Abstract
Background:
Mutations in Janus kinase 3 (JAK3) are a cause of severe combined immunodeficiency, but hypomorphic JAK3 defects can result in a milder clinical phenotype, with residual development and function of autologous T cells. Maternal T-cell engraftment is a common finding in infants with severe combined immunodeficiency but is not typically observed in patients with residual T-cell development.
Objective:
We sought to study in detail the molecular, cellular, and humoral immune phenotype and function of 3 patients with hypomorphic JAK3 mutations.
Methods:
We analyzed the distribution and function of T and B lymphocytes in 3 patients and studied the in vitro and in vivo responses of maternal T lymphocytes in 1 patient with maternal T-cell engraftment and residual production of autologous T lymphocytes.
Results:
B cells were present in normal numbers but with abnormal distribution of marginal zone–like and memory B cells. B-cell differentiation to plasmablasts in vitro in response to CD40 ligand and IL-21 was abolished. In 2 patients the T-cell repertoire was moderately restricted. Surprisingly, 1 patient showed coexistence of maternal and autologous T lymphocytes. By using an mAb recognizing the maternal noninherited HLA-A2 antigen, we found that autologous cells progressively accumulated in vivo but did not compete with maternal cells in vitro.
Conclusion:
The study of 3 patients with hypomorphic JAK3 mutations suggests that terminal B-cell maturation/ differentiation requires intact JAK3 function, even if partially functioning T lymphocytes are present. Maternal T-cell engraftment can occur in patients with JAK3 mutations despite the presence of autologous T cells.
Keywords: Severe combined immunodeficiency, cytokine signaling, maternal engraftment
Null mutations in genes critical to T-cell development typically result in severe combined immunodeficiency (SCID) disease.1,2 Defects of the IL-2 receptor common γ chain (IL2RG, γc),3,4 Janus kinase 3 (JAK3),5,6 and IL-7 receptor α (IL7R)7 genes affect cytokine signaling, account for a large proportion of SCID,1 and are typically associated with a T−B+phenotype.8 Failure of T-cell development in patients with SCID often allows the engraftment of maternal T lymphocytes in utero.9–11 These maternal T cells are typically oligoclonal, poorly functional in vitro, nonprotective in vivo, and can cause graft-versus-host disease (GVHD) or graft rejection after T cell–depleted, unconditioned, haploidentical hematopoietic stem cell transplantation from a donor other than the mother.9,11–15
In contrast, missense and splice-site mutations in SCID-causing genes can lead to partial preservation of protein expression, function, or both, allowing residual development of autologous T lymphocytes. Omenn syndrome (OS), which is characterized by erythroderma, hepatosplenomegaly, and lymphadenopathy and associated with oligoclonal and activated autologous T cells, is associated with hypomorphic mutations of SCID-causing genes. In other cases, often referred to as “atypical” or “leaky” SCID, autologous T cells of varying number are observed in the absence of clinical features of OS. Most cases of OS and of atypical SCID in human subjects are caused by hypomorphic recombination-activating gene 1 (RAG1) or RAG2 mutations.16,17 However, in a recent series hypomorphic Abbreviations used mutations in genes encoding components of the γc-dependent signaling pathway accounted for 15 of 73 patients with atypical SCID.18 In particular, hypomorphic IL2RG mutations have been demonstrated in patients with clinical features of combined immunodeficiency, low to normal numbers of poorly functional autologous T cells, and survival that can extend into late childhood or even adulthood.19–22 An even broader range of clinical phenotypes has been described in patients with hypomorphic JAK3 mutations,23–28 including presentation in infancy with features of SCID, milder infectious history later in childhood, delayed-onset immunodeficiency, lymphoproliferative disorder, persistent warts, and even asymptomatic presentation in young adulthood. Interestingly, significant clinical and immunologic heterogeneity has been noted in siblings carrying the same JAK3 mutations,25 suggesting that modifying genes or environmental factors can influence the phenotype.
We studied 3 patients with hypomorphic JAK3 mutations and a spectrum of humoral and cellular function. Surprisingly, in 1 patient the presence of maternally engrafted T lymphocytes was associated with autologous T cells that retain some residual function in vitro and in vivo.
METHODS
Subjects
Medical record review and research blood samples were obtained with written consent from parents or guardians and approved by the Boston Children’s Hospital Institutional Review Board.
Cell purification and culture
PBMCs were purified by using Ficoll gradient. B-lymphoblast cell lines (B-LCLs) and T-cell lines were derived by means of immortalization with EBV and herpesvirus saimiri, respectively. Cells were cultured in RPMI medium supplemented with 10% FCS and penicillin-streptomycin. Where indicated, IL-2 (National Institutes of Health Biorepository, Rockville, Md) at 100 U/mL, IL-4 (eBioscience, San Diego, Calif) at 25 ng/mL, CD40 ligand (CD40L) at 5 mg/mL, and/or IL-21–Fc were added to the culture.29 For mixed lymphocyte culture, magnetically purified T cells were cultured 1:1 with irradiated PBMCs from the indicated donors for 7 days, including tritiated thymi-dine during the last 18 hours.
Flow cytometry
Immunophenotyping was performed with the LSR II or FACSCanto flow cytometer (BD Biosciences, San Jose, Calif) by using the FACSDiva (BD Biosciences) and FlowJo software (TreeStar, Ashland, Ore). The following mAbs were used: CD3 (UCHT1 or HIT3a), CD4 (OKT4), CD8 (SK1), CD19 (HIB19), CD24 (ML5), CD38 (HIT2), CD27(0323), IgM (MHM-88), CD45RA (HI100), HLA-A2 (BB7.2), and HLA-DR (L243) from BioLegend (San Diego, Calif) and CD8 (SK1 or RPA-T8), CD38 (HIT2), IgD (IA6–2), HLA-DR (L243), and Ki67 (B56) from BD Biosciences. Percentages of transitional (CD19+CD24hiCD38hi), marginal zone–like (CD19+CD24hiCD38lo), and switched memory (CD19+CD27+IgD−) B cells were compared with published age-specific normal values.30,31
Expression of T-cell receptor (TCR) Vβ families was studied with the IOTest Beta Mark kit (Beckman Coulter, Indianapolis, Ind).
T-cell receptor excision circle analysis
T-cell receptor excision circle (TREC) levels were determined by using real-time quantitative PCR of genomic DNA with RNAse P as the control gene32,33 and newborn screening dried blood spot specimens retrieved with informed consent (patient 1) or in the course of universal pilot screening (patient 2). Determination of TREC levels in patient 3 was performed at age 23 months by using quantitative PCR of genomic DNA from PBMCs with albumin as the internal control of genomic DNA amplification.34
In vitro cell proliferation and plasmablast differentiation
For analysis of T-cell proliferation, PBMCs were incubated with 5 mmol/L carboxyfluorescein diacetate succinimidyl ester (CFSE) and then cultured as indicated with medium only or with soluble anti-CD3 (clone OKT3, eBioscience) plus soluble anti-CD28 (eBioscience) in the presence or absence of 100 U/mL IL-2 (NIH Biorepository) and analyzed after 3 days, gating on CD3, CD4, CD8, HLA-A2, and/or HLA-DR. For analysis of B-cell proliferation, PBMCs were incubated with 5 mmol/L CFSE, cultured as indicated, and analyzed after 5 days, gating on CD19+ cells. In vitro plasmablast differentiation in response to CD40L plus IL-21 was measured, as previously described.35
Sequence and cDNA analysis
RNA was isolated from B-LCLs with the mirVana miRNA isolation kit (Ambion from Applied Biosystems, Foster City, Calif). Reverse transcription was performed with qScript cDNA SuperMix (QuantaBioSciences, Gaithersburg, Md) with 1 μg of RNA. JAK3 cDNA (ENST00000527670) was amplified with the primers JAK3–1353F and JAK3–1838R (exons 9–13) or JAK3–1353F and JAK3–2167R (exons 9–15). Sequences and amplification conditions are available on request. PCR products were analyzed after cycle sequencing (Big-Dye Terminator, Applied Biosystems) on an ABI3130 Genetic analyzer (Applied Biosystems). For real-time PCR analysis, TaqMan primers and probes spanning exon 22–23 were used (Hs00169663_m1; Life Technologies, Grand Island, NY).
Protein analysis
After stimulation with 1200 μg/mL IL-2 for 12 minutes at 378C, cells were lysed in cold buffer (300 mmol/L NaCl, 50 mmol/L Tris-HCl [pH 7.4], 0.5% Triton, 2 mmol/L EDTA [pH 8], and protease inhibitors; Roche, Mannheim, Germany) on ice for 30 minutes. Western blotting of cytoplasmic cell extracts was performed with antibodies to phosphotyrosine signal transducer and activator of transcription (STAT) 5 (pY694) and STAT5 (BD Biosciences), JAK3 (C-21; Santa Cruz Biotechnology, Santa Cruz, Calif), b-actin (A5060; Sigma-Aldrich, St Louis, Mo), horseradish peroxidase–conjugated anti-mouse or anti-rabbit IgG, and the ECL system (Amersham Biosciences, Piscataway, NJ).
RESULTS
Clinical and immunologic findings
Patients 1 and 2 are sister and brother born to nonconsanguineous Northern European parents. Patient 1 had eczema at 1 month of age. Lymphopenia was first demonstrated at age 9 months and persisted (Table I). Skin rash and high IgE levels improved within several months with an elimination diet and topical therapy. She did not make protective titers to tetanus after the primary vaccination series, and despite repeated boosting, she did not maintain protective titers to pneumococcus (Table I and data not shown). She was started on sulfamethoxazole-trimethoprim and intravenous immunoglobulin substitution. With this therapy, she has been thriving without infections up to the current age of 4 years.
TABLE I.
Immunologic phenotype
| Patient 1 (female) | Patient 2 (male) | Patient 3 (male) | Normal values | |||
|---|---|---|---|---|---|---|
| Age (mo) | 9 | 15 | 33 | 1 | 23 | — |
| Lymphocytes | ||||||
| CD3+ cells/μL | 546 | 302 | 541 | 10 | 5,670 | 1,900–6,200 |
| CD4+ cells/μL | 304 | 192 | 191 | 4 | 400 | 1,300–4,300 |
| CD8+ cells/μL | 228 | 101 | 276 | 8 | 3,263 | 500–2,000 |
| CD19+ cells/μL | 886 | 546 | 675 | 1,010 | 2,132 | 300–3,000 |
| CD56+ cells/μL | 141 | 89 | 58 | 55 | 53 | 170–1,100 |
| γ/δ TCR+ (%) | — | — | — | — | 45 | |
| Naive CD4 (%) | — | 3.1 | 0.7 | — | 1 | 63–95 |
| Naive CD8 (%) | — | 1.3 | 0.4 | — | 20 | 71–99 |
| Proliferation (cpm) | ||||||
| PHA | — | 35,545 | 25,376 | 2,313 | 1,871 | >41,000 |
| Tetanus* | — | 2,344 | 6,788 | — | 346 | — |
| Normal for laboratory | — | >4,000 | >4,000 | — | >2,000 | — |
| TRECs | Less than limit of detection† | Less than limit of detection† | Less than limit of detection‡ | †,‡ | ||
| Maternal engraftment (%) | ||||||
| CD3+ | — | 39 | 5 | 84 | 0 | 0 |
| CD4+ | — | 31 | 12 | 79 | — | 0 |
| CD8+ | — | 48 | 1 | 97 | — | 0 |
| Immunoglobulins | ||||||
| IgG (mg/dL) | 974 (300–1,500) | 571 (400–1,300) | 681§ (400–1,300) | 423 (280–750) | 1200§ (400–1,600) | — |
| IgA (mg/dL) | 264 (30–327) | 224 (16–100) | 85 (20–230) | — | 155 (15–200) | — |
| IgM (mg/dL) | 146 (25–115) | 117 (25–115) | 43 (30–120) | — | 83 (40–200) | — |
| IgE (IU/mL) | 10,965 (0–30) | 2,915 (0–30) | 78 (0–30) | — | <2 (0–115) | — |
| Specific antibody | ||||||
| Tetanus (IU/mL) | 0.05 | — | — | — | <0.07 | 0.15–7.0 |
| Pneumococcus | — | 0/7 | —. | —. | 0/14 | ¶ |
| B-cell subpopulation (%) | ||||||
| CD27− (naive) | — | 82.7 | 83.5∥ | 99.1 | 71.2 to 86.6 | |
| CD27+IgD+ | — | 5.9 | 5.5∥ | — | 0.6 | 4.6 to 15 |
| CD27+IgD– (switched) | — | 8.4 | 9.5∥ | — | 0.3 | 3.9 to 8.5 |
The adult control subject assessed on the same day showed 68,151, 60,217, and 10,968 cpm, respectively.
Measured in a newborn dried blood spot (normal value, >252 copies/mL whole blood).
Measured in peripheral blood (normal, >801 copies/million PBMCs).
Value while undergoing IgG replacement therapy.
Measured at 24 months of age.
Value expressed as the number of serotypes with protective titers over the total number of serotypes tested.
Patient 2 was found to have undetectable TRECs and very severe T-cell lymphopenia at birth.32 He was placed in protective isolation at home and treated with sulfamethoxazoletrimethoprim starting at age 6 weeks. At 76 days of age, he underwent 9/10 antigen HLA-A–mismatched unrelated donor bone marrow transplantation (BMT) after busulfan, cyclophosphamide, and antithymocyte globulin conditioning. Cyclosporine, methotrexate, and corticosteroids were administered as GVHD prophylaxis. He is now 19 months after BMTand off immunosuppression and off immunoglobulin substitution, with 100% donor chimerism in CD3, CD15, and CD19 lineages after BMT, and he lives at home free of infections and without signs of GVHD
Patient 3 was born to nonconsanguineous South Asian parents and was an only child. He had multiple infections while living in India, including disseminated mycobacterium not further speciated, Pneumocystis jiroveci, and disseminated varicella zoster infection soon after vaccination with live attenuated varicella zoster vaccine. He was started on immunoglobulin substitution and moved to the United States at approximately 18 months of age. He had and cleared respiratory syncytial virus infection after treatment with ribavirin. At the age of 25 months, he underwent fully matched, unrelated donor BMT after conditioning with busulfan, cyclophosphamide, and antithymocyte globulin. Cyclosporine and methotrexate were administered as GVHD prophylaxis. He is now 12 months after BMTand off immunosuppression and immunoglobulin substitution with mixed donor chimerism; he is free of infections and GVHD.
Mutational and expression analysis of JAK3
Patient 1 had marked T lymphopenia and normal numbers of B cells for age, and therefore the possibility of a defect in JAK3 was considered. Sequencing of the JAK3 gene in patient 1 and her parents revealed compound heterozygous mutations: c.578G>A on the maternal allele predicted to result in p.Cys193Tyr and c.17861+3G>T within the donor splice site of intron 12 of the paternal allele (Fig 1, A and B). The same mutations were confirmed in patient 2. Sequencing of the JAK3 gene in patient 3 revealed compound heterozygosity for the following mutations:c.1767C>T, which was predicted to result in a splicing defect,23and c.678_679delCT, which was predicted to result in a frame-shift with premature termination (p.Cys227fsX49; Fig 1, B).
FIG 1.
Analysis of patients with JAK3 mutations. A, Pedigrees of patient 1 (P1), patient 2 (P2) and patient 3 (P3). B, Schematic representation of the JAK3 gene with the location of primers used to amplify cDNA and of mutations (*patients 1 and 2; #patient 3). C, Expression of JAK3 protein by Western blotting on B-LCLs of a null JAK3 patient, a positive control subject (C), and patient 1 (Pt). D, Phosphorylated STAT5 by Western blotting with or without stimulation with IL-2 of B-LCLs from patient 1 (Pt), a JAK3 null patient (JAK3−/−), and a healthy control subject (C). E, Quantification of JAK3 message by means of real-time PCR in B-LCLs from patient 1 (Pt) and a control subject (C). F, RT-PCR analysis of JAK3 RNA spanning exons 9 to 13 and 9 to 15, showing a lower-sized band in the patient. G, Direct sequencing of the lower-sized band obtained by using RT-PCR in patient 1 revealed the complete skipping of exons 12 and 13 of the JAK3 cDNA.
We further analyzed the expression and function of JAK3 protein in patient 1. Western blotting of B-LCLs from patient 1 revealed reduced JAK3 protein expression with 2 bands, one of normal size and one smaller (Fig 1, C and D). Stimulation of B-LCLs from patient 1 with IL-2 induced detectable but markedly reduced levels of phosphorylated STAT5 (Fig 1, D). Quantification of JAK3 mRNA by means of real-time PCR in B-LCLs from patient 1 revealed approximately 10% of the normal level of message (Fig 1, E). We hypothesized that the lower-molecular-weight JAK3 band was derived from a splicing defect associated with the c.17861+3G>T allele; however, whether the normal-sized band was derived from the missense allele or was also generated by residual correct splicing of the c.17861+3G>T allele was unclear. We analyzed cDNA from patient 1 by using 2 different pairs of primers (Fig 1, B), one spanning exons 9 to 13 (expected product, 503 bp) and one spanning exons 9 to 15 (expected product, 832 bp). Amplification performed with the exon 13 reverse primer resulted in a single normal-sized band. On the contrary, amplification with the exon 15 reverse primer resulted in 2 major bands in patient 1, one the same size as in the healthy control subject and a lower one approximately 200 bp smaller (Fig 1, F). Sequencing of the lower band revealed complete skipping of both exon 12 and exon 13 (Fig 1, G), which are presumed to cause an in-frame deletion of 71 amino acids (Ser568-Leu638). Overall, these data demonstrate that the JAK3 mutations in patient 1 dramatically affect the mRNA levels and allow expression of a low amount of full-length protein and a more abundant smaller-sized variant, reflecting the splicing defect.
Phenotypic and functional abnormalities of JAK3-deficient B cells
Circulating B cells from the 3 patients were present in normal numbers (Table I). At 15 and 25 months of age, respectively, both patient 1 and patient 3 had transitional B-cell percentages similar to published normal values for age (Fig 2, A).30 The proportion of marginal zone–like B cells was in the normal range for age in patient 1 (8.8% vs 4.6% to 15%) but significantly decreased in patient 3 (2.2% vs 5.1% to 12.3%; Fig 2, A). Switched memory B cells were nearly absent in patient 3 but present in the normal range in patient 1 (Table I). These findings imply partial function of JAK3 in patient 1, which is consistent with low levels of residual STAT5 phosphorylation in B-LCLs in response to IL-2 (Fig 1, D).
FIG 2.
Phenotypic and functional studies of peripheral B cells. A, Percentages of transitional (CD24hiCD38hi) and marginal zone–like (CD24hiCD38low) B cells for adult control subjects, patient 1 (P1), and patient 3 (P3). B, Percentage of CD19+ B cells from an adult control subject (mother of patient 1) or patient 1 that diluted CFSE in response to the stimuli indicated. C, Percentage of plasmablasts (CD19+CD27+CD38++) from an adult control subject, patient 1 (P1; left panel), and patient 3 (P3; right panel) expressing high levels of CD27 and CD38 after culture with the indicated stimuli.
Patient 1 had low serum IgG levels and did not mount an antigen-specific antibody response, despite boosting. We therefore examined in vitro plasmablast differentiation and immunoglobulin secretion in response to a γc-dependent stimulus. As shown in Fig 2, B, PBMCs from patient 1 cultured in the presence of CD40L and IL-21 did not proliferate. Furthermore, PBMCs from both patient 1 and patient 3 did not differentiate into plasma-blasts in the presence of CD40L and IL-21 (Fig 2, C). This result might reflect the low number of memory B cells in patient 3; however, a normal number of class-switched memory B cells (defined as CD19+CD27+IgD−) was present in patient 1, yet this patient did not generate plasmablasts in vitro. Therefore these data indicate that integrity of γc-JAK3 signaling is required for terminal differentiation of B lymphocytes in response to T-dependent signals, which is consistent with our recent observations.35
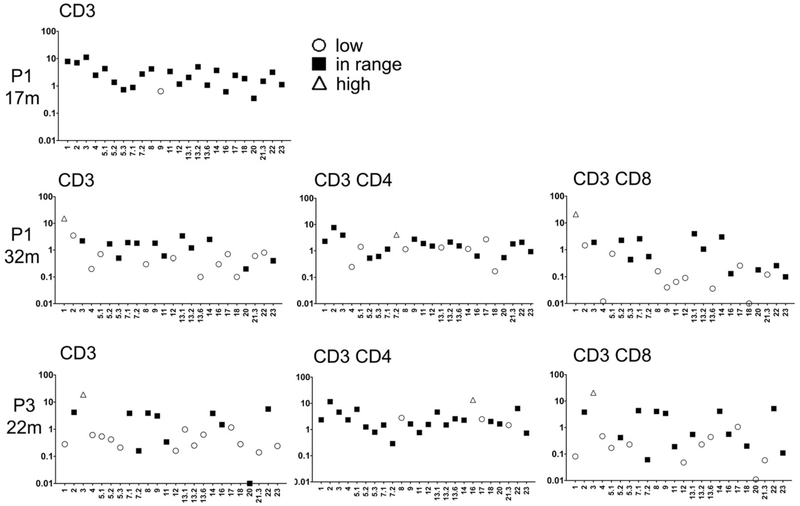
TCR Vβ repertoire and T-cell function
Patient 2 had a classic SCID phenotype and had few T cells to examine. In contrast, patient 1 had proportional lymphopenia in both CD4+ and CD8+ T cells, whereas patient 3 had CD4+ T-cell lymphopenia with expansion of CD8+ T cells (Table I). Analysis of the TCRVβ repertoire showed that in patient 1 the Vβ families were initially represented in normal proportions but that both CD4+ and CD8+ T-cell repertoires became aberrant with time (Fig 3). In patient 3, 3 and 10 Vβ families were underrepresented in CD4+ and CD8+ T cells, respectively (Fig 3). In keeping with the clinical phenotype, patient 1, who has had no infections, has low but not absent proliferation to PHA and tetanus toxoid. In contrast, patient 3, who has had many infections, has greatly reduced proliferation to both PHA and tetanus toxoid. Markedly reduced T-cell responses were observed in patient 2, who presented with a classic SCID phenotype (Table I).
FIG 3.
TCR Vβ repertoire in peripheral T cells from patient 1 (P1) and patient 3 (P3). The percentages of CD3+, CD3+CD4+, or CD3+CD8+T cells expressing the indicated TCR Vβ families are depicted compared with published norms for adults. Vβ families were expressed at less than (open circles), within (solid squares), or greater than (open triangles) the normal range.
Partial maternal engraftment
Maternal engraftment typically occurs in young infants with classic SCID. Short tandem repeat–based analysis of maternal engraftment in patient 3 from whole blood revealed no maternal DNA (Table I). In patient 1, however, short tandem repeat genotyping of magnetically purified CD3+ lymphocytes surprisingly showed 34% maternal chimerism (Table I). Unlike 2 previous reports of long-term maternal engraftment,36,37 HLA typing showed that patient 1 and her mother are haploidentical (Fig 1, A). To confirm this finding, we analyzed HLA-A2 expression in patient 1’s lymphocytes to distinguish autologous (HLA-A2−) cells from maternally engrafted (HLA-A2+) T lymphocytes. As shown in Fig 4, A, partial maternal engraftment of both CD4+ and CD8+ T cells was confirmed, with 30.7% and 48.4% of the cells being of maternal origin, respectively. As expected, CD19+ B cells were entirely of autologous origin. Serial measurement over time showed persistence of maternal engraftment, albeit with a lower percentage, at 33 months (Table I). Her younger brother, patient 2, had 80.4% and 97.5% of CD4+ and CD8+ T cells of maternal origin, respectively (Fig 4, A).
FIG 4.
Phenotypic characteristics of maternally engrafted and host-derived autologous peripheral T cells in patient 1 (P1) and patient 2 (P2). A, The left 2 panels show that CD3+ cells from patients 1 and 2 contain both HLA-A2+ (maternally derived) and HLA-A2− (autologous) populations. The proportion of maternally derived and autologous CD4+, CD8+, and CD19+ cells in patients 1 and 2 is shown in the right 8 panels. B, HLA-A2+ and HLA-A2− cells from patient 1 were electronically gated, and expression of CD45RA is shown (left panel). Control cells from an HLA-A2+ adult is shown for comparison (right panel). C, CD45RA and CD31 expression in CD4 cells from patient 1 and an adult control subject are shown. D, CD3+ T cells from patient 1 were electronically gated according to HLA-A2 expression, and Vβ repertoire is depicted as in Fig 3. E, CD4+ CD45RA− T cells and CD8+ CD45RA− T cells from patient 1 (left panel) were analyzed for expression of HLA-A2, HLA-DR, and CD62L. Control cells from an HLA-A2+ adult are shown in the right panel. F, CD3+ T cells from patient 1 were analyzed for expression of HLA-A2 and Ki67.
Analysis of autologous and maternal T cells
The excellent clinical status in patient 1, with no opportunistic infections, prompted us to analyze the phenotype of autologous and maternal T cells in vivo. We hypothesized that CD4+ T cells with a naive phenotype would be entirely derived from the patient. Indeed, CD4+ CD45RA+ naive T cells were only found within the HLA-A2− subset and were therefore of autologous origin (Fig 4, B). Furthermore, most of the very few CD4+ CD45RA+ T cells expressed CD31, which is typical of recent thymic emigrants, implying a low level of ongoing active thymopoiesis in vivo (Fig 4, C). We hypothesized that autologous T cells, although few in number, would have a polyclonal repertoire, whereas maternally engrafted cells would be less diverse, as previously reported.38 Indeed, autologous HLA-A2− T cells had a diversified TCR Vβ repertoire (Fig 4, D). In contrast, among maternally engrafted T cells, 9 of the 24 TCR Vβ families were underrepresented or overrepresented compared with published normal values (Fig 4, D). T cells that have responded to antigen recently in vivo should show evidence of activation and proliferation. Autologous CD4+ and CD8+ CD45RA− T cells were effector memory cells (CD45RA−, CD62L−), central memory cells (CD45RA−, CD62L−) and activated T cells (HLA-DR+). In contrast, maternally derived CD4+ and CD8+ CD45RA+ T cells were effector memory and central memory phenotypes and were HLA-DR− (Fig 4, E). Only autologous T cells expressed the Ki67 proliferation antigen (Fig 4, F). Thus we concluded that only autologous T cells were proliferating and activated in vivo.
IL-2 receptor signaling through JAK3 is required for proliferation and survival of T cells, and mutations in JAK3 lead to increased apoptosis.24 We reasoned that JAK3 mutant cells might have increased susceptibility to activation-induced cell death compared with maternal cells. Freshly isolated autologous T cells from patient 1 had more expression of Annexin V ex vivo than maternally engrafted T cells (Fig 5, A). Annexin V expression was prominent in both patient-derived cells and maternally engrafted cells after 72 hours of culture with anti-CD3 and anti-CD28 compared with that seen in control cells (Fig 5, B). Consistent with the finding of partial JAK3 function, we also saw partial rescue of apoptosis in both autologous and maternally engrafted T cells when high doses of IL-2 were added (Fig 5, B).
FIG 5.
Analysis of apoptosis and proliferation of maternally engrafted and autologous peripheral T cells in patient 1 (P1). A, Staining with Annexin V reagent in freshly isolated CD4+ and CD8+ T cells from patient 1. B, Staining of CD4+ and CD8+ T cells with Annexin V reagent after 3 days of culture in the indicated stimuli for an adult control subject (red), HLA-A2− autologous cells from patient 1 (blue), and HLA-A2+ maternally engrafted cells from patient 1 (green). C, Cells from an adult control subject, patient 1 cultured in bulk, or patient 1 magnetically separated into HLA-A2–expressing and nonexpressing fractions were cultured for 3 days after CFSE labeling. HLA-A2 expression profile after culture (left) and CFSE and HLA-DR expression (right). D, Proliferation of T cells from patient 1 and her mother to irradiated allogeneic targets from the indicated donors in mixed lymphocyte culture.
Next we compared the proliferative capacity of the maternal and autologous T cells cultured together or separately. When PBMCs from patient 1 were labeled in vitro with CFSE and then stimulated with agonistic anti-CD3 and anti-CD28 mAb for 72 hours, both autologous (HLA-A2−) and maternally engrafted (HLA-A2+) CD4+ and CD8+ T cells diluted CFSE vigorously (Fig 5, C), which is contrary to reports of poor in vitro proliferation of maternally engrafted cells.11 This finding was confirmed when HLA-A2+ and HLA-A2+ T cells from the patient were purified magnetically (>94% purity), loaded with CFSE, and cultured separately (Fig 5, C). These data suggest that both autologous and maternally engrafted T cells retained short-term functional capacity in vitro, which is in contrast to what we observed in vivo, where only freshly isolated HLA-A2−autologous T cells expressed Ki67 (Fig 4, F). In long-term culture, however, JAK3-deficient T cells in patient 1 ultimately did not compete with maternal T cells. All polyclonally generated herpesvirus saimiri T-cell lines were HLA-A2+ and hence exclusively maternally derived, indicating that maternal T cells outcompeted autologous T cells in vitro (data not shown).
Finally, we examined the allogeneic responsiveness of T cells from patient 1 in mixed lymphocyte culture to investigate the hypothesis that partial function of these cells could explain the patient’s excellent clinical status and lack of infections. At the time of testing, at 3 years and 8 months of age, maternal T cells in peripheral blood had waned to almost nil (data not shown). Purified T cells from patient 1 cultured in mixed lymphocyte culture with irradiated PBMCs from 3 different normal donors proliferated, on average, 51% (37% to 53%) compared with T cells from her mother (Fig 5, D). Of note, T cells from patient 1 also exhibited similar proliferation in response to irradiated PBMCs of her mother (Fig 5).
DISCUSSION
JAK3 mutations generally result in complete absence of T-cell development but partial function with a leaky phenotype, and different phenotypes in siblings with the same mutation have been described.25 Here we show that marginal zone–like B-cells, memory B-cells, T-cells, and T-cell in vitro proliferation to mitogens and antigens were variably deficient in 3 patients with JAK3 mutations. STAT5 phosphorylation in response to IL-2 was severely reduced but detectable in B-LCLs from patient 1. Plasma-blast formation in response to CD40L and IL-21 was absent in both patient 1 and patient 3, despite patient 1 having a normal percentage of circulating switched memory B cells. From these data, it is tempting to speculate that the requirement for JAK3 function is least stringent for T-cell generation and in vitro proliferation and most stringent, perhaps absolute, for terminal B-cell differentiation and function. This intrinsic B-cell defect in patients with the γc-JAK3 defect might account for the poor reconstitution of humoral immunity that is typically observed after unconditioned, haploidentical hematopoietic stem cell transplantation, with donor T-cell chimerism and persistence of autologous B cells.39–41 Our recent data in a series of patients undergoing transplantation with γc or JAK3 defects support this hypothesis.35
To our knowledge, this is the first definitive demonstration of the long-term coexistence of autologous T cells with a high level of engraftment of haploidentical maternal T cells. Long-term engraftment of maternal cells has been previously described in 2 patients with SCID, one of whom carried JAK3 mutations; however, in both cases coexistence of autologous and maternal T cells was associated with a high degree of sharing of HLA alleles. In particular, Tezcan et al36 reported an 8-year-old boy with repeated infections, severe failure to thrive, and dermatitis, who nevertheless maintained normal total immunoglobulin levels and residual specific antibody production. The authors speculated that a high degree of matching between patient and mother (7/8 in the GVHD direction and 5/8 in the rejection direction caused by shared alleles and crossover) might have played a role in the persistence and possibly the function of these cells. Al-Muhsen37 reported the case of a 9-year-old boy with chronic pneumonia, bronchiectasis, and failure to thrive who also manifested long-term maternal engraftment of both T cells and a small granulocyte percentage in the setting of full HLA compatibility between mother and patient. Our finding defies the dogma that engraftment of haploidentical maternal T cells can only occur in the complete absence of host T cells.11,15 Although we did not discern any evidence that maternally engrafted T cells were functional in vivo, it is possible that patient 1’s excellent clinical status and freedom from infection to date has been mediated in part by maternal T-cell function. However, we favor the hypothesis that this is due to residual function and a diversified repertoire of autologous, JAK3-deficient T cells in addition to intravenous immunoglobulin substitution and antimicrobial prophylaxis. In fact, we demonstrated approximately half-normal allogeneic responsiveness of T cells from patient 1 against third-party donor and also against maternal PBMCs.
Our data add to the spectrum of clinical and immunologic phenotypes associated with JAK3 mutations in human subjects and demonstrate that long-term maternal engraftment can occur despite the presence of host T cells. Hypomorphic mutations in JAK3 should be considered in all patients with T-cell lymphopenia in association with poor antigen-specific antibody responses.
We thank the patients and their families for participating in the research; Ms Amelia Sabadini, Mr Gregory Hopkins, and Dr Ottavia Delmonte for technical assistance; Dr Raif S. Geha for helpful discussion; and Dr Anne M. Comeau for assistance with TREC assays from newborn dried blood spots.
Acknowledgments
Supported by the Translational Investigator Service at Boston Children’s Hospital (to S.-Y.P.), the Manton Foundation (to L.D.N.), the National Institutes of Health (U54-AI082973 to S.-Y.P. and L.D.N.), and Fondazione Nocivelli (to S.G). M.R. was supported by the Swiss National Science Foundation (SNSF/SSMBS) grant PASMP3–127678.
Abbreviations used
- B-LCL
B-lymphoblast cell line
- BMT
Bone marrow transplantation
- CD40L
CD40 ligand
- CFSE
Carboxyfluorescein diacetate succinimidyl ester
- GVHD
Graft-versus-host disease
- JAK3
Janus kinase 3
- OS
Omenn syndrome
- SCID
Severe combined immunodeficiency
- STAT
Signal transducer and activator of transcription
- TCR
T-cell receptor
- TREC
T-cell receptor excision circle
Footnotes
Clinical implications: Persistence of JAK3-deficient B cells might preclude successful reconstitution of humoral immunity after transplantation. Coexistence of autologous and maternally engrafted T cells should be sought in patients with hypomorphic mutations in JAK3.
Disclosure of potential conflict of interest: M. Recher has been supported by one or more grants from the Swiss National Science Foundation (SNSF/SSMBS; Nrz PASMP3–127678). C. A. Wysocki has been supported by one or more grants from the National Institutes of Health. L. D. Notarangelo has received one or more grants from the National Institutes of Health and from the Manton Foundation, is a Board member for the Immune Disease Institute, is employed by Boston Children’s Hospital, has received one or more grants from or has one or more grants pending with the Wiskott-Aldrich Foundation and with the March of Dimes, and has received royalties from UpToDate. S.-Y. Pai has been supported by one or more grants from the National Institute of Allergy and Infectious Diseases and from Translational Research Program at Boston Children’s Hospital. The rest of the authors declare that they have no relevant conflicts of interest.
REFERENCES
- 1.Buckley RH. Molecular defects in human severe combined immunodeficiency and approaches to immune reconstitution. Annu Rev Immunol 2004;22:625–55. [DOI] [PubMed] [Google Scholar]
- 2.Fischer A, Le Deist F, Hacein-Bey-Abina S, Andr e-Schmutz I, Basile Gde S, De Villartay JP, et al. Severe combined immunodeficiency. A model disease for molecular immunology and therapy. Immunol Rev 2005;203:98–109. [DOI] [PubMed] [Google Scholar]
- 3.Noguchi M, Yi H, Rosenblatt HM, Filipovich AH, Adelstein S, Modi WS, et al. Interleukin-2 receptor gamma chain mutation results in X-linked severe combined immunodeficiency in humans. Cell 1993;73:147–57. [DOI] [PubMed] [Google Scholar]
- 4.Puck JM, Deschenes SM, Porter JC, Dutra AS, Brown CJ, Willard HF, et al. The interleukin-2 receptor gamma chain maps to Xq13.1 and is mutated in X-linked severe combined immunodeficiency, SCIDX1. Hum Mol Genet 1993;2: 1099–104. [DOI] [PubMed] [Google Scholar]
- 5.Macchi P, Villa A, Giliani S, Sacco MG, Frattini A, Porta F, et al. Mutations of Jak-3 gene in patients with autosomal severe combined immune deficiency (SCID). Nature 1995;377:65–8. [DOI] [PubMed] [Google Scholar]
- 6.Russell SM, Tayebi N, Nakajima H, Riedy MC, Roberts JL, Aman MJ, et al. Mutation of Jak3 in a patient with SCID: essential role of Jak3 in lymphoid development. Science 1995;270:797–800. [DOI] [PubMed] [Google Scholar]
- 7.Puel A, Ziegler SF, Buckley RH, Leonard WJ. Defective IL7R expression in T(−) B(1)NK(1) severe combined immunodeficiency. Nat Genet 1998;20:394–7. [DOI] [PubMed] [Google Scholar]
- 8.Casanova J-L, Holland SM, Notarangelo LD. Inborn errors of human JAKs and STATs. Immunity 2012;36:515–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pollack MS, Kirkpatrick D, Kapoor N, Dupont B, O’Reilly RJ. Identification by HLA typing of intrauterine-derived maternal T cells in four patients with severe combined immunodeficiency. N Engl J Med 1982;307:662–6. [DOI] [PubMed] [Google Scholar]
- 10.Stephan JL, Vlekova V, Le Deist F, Blanche S, Donadieu J, De Saint-Basile G, et al. Severe combined immunodeficiency: a retrospective single-center study of clinical presentation and outcome in 117 patients. J Pediatr 1993;123: 564–72. [DOI] [PubMed] [Google Scholar]
- 11.M€uller SM, Ege M, Pottharst A, Schulz AS, Schwarz K, Friedrich W. Transplacen-tally acquired maternal T lymphocytes in severe combined immunodeficiency: a study of 121 patients. Blood 2001;98:1847–51. [DOI] [PubMed] [Google Scholar]
- 12.Thompson LF, O’Connor RD, Bastian JF. Phenotype and function of engrafted maternal T cells in patients with severe combined immunodeficiency. J Immunol 1984;133:2513–7. [PubMed] [Google Scholar]
- 13.Cowan MJ, Wara DW, Weintrub PS, Pabst H, Ammann AJ. Haploidentical bone marrow transplantation for severe combined immunodeficiency disease using soybean agglutinin-negative, T-depleted marrow cells. J Clin Immunol 1985;5: 370–6. [DOI] [PubMed] [Google Scholar]
- 14.Ocejo-Vinyals JG, Lozano MJ, S anchez-Velasco P, Escribano de Diego J, Paz-Miguel JE, Leyva-Cobi an F. An unusual concurrence of graft versus host disease caused by engraftment of maternal lymphocytes with DiGeorge anomaly. Arch Dis Child 2000;83:165–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Palmer K, Green TD, Roberts JL, Sajaroff E, Cooney M, Parrott R, et al. Unusual clinical and immunologic manifestations of transplacentally acquired maternal T cells in severe combined immunodeficiency. J Allergy Clin Immunol 2007;120:423–8. [DOI] [PubMed] [Google Scholar]
- 16.Villa A, Santagata S, Bozzi F, Giliani S, Frattini A, Imberti L, et al. Partial V(D)J recombination activity leads to Omenn syndrome. Cell 1998;93:885–96. [DOI] [PubMed] [Google Scholar]
- 17.Villa A, Sobacchi C, Notarangelo LD, Bozzi F, Abinun M, Abrahamsen TG, et al. V(D)J recombination defects in lymphocytes due to RAG mutations: severe immunodeficiency with a spectrum of clinical presentations. Blood 2001;97:81–8. [DOI] [PubMed] [Google Scholar]
- 18.Felgentreff K, Perez-Becker R, Speckmann C, Schwarz K, Kalwak K, Markelj G, et al. Clinical and immunological manifestations of patients with atypical severe combined immunodeficiency. Clin Immunol 2011;141:73–82. [DOI] [PubMed] [Google Scholar]
- 19.DiSanto JP, Rieux-Laucat F, Dautry-Varsat A, Fischer A. de Saint Basile G. Defective human interleukin 2 receptor gamma chain in an atypical X chromosome-linked severe combined immunodeficiency with peripheral T cells. Proc Natl Acad Sci U S A 1994;91:9466–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mella P, Imberti L, Brugnoni D, Pirovano S, Candotti F, Mazzolari E, et al. Development of autologous T lymphocytes in two males with X-linked severe combined immune deficiency: molecular and cellular characterization. Clin Immunol 2000; 95:39–50. [DOI] [PubMed] [Google Scholar]
- 21.Ursini MV, Gaetaniello L, Ambrosio R, Matrecano E, Apicella AJ, Salerno MC, et al. Atypical X-linked SCID phenotype associated with growth hormone hyporesponsiveness. Clin Exp Immunol 2002;129:502–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wada T, Yasui M, Toma T, Nakayama Y, Nishida M, Shimizu M, et al. Detection of T lymphocytes with a second-site mutation in skin lesions of atypical X-linked severe combined immunodeficiency mimicking Omenn syndrome. Blood 2008;112: 1872–5. [DOI] [PubMed] [Google Scholar]
- 23.Candotti F, Oakes SA, Johnston JA, Giliani S, Schumacher RF, Mella P, et al. Structural and functional basis for JAK3-deficient severe combined immunodeficiency. Blood 1997;90:3996–4003. [PubMed] [Google Scholar]
- 24.Brugnoni D, Notarangelo LD, Sottini A, Air o P, Pennacchio M, Mazzolari E, et al. Development of autologous, oligoclonal, poorly functioning T lymphocytes in a patient with autosomal recessive severe combined immunodeficiency caused by defects of the Jak3 tyrosine kinase. Blood 1998;91:949–55. [PubMed] [Google Scholar]
- 25.Frucht DM, Gadina M, Jagadeesh GJ, Aksentijevich I, Takada K, Bleesing JJ, et al. Unexpected and variable phenotypes in a family with JAK3 deficiency. Genes Immun 2001;2:422–32. [DOI] [PubMed] [Google Scholar]
- 26.Notarangelo LD, Mella P, Jones A, de Saint Basile G, Savoldi G, Cranston T, et al. Mutations in severe combined immune deficiency (SCID) due to JAK3 deficiency. Hum Mutat 2001;18:255–63. [DOI] [PubMed] [Google Scholar]
- 27.Mella P, Schumacher RF, Cranston T, de Saint Basile G, Savoldi G, Notarangelo LD. Eleven novel JAK3 mutations in patients with severe combined immunodeficiency-including the first patients with mutations in the kinase domain. Hum Mutat 2001;18:355–6. [DOI] [PubMed] [Google Scholar]
- 28.Li J, Nara H, Rahman M, Juliana FM, Araki A, Asao H. Impaired IL-7 signaling may explain a case of atypical JAK3-SCID. Cytokine 2010;49:221–8. [DOI] [PubMed] [Google Scholar]
- 29.Warnatz K, Salzer U, Rizzi M, Fischer B, Gutenberger S, B€ohm J, et al. B-cell activating factor receptor deficiency is associated with an adult-onset antibody deficiency syndrome in humans. Proc Natl Acad Sci U S A 2009;106:13945–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van Gent R, van Tilburg CM, Nibbelke EE, Otto SA, Gaiser JF, Janssens-Korpela PL, et al. Refined characterization and reference values of the pediatric T- and B-cell compartments. Clin Immunol 2009;133:95–107. [DOI] [PubMed] [Google Scholar]
- 31.Shearer WT, Rosenblatt HM, Gelman RS, Oyomopito R, Plaeger S, Stiehm ER, et al. Lymphocyte subsets in healthy children from birth through 18 years of age: the Pediatric AIDS Clinical Trials Group P1009 study. J Allergy Clin Immunol 2003;112:973–80. [DOI] [PubMed] [Google Scholar]
- 32.Hale JE, Bonilla FA, Pai S-Y, Gerstel-Thompson JL, Notarangelo LD, Eaton RB, et al. Identification of an infant with severe combined immunodeficiency by newborn screening. J Allergy Clin Immunol 2010;126:1073–4. [DOI] [PubMed] [Google Scholar]
- 33.Gerstel-Thompson JL, Wilkey JF, Baptiste JC, Navas JS, Pai S-Y, Pass KA, et al. High-throughput multiplexed T-cell-receptor excision circle quantitative PCR assay with internal controls for detection of severe combined immunodeficiency in population-based newborn screening. Clin Chem 2010;56:1466–74. [DOI] [PubMed] [Google Scholar]
- 34.Mayo Medical Laboratories. T-cell receptor excision circles (TREC) analysis for-immune reconstitution. Available at:http://www.mayomedicallaboratories.com/test-catalog/Overview/87959. Accessed January 17, 2013.
- 35.Recher M, Berglund LJ, Avery DT, Cowan MJ, Gennery AR, Smart J, et al. IL-21 is the primary common g chain-binding cytokine required for human B-cell differentiation in vivo. Blood 2011;118:6824–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tezcan I, Ersoy F, Sanal O, Turul T, Uckan D, Balci S, et al. Long-term survival in severe combined immune deficiency: the role of persistent maternal engraftment. J Pediatr 2005;146:137–40. [DOI] [PubMed] [Google Scholar]
- 37.Al-Muhsen SZ. Delayed presentation of severe combined immunodeficiency due to prolonged maternal T cell engraftment. Ann Saudi Med 2010;30:239–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Knobloch C, Goldmann SF, Friedrich W. Limited T cell receptor diversity of transplacentally acquired maternal T cells in severe combined immunodeficiency. J Immunol 1991;146:4157–64. [PubMed] [Google Scholar]
- 39.Haddad E, Landais P, Friedrich W, Gerritsen B, Cavazzana-Calvo M, Morgan G, et al. Long-term immune reconstitution and outcome after HLA-nonidentical T-cell-depleted bone marrow transplantation for severe combined immunodeficiency: a European retrospective study of 116 patients. Blood 1998;91:3646–53. [PubMed] [Google Scholar]
- 40.Buckley RH. B-cell function in severe combined immunodeficiency after stem cell or gene therapy: a review. J Allergy Clin Immunol 2010;125:790–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Railey MD, Lokhnygina Y, Buckley RH. Long-term clinical outcome of patients with severe combined immunodeficiency who received related donor bone marrow transplants without pretransplant chemotherapy or post-transplant GVHD prophylaxis. J Pediatr 2009;155:834–40.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]