonia in our community.
Keywords: Mycoplasma pneumoniae, epidemiology, culture techniques, polymerase chain reaction (PCR)
Introduction
Nowadays, community-acquired pneumonia (CAP) is one of the common causes of morbidity and mortality worldwide, particularly in children and the elderly with depressed immune functions.1 Clinical manifestations and biochemical parameters hardly provide precise diagnosis of CAP.2 Laboratory diagnosis is most frequently done when there is serious illness and a need for hospitalization due to the presence of extra-pulmonary manifestations, failure to respond to antimicrobial treatments, occurrence in immunocompromised patients, and/or outbreak in the community.3 Studies have shown that a wide variety of viral and bacterial pathogens can cause CAP.2
Human respiratory infections, such as pharyngitis, tracheobronchitis, and CAP may be commonly caused by Mycoplasma pneumoniae at any ages especially among school-aged children.4 M. pneumoniae infections have been frequently reported, especially in closed settings. Transmission is known to usually occur between household contacts. Its outbreaks are often neglected since it has a relatively long incubation period (up to 3 weeks), during which indolent infection can occur before the emergence of shedding symptoms.5 During the past years, reports from Europe and Asia have shown significant increases in the frequency of M. pneumoniae infections.6 In contrast to its subclinical (mild) manifestations, M. pneumoniae accounts for 33% of hospitalizations among adults and children with bacterial pneumonia.7 The confirmation of M. pneumoniae infection is clinically challenging, and its delay may lead to intense clinical syndromes associated with dermatological and neurological manifestations as well as involvement of other organ systems, such as the cardiovascular, musculoskeletal, hematopoietic and urogenital systems.8 However, the frequency of direct invasion of these organs is unknown because the microorganism is rarely sought for clinical purposes. Therefore, techniques for accurate detection and identification of the bacteria in the body are necessary.
Culturing this pathogen is difficult and time-consuming, although it has remained the gold standard for the laboratory confirmation of M. pneumoniae.8 Serological tests including acute and convalescent-phase serum samples need appropriate interpretations, while more advanced diagnostic techniques like PCR, which can specifically amplify M. pneumoniae DNA from nucleic acid extracts, allow more rapid and sensitive detections of the pathogen compared to culture techniques and serological tests.9 During the past decade, different molecular methods in the field of molecular biology have been employed for the diagnosis of M. pneumoniae. The availability of PCR has greatly enhanced the understanding of how M. pneumoniae can disseminate throughout the body.8
Considering the scarcity of data on frequency of M. pneumoniae obtainable from Iran, this investigation was conducted to determine the frequency of M. pneumoniae among patients with atypical pneumonia. We also compared the results of Mycoplasma culture and PCR assays for the clinical specimens collected from patients with CAP in a laboratory setting.
Methods
Patients and clinical specimens
A cross-sectional study was designed and conducted between January and June 2017, at the outpatients clinics of three university hospitals in Iran (Mostafa Khomeini Hospital [Italia St, Naderi St, Keshavarz Blvd, Tehra], Mofid Children's Hospital [Shariati St, Tehran, Iran], and Imam Khomeini's Hospital [Baqerkhan St, Tehran, Iran]) to estimate the frequency of M. pneumoniae among patients with atypical pneumonia. Out of 520 patients who presented with symptoms of CAP, a total of 110 were enrolled in this study. The patients were classified into four main groups according to the previous studies.10 The diagnosis was based on clinical signs and symptoms (such as chest pain, non-productive cough, dyspnea, fever (≥38.3°C), or abnormal breathing sounds) and radiographic pulmonary abnormalities that were not induced by a pre-existing or any other known condition under the supervision of medical doctors.
Criteria for inclusion were: presence of cough with breathlessness of fewer than 30 days duration, or a non-productive cough, increased respiratory rate (with/without features of respiratory distress) on examination, and presence of abnormal breath sounds on auscultation.
Criteria for exclusion were hospital-acquired pneumonia, i.e., pneumonia that developed 72 hours after hospitalization or within 7 days of discharge, severe concomitant disease, and use of macrolide antibiotics in the 48 hours preceding enrollment.
None of the patients had tuberculosis, asthma, chronic bronchitis, or lung cancer. After obtaining an informed consent from the patients and/or their parents or guardians, clinical throat specimens were collected using a sterile rayon swab (Dry swab™ ENT, Corsham, Wiltshire, UK). The specimens were immediately placed in the test tubes containing the transport medium for M. pneumoniae (PPLO broth, HiMedia, Mumbai, India), and then transferred at 4°C to the Mycoplasma Laboratory of the Division of Microbiology, Tehran University of Medical Sciences, for immediate processing.
Cultivation of M. pneumoniae
The specimens were incubated in PPLO broth, enriched with 5% CO2 in humid air at 37°C for 8-16 hours. After filtration with 0.45 µm-PPLO broth filter paper, the obtained cultures were incubated with 5% CO2 in a humid atmosphere at 37°C for 12-20 days, together with the negative and positive (ATCC 29342) controls according to Pharmacopoeia (2005). When the broth cultures were suspected to show Mycoplasma positivity, 500 µL of the obtained specimens were spread directly onto PPLO agar plates for colony isolation. PPLO agar was used for counting M. pneumoniae colonies. The remaining specimens were stored at -80°C for the subsequent identification of M. pneumoniae.
Biochemical identification
Biochemical identification was applied for further testing of Mycoplasma spp. through glucose fermentation, arginine deamination, urea hydrolysis, hemadsorption, and hemolysis tests as previously described (Table 1).11
Table 1. Biological and biochemical reactions of members of the human Mycoplasma flora11.
| Species | Requirement for yeast extracts | Inhibition of growth by | Hemolysis | Hemadsorption | Acid from glucose | Arginine | Urea | Aerobic reduction of tetrazolium | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Thallium acetate (0.001% w/v) | Methylene blue (0.001% w/v) | Erythromycin (100 µg/mL) | Lincomycin (100 µg/mL) | ||||||||
| M. pneumoniae | + | - | - | + | - | β | + | + | - | - | + |
| M. fermentans | - | - | + | + | + | - | - | + | + | - | - |
| M. hominis | - | - | + | - | + | - or x | - | - | + | - | - |
| M. orale 1 (M. orale) | + | - | + | - | + | - | +* | - | + | - | - |
| M. orale 2 (buccale) | - | - | + | - | - | - | - | - | + | - | - |
| M. orale 3 (faucium) | + | - | + | - | - | - | +* | - | + | - | - |
| M. salivarium | - | - | + | - | - | - | - | - | + | - | - |
| M. genitalium | + | + | … | … | … | … | + | + | - | - | ± |
| Ureaplasma urealyticum | + | + | - | + | - | β | - | - | - | + | - |
With chicken erythrocytes, not guinea-pig or human, but see Purcell & Chanock (1969)
“…” – results not available; “+” – positive, “-“ – negative, “β” – complete hemolysis of erythrocytes (β hemolysis); “- or x” – no or partial hemolysis.
DNA extraction
The frozen respiratory specimens were thawed on the day of performing the assays out of which 500 µL of each specimen were used for Mycoplasma DNA extraction using the Tissue Genomic DNA Extraction Kit (YTA Genomic DNA Extraction Mini Kit, Yekta Tazhiz Azma, Tehran, Iran) according to the manufacturer’s instructions.
Molecular identification
A number of 110 isolates were examined to evaluate the presence of 16S rRNA gene from Mycoplasma species.12 The final volume of the reaction set was 25 µL of Taq master mix kit (Ampliqon, Odense, Denmark), including 12.5 µL of 1x Taq master mix (containing 1.5 mM of MgCl2, 0.22 mM of each dNTP, and 0.11 units/µL of Taq DNA polymerase), 2 µL of genomic DNA, 0.5 µL (10 pmol) of each primer, and 9.5 µL of ddH2O. The PCR reactions for the 16S rRNA gene were conducted under the following conditions: DNA denaturation at 94°C for 5 min, 30 cycles of denaturation at 94°C for 30 s, annealing at 56°C for 35 s, extension at 72°C for 30 s, and the final extension step at 72°C for 5 min. The amplification products were electrophoresed in 2% agarose gel at 106 V for 45 min, stained with KBC (0.5 mg/mL) (Kawsar, Tehran, Iran), and photographed under UV light.
The PCR reactions were conducted for the detection of the 450-bp cytadhesin p1 gene from M. pneumoniae according to the techniques previously described13 under the following conditions: DNA denaturation at 95°C for 5 min, 35 cycles of denaturation at 95°C for 35 s, annealing at 58°C for 35 s, extension at 72°C for 30 s, and the final extension step at 72°C for 7 min. The amplification products were electrophoresed in 1% agarose gel at 106 V for 45 min, stained with KBC (0.5 mg/mL) (Kawsar), and photographed under UV light.
Statistical analysis
Comparisons between the groups of patients (by age, by gender, by symptoms and by comorbid disease) and associations between clinical features and presence of M. pneumoniae were performed by multi-variable logistic regression test, while Cohen’s kappa value (k) was calculated to assess the degree of agreement between results achieved by culture and PCR assay. Kappa values of <0.00, 0.00-0.20, 0.21-0.4, 0.41-0.6, 0.61-0.8, 0.81-1.00 indicate ‘poor’, ‘slight’, ‘moderate’, ‘substantial’ and ‘almost perfect’, respectively. All the tests of significance were 2-tailed with the alpha set at 0.05. The sensitivity, specificity, the positive and negative predictive values for molecular methods (PCR) were calculated according to the formula at the eighth chapter of Bailey & Scott's Diagnostic Microbiology book.14 All the analyses were done with SPSS software (SPSS 20, IBM, Armonk, NY, USA).
Results
General characteristics of the study population
Out of 520 patients who presented to the outpatients clinics with CAP, 110 patients with atypical pneumonia were included in our study. There were 43 males and 67 females with the median ages of 51.50 years (IQR 65-42). The comorbid diseases included diabetes mellitus, cardiovascular disease, and kidney failure in 16, 24, and 5 patients, respectively. The most prominent symptoms in all patients were dyspnea (67.3%), cough (64.5%), sore throat (64.5%), chest pain (60.9%), lethargy (49.1%), sputum production (39.1%), headache (29.1%), anxiety (23.6%), and nausea (15.5%). The clinical features and PCR assay results of Mycoplasma pneumoniae pneumonia (MPP) in patients with atypical pneumonia were statistically analyzed and presented in Table 2.
Table 2. The clinical features and PCR assay results of Mycoplasma pneumoniae pneumonia in patients with atypical pneumonia.
| M. pneumoniae positive No. (%) | M. pneumoniae negative No. (%) | Total No. (%) | P-value | Odds ratio | 95% confidence interval | ||
|---|---|---|---|---|---|---|---|
| Age | 1-20 | 5 (41.7) | 7 (58.3) | 12 (10.9) | 0.44 | 1.93 | (0.35-10.49) |
| 20-40 | 3 (20) | 12 (80) | 15 (13.6) | 0.19 | 0.29 | (0.04-1.88) | |
| 40-60 | 9 (18.8) | 39 (81.3) | 48 (43.6) | 0.17 | 0.41 | (0.11-1.45) | |
| Sex | Male | 11 (25.6) | 32 (74.4) | 43 (39.1) | 0.46 | 1.50 | (0.05-4.46) |
| Female | 18 (26.9) | 49 (73.1) | 67 (60.9) | ||||
| Chest pain | Yes | 18 (26.9) | 49 (73.1) | 67 (60.9) | 0.003 | 6.45 | (1.91-21.7) |
| No | 10 (17.2) | 48 (82.8) | 58 (52.7) | ||||
| Fever | Yes | 3 (18.8) | 13 (81.3) | 16 (14.5) | 0.59 | 0.61 | (0.10-3.67) |
| No | 26 (27.7) | 68 (72.3) | 94 (85.5) | ||||
| Lethargy | Yes | 13 (24.1) | 41 (75.9) | 54 (49.1) | 0.560 | 1.45 | (0.414-1.85) |
| No | 16 (28.6) | 40 (71.4) | 56 (50.9) | ||||
| Headache | Yes | 6 (18.8) | 26 (81.3) | 32 (29.1) | 0.08 | 0.26 | (0.06-1.20) |
| No | 23 (29.5) | 55 (70.5) | 78 (70.9) | ||||
| Nausea | Yes | 1 (5.1) | 16 (94.1) | 17 (15.5) | 0.09 | 0.12 | (0.01-1.38) |
| No | 28 (30.1) | 65 (69.9) | 93 (84.5) | ||||
| Sore throat | Yes | 6 (19.4) | 25 (80.6) | 31 (64.5) | 0.30 | 2.19 | (0.49-9.71) |
| No | 23 (29.1) | 56 (70.9) | 39 (35.5) | ||||
| Cough | Yes | 19 (26.8) | 52 (73.2) | 71 (64.5) | 0.84 | 1.13 | (0.31-4.11) |
| No | 10 (25.6) | 29 (74.4) | 39 (35.5) | ||||
| Sputum production | Yes | 10 (23.3) | 33 (76.7) | 43 (39.1) | 0.57 | 0.69 | (0.18-2.53) |
| No | 19 (28.4) | 48 (61.1) | 67 (60.9) | ||||
| Dyspnea | Yes | 16 (21.6) | 58 (78.4) | 74 (67.3) | 0.08 | 0.37 | (0.12-1.12) |
| No | 13 (36.1) | 23 (63.9) | 36 (32.7) | ||||
| Anxiety | Yes | 5 (19.2) | 21 (80.8) | 26 (23.6) | 0.98 | 1.01 | (0.21-4.88) |
| No | 24 (28.6) | 60 (71.4) | 84 (76.4) | ||||
| Diabetes | Yes | 7 (43.8) | 9 (56.3) | 16 (14.5) | 0.41 | 1.80 | (0.43-7.49) |
| No | 22 (23.4) | 72 (76.8) | 94 (85.5) | ||||
| Cardiovascular disease | Yes | 6 (25) | 18 (75) | 24 (21.8) | 0.59 | 0.70 | (0.19-2.53) |
| No | 23 (26.7) | 63 (73.3) | 86 (78.2) | ||||
| Kidney failure | Yes | 2 (40) | 3 (60) | 5 (4.5) | 0.57 | 1.99 | (0.17-22.90) |
| No | 27 (25.7) | 78 (74.3) | 105 (95.5) |
Culture characteristics and biochemical tests
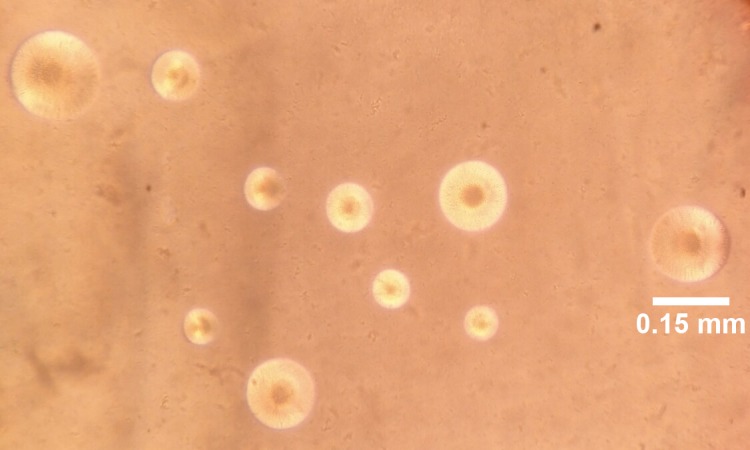
Microscopic examinations of the positive culture plates demonstrated spherical colonies with a fried-egg appearance, in which a dark central zone was usually surrounded by a lighter peripheral zone or finely granular berry-like colonies penetrating into the agar surface, while some Mycoplasma strains had formed tiny pinpoint ball-like colonies (Figure 1). The isolates underwent 3 biochemical tests (glucose fermentation, arginine deamination, and urea hydrolysis) and two biological tests (hemadsorption and hemolysis), through which 25 (22.7%) were confirmed to be M. pneumoniae. The culture positive patients for M. pneumoniae included 10 (23.3%) males and 15 (22.4%) females.
Figure 1. Spherical colonies of M. pneumoniae growing on PPLO agar (magnification ×160).
Molecular detection
The presence of Mycoplasma spp. in the clinical specimens was confirmed by PCR amplification of the 16S rRNA gene directly from the specimens. The presence of M. pneumoniae was further corroborated by the PCR amplification of the p1 cytadhesin gene. Twenty-nine (26.4%) specimens were PCR positive for M. pneumoniae among which 11 (25.6%) were from male and 18 (26.9%) from female patients. Four (3.64%) of the culture negative specimens were identified as positive for M. pneumoniae by PCR assay. The sensitivity of PCR was 100%, with a specificity of 95.5%; the positive and negative predictive values were 100% and 86.2%, respectively.
The nucleotide sequence data reported in this research were submitted to the GenBank sequence database and the GenBank accession number MH257275 was assigned to M. pneumoniae.
Comparison of PCR and culture
For the diagnosis of M. pneumoniae infection we compared the PCR assay with the culture method. Among 110 patients with atypical pneumonia, 25 patients (22.4%) were culture positive and 29 (26.4%) were positive by PCR assay.
The concordance rate between the two methods was calculated and the PCR assay showed an ‘almost perfect’ agreement with the culture method (κ=0.90, p<0.001).
Discussion
In this research, patients with clinical signs and symptoms of atypical pneumonia were examined at the hospitals of Tehran University. A quarter of the patients were diagnosed to have M. pneumoniae through culture and molecular methods. Molecular methods were able to also identify M. pneumoniae among the culture negative specimens. This finding is consistent with the findings of another study.15
Previous studies have shown that culture is the gold standard for the diagnosis of M. pneumoniae despite being time-consuming; M. pneumoniae is a fastidious pathogen and may require several weeks to grow.8 Culture is scarcely applied for the diagnosis of M. pneumoniae infections in the emergency care hospital setting; however, it can facilitate obtainment of isolates for antimicrobial susceptibility testing and/or typing, and consequently provide documented evidence for epidemiological purposes and antibiotic hospital stewardship. Nucleic acid-based tests, such as PCR, provide fast and sensitive results. Furthermore, the PCR technique has been shown to have a greater sensitivity than the culture method.15 In our study, the sensitivity of the PCR method was greater than that of the culture technique and significant differences were observed between the two methods. Rosemary et al. reported lower culture positivity rates and did not recommend the latter approach for identifying this pathogen.15 Early identification of causative pathogens in patients with severe infections is vital for patient survival.
Various studies have reported the prevalence of M. pneumoniae infection among patients with CAP to be between 10% and 40%.16 Even though we recorded a high frequency (26.4%) of M. pneumoniae in our study, low frequencies (1-6.15%) have been reported in several studies in Iran.17,18 The research conducted by Oskooee et al. involving 102 CAP patients displayed the positivity rate of only 1% for M. pneumoniae.18 Also, the study of Ghotaslou et al. carried out on 51 patients with lower respiratory tract infection in Azerbaijan revealed that about 6.15% of them had M. pneumoniae.17 Similar frequencies of this pathogen have been reported in other studies from other parts of the world, like those of Elkholy et al. and Meijer et al. involving 111 Egyptian children and 557 Dutch patients with the positivity rates of only 4.5%19 and 1.3%,20 respectively. However, a relatively high frequency of the infection (>19%) has been reported in our neighboring countries, such as Turkey.21 Still, there are several studies reporting higher frequencies of M. pneumoniae, ranging from 27.5% to 35%.22,23 The prevalence rate of respiratory infection due to this pathogen varies from one country to another due to seasonal variation and differences in geographical locations.24 The substantial part of our study was conducted during the winter season (January to June), in Tehran, a megacity characterized by a high level of air pollution; these are two factors which might have a positive effect on the frequency of respiratory tract infections, including MPP. Higher frequencies of M. pneumoniae detection have been reported by other researchers around the world.4
The clinical manifestations of M. pneumoniae range from asymptomatic infections to severe pneumonia associated with extra-pulmonary complications. Previous studies have shown that the percentages of clinical signs and symptoms do not vary significantly among patients.2 In this research, we found a relationship between chest pain and Mycoplasma infection (p=0.003); the odds of chest pain were 6.45-fold greater for MP pneumonia.
Studies have shown that underlying conditions such as renal, cardiovascular diseases and diabetes mellitus increase the risk and severity of CAP in individuals.1,25 This may serve as a prognostic factor for the physicians to make better decisions on the diagnosis and overall patient management.2 Although this study does not show any statistical difference between the presence of M. pneumoniae and underlying diseases (p value >0.05), the odds ratio of MPP were 1.99, 1.80 and 0.7 in kidney disease, diabetes and cardiovascular disease respectively. The high odds of MPP in the patients with comorbid condition would be better explained with higher sample size. The small sample size is one of the drawbacks of our study. On the other hand, investigating only M. pneumoniae frequency without exploring the causes of atypical pneumonia is considered another limitation of our study. Furthermore, the lack of a national epidemiological surveillance system for M. pneumoniae infections in Iran might explain the unduly high frequency of the infection as recorded in our study.
Conclusion
To the best of our knowledge, this is the first report on the high frequency of M. pneumoniae in the capital city of Iran. We demonstrated that the PCR assay is more sensitive than the culture method, which is still regarded as the gold standard technique for the diagnosis of M. pneumoniae, although we found a high level of congruency between the two methods. Recognition of the high frequency of M. pneumoniae among CAP patients in Iran is the baseline information that can be utilized by the physicians, the epidemiologists and the government in order to set up mechanisms for the confirmation, prevention and control of the potential epidemics of MPP in our community.
Footnotes
Authors’ contributions statement: MA undertook the laboratory tests and writing of the manuscript. FA, AA, SAP and EM assisted with the laboratory examination and sample processing. GA acted as a clinician responsible for patients’ management and collection of throat swab samples. MRP was the laboratory manager and supervisor of the entire work. All authors read and approved the final version of the manuscript.
Conflicts of interest: All authors – none to disclose.
Acknowledgment and funding support: This research was supported by the Tehran University of Medical Sciences and Health Services (Tehran, Iran) [grant no. 34054].
References
- 1.Restrepo MI, Reyes LF, Anzueto A. Complication of community-acquired pneumonia (including cardiac complications) Semin Respir Crit Care Med. 2016;37:897–904. doi: 10.1055/s-0036-1593754. [DOI] [PubMed] [Google Scholar]
- 2.Foering A. Community acquired pneumonia. Kabod. 2017;3:8. [Google Scholar]
- 3.Ratliff AE, Duffy LB, Waites KB. Comparison of the illumigene Mycoplasma DNA amplification assay and culture for detection of Mycoplasma pneumoniae. J Clin Microbiol. 2014;52:1060–3. doi: 10.1128/JCM.02913-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Meyer Sauteur PM, Unger WW, Nadal D, Berger C, Vink C, van Rossum A. Infection with and carriage of Mycoplasma pneumoniae in children. Front Microbiol. 2016;7:329. doi: 10.3389/fmicb.2016.00329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Winchell JM. Mycoplasma pneumoniae – a national public health perspective. Curr Pediatr Rev. 2013;9:324–33. [Google Scholar]
- 6.Dumke R, Schnee C, Pletz MW, et al. Mycoplasma pneumoniae and Chlamydia spp. infection in community-acquired pneumonia, Germany, 2011–2012. Emerg Infect Dis. 2015;21:426–34. doi: 10.3201/eid2103.140927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Petrone BL, Wolff BJ, DeLaney AA, Diaz MH, Winchell JM. Isothermal detection of Mycoplasma pneumoniae directly from respiratory clinical specimens. J Clin Microbiol. 2015;53:2970–6. doi: 10.1128/JCM.01431-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Waites KB, Talkington DF. Mycoplasma pneumoniae and its role as a human pathogen. Clin Microbiol Rev. 2004;17:697–728. doi: 10.1128/CMR.17.4.697-728.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ieven MM, Loens K. Should serology be abolished in favor of PCR for the diagnosis of Mycoplasma pneumoniae infections? Curr Pediatr Rev. 2013;9:304–13. [Google Scholar]
- 10.Whistler T, Sawatwong P, Diaz MH, et al. Molecular characterization of Mycoplasma pneumoniae infections in two rural populations of Thailand from 2009-2012. J Clin Microbiol. 2017;55:2222–33. doi: 10.1128/JCM.00350-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Colle JG, Marmion BP, Fraser AG, Simmons A, editors. Mackie & McCartney Practical Medical Microbiology. Thirteenth Edition ed. New York: Churchill Livingstone; 1996. [Google Scholar]
- 12.Golmohammadi R, Ataee RA, Alishiri GH, et al. Molecular diagnosis of Mycoplasma pneumoniae in synovial fluid of rheumatoid arthritis patients. Iran J Med Microbiol. 2014;8:1–8. [Google Scholar]
- 13.Pooladgar AR, Looni R, Ghaemmaghami S, Pourbakhsh A, Ashtari A, Shirudi A. Isolation and identification of Mycoplasma agalactiae by culture and polymerase chain reaction (PCR) from affected sheep to contagious agalactia of Khuzestan province, Iran. Arch Razi Inst. 2015;70:21–7. [Google Scholar]
- 14.Clinical Laboratory Management . In: Bailey & Scott's Diagnostic Microbiology, 12th Edition. St. Louis, MO. Forbes B, Sahm D, Weissfeld A, editors. USA: Mosby; 2007. p. 1056. [Google Scholar]
- 15.She RC, Thurber A, Hymas WC, et al. Limited utility of culture for Mycoplasma pneumoniae and Chlamydophila pneumoniae for diagnosis of respiratory tract infections. J Clin Microbiol. 2010;48:3380–2. doi: 10.1128/JCM.00321-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bajantri B, Venkatram S, Diaz-Fuentes G. Mycoplasma pneumoniae: a potentially severe infection. J Clin Med Res. 2018;10:535–44. doi: 10.14740/jocmr3421w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ghotaslou R, Sharifi S, Akhi MT, Soroush MH. Epidemiology, clinical features, and laboratory detection of Mycoplasma pneumoniae infection in East Azerbaijan, Iran. Turk J Med Sci. 2013;43:521–4. [Google Scholar]
- 18.Oskooee MB, Karimi A, Shiva F, et al. Detection of Mycoplasma pneumoniae and Chlamydia trachomatis in Iranian children with acute lower respiratory infections by polymerase chain reaction. Asian Pac J Trop Dis. 2014;4:S302–6. [Google Scholar]
- 19.Elkholy A, Elkaraksy H, Fattouh A, Bazaraa H, Hegazy R, AbdElhalim M. Acute lower respiratory tract infection due to Chlamydia and Mycoplasma spp. in Egyptian children under 5 years of age. J Trop Pediatr. 2009;55:195–7. doi: 10.1093/tropej/fmn102. [DOI] [PubMed] [Google Scholar]
- 20.Meijer A, Dagnelie C, De Jong J, et al. Low prevalence of Chlamydia pneumoniae and Mycoplasma pneumoniae among patients with symptoms of respiratory tract infections in Dutch general practices. Eur J Epidemiol. 2000;16:1099–106. doi: 10.1023/A:1010912012932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bosnak M, Dikici B, Bosnak V, et al. Prevalence of Mycoplasma pneumoniae in children in Diyarbakir, the south-east of Turkey. Pediatr Int. 2002;44:510–2. doi: 10.1046/j.1442-200x.2002.01606.x. [DOI] [PubMed] [Google Scholar]
- 22.Maltezou HC, La-Scola B, Astra H, et al. Mycoplasma pneumoniae and Legionella pneumophila in community-acquired lower respiratory tract infections among hospitalized children: diagnosis by real time PCR. Scand J Infect Dis. 2004;36:639–42. doi: 10.1080/00365540410020884. [DOI] [PubMed] [Google Scholar]
- 23.Tsolia MN, Psarras S, Bossios A, et al. Etiology of community-acquired pneumonia in hospitalized school-age children: evidence for high prevalence of viral infections. Clin Infect Dis. 2004;39:681–6. doi: 10.1086/422996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Del Valle-Mendoza J, Orellana-Peralta F, Marcelo-Rodríguez A, et al. High prevalence of Mycoplasma pneumoniae and Chlamydia pneumoniae in children with acute respiratory infections from Lima, Peru. PloS One. 2017;12:e0170787. doi: 10.1371/journal.pone.0170787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fisher-Hoch SP, Mathews CE, McCormick JB. Obesity, diabetes and pneumonia: the menacing interface of non-communicable and infectious diseases. Trop Med Int Health. 2013;18:1510–9. doi: 10.1111/tmi.12206. [DOI] [PubMed] [Google Scholar]


