Abstract
Drosophila has long been an excellent model organism for studying stem cell biology. Notably, studies of Drosophila’s germline stem cells have been instrumental in developing the stem cell niche concept. The recent discovery of somatic stem cells in adult Drosophila, particularly the intestinal stem cells (ISCs) of the midgut, has established Drosophila as an exciting model to study stem cell-mediated adult tissue homeostasis and regeneration. Here, we review the major signaling pathways that regulate the self-renewal, proliferation and differentiation of Drosophila ISCs, discussing how this regulation maintains midgut homeostasis and mediates regeneration of the intestinal epithelium after injury.
Keywords: Intestinal stem cell, Drosophila midgut, Tissue regeneration, Tissue homeostasis
Origin of Drosophila intestinal stem cells (ISCs)
The embryonic development of the Drosophila midgut, which is the endodermally derived portion of the intestine, has been studied extensively, and shares many evolutionarily conserved regulatory signals with that of vertebrate endoderm-derived organs [1]. In both systems, members of the Forkhead and Gata transcription factors play conserved roles in endoderm specification. Conserved Hox homeobox genes regulate the anterior-posterior patterning of the endoderm. Multiple signaling pathways, such as the Notch, Wnt and TGF-β pathways, further regulate the specification of different cell types in the endoderm to form a functional digestive system. The precursors for intestinal stem cells (ISCs), called adult midgut progenitors (AMPs), first appear in the embryonic Drosophila midgut epithelium amongst a small number of diploid cells that are set aside to generate the future adult midgut epithelium during metamorphosis. The rest of the epithelial cells in the embryonic midgut terminally differentiate into functional enterocytes (ECs) or enteroendocrine cells (EEs) of the larval midgut [2].
During Drosophila larval development, the midgut grows substantially, mainly due to the very dramatic increase in cell size and ploidy of the larval ECs. Meanwhile, the small diploid AMPs proliferate extensively to increase their numbers. At the onset of metamorphosis, AMPs form distinctive clusters in the midgut [3–6]. During early pupal development, the larval midgut contracts and shortens itself dramatically, which brings the AMP clusters together to form two new epithelial tubes. The AMPs in the centers of the clusters form the future adult midgut epithelium. Inside this newly formed adult midgut, the lateral AMPs, also called peripheral cells (PCs), form a transient epithelium that wraps around the histolyzing larval midgut [4]. Following this, the majority of the AMPs differentiate into adult ECs in the newly formed adult midgut epithelium. A small number of these AMPs remain undifferentiated, however, and during metamorphosis they divide and expand their numbers to become the ISCs of the adult midgut.
Structurally, the adult Drosophila midgut comprises a simple, essentially monolayer cell epithelium enveloped by two layers of mesodermally derived visceral muscle with orthogonally oriented actin-myosin fibers (one circular and one longitudinal). Reference [7] provides a fine ultrastructural description. While the Drosophila midgut lacks the characteristic crypt-villus structure of the mammalian intestine, it is worth pointing out that the midgut epithelia of some large insects are organized in a similar fashion to that of the mammalian intestine, where the regenerative stem cells are located at the bottom of “crypt”-like structures [8]. Inside the Drosophila midgut lumen, a chitinous membrane, called the peritrophic membrane or matrix, separates the epithelium from the ingested food and serves as a barrier against the gut bacteria. Within the epithelium, the ISCs reside basally just above the basement membrane, which separates the epithelium from the underlying visceral muscle cells (Fig. 1A). Similar to the mammalian intestine and colon, the Drosophila adult midgut also undergoes dynamic self-renewal [9,10]. ISCs divide to self renew themselves and to give rise to committed progenitor cells, called enteroblasts (EBs). Unlike the mammalian intestinal transient amplifying cells (TAs), Drosophila midgut enteroblasts rarely, if ever, divide. Instead, they directly differentiate into two conserved functional gut cell types, the absorptive EC and secretory enteroendocrine cells (EEs). As part of their differentiation program, ECs grow very large and endoreplicate their genomes several times, to ploidy levels of 16–32C. Hence these cells make up the bulk of the midgut epithelium. Newly generated gut cells presumably replenish the loss of aging or damaged gut cells and thereby maintain midgut homeostasis. ISC lineage tracing experiments using mitotic recombination and the FLP/FRT technique suggest that the midgut turns over at a rate of about once per 1–2 weeks [10]. However, another ISC lineage tracing system revealed that adult midgut has a slower turnover rate, of 2–3 weeks in females and longer in males [11]. The gut turnover rate in healthy animals also appears to vary greatly according to age and diet, though specific measurements have not yet been published.
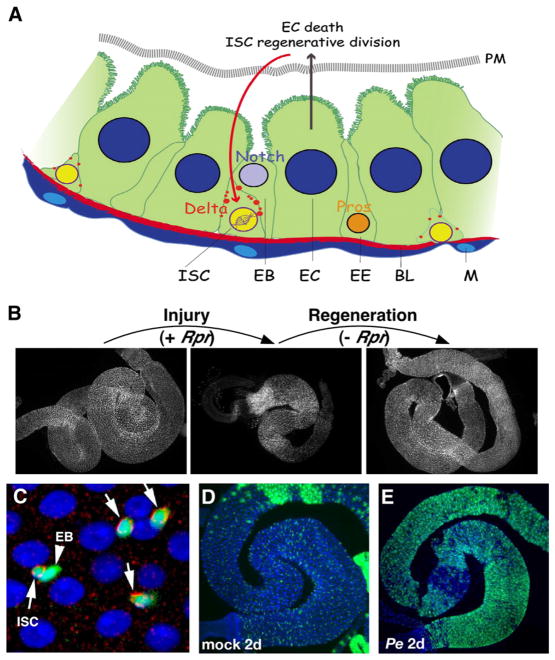
Fig. 1.
Adult midgut regeneration models in Drosophila. A. Schematic of adult Drosophila midgut. ISC, intestinal stem cells; EB, enteroblasts; EC, enterocytes; EE, enteroendocrine cells; M, visceral muscle cells; BL, basal lamina; PM, peritrophic membrane. Delta is noted as red spots. B. Fly midgut regeneration induced by EC apoptosis. EC-specific expression of the cell death gene reaper (+Rpr) induces EC apoptosis and the midgut shrinks dramatically. Upon ceasing Rpr expression (−Rpr), the midgut rapidly regenerates itself. C. Adult Drosophila midgut epithelium. ISCs (arrows) and EBs (arrowhead) are marked by esgGal4-driven GFP (green). ISCs are also marked by Notch ligand Delta staining (arrow, red). ECs are marked by their large nuclei (Hoechst stain, blue). D, E. Fly midgut regeneration induced by Pseudomonas entomophila (Pe) infection. Feeding adult flies with pathogenic bacteria (such as Pe) for 2 days (E, Pe 2d) induces complete renewal of the adult midgut epithelium, as indicated by GFP expression using an ISC lineage tracing system, called esgtsF/O, which marks midgut progenitor cells and all of their newborn progeny. In contrast, only the original progenitor cells are marked by GFP in a mock-treated (not infected) midgut (D, Mock 2d), indicating minimal epithelial renewal within 2 days in un-infected midguts.
A feedback mechanism for midgut homeostasis and regeneration
In response to midgut injury, caused either by killing ECs directly (by expressing cell death genes, such as reaper, in ECs), by ingestion of cytotoxic agents such as bleomycin, paraquat, or dextran sodium sulfate (DSS), or by enteric infection with bacteria such as Pseudomonas entomophila or Erwinia carotovora carotovora, the midgut mitotic index of ISCs increases 10–100 fold [11–17]. Following this mitotic activation of ISCs, newborn EBs and perhaps also previously present ones, quickly differentiate into mature midgut enterocytes and endocrine cells to replenish the loss of damaged cells, and thus the midgut epithelium regenerates itself (Fig. 1B, D, E). We have proposed a feedback mechanism to describe this system of injury-induced tissue regeneration (Fig. 2) [11]. In this model, the degree of cell loss in the tissue is sensed and generates signals that feed back to the resident stem cells, allowing them to adjust their corresponding proliferation and differentiation rates. This feedback is envisioned to provide a truly homeostatic mechanism that mediates both tissue maintenance in healthy animals and tissue regeneration after injury. In the following sections we discuss the signaling pathways involved in this feedback mechanism, and how their regulation and interactions facilitate homeostatic growth in the fly’s intestinal epithelium.
Fig. 2.
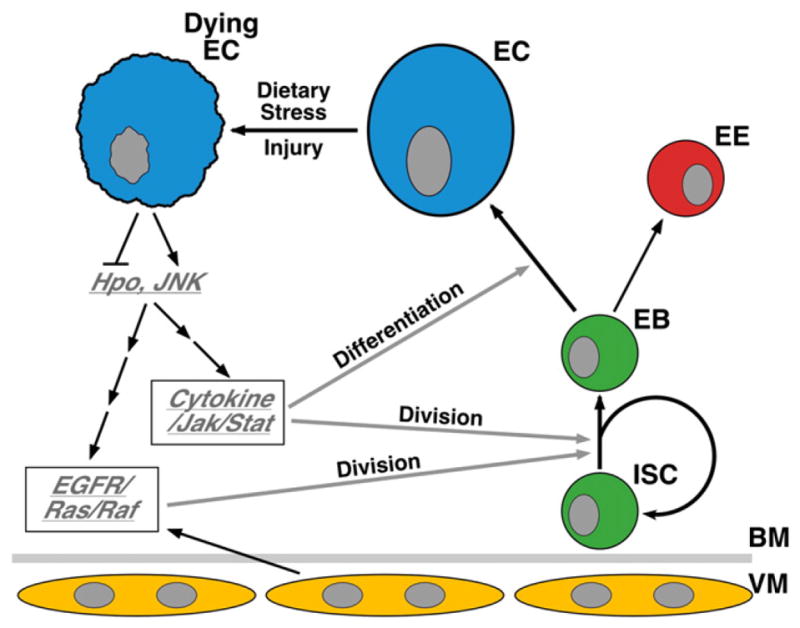
Feedback mechanisms regulating adult midgut homeostasis and regeneration in Drosophila. Mature midgut cell loss (such as EC losses caused by pathogenic infection) trigger the activation of the JNK pathway and inactivation of the Hpo pathway in the ECs, both of which are able to induce the expression of mitogenic cytokines (Upds) and growth factors (fly EGFs) in the damaged midgut. Upd cytokines and fly EGFs (spi, krn, vn) activate the Jak/Stat and EGFR/Ras/MapK pathways respectively in the progenitor cells, where these stimuli function synergistically to promote ISC division. In addition, the Jak/Stat signaling also promotes EB differentiation. Thus, in response to damage, the ISCs rapidly generate new midgut cells to maintain epithelial homeostasis.
Delta/Notch signaling regulates differentiation of midgut progenitor cells
Notch signaling is instrumental in regulating the specification and differentiation of midgut progenitors during multiple stages of midgut development. During formation of the embryonic midgut, Notch activity levels determine the fate of midgut epithelial cells. High Notch activity induces the differentiation of enterocytes (ECs), and low Notch activity leads to the formation of enteroendocrine cells (EE) or adult midgut progenitors (AMPs) [4]. Similarly, Notch signaling also controls midgut cell fate decision during larval and pupal development. During larval development, the Notch ligand Dl is expressed in the central cells of AMP clusters, which activates Notch signaling in the peripheral cells of the cluster to specify them as peripheral cells (PCs) [4,6]. The PCs have been proposed to function as a transient niche for AMPs [6]. They are also proposed to form a transient pupal midgut epithelium that wraps around the histolyzing larval midgut cells during metamorphosis [4]. PCs express a marker found in mature enterocytes, MyoIA-lacZ (H.J. and B.A. E. unpublished data). These cells likely also function to aid the histolysis of the larval midgut epithelium that is discarded during metamorphosis. In the newly formed adult midgut epithelium, the future adult ISCs express low levels of Dl. The resulting low Notch activation in turn facilitates the expansion of ISCs and subsequent emergence of adult EEs in the epithelium [4].
In the adult midgut epithelium, Notch signaling is essential for differentiation of ISC progeny into ECs [9,10]. Dl is specifically expressed in ISCs, where its expression seems to fluctuate (Fig. 1C). High levels of Dl in ISCs (presumably leading to high level of Notch activation in EBs) induces EBs to differentiate into the EC cell fate, while low Dl expression has been proposed to promote the EE cell fate [18]. However, experiments in which Dl/N signaling are suppressed, by either RNAi or clonal deletion of Dl or N, suggest that EEs can be generated even in the absence of Notch activity. The mechanism underlying the fluctuations of Dl expression in ISCs is still unknown, but similar oscillations of Notch ligand Dll1 expression have been reported in mammalian neural progenitors [19].
Whereas Notch signaling’s role in promoting absorptive cell fate determination is conserved between the fly midgut and the mammalian intestine, the effect of Notch signaling on ISC self renewal and proliferation is rather opposite in the two systems. In the mammalian intestine, ectopic Notch activation promotes the proliferation of intestinal epithelial cells and the expansion of progenitor cells [20], whereas inhibiting Notch suppresses cell proliferation and induces the formation of ectopic goblet cells, depleting progenitor cells [21]. In Drosophila, in contrast, Notch activation in ISCs inhibits their proliferation and promotes their differentiation into mature polyploid ECs, leading rapidly to stem cell depletion [9,10]. Conversely, loss of Notch in Drosophila ISCs and EBs results in the rampant expansion of small Delta-positive ISC-like cells and EE pools, and blocks EC production [9,10]. Suppressing Notch activity also induces higher ISC proliferation in Drosophila midgut. This is most likely a result of frequent ISC duplications, leading to exponential expansion, but might also involve positive feedback by autocrine mitogens (Spi, Upd) produced by these cells. The fact that the Drosophila midgut lacks the transient amplifying cells seen in mammals may explain some of Notch signaling’s different effects on cell proliferation in this system.
In the Drosophila midgut, differential Notch activation between the two daughter cells of an ISC division is generated by asymmetric Dl expression [18,22]. During mitosis, Dl is expressed in both daughter cells. However, just after the completion of the division, only one daughter cell retains punctuated Dl expression while the other daughter cell loses Dl expression but activates Notch target genes and reporters. It has been proposed that the more basally located cell retains Delta expression and self-renewing ISC identity, whereas the more apically localized daughter cell will lose Delta expression and become the committed progenitor EB [18]. The asymmetry of Dl expression between ISC and EB is further reinforced by the repression of Dl transcription in EBs. The precise mechanism for establishing asymmetric Dl expression in the midgut progenitors is still unclear and multiple models have been proposed [18,22].
In the ISCs, downstream Notch target genes are repressed by the Hairless and Suppress of Hairless (Su(H)) complex, and this repression is critical for maintaining their stem cell fate [22]. In the EBs, activated Notch binds to Su(H) and competes with Hairless to relieve the suppression of Notch target genes, including bHLH transcription factors encoded by the Enhancer of split Complex (E(spl)-C). E(spl)-C gene products then inhibit the expression of another bHLH transcription factor, daughterless (Da), which in turn inactivates genes that are required for maintaining stem cell fate. This commits the EB to differentiate [22]. Similarly, the mammalian Da homologs E2A and HEB are both expressed in lgr5+ ISCs in mice. They form heterodimers with acheate-scute like 2 (Ascl2), which is required for the maintenance of ISCs [23]. Thus, a conserved cascade of bHLH transcriptional factors downstream of Notch regulates the self renewal and differentiation of ISCs in both the mammalian and fly gut.
WNT signaling, ISC proliferation, and ISC maintenance
Given the critical role of Wnt signaling in regulating mammalian ISC proliferation and maintenance [24], it is surprising that manipulation of Wnt/Wingless signaling has relatively mild effects in the Drosophila adult midgut [25,26]. ISCs defective for Drosophila Wg pathway components, such as shaggy (GSK3β), dAxin or Apc, which lead to the activation of Wg signaling, have a higher proliferation rate [25,26]. Similarly, ectopic expression of Drosophila Wnt ligand, Wingless, or a constitutively active form of β-Catenin, ArmS10, also promotes ISC proliferation [25,26]. However, compared to other mitogenic signals for ISCs (such as Jak/Stat and EGFR pathways, see below), Wg signaling’s mitogenic activity in the Drosophila midgut is rather mild.
While two published reports on this topic agree on Wg signaling’s function in promoting ISC proliferation, they differ regarding its role in ISC self-renewal and EB differentiation. Lin et. al. (2008) proposed that Wg signaling positively regulates ISC self-renewal [25], and prolonged activation of Wg signaling resulted in ISC expansion. They also reported that ISCs defective in Wg pathway components, such as frizzled and fz2, disheveled, or armadillo, were poorly maintained in long-term assays. Based on an epistatic analysis, Lin et al. proposed a hierarchical model in which Wg and Notch signaling regulate ISC self-renewal and EB differentiation. However, an independent study by the Micchelli lab disputed this model and reported that loss of Drosophila adenomatous polyposis coli, Apc, had no effect on rates of ISC self-renewal or EB differentiation [26]. Also mysterious is the source of Wnt ligands in the fly midgut. Wg is expressed in epithelial cells of foregut/midgut and midgut/hindgut boundaries [27,28], and also in a small band of visceral muscle cells of the midgut [25,29]. While Wg expression at the midgut boundaries likely regulates the development of foregut and hindgut during metamorphosis [27,28], the function of its expression in the small band of visceral muscle cells is not clear. Moreover, a source of Wg that could regulate all of the widely distributed ISCs in the midgut has not been reported, raising the possibility that some of the effects of experimental Apc or Axin deletion might reflect ligand-independent functions of this pathway. Hence further studies are needed to reveal the identity, source, and function of Wnt ligand in the Drosophila midgut.
Cytokine/Jak/Stat signaling regulates ISC proliferation
The Drosophila Jak/Stat pathway has emerged as a major mitogenic signal for ISCs during Drosophila midgut homeostasis and regeneration [11,30–34]. In response to diverse types of gut epithelial injury, the damaged midgut induces the expression of Drosophila cytokines, Unpaireds (Upd, Upd2 and Upd3). Upd3 is likely directly secreted by ECs in the compromised epithelium [11,34], while the exact sources of Upd and Upd2 are less clear. Upd has been reported to be expressed in midgut epithelial cells, including ISCs [11,33], and has also been reported to be expressed by visceral muscle, where it functions as a niche signal for ISC proliferation and maintenance [31]. The Upd cytokines activate Drosophila Jak/Stat signaling in the midgut progenitors, including ISCs and EBs, and this promotes both ISC proliferation and EB differentiation, facilitating the rapid generation of new midgut epithelial cells (mostly ECs) to replace damaged ones. Ectopic activation of Jak/Stat signaling in ISCs can drastically increase ISC division and differentiation, resulting in massive midgut hyperplasia. Conversely, the depletion of Jak/Stat signaling components such as domeless (Drosophila cytokine receptor), hopscotch (Drosophila Jak) or stat92E (Drosophila Stat) in the ISCs and EBs, using RNAi or mutant alleles, impairs the ability of the midgut to regenerate [11,34].
Jak/Stat signaling is also required for the proper differentiation of ISC progeny in the adult midgut. ISC clones mutant for domeless, hopscotch or Stat92E generate reduced numbers of mature midgut cells, and instead are comprised mostly of small midgut progenitors [11,30,31]. These mutant ISC clones lack Pdm-1 and Pros, markers for mature ECs and EEs, respectively, but express normal or reduced Dl, a marker for ISCs [30]. These results indicate that ISC clones mutant for Jak/Stat signaling fail to undergo binary differentiation, resulting in the accumulation of EB-like progenitor cells. Epigenetic analyses place Jak/Stat signaling downstream of Notch signaling in regulating EB differentiation [30]. Although genetic tests suggest that Jak/Stat signaling is not essential for ISC divisions and midgut maintenance in healthy animals, its essential role in differentiation, and the observation that Stat signaling is always detectable in ISCs and EBs, indicate important functions apart from regeneration after extreme injury.
The cytokine/Jak/Stat pathway’s role in inducing ISC proliferation upon midgut damage is reminiscent of IL-6/Stat3’s function in inducing compensatory proliferation following tissue damage in mammals. For example, in a classic model of compensatory proliferation induced by tissue injury, IL-6 is induced in the damaged liver and functions to promote the proliferation of hepatocytes [35]. A similar role for IL-6/Stat3 has also been reported in mammalian small intestine and colon [36]. Interestingly, Drosophila Upds encode leptin-like cytokines, which belong to IL-6 family, and Domeless encodes a cytokine receptor that closely resembles mammalian IL-6 receptor (IL-6R). Hence we postulate that compensatory stem cell proliferation induced by Cytokine/Jak/Stat signaling may be a conserved mechanism mediating tissue regeneration in insects and vertebrates.
EGFR signaling also regulates ISC proliferation
A second key regulator of Drosophila ISCs is the EGFR/RAS/MAPK signaling pathway. ISCs and EBs specifically express high levels of di-phospho-ERK, the activated MAPK, and mutation or depletion of many components in this highly conserved signaling pathway arrest ISC division and compromises ISC survival [37–40]. As with the Upd cytokines, multiple EGF-like growth factors are also induced in the Drosophila midgut following damage, and like Jak/Stat signaling, these signals are powerful promoters of compensatory ISC divisions [37–40]. One of these EGFR ligands, Vn, is specifically expressed in the visceral muscle, at low levels in healthy animals and at much higher levels following enteric infection or injury [37–40]. Two additional EGFR ligands, spitz and Keren, are expressed in midgut epithelial cells and are likewise induced by infection or midgut damage [37–40]. These EGFR ligands function redundantly to activate the EGFR/RAS/RAF/MAPK pathway in the ISCs to promote their division. Similar to Upds, overexpression of EGFR ligands induces massive ISC division and results in midgut hyperplasia. EGFR signaling is required for compensatory ISC proliferation and midgut regeneration in gut injury models [37,38,40] and is also required for ISC division and midgut homeostasis under normal culture conditions [37–40]. Furthermore, EGFR signaling is required for ectopic ISC proliferation induced by Jak/Stat signaling [37,38,40], and for the expansion of ISC pools induced by Notch inhibition, a result of symmetric ISC division [32]. Finally, EGFR signaling is also required for ISC proliferation induced by JNK signaling (see below) [39]. Unlike Jak/Stat signaling, genetic test suggest that EGFR signaling has little or no role in fate specification or differentiation in the intestinal stem cell lineage, but rather acts as a dedicated cell growth and proliferation factor.
A similar role for EGFR signaling has also been reported for the development, homeostasis and tumorigenesis of the mucosa epithelium in the mouse intestine and colon [41–43]. Recent in vitro ISC culture experiments further underscore the critical requirement for EGF growth factors in promoting ISC proliferation [44,45]. Most importantly, several therapies targeting EGFR have been clinically approved for colon cancer treatment, including two anti-EGFR monoclonal antibodies, Cetuximab and Panitumumab [46,47]. Given the conserved role of EGFR signaling in regulating ISC proliferation in the fly and mammalian gut, further study of its function during fly midgut homeostasis and regeneration may provide important clues to how mammalian EGFR signaling drives CRC development, and to design therapies better targeting this pathway. Of critical importance is the need to devise targeted therapies for patients with activated KRAS, who are resistant to the current EGFR therapies but account for about 40% of all CRC patients.
JNK signaling coordinates stress response and ISC proliferation
Drosophila Jun N-terminal kinase (JNK) signaling pathway is also activated in the midgut by a variety of stress signals such as oxidative damage or pathogenic infection [11,15,34]. In turn, active JNK signaling promotes midgut expression of the Upd cytokines and EGFR ligands, promoting ISC proliferation and regeneration upon damage. Although components of this signaling pathway were found not to be essential for ISC activation and division following EC ablation by apoptosis or P. entomophila infection [11], JNK signaling was reported to be required for ISC mobilization following damage by oxidative stress [15]. Thus the JNK pathway appears to be used selectively in sensing certain types of gut epithelial damage or stress. The JNK feedback inhibitor, puckered (Jun Kinase Phosphatase) is required to restrain unchecked ISC proliferation in healthy animals, suggesting that tight control of this pathway is essential for normal gut homeostasis. In infection models, the pattern of JNK activation in the midgut epithelium depends on the type of pathogen. Infection by pathogens that directly damage mature midgut cells, such as P. entomophila or aeruginosa, activate JNK signaling exclusively in mature midgut cells, mostly ECs [11,16]. However, infection by a gram-negative bacterium, Erwinia carotovora carotovora 15 (Ecc15), which damages the gut by inducing an oxidative burst, activates JNK signaling in both mature ECs and progenitor cells [34] Consistent with this, feeding the oxidative damaging agent, paraquat, to adult flies also activates JNK signaling in both mature midgut cells and progenitors [15]. The activation of JNK signaling in mature cells likely facilitates their elimination after damage, thus inducing compensatory ISC proliferation [11,16]. Indeed, ectopic activation of JNK signaling in ECs leads to a dramatic increase in ISC proliferation and midgut turnover [11]. JNK activation in ISCs likely has three functions: First, it activates stress response genes in ISCs that protect them from oxidative damage. In fact, JNK signaling is required for the survival of ISCs after Ecc15 infection [34]. Second, it leads to increased ISC proliferation, as indicated by the observation that ectopic activation of JNK signaling in ISCs induces their division [15,34,39]. Third, it leads to the mis-differentiation of ISCs by inducing higher Dl expression in the progenitor cells. The utility of this is not clear, but similar ISC mis-differentiation is also observed in the midgut of aging flies, suggesting that JNK signaling may mediate the loss of midgut homeostasis in the aging midgut [15].
The mitogenic activity of JNK signaling in ISCs is somewhat surprising. Drosophila JNK signaling has previously been implicated in cellular stress responses or the morphogenesis of multiple tissues during development [48]. However, JNK signaling has also been reported to induce ectopic proliferation of intestinal crypts and enhance tumor development in a mouse model of colon cancer [49]. In both systems, JNK’s mitogenic activity is mediated through the conserved downstream transcription factors AP-1, comprised of Jun and Fos. In Drosophila the Fos homolog, kayak (kay), is required for JNK-induced ISC proliferation [39]. Kay is also required for EGFR-induced ISC proliferation in ISCs, suggesting that the JNK and EGFR signaling pathways likely converge on AP-1 transcription factors to promote ISC proliferation. Although a variety of insults can activate JNK signaling in the Drosophila midgut, the molecular sensing mechanisms that regulate this activation remain unknown. Further study is required to identify the endogenous signals that activate JNK signaling in the midgut.
Hippo/Salvador/Warts signaling regulates midgut regeneration
The Hippo/Wts/Yorkie signaling pathway was originally discovered in genetic screens for genes regulating organ size in Drosophila [50]. It has emerged as an evolutionary conserved pathway that senses cell adhesion or structural integrity, and regulates cell proliferation and cell survival accordingly [51]. This pathway controls cell number in developing Drosophila appendages, the mouse liver and small intestine, and during contact inhibition of mammalian epithelial cells in culture. The upstream signals that feed into this pathway are diverse, and include the protocadherins Fat and Dachsous in flies and ERM family members such as Merlin/NF2, but secreted signals and receptors for such have not been identified. These cell adhesion/cytostructural regulators control a central kinase complex consisting of the Hippo/MST and Warts/LATS kinases, which phosphorylate and prevent the nuclear translocation of the transcriptional co-activator Yorkie (Yki, YAP in mammals). Inactivation of the Hippo/Warts kinases leads to the nuclear translocation of Yki, where it acts with Sd or TEAD DNA binding proteins to induce multiple downstream targets to promote cell growth, proliferation and to inhibit apoptosis.
Recently, flurries of studies have implicated Hippo signaling in the regulation of intestinal homeostasis and regeneration in Drosophila [40,52–54] and mice [55]. The Drosophila studies show that loss of Hippo signaling in ISCs can promote their proliferation, though this effect is mild. More strikingly, inactivating the Hippo pathway in ECs consistently induces dramatic ISC over-proliferation, leading to hyperplastic intestinal epithelia containing an excess of ISC-like cells. This non-cell-autonomous effect appears to be achieved through the induction of multiple Upd cytokines and EGFR ligands, suggestive of functions in regenerative growth. Indeed, enteric infection was shown to up-regulate Yki levels and target-gene expression [52,53], and loss of Yki in ISCs was found in some cases to block their mitotic response. The requirement for Hpo/Yki signaling in enterocytes, however, was less clear, with some groups reporting a partial requirement for Yki in ECs for stem cell activation following infection or bleomycin induced damage [52,53], and others finding no requirement [40]. Overall, these results present the interesting possibility that Hpo signaling is used in ECs to sense damage or a loss of epithelial integrity, and to activate cytokine and growth factor expression and consequently regenerative growth.
An apparently similar role for the Hippo pathway during tissue regeneration in the mouse intestine has also been reported by Cai et al., who showed that loss of Yap (the mammalian Yorkie homlog) severely impairs intestinal regeneration in a DSS-induced colonic regeneration model, and that higher Yap activity leads to crypt hyperplasia and enhanced polyp development induced by DSS [55].
Cellular redox state regulates ISC proliferation in Drosophila midgut
Stem cells have been proposed to maintain low levels of reactive oxygen species (ROS) in order to protect themselves from oxidative damage [56]. A recent study in the Drosophila midgut revealed that a master regulator of cellular redox state, Nrf2, and its negative regulator Keap1, play important roles in regulating the redox state in ISCs [57]. Nrf2 is active in the quiescent ISCs, and this induces the expression of several key redox regulators to maintain a low redox state. In response to gut damage Nrf2 is inhibited by Keap1, leading to increased ROS levels in ISCs. High ROS levels in turn provide a permissive condition for ISC proliferation. Although it remains unknown how ROS regulate stem cell proliferation, this result from Drosophila adds to accumulating evidence that cellular redox state is an important regulator of stem cell behavior. While transiently high ROS induces stem cell proliferation, persistently high ROS can contribute to ectopic stem cell divisions and misdifferentiation of progenitors, disrupting midgut homeostasis, a phenotype common in aging midguts. Indeed, the expression of Jafrac1, a peroxiredoxin that detoxifies ROS, specifically in the midgut progenitor cells has been shown to delay age-dependent dysplasia in the gut [58]. Remarkably, this treatment also extended the animals’ lifespan 20–25%. Thus, Nrf2, Keap1, and Jafrac1 appear to maintain a delicate balance between the need to protect ISCs from oxidative damage and the need to activate ISCs after midgut damage.
Other pathways that regulate ISC proliferation
Several additional pathways have been reported to regulate ISC proliferation and midgut regeneration in adult Drosophila, including insulin receptor (InR)/Tor signaling and PDGF/VEGF receptor (PVR) signaling [13,59]. Pvf2, one of the PVR ligands and an effector of Ras/Mapk signaling, was found to be upregulated in midgut progenitor cells in aging animals, and to induce ISC proliferation in this situation [59]. The insulin receptor and several downstream effectors have been shown to be required for ISC growth and the activation of their division following gut epithelial damage, and overexpression of the InR or depletion of the tumor suppressor PTEN can promote increased ISC division [13,60]. The InR/PI3K signaling pathway has been characterized in the fly as linkage between nutrient intake and cell growth, with the ligands (Drosophila insulin like peptides; dILPs) being expressed primarily by neurosecretory cells in the brain, following feeding. Other tissues including the CNS glia and larval gut have been reported to express some of the dILPs, however. The source of dILPs that regulate midgut cells, and the downstream effectors that promote ISC proliferation, have not yet been determined. Interestingly, suppressing InR/Pi3K signaling in the intestinal progenitor cells, by expressing inhibitors of PI3K, Akt or activating FOXO, can delay age-dependent dysplasia in the fly intestine and extend the animal’s lifespan [58]. How the InR and PVR signals interface with the other pathways described above remains to be investigated.
Other stem cells in the Drosophila digestive system
The Drosophila adult digestive system is comprised of ectoderm-derived foregut and hindgut, endoderm-derived midgut, and mesoderm-derived malpighian tubules (MT) that act similarly to kidneys. At the junctions of foregut/midgut and hindgut/midgut, the progenitors for adult foregut and hindgut form distinctive rings, called larval imaginal rings. These progenitors proliferate extensively to form adult the foregut and crop (from the foregut imaginal ring) and anterior adult hindgut (from the hindgut imaginal ring) during metamorphosis [61]. It was originally reported that active stem cells exist in the pylorus region of the hindgut and mediate dynamic adult hindgut homeostasis [28]. However, using a more robust cell lineage tracing system, Fox et. al. found that there are no active stem cells in the adult hindgut [62]. Nevertheless, severe damage to the hindgut epithelium can induce the proliferation of putative quiescent stem cells in the anterior pylorus region of the adult hindgut. Recently, active stem cells (called gastric stem cells) at the foregut/midgut junction have been reported to maintain the adult foregut, crop and cardia [27]. Similarly, active renal stem cells in the lower malpighian tubules have been reported to maintain the adult malpighian tubules [63]. With the previous caution about adult hindgut stem cells in mind, independent studies will be necessary to verify these reports. The regulation of these other stem cell types is relatively uncharted and studies focused therein may reveal novel insights into stem cell function.
Acknowledgments
We thank Alexander Kohlmaier for Fig. 1A and Jeffrey Rosa for comments. B.A.E was supported by NIH grant GM51186, the European Research Council, and the DKFZ.
References
- 1.Stainier DY. No organ left behind: tales of gut development and evolution. Science. 2005;307:1902–1904. doi: 10.1126/science.1108709. [DOI] [PubMed] [Google Scholar]
- 2.Hartenstein AY, Rugendorff A, Tepass U, Hartenstein V. The function of the neurogenic genes during epithelial development in the Drosophila embryo. Development. 1992;116:1203–1220. doi: 10.1242/dev.116.4.1203. [DOI] [PubMed] [Google Scholar]
- 3.Jiang H, Edgar BA. EGFR signaling regulates the proliferation of Drosophila adult midgut progenitors. Development. 2009;136:483–493. doi: 10.1242/dev.026955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Takashima S, Adams KL, Ortiz PA, Ying CT, Moridzadeh R, Younossi-Hartenstein A, Hartenstein V. Development of the Drosophila entero-endocrine lineage and its specification by the Notch signaling pathway. Dev Biol. 2011;353:161–172. doi: 10.1016/j.ydbio.2011.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Micchelli CA, Sudmeier L, Perrimon N, Tang S, Beehler-Evans R. Identification of adult midgut precursors in Drosophila. Gene Expr Patterns. 2010;11:12–21. doi: 10.1016/j.gep.2010.08.005. [DOI] [PubMed] [Google Scholar]
- 6.Mathur D, Bost A, Driver I, Ohlstein B. A transient niche regulates the specification of Drosophila intestinal stem cells. Science. 2010;327:210–213. doi: 10.1126/science.1181958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shanbhag S, Tripathi S. Epithelial ultrastructure and cellular mechanisms of acid and base transport in the Drosophila midgut. J Exp Biol. 2009;212:1731–1744. doi: 10.1242/jeb.029306. [DOI] [PubMed] [Google Scholar]
- 8.Nardi JB, Bee CM, Miller LA. Stem cells of the beetle midgut epithelium. J Insect Physiol. 2010;56:296–303. doi: 10.1016/j.jinsphys.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 9.Micchelli CA, Perrimon N. Evidence that stem cells reside in the adult Drosophila midgut epithelium. Nature. 2006;439:475–479. doi: 10.1038/nature04371. [DOI] [PubMed] [Google Scholar]
- 10.Ohlstein B, Spradling A. The adult Drosophila posterior midgut is maintained by pluripotent stem cells. Nature. 2006;439:470–474. doi: 10.1038/nature04333. [DOI] [PubMed] [Google Scholar]
- 11.Jiang H, Patel PH, Kohlmaier A, Grenley MO, McEwen DG, Edgar BA. Cytokine/Jak/Stat signaling mediates regeneration and homeostasis in the Drosophila midgut. Cell. 2009;137:1343–1355. doi: 10.1016/j.cell.2009.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chatterjee M, Ip YT. Pathogenic stimulation of intestinal stem cell response in Drosophila. J Cell Physiol. 2009;220:664–671. doi: 10.1002/jcp.21808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Amcheslavsky A, Jiang J, Ip YT. Tissue damage-induced intestinal stem cell division in Drosophila. Cell Stem Cell. 2009;4:49–61. doi: 10.1016/j.stem.2008.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Buchon N, Broderick NA, Poidevin M, Pradervand S, Lemaitre B. Drosophila intestinal response to bacterial infection: activation of host defense and stem cell proliferation. Cell Host Microbe. 2009;5:200–211. doi: 10.1016/j.chom.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 15.Biteau B, Hochmuth CE, Jasper H. JNK activity in somatic stem cells causes loss of tissue homeostasis in the aging Drosophila gut. Cell Stem Cell. 2008;3:442–455. doi: 10.1016/j.stem.2008.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Apidianakis Y, Pitsouli C, Perrimon N, Rahme L. Synergy between bacterial infection and genetic predisposition in intestinal dysplasia. Proc Natl Acad Sci U S A. 2009;106:20883–20888. doi: 10.1073/pnas.0911797106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cronin SJ, Nehme NT, Limmer S, Liegeois S, Pospisilik JA, Schramek D, Leibbrandt A, de Simoes RM, Gruber S, Puc U, Ebersberger I, Zoranovic T, Neely GG, von Haeseler A, Ferrandon D, Penninger JM. Genome-wide RNAi screen identifies genes involved in intestinal pathogenic bacterial infection. Science. 2009;325:340–343. doi: 10.1126/science.1173164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ohlstein B, Spradling A. Multipotent Drosophila intestinal stem cells specify daughter cell fates by differential notch signaling. Science. 2007;315:988–992. doi: 10.1126/science.1136606. [DOI] [PubMed] [Google Scholar]
- 19.Shimojo H, Ohtsuka T, Kageyama R. Oscillations in notch signaling regulate maintenance of neural progenitors. Neuron. 2008;58:52–64. doi: 10.1016/j.neuron.2008.02.014. [DOI] [PubMed] [Google Scholar]
- 20.Fre S, Huyghe M, Mourikis P, Robine S, Louvard D, Artavanis-Tsakonas S. Notch signals control the fate of immature progenitor cells in the intestine. Nature. 2005;435:964–968. doi: 10.1038/nature03589. [DOI] [PubMed] [Google Scholar]
- 21.van Es JH, van Gijn ME, Riccio O, van den Born M, Vooijs M, Begthel H, Cozijnsen M, Robine S, Winton DJ, Radtke F, Clevers H. Notch/gamma-secretase inhibition turns proliferative cells in intestinal crypts and adenomas into goblet cells. Nature. 2005;435:959–963. doi: 10.1038/nature03659. [DOI] [PubMed] [Google Scholar]
- 22.Bardin AJ, Perdigoto CN, Southall TD, Brand AH, Schweisguth F. Transcriptional control of stem cell maintenance in the Drosophila intestine. Development. 2010;137:705–714. doi: 10.1242/dev.039404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van der Flier LG, van Gijn ME, Hatzis P, Kujala P, Haegebarth A, Stange DE, Begthel H, van den Born M, Guryev V, Oving I, van Es JH, Barker N, Peters PJ, van de Wetering M, Clevers H. Transcription factor achaete scute-like 2 controls intestinal stem cell fate. Cell. 2009;136:903–912. doi: 10.1016/j.cell.2009.01.031. [DOI] [PubMed] [Google Scholar]
- 24.van der Flier LG, Clevers H. Stem cells, self-renewal, and differentiation in the intestinal epithelium. Annu Rev Physiol. 2009;71:241–260. doi: 10.1146/annurev.physiol.010908.163145. [DOI] [PubMed] [Google Scholar]
- 25.Lin G, Xu N, Xi R. Paracrine Wingless signalling controls self-renewal of Drosophila intestinal stem cells. Nature. 2008;455:1119–1123. doi: 10.1038/nature07329. [DOI] [PubMed] [Google Scholar]
- 26.Lee WC, Beebe K, Sudmeier L, Micchelli CA. Adenomatous polyposis coli regulates Drosophila intestinal stem cell proliferation. Development. 2009;136:2255–2264. doi: 10.1242/dev.035196. [DOI] [PubMed] [Google Scholar]
- 27.Singh SR, Zeng X, Zheng Z, Hou SX. The adult Drosophila gastric and stomach organs are maintained by a multipotent stem cell pool at the foregut/midgut junction in the cardia (proventriculus) Cell Cycle. 2011;10:1109–1120. doi: 10.4161/cc.10.7.14830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Takashima S, Mkrtchyan M, Younossi-Hartenstein A, Merriam JR, Hartenstein V. The behaviour of Drosophila adult hindgut stem cells is controlled by Wnt and Hh signalling. Nature. 2008;454:651–655. doi: 10.1038/nature07156. [DOI] [PubMed] [Google Scholar]
- 29.Thuringer F, Bienz M. Indirect autoregulation of a homeotic Drosophila gene mediated by extracellular signaling. Proc Natl Acad Sci U S A. 1993;90:3899–3903. doi: 10.1073/pnas.90.9.3899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Beebe K, Lee WC, Micchelli CA. JAK/STAT signaling coordinates stem cell proliferation and multilineage differentiation in the Drosophila intestinal stem cell lineage. Dev Biol. 2010;338:28–37. doi: 10.1016/j.ydbio.2009.10.045. [DOI] [PubMed] [Google Scholar]
- 31.Lin G, Xu N, Xi R. Paracrine unpaired signaling through the JAK/STAT pathway controls self-renewal and lineage differentiation of Drosophila intestinal stem cells. J Mol Cell Biol. 2009;2:37–49. doi: 10.1093/jmcb/mjp028. [DOI] [PubMed] [Google Scholar]
- 32.Xu N, Wang SQ, Tan D, Gao Y, Lin G, Xi R. EGFR, Wingless and JAK/STAT signaling cooperatively maintain Drosophila intestinal stem cells. Dev Biol. 2011;354 doi: 10.1016/j.ydbio.2011.03.018. [DOI] [PubMed] [Google Scholar]
- 33.Liu W, Singh SR, Hou SX. JAK-STAT is restrained by Notch to control cell proliferation of the Drosophila intestinal stem cells. J Cell Biochem. 2010;109:992–999. doi: 10.1002/jcb.22482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Buchon N, Broderick NA, Chakrabarti S, Lemaitre B. Invasive and indigenous microbiota impact intestinal stem cell activity through multiple pathways in Drosophila. Genes Dev. 2009;23:2333–2344. doi: 10.1101/gad.1827009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Prieto J. Inflammation, HCC and sex: IL-6 in the centre of the triangle. J Hepatol. 2008;48:380–381. doi: 10.1016/j.jhep.2007.11.007. [DOI] [PubMed] [Google Scholar]
- 36.Grivennikov S, Karin E, Terzic J, Mucida D, Yu GY, Vallabhapurapu S, Scheller J, Rose-John S, Cheroutre H, Eckmann L, Karin M. IL-6 and Stat3 are required for survival of intestinal epithelial cells and development of colitis-associated cancer. Cancer Cell. 2009;15:103–113. doi: 10.1016/j.ccr.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Buchon N, Broderick NA, Kuraishi T, Lemaitre B. Drosophila EGFR pathway coordinates stem cell proliferation and gut remodeling following infection. BMC Biol. 2010;8:152. doi: 10.1186/1741-7007-8-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jiang H, Grenley MO, Bravo MJ, Blumhagen RZ, Edgar BA. EGFR/Ras/MAPK signaling mediates adult midgut epithelial homeostasis and regeneration in Drosophila. Cell Stem Cell. 2011;8:84–95. doi: 10.1016/j.stem.2010.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Biteau B, Jasper H. EGF signaling regulates the proliferation of intestinal stem cells in Drosophila. Development. 2011;138:1045–1055. doi: 10.1242/dev.056671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ren F, Wang B, Yue T, Yun EY, Ip YT, Jiang J. Hippo signaling regulates Drosophila intestine stem cell proliferation through multiple pathways. Proc Natl Acad Sci U S A. 2010;107:21064–21069. doi: 10.1073/pnas.1012759107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Roberts RB, Min L, Washington MK, Olsen SJ, Settle SH, Coffey RJ, Threadgill DW. Importance of epidermal growth factor receptor signaling in establishment of adenomas and maintenance of carcinomas during intestinal tumorigenesis. Proc Natl Acad Sci U S A. 2002;99:1521–1526. doi: 10.1073/pnas.032678499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Threadgill DW, Dlugosz AA, Hansen LA, Tennenbaum T, Lichti U, Yee D, LaMantia C, Mourton T, Herrup K, Harris RC, Barnard JA, Yuspa SH, Coffey RJ, Magnuson T. Targeted disruption of mouse EGF receptor: effect of genetic background on mutant phenotype. Science. 1995;269:230–234. doi: 10.1126/science.7618084. [DOI] [PubMed] [Google Scholar]
- 43.Troyer KL, Luetteke NC, Saxon ML, Qiu TH, Xian CJ, Lee DC. Growth retardation, duodenal lesions, and aberrant ileum architecture in triple null mice lacking EGF, amphiregulin, and TGF-alpha. Gastroenterology. 2001;121:68–78. doi: 10.1053/gast.2001.25478. [DOI] [PubMed] [Google Scholar]
- 44.Sato T, van Es JH, Snippert HJ, Stange DE, Vries RG, van den Born M, Barker N, Shroyer NF, van de Wetering M, Clevers H. Paneth cells constitute the niche for Lgr5 stem cells in intestinal crypts. Nature. 2011;469:415–418. doi: 10.1038/nature09637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sato T, Vries RG, Snippert HJ, van de Wetering M, Barker N, Stange DE, van Es JH, Abo A, Kujala P, Peters PJ, Clevers H. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature. 2009;459:262–265. doi: 10.1038/nature07935. [DOI] [PubMed] [Google Scholar]
- 46.Amado RG, Wolf M, Peeters M, Van Cutsem E, Siena S, Freeman DJ, Juan T, Sikorski R, Suggs S, Radinsky R, Patterson SD, Chang DD. Wild-type KRAS is required for panitumumab efficacy in patients with metastatic colorectal cancer. J Clin Oncol. 2008;26:1626–1634. doi: 10.1200/JCO.2007.14.7116. [DOI] [PubMed] [Google Scholar]
- 47.Di Nicolantonio F, Martini M, Molinari F, Sartore-Bianchi A, Arena S, Saletti P, De Dosso S, Mazzucchelli L, Frattini M, Siena S, Bardelli A. Wild-type BRAF is required for response to panitumumab or cetuximab in metastatic colorectal cancer. J Clin Oncol. 2008;26:5705–5712. doi: 10.1200/JCO.2008.18.0786. [DOI] [PubMed] [Google Scholar]
- 48.Goberdhan DC, Wilson C. JNK, cytoskeletal regulator and stress response kinase? A Drosophila perspective. Bioessays. 1998;20:1009–1019. doi: 10.1002/(SICI)1521-1878(199812)20:12<1009::AID-BIES7>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 49.Sancho R, Nateri AS, de Vinuesa AG, Aguilera C, Nye E, Spencer-Dene B, Behrens A. JNK signalling modulates intestinal homeostasis and tumourigenesis in mice. EMBO J. 2009;28:1843–1854. doi: 10.1038/emboj.2009.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pan D. Hippo signaling in organ size control. Genes Dev. 2007;21:886–897. doi: 10.1101/gad.1536007. [DOI] [PubMed] [Google Scholar]
- 51.Pan D. The hippo signaling pathway in development and cancer. Dev Cell. 2010;19:491–505. doi: 10.1016/j.devcel.2010.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Staley BK, Irvine KD. Warts and Yorkie mediate intestinal regeneration by influencing stem cell proliferation. Curr Biol. 2010;20:1580–1587. doi: 10.1016/j.cub.2010.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shaw RL, Kohlmaier A, Polesello C, Veelken C, Edgar BA, Tapon N. The Hippo pathway regulates intestinal stem cell proliferation during Drosophila adult midgut regeneration. Development. 2010;137:4147–4158. doi: 10.1242/dev.052506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Karpowicz P, Perez J, Perrimon N. The Hippo tumor suppressor pathway regulates intestinal stem cell regeneration. Development. 2010;137:4135–4145. doi: 10.1242/dev.060483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cai J, Zhang N, Zheng Y, de Wilde RF, Maitra A, Pan D. The Hippo signaling pathway restricts the oncogenic potential of an intestinal regeneration program. Genes Dev. 2010;24:2383–2388. doi: 10.1101/gad.1978810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kobayashi CI, Suda T. Regulation of reactive oxygen species in stem cells and cancer stem cells. J Cell Physiol. 2011 doi: 10.1002/jcp.22764. [DOI] [PubMed] [Google Scholar]
- 57.Hochmuth CE, Biteau B, Bohmann D, Jasper H. Redox regulation by Keap1 and Nrf2 controls intestinal stem cell proliferation in Drosophila. Cell Stem Cell. 2011;8:188–199. doi: 10.1016/j.stem.2010.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Biteau B, Karpac J, Supoyo S, Degennaro M, Lehmann R, Jasper H. Lifespan extension by preserving proliferative homeostasis in Drosophila. PLoS Genet. 2010;6:e1001159. doi: 10.1371/journal.pgen.1001159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Choi NH, Kim JG, Yang DJ, Kim YS, Yoo MA. Age-related changes in Drosophila midgut are associated with PVF2, a PDGF/VEGF-like growth factor. Aging cell. 2008;7:318–334. doi: 10.1111/j.1474-9726.2008.00380.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Amcheslavsky A, Ito N, Jiang J, Ip YT. Tuberous sclerosis complex and Myc coordinate the growth and division of Drosophila intestinal stem cells. J Cell Biol. 2011;193:695–710. doi: 10.1083/jcb.201103018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bodenstein D. The postembryonic development of Drosophila. In: Demerec M, editor. Biology of Drosophila. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, NY: 1994. pp. 275–367. [Google Scholar]
- 62.Fox DT, Spradling AC. The Drosophila hindgut lacks constitutively active adult stem cells but proliferates in response to tissue damage. Cell Stem Cell. 2009;5:290–297. doi: 10.1016/j.stem.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Singh SR, Liu W, Hou SX. The adult Drosophila malpighian tubules are maintained by multipotent stem cells. Cell Stem Cell. 2007;1:191–203. doi: 10.1016/j.stem.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]