Abstract
Aim:
Broilers’ optimum performance in response to their genetic potential depends on litter environment which is ideal for bacterial survival and coccidian oocyst sporulation. An in vitro evaluation was conducted for the effectiveness of superphosphate, meta-bisulfide, and charcoal litter amendments in minimizing Escherichia coli O157:H7 and Salmonella Typhimurium survival, Eimeria oocyst count, and sporulation.
Materials and Methods:
Three groups of 16 litter trays were prepared and inoculated with E. coli O157:H7, S. Typhimurium, and Eimeria non-sporulated oocyst. A set of four trays in each group was designed for each one of the chemical amendments. A total of 720 litter samples were collected and examined for bacterial counts, Eimeria oocyst count, and sporulation during the experimental period (35 days).
Results:
Litter moisture and pH revealed a highly significant (p<0.001) reduction in all treated litter trays compared to control. Total bacterial count (TBC), total Enterobacteriaceae count, and S. Typhimurium count showed a highly significant (p<0.001) reduction in meta-bisulfide-treated trays compared to other amendments and positive control. Meanwhile, Eimeria oocyst count and sporulation revealed a highly significant (p<0.001) reduction in superphosphate, meta-bisulfide, and charcoal-treated trays, respectively. Temperature revealed a highly significant (p<0.001) weak positive correlation with pH of all inoculated trays, a highly significant (p<0.001) weak negative correlation with moisture percentage of E. coli O157:H7 and S. Typhimurium inoculated trays, and a highly significant (p<0.001) weak negative correlation with TBC. Meanwhile, relative humidity revealed significant (p≤0.005) weak positive correlation with moisture percentage of E. coli O157:H7 inoculated trays.
Conclusion:
The study concluded that regular usage with periodical reapplication of litter amendments as meta-bisulfide or superphosphate in poultry farms is one of the indispensable managemental and preventive measures for minimizing bacterial survival and inhibiting Eimeria oocyst maturation and sporulation.
Keywords: charcoal, Eimeria, Escherichia coli, litter, meta-bisulfide, Salmonella, superphosphate
Introduction
Litter in poultry farms is a representative ecosystem in which birds remain in contact for almost all of their life. Litter carry important characteristic such as: moisture absorption [1], thermal insulation, protection, and reduction of ammonia [2]. Type of litter material including hay, straw, wood shaving, sawdust, and rice hull can exert an impact on microbial colonization and influence the growth and performance of birds [3].
Litter quality in poultry farms is a major concern, and it may act as a potential vehicle and source for the transmission of pathogenic microorganisms depending on the prevailing temperature, humidity, pH, and moisture content. Litter may allow the survival of many microorganisms incriminated in viral diseases as avian influenza, gumboro, reovirus, laryngotracheitis, and bronchitis; bacterial diseases as colibacillosis and salmonellosis; fungal diseases as aspergillosis and mycoses; and parasitic diseases caused by roundworms, tapeworms, and coccidia that depend on wet litter to provide the proper environment for oocysts to sporulate [4].
Moisture and temperature represent the two most important factors affecting fecal coliform survival. Some stains of coliform as Escherichia coli O157:H7 can survive in broilers’ litter for 42-49 days at 37°C, for 49-56 days at 22°C, and for 63-70 days at 5°C [5]. Litter in broiler farms can be considered as one of the most favorable media for the growth and transmission of Salmonella Typhimurium, increased litter pH, and moisture percentage presented optimal conditions for the increased survival of S. Typhimurium. Coccidiosis is one of the most important parasitic diseases in poultry industry [6], and it caused highly economic losses in poultry production represented in mortalities, morbidities, and the cost of preventative or therapeutic drugs and vaccinations [7]. Coccidian mortalities ranked as the second after viral disease mortalities [8]. Control of coccidiosis is usually depended on the use of anticoccidial drugs in feed and litters to prevent and minimize the developmental stages of the Eimeria [9].
Chemical litter amendments have numerous benefits as lowering litter pH, prevent transformation of nitrogen into ammonia [10], reduce moisture percentage, absorb odors [11], enhance litter chemical composition, and inhibit enzymatic production and microbial growth. Proper broilers’ farm preparation, chemical litter amendment correct application, and litter management are proper influences which have to be fulfilled to obtain maximum effectiveness.
The present study aimed for evaluating and comparing the effectiveness of some chemical litter amendments (superphosphate, meta-bisulfide, and charcoal) on rates of bacterial survival (E. coli O157:H7 and S. Typhimurium) and Eimeria tenella count and sporulation in poultry litter.
Materials and Methods
Ethical approval
The international, national, and institutional applicable guidelines were followed in care and use of birds. The number of birds, as well as the un-necessary repetition, was minimized to avoid excessive stress, discomfort, and suffering. Birds’ requirements were completely satisfied (physiological, psychological, and nutritional).
Experimental design
Three groups of 16 trays (four sets of four replicates per each inoculum), each 1 m3 (3×4×4, 1 m3), were prepared. Each tray was filled with poultry litter (Hay). All trays were sterilized by autoclaving at 121°C/20 min. The sterilization was confirmed by sampling 3 g randomly from the litter depth of each tray and added to 27 mL buffered peptone water, mixed thoroughly, and then filtrated; the filtrate tubes were incubated at 37°C for 24 h. About 10 μL from each tube was dropped onto a standard plate count agar (SPCA) and let to dry and incubated at 37°C for 24 h to demonstrate a state of freedom from microbial contamination. Litter trays were supplemented with fresh poultry dropping (500 g/tray) after being sterilized by autoclaving at 121°C/20 min as a source of organic matter to simulate the biological conditions in poultry farms; the supplementation was repeated twice weekly.
Litter amendments
A set of four litter trays in each group was designed for each one of the chemical amendments. In each group, the first set of four trays was treated with superphosphate, the second set of four trays was treated with meta-bisulfide, the third set of four trays was treated with charcoal, and the fourth set of four litter trays was used as control positive (inoculated untreated trays). Superphosphate, meta-bisulfide, and charcoal were added by 0.5 g, 0.05 g, and 0.05 g/m2, respectively. The amendments were added once at the beginning of the experiment, which was designed to last for 35 days.
Bacterial cultures and coccidium oocyst
E. coli O157:H7 2.5×107 colony-forming units (CFU)/mL culture was purchased from Animal Health Research Institute, Dokki, and propagated using MacConkey broth at 44°C/24 h. 10 μL was transferred aseptically and dropped onto eosin methylene blue (EMB) agar and incubated at 37°C/24 h [12]. Metallic green colonies were counted and picked up. The sixteen trays of the 1st group were inoculated with E. coli O157:H7 1.6×108 CFU/mL and mixed thoroughly to ensure even distribution.
S. Typhimurium lyophilized vial 2.2×104 CFU was purchased from Animal Health Research Institute, Dokki, reconstituted and propagated using tetrathionate broth, and incubated at 37°C/24 h, and the pre-enrichment was repeated daily for 7 successive days. About 10 μL was dropped under complete aseptic conditions onto CHROMagar [12], and the plates were incubated at 37°C/24 h. Pink colonies were counted and pick up. The sixteen trays of the second group were inoculated with S. Typhimurium 5.8×107 CFU/mL and mixed thoroughly.
E. tenella sporulated oocyst 1×104 oocyst per gram (OPG) in potassium dichromate 2.5% suspension was provided kindly by Animal Health Research Institute, Ismailia. E. tenella sporulated oocyst suspension was washed 3 times with centrifugation, resuspended in distilled water, and propagated experimentally in broilers. Ten broilers of two-weeks old were purchased from Ismailia-Egypt Poultry Co, housed in metal galvanized battery, given ad libitum access to water, and supplied with a standard corn-soybean basal diet to meet their dietary requirements according to the National Research Council, [13]. Birds were challenged with E. tenella sporulated oocyst 1×104 OPG by oral gavage and kept under observation for the development of clinical manifestation (bloody diarrhea). The fecal material was collected daily for 7 successive days from the onset of clinical manifestation and examined. Positive fecal material revealed the presence of non-sporulated E. tenella oocysts was kept in sterilized screw capped bottle in the refrigerator at 4°C. Non-sporulated E. tenella oocyst 3×104 OPG was added to the sixteen trays of the third group.
Sampling and measurements
A total of 720 Litter samples were collected during the study period (three samples weekly from each tray for 5 consecutive weeks). Litter samples were collected by receiving a handful amount from the depth of the litter tray in polyethylene bags. Samples were preserved at 4°C for bacteriological and parasitological examination.
Microclimatic measures
Microclimatic temperature and relative humidity were recorded daily during the entire length of the experiment using Clock and Hygro-Thermometer (Boeco Germany, BOE 325).
Litter chemical examination
Litter pH was recorded daily using Jenway 370 pH meter. Meanwhile, moisture content was determined 3 times weekly by weighting litter samples (W1) using JJ223BC 0.001 g 220 g electronic analytical balance and dried in LABTECH digital hot air oven at 100°C for 36-48 h, and the dry weight (W2, two equal successive weights) was subtracted from the initial weight (W1) to detect changes in litter’s moisture.
Bacteriological examination
Litter samples were prepared by adding 3 g sample to 27 mL buffered peptone water, and the mixture was vortexed and filtered. 1 mL filtrate was transferred aseptically to a sterilized tube containing 9 mL of physiological saline. Ten-fold serial dilutions up to 10−6 were prepared. Total bacterial count (TBC), total Enterobacteriaceae count (TEC), and S. Typhimurium count were applied using a drop plate technique [12,14]. SPCA was inoculated with different filtrate concentrations for TBC at 37°C for 24-48 h, EMB agar was used for TEC at 37°C for 24-48 h, and CHROMagr for S. Typhimurium count at 37°C for 24-48 h. Plates showed 30-300 CFU were counted using Dark-field Colony Counter [15].
Parasitological examination
Samples were examined by sugar flotation technique [16,17] for the detection of oocyst. The quantification of oocyst was carried out using McMaster[18], multiplied by the dilution factor (100×), and expressed by OPG.
Statistical analysis
Statistical analysis was carried out using the Statistical Package for the Social Sciences [19] and statistical analysis system [20]. E. coli O157:H7, S. Typhimurium, and Eimeria oocyst counts were expressed as logarithm using Microsoft Excel. The obtained data were analyzed statistically using multifactorial analysis of variance with multivariate general linear model procedures (generalized linear modeling) for treated and inoculated untreated trays (positive control), time, temperature, relative humidity, and their interactions [21].
Results
Meta-bisulfide and superphosphate litter amendments revealed as shown in Table-1 a highly significant (p<0.001) reduction in litter moisture percentage rather than charcoal compared to positive control (untreated) trays. Meta-bisulfide revealed a highly significant (p<0.001) reduction of moisture percentage (Table-1) at the 3rd week in all inoculated litter trays, superphosphate showed a highly significant (p<0.001) reduction of moisture percentage (Table-1) at the 3rd week in E. coli O157:H7 and S. Typhimurium inoculated trays and at the 4th week in E. tenella inoculated trays, and charcoal revealed a highly significant (p<0.001) reduction of moisture percentage (Table-1) at the 4th week in all inoculated litter trays.
Table-1.
Moisture percentage (mean±SE) of treated and untreated litter inoculated with E. coli O157:H7, S. Typhimurium, and E. tenella.
| Litter amendments | Time/week | Litter inoculums | ||
|---|---|---|---|---|
| E. coli inoculum | Salmonella inoculum | E. tenellainoculum | ||
| Superphosphate | 31.80c±0.16 | 30.34d±0.05 | 30.67c±0.18 | |
| Meta-bisulfide | 31.59c±0.33 | 31.14c±0.21 | 30.92c±0.19 | |
| Charcoal | 35.31b±0.18 | 36.05b±0.22 | 34.21b±0.15 | |
| Control positive | 41.68a±0.26 | 41.34a±0.25 | 39.88a±0.21 | |
| p-value | 0.001 | 0.001 | 0.001 | |
| Amendment* time | ||||
| Superphosphate | 1st | 29.83c±1.92 | 30.12b±1.72 | 29.30c±1.49 |
| 2nd | 33.16b±1.71 | 31.95a±0.80 | 32.62a±1.13 | |
| 3rd | 34.29a±1.19 | 31.91a±0.82 | 31.33b±1.04 | |
| 4th | 29.91c±1.52 | 27.37c±1.24 | 29.37c±2.18 | |
| 5th | 29.18c±1.48 | 28.00c±1.15 | 29.19c±2.01 | |
| Metabisulfide | 1st | 31.62b±0.76 | 31.66c±0.76 | 31.12b±0.85 |
| 2nd | 34.95a±3.51 | 33.37a±5.20 | 34.04a±3.58 | |
| 3rd | 31.37b±0.82 | 32.54b±3.98 | 31.33b±0.48 | |
| 4th | 28.41c±0.58 | 27.00d±1.25 | 27.20c±1.58 | |
| 5th | 28.40c±0.49 | 27.14d±1.05 | 27.28c±1.18 | |
| Charcoal | 1st | 37.12a±0.85 | 39.66a±2.07 | 33.16c±1.71 |
| 2nd | 35.37c±0.68 | 34.79c±3.20 | 34.25b±0.94 | |
| 3rd | 36.87b±0.85 | 36.91b±1.61 | 36.91a±0.77 | |
| 4th | 31.87d±0.85 | 32.83d±1.12 | 32.54d±1.17 | |
| 5th | 31.18d±0.81 | 32.71d±1.01 | 32.44d±1.05 | |
| Control positive | 1st | 45.50a±3.03 | 44.41b±1.38 | 38.45b±0.50 |
| 2nd | 44.75b±1.22 | 45.50a±2.32 | 40.66a±0.70 | |
| 3rd | 38.45c±2.97 | 37.50c±0.51 | 40.00a±1.53 | |
| 4th | 38.04c±0.85 | 37.95c±0.85 | 40.41a±1.50 | |
| 5th | 38.16c±0.79 | 37.66c±0.81 | 40.44a±1.54 | |
| p-value | 0.001 | 0.001 | 0.001 | |
Means carrying different superscripts in the same column are significantly different at (p≤0.05) or highly significantly different at (p<0.01). Means carrying the same superscripts in the same column are non-significantly different at (p<0.05). SE: Standard error, E. coli=Escherichia coli, S. Typhimurium=Salmonella Typhimurium, E. tenella=Eimeriatenella
Litter pH as shown in Table-2 revealed a highly significant (p<0.001) overall decrease in meta-bisulfide, superphosphate, and charcoal-treated litter trays, respectively. Superphosphate, meta-bisulfide, and charcoal as shown in Table-2 produced a highly significant (p<0.001) decrease of pH from the 1st week, and as the experiment proceeds, pH showed a constant increase without achieving the neutral pH once again.
Table-2.
pH (mean±SE) of treated and untreated litter inoculated with E. coli O157:H7, S. Typhimurium, and E. tenella.
| Litter amendments | Time/week | Litter inoculums | ||
|---|---|---|---|---|
| E. coli inoculum | Salmonella inoculum | E. tenella inoculum | ||
| Superphosphate | 5.73b±0.44 | 5.67c±0.04 | 5.72b±0.05 | |
| Meta-bisulfide | 5.35c±0.24 | 5.31d±0.03 | 5.33c±0.12 | |
| Charcoal | 5.80b±0.63 | 5.95b±0.04 | 5.73b±0.09 | |
| Control Positive | 7.73a±0.81 | 6.51a±0.05 | 6.32a±0.04 | |
| P-value | 0.001 | 0.001 | 0.001 | |
| Amendment* time | ||||
| Superphosphate | 1st | 4.53d±0.70 | 4.67d±0.46 | 4.67c±0.44 |
| 2nd | 5.56c±0.28 | 5.84c±0.26 | 5.69b±0.43 | |
| 3rd | 6.35b±0.45 | 6.05b±0.56 | 6.30a±0.50 | |
| 4th | 6.49a±0.42 | 6.11a±0.31 | 6.24a±0.21 | |
| 5th | 6.41a±0.41 | 6.10a±0.28 | 6.23a±0.22 | |
| Meta-bisulfide | 1st | 4.65d±0.40 | 4.46d±0.61 | 4.44d±0.44 |
| 2nd | 5.21c±0.24 | 5.31c±0.28 | 5.46c±0.28 | |
| 3rd | 5.64b±0.21 | 5.49b±0.18 | 5.58b±0.45 | |
| 4th | 5.89a±0.29 | 5.99a±0.28 | 5.85a±0.30 | |
| 5th | 5.86a±0.23 | 5.98a±0.23 | 5.82a±0.28 | |
| Charcoal | 1st | 5.51d±0.08 | 5.16c±0.61 | 5.46c±0.94 |
| 2nd | 5.66c±0.20 | 5.94b±0.50 | 5.62b±0.43 | |
| 3rd | 5.78b±0.17 | 6.39a±0.64 | 5.70b±0.22 | |
| 4th | 6.27a±0.44 | 6.31a±0.26 | 6.16a±0.32 | |
| 5th | 6.22a±0.32 | 6.30a±0.18 | 6.12a±0.16 | |
| Control positive | 1st | 6.92d±0.56 | 6.67b±0.45 | 5.73d±0.70 |
| 2nd | 7.77c±0.80 | 6.86a±0.63 | 6.17c±0.77 | |
| 3rd | 8.26a±0.10 | 6.29c±0.17 | 7.07a±0.57 | |
| 4th | 7.95b±0.31 | 6.23c±0.29 | 6.31b±0.14 | |
| 5th | 7.92b±0.32 | 6.22c±0.35 | 6.30b±0.17 | |
| p-value | 0.001 | 0.001 | 0.001 | |
Means carrying different superscripts in the same column are significantly different at (p≤0.05) or highly significantly different at (p<0.01). Means carrying the same superscripts in the same column are non-significantly different at (p<0.05). SE: Standard error, E. coli=Escherichia coli, S. Typhimurium=Salmonella Typhimurium, E. tenella=Eimeriatenella
A highly significant (p<0.01) reduction of total bacterial and S. Typhimurium counts was detected (Table-3) in litter trays treated with meta-bisulfide, superphosphate, and charcoal, respectively, compared to control positive trays. Meanwhile, TEC revealed a highly significant (p<0.01) reduction in meta-bisulfide-treated litter trays, with no significant differences between superphosphate and charcoal-treated litter trays.
Table-3.
Logarithmic bacterial counts (mean±SECFU/mL) in treated and untreated poultry litter inoculated with E. coli O157:H7 and S. Typhimurium.
| Litter Amendments | Time/week | Log TBC CFU/ml | Log TEC CFU/ml | Log Salmonella count CFU/ml |
|---|---|---|---|---|
| Superphosphate | 6.079c±0.052 | 3.500b±0.139 | 2.637c±0.077 | |
| Meta-bisulfide | 4.935d±0.110 | 2.450c±0.088 | 2.179d±0.081 | |
| Charcoal | 6.279b±0.098 | 3.786b±0.112 | 3.916b±0.093 | |
| Control positive | 6.804a±0.116 | 5.290a±0.359 | 5.133a±0.132 | |
| p-value | 0.001 | 0.001 | 0.001 | |
| Amendment* time | ||||
| Superphosphate | 1st | 6.940a±0.104 | 6.440a±0.081 | 4.469a±0.124 |
| 2nd | 6.619b±0.028 | 3.617b±0.102 | 2.791b±0.012 | |
| 3rd | 5.501c±0.013 | 3.064c±0.221 | 2.549c±0.024 | |
| 4th | 5.255d±0.098 | 0.880d±0.312 | 0.738d±0.343 | |
| 5th | 3.306e±0.018 | 0.000e±0.312 | 0.000e±0.343 | |
| Meta-bisulfide | 1st | 5.692a±0.016 | 2.394c±0.102 | 2.097c±0.041 |
| 2nd | 5.517b±0.156 | 3.900a±0.312 | 3.336a±0.414 | |
| 3rd | 4.823c±0.330 | 3.506b±0.432 | 3.284b±0.310 | |
| 4th | 3.706d±0.083 | 0.000d±0.000 | 0.000d±0.000 | |
| 5th | 1.517e±0.051 | 0.000d±0.000 | 0.000d±0.000 | |
| Charcoal | 1st | 5.843d±0.122 | 4.908a±0.251 | 4.860a±0.320 |
| 2nd | 6.133c±0.589 | 4.800b±0.223 | 3.879b±0.031 | |
| 3rd | 6.733a±0.079 | 3.510c±0.129 | 3.710c±0.231 | |
| 4th | 6.408b±0.178 | 1.925d±0.038 | 3.213d±0.031 | |
| 5th | 5.751d±0.118 | 1.780d±0.321 | 2.117e±0.105 | |
| Control positive | 1st | 7.609a±0.284 | 6.586a±0.341 | 6.155a±0.052 |
| 2nd | 6.336d±0.089 | 5.382b±0.112 | 6.186a±0.084 | |
| 3rd | 6.730b±0.158 | 4.657c±0.241 | 4.060b±0.139 | |
| 4th | 6.542c±0.030 | 4.533c±0.131 | 4.128b±0.039 | |
| 5th | 6.735b±0.036 | 6.465c±0.124 | 4.133b±0.124 | |
| p-value | 0.001 | 0.001 | 0.001 |
Means carrying different superscripts in the same column are significantly different at (p≤0.05) or highly significantly different at (p<0.01). Means carrying the same superscripts in the same column are non-significantly different at (p<0.05). SE: Standard error, TBC=Total bacterial count, TEC=Total Enterobacteriaceae count, CFU=Colony-forming unit, E. coli=Escherichia coli, S. Typhimurium=Salmonella Typhimurium
On a weekly basis, superphosphate revealed (Table-3) a uniform pattern of highly significant (p<0.001) reduction in total bacterial, Enterobacteriaceae, and S. Typhimurium counts from the 1st week. Meta-bisulfide as shown in Table-3 revealed a highly significant (p<0.001) reduction in TBC from the 1st week and in total Enterobacteriaceae and S. Typhimurium counts from the 2nd week and achieved zero counts at the 4th week compared to positive control. Charcoal revealed, in Table-3, a uniform pattern of a highly significant (p<0.001) reduction in Enterobacteriaceae and S. Typhimurium counts from the 1st week, while TBC did not respond for the amendment until the 4th week compared to the positive control trays.
E. tenella oocyst sporulation and count as shown in Table-4 and Figure-1 revealed a highly significant (p<0.001) extinction and destruction of Eimeria oocyst from the 1st week in superphosphate-treated trays compared to intact non-sporulated Eimeria oocyst in control positive untreated trays (Figure-2). Meta-bisulfide revealed, in Table-4 and Figure-3, a highly significant (p<0.001) reduction in E. tenella oocyst counts with cessation of sporulation from the 1st week, and zero counts with complete destruction of all Eimeria oocyst were achieved by the 4th week compared to control positive (inoculated untreated litter) in Figure-2. Meanwhile, charcoal revealed a highly significant (p<0.001) reduction in E. tenella oocyst count (Table-4 and Figure-4) from the 1st week and achieved zero counts at the 4th week, but it did not have the ability to cease the oocyst sporulation activity in litter that continued until the end of the 3rd week compared to control positive (inoculated untreated litter) in Figure-2 which revealed the intact non-sporulated E. tenella oocyst.
Table-4.
Eimeria oocyst sporulation and logarithmic count (mean±SE OPG) in treated and untreated poultry litter inoculated with E. tenella.
| Time/weeks | Parameter | Litter amendments | |||
|---|---|---|---|---|---|
| Superphosphate | Meta-bisulfide | Charcoal | Positive control | ||
| 1st | Oocyst | Negative | NS | NS | NS |
| Count/OPG | 0a | 3.669a | 3.845a | 3.903a | |
| 2nd | Oocyst | Negative | NS | S | S |
| Count/OPG | 0a | 3.301b | 3.000b | 3.301b | |
| 3rd | Oocyst | Negative | NS | S | S |
| Count/OPG | 0a | 3.000c | 3.000b | 3.000c | |
| 4th | Oocyst | Negative | Negative | Negative | Negative |
| Count/OPG | 0a | 0d | 0c | 0d | |
| 5th | Oocyst | Negative | Negative | Negative | Negative |
| Count/OPG | 0a | 0d | 0c | 0d | |
Means carrying different superscripts in the same column are significantly different at (P≤0.05) or highly significantly different at (p<0.01). Means carrying the same superscripts in the same column are non-significantly different at (p<0.05). NS=Non-sporulated oocyst, S=Sporulated oocyst, OPG=Oocyst per gram, SE: Standard error, E. tenella=Eimeriatenella
Figure-1.
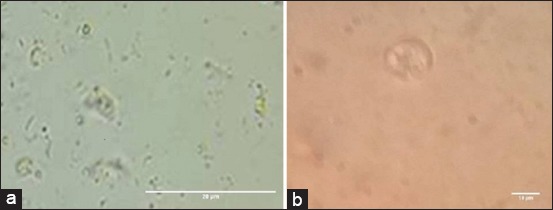
Light microscopic picture of Eimeria tenella oocyst in superphosphate treated litter. (a) Remnant parts of E. tenella oocyst. (b) Broken E. tenella oocyst.
Figure-2.
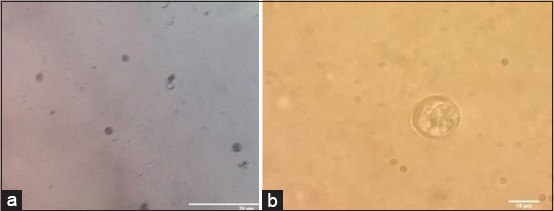
Light microscopic picture of Eimeria tenella oocyst in positive control (inoculated untreated litter). (a) Non-sporulated E. tenella oocyst. (b) Sporulated E. tenella oocyst.
Figure-3.
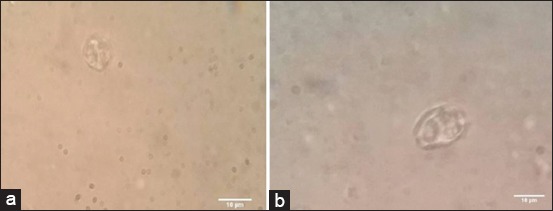
Light microscopic picture of Eimeria tenella oocyst in meta-bisulfide-treated litter. (a) Disrupted shaped E. tenella oocyst. (b) Broken E. tenella oocyst.
Figure-4.
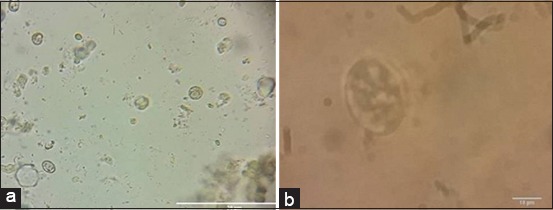
Light microscopic picture of Eimeria tenella oocyst in charcoal-treated litter. (a) Intact non-sporulated E. tenella oocyst. (b) Sporulated E. tenella oocyst.
Temperature revealed highly significant weak positive correlations (r=0.186, 0.176, 0.281, p<0.001) with pH of E. coli O157:H7, S. Typhimurium, and E. tenella inoculated litter trays, respectively, as shown in Table-5. Although temperature revealed highly significant weak negative correlations (r=−0.207, −0.210, p<0.001) with moisture percentage of E. coli O157:H7 and S. Typhimurium inoculated litter trays (Table-5) and a highly significant weak negative correlation (r=−0.312, p<0.001) with TBC (Table-6). Relative humidity in Table-5 revealed a significant weak positive correlation (r=0.103, p<0.001) with moisture percentage of E. coli O157:H7 inoculated litter trays.
Table-5.
Correlation coefficient between chemical characteristics of litter inoculated with E. coli O157:H7, S. Typhimurium, and E. tenella with ambient temperature (above diagonal) and relative humidity (below diagonal).
| r | Temperature | E. coli inoculum | Salmonella inoculum | Eimeria inoculum | |||
|---|---|---|---|---|---|---|---|
| pH | Moisture | pH | Moisture | pH | Moisture | ||
| RH | 1 | 0.186** | −0.207** | 0.176** | −0.210** | 0.261** | 0.063 |
| E. coli inoculum | |||||||
| pH | −0.059 | 1 | 0.564** | 0.683** | 0.469** | 0.680** | 0.659** |
| Moisture | 0.103* | 0.564** | 1 | 0.474** | 0.910** | 0.217** | 0.821** |
| Salmonella inoculum | |||||||
| pH | −0.080 | 0.683** | 0.474** | 1 | 0.384** | 0.597** | 0.475** |
| Moisture | 0.076 | 0.469** | 0.910** | 0.384** | 1 | 0.129* | 0.807** |
| Eimeria inoculum | |||||||
| pH | 0.034 | 0.680** | 0.217** | 0.597** | 0.129** | 1 | 0.332** |
| Moisture | 0.042 | 0.659** | 0.821** | 0.475** | 0.807** | 0.332** | 1 |
r = Pearson’s correlation,
Correlation is significant at the 0.01 level.
Correlation is significant at the 0.05 level.
NSCorrelation is non−significant at the 0.05 level. E. coli=Escherichia coli, E. tenella=Eimeriatenella, S. Typhimurium=Salmonella Typhimurium, SE: Standard error
Table-6.
Correlation coefficient between bacterial and coccidian oocyst load in litter inoculated with E. coli O157:H7, S. Typhimurium, and E. tenella with ambient temperature (above diagonal) and relative humidity (below diagonal).
| r | Temperature | TBC | TEC | Salmonella count | Eimeria oocyst |
|---|---|---|---|---|---|
| RH | 1 | −0.312** | −0.043 | −0.078 | 0.095 |
| TBC | 0.122 | 1 | 0.699** | 0.704** | 0.235** |
| TEC | 0.117 | 0.699** | 1 | 0.903** | 0.367** |
| Salmonella count | 0.090 | 0.704* | 0.903** | 1 | 0.451** |
| Eimeria oocyst | 0.117 | 0.235** | 0.367** | 0.451** | 1 |
r=Pearson’s correlation,
Correlation is significant at the 0.01 level.
Correlation is significant at the 0.05 level.
NSCorrelation is non-significant at the 0.05 level. TBC=Total bacterial count, TEC=Total Enterobacteriaceae count, S. Typhimurium=Salmonella Typhimurium
Discussion
Deep litter system is one of the most common housing systems in broilers’ production worldwide. Litter is a mixture of pathogen-free bedding material (hay, straw, wood shaving, sawdust, and rice hull), bird’s excreta that contained high levels of nitrogen from dietary protein, feathers, and wasted feed. Thus, by the end of each broilers’ cycle, litter becomes seeded with high microbial load up to 1010 CFU/g [22]. A variety of pathogenic microorganisms that affect bird’s growth and performance can be isolated from broilers’ litter as Actinobacillus spp., Campylobacter spp., Clostridium spp., Corynebacterium spp., Escherichia coli, Listeria spp., Mycobacterium spp., Salmonella spp., Staphylococcus spp., and Streptococcus spp [23]. In an epidemiological survey in broilers’ farms, Soliman et al. [24] isolated and identified a wide variety of bacterial microorganisms from broilers’ litter including E. coli, Salmonella spp., Klebsiella oxytoca, Pseudomonas aeruginosa, Shigella spp., Citrobacter spp., Proteus vulgaris, Streptococcus faecalis, Streptococcus pneumoniae, and Staphylococcus aureus. Microbial load (aerobic, anaerobic, and coliform) usually increased in the superficial layers of poultry litter by the direct deposition of fecal material that contains a high level of bacterial and protozoal organisms and decreased as litter depth increased [25]. Chen and Jiang [26] stated that microbial concentration in litter can achieve 1010 CFU/g, from which Gram-positive bacteria represent about 90%. Microorganisms as Clostridium, Escherichia coli, Salmonella, Streptococcus, Staphylococcus, and Listeria can exert different metabolic activities in litter causing interference with growth and production of poultry.
Litter conditions are the principal key to overall management of poultry farm. Broilers’ litter is naturally a hostile environment for survival and persistence of many microorganisms as it becomes dry and heats up rapidly in normal and securely managed broilers’ houses. Litter pH, moisture percentage, temperature, and relative humidity levels are responsible for microbial growth in poultry litter [27]. Daí Pra et al. [28] found that an increase in the litter’s temperature might contribute a significant reduction in microbial load. Heat treatment of litter studied by Stringfellow et al. [29] caused an increase in litter pH that contributed a significant reduction in microbial colonization of S. Typhimurium. Wilkinson et al. [30] found a great reduction in E. coli and S. Typhimurium counts by 99% in 1 h at 55 and 65°C, and they were able to survive for longer periods at lower temperature (35°C) accompanied with high moisture percentage (65%) in a laboratory trial. Meanwhile, in field trials, they examined four broilers’ litter samples and found that initial fecal coliform counts were 5.07, 5.21, 4.64, and 5.91 log10 CFU/g, these counts declined by 96% after 2-16 weeks to 3.65, 3.78, 3.20, and 4.47 log10 CFU/g, respectively.
Chemical and biological litter amendments enforced itself into poultry industry in many shapes as acidifying agents that reduce litter pH, clay-based products that absorb odors, especially ammonia, and microbial inhibitors that inhibit enzyme synthesis, microbial growth, and multiplication. Variables such as litter stocking, litter moisture, litter pH, breed, temperature, relative humidity, and concurrent disease threats can influence the choice of litter amendment type. The choice of chemical litter amendments was more successful compared to other forms of amendments, as it reduces litter pH and moisture percentage creating unfavorable conditions for bacterial and viral survival as well as coccidian sporulation and maturation [31]. Chemical amendments cannot provide solutions for inadequate ventilation, small air inlet, and high bird stocking density. That is why broilers’ houses should be provided with strict management system before application of these amendments.
Our results indicated that meta-bisulfide and superphosphate were relatively acidic amendments and, when applied to litter trays, contributed a sharp drop in litter pH up to 5.6:5.7 in superphosphate-treated trays and 5.3 in meta-bisulfide-treated trays. Meanwhile, Charcoal was able to decrease litter pH up to 5.7:5.9 compared to superphosphate and meta-bisulfide. Chemical amendments were able to maintain such acidic pH for the entire duration of the experiment with a little fluctuation as a trial to achieve a neutral pH starting from the 3rd week up to the end of the experiment. These results agreed with those reported by McWard and Taylor [32], who revealed that poultry guard acidifying clay, alum, and sodium bisulfate (SBTL) significantly reduced litter pH and ammonia volatilization from the litter. The results agreed with those of Medeiros et al. [33] studied the influence of superphosphate in litter amendment using 0, 5, 10, 15, 20, and 25% in litters from four cycles and found that pH sharply reduced from 8.4 to 5.8.
Chemical amendment application in litter trays caused a dramatic decline in moisture percentage, which was more prominent in superphosphate and meta-bisulfide rather than charcoal-treated trays. Our results agreed with those of Sahoo et al. [34], who examined the efficiency of alum sulfate (ATL at dose rate of 90 g.f-2) and sodium bisulfate (SBTL at dose of 25 g.f-2) litter amendments on litter quality, broilers’ performance, carcass characteristics, and broilers’ welfare during winter season; they found that litter treated with SBTL had more moisture and lower pH than that treated with ATL compared to control; the acidification of litter contributed to a great improvement in litter quality and enhanced productivity and broilers’ welfare. Furthermore, the results agreed with those of Schneider et al. [35], who found that the addition of zeolite 10% to sawdust litter was able to reduce litter moisture contents up to 32% so that it can be used for three consecutive broilers’ flocks safely. The current results also agreed with those of Soliman and Hassan [36], who reported a highly significant (p<0.001) reduction of aerial ammonia concentration in both laboratory and field trials caused by a significant reduction in litter moisture percentage and pH in superphosphate and sodium meta-bisulfide-treated litter, respectively.
E. coli have a great ability to survive in different environments including air, water, manure, and soil with great possibilities to migrate between these habitats [37]. The survival capabilities of E. coli in litter depend on numerous factors including energy and nutrients availability, pH, moisture, and temperature. Van Elsas et al. [38] stated that E. coli O157:H7 have great survival capabilities for long periods (130 days) in manure soil at dry conditions (1% moisture) and 18°C. S. Typhimurium can survive for significantly more extended periods compared to E. coli, and this might be attributed to the genome structure represented in 1-4% increase in guanine plus cytosine content in S. Typhimurium [38].
Total bacterial, Enterobacteriaceae and S. Typhimurium counts in our study were significantly reduced due to the drastic decline in litter pH and moisture content ensured by the highly significant (p<0.001) correlations between pH and moisture percentage in all inoculated litter trays, making the litter an unfavorable media for microbial survival and growth. The results agreed with those of Soliman et al. [39], who found a significant reduction in survival of S. Typhimurium caused by a collaboration of some condition including a decline of litter pH, an increase of ambient temperature, and a decrease of relative humidity irrespective to the presence or absence of organic matter source. Bennett et al. [40] findings were agreed to our results, as they found that 5%, 10%, and 20% hydrated lime significantly reduced the survival of Salmonella enteritidis in in vitro experiment after 24 h from the application in poultry litter. On the other hand, Bennett et al. [41] did not agree with our results as they revealed that 5% hydrated lime was not able to suppress the survival of Salmonella and Campylobacter, but it did cause a low significant reduction in the total bacterial aerobic count.
Line and Bailey [42] also agreed with our results and found that aluminum sulfate and SBTL caused a little degree of delay in Campylobacter colonization in broiler chicks, while Salmonella levels remained unaffected. Vicente et al. [43] also agreed with our results and revealed that using low dose (360 g.m−2) as well as high dose (720 g.m-2) of acidified clay minimized Salmonella enteritidis recovery in poultry litter to 0 and 3%, respectively, after 11 days and 23 and 18%, respectively, after 21 days. Lopes et al. [44] agreed with our results and found that adding 300 g quicklime per square meter litter caused a significant reduction in CFU counted on brain heart infusion, Chapman, and MacConkey agar by 57.2, 66.9, and 92.1%, respectively, and they contributed this reduction to the decline in litter pH. Furthermore, Soliman and Hassan [36] results agreed with our results as they recorded a highly significant (p<0.001) reduction in total bacterial and TECs with an increase in body weight, performance index, and bursa’s weight in birds raised on superphosphate-treated litter compared to a highly significant (p<0.001) increase in weight gain, spleen, and thymus weight in birds raised on meta-bisulfide-treated litter.
Coccidiosis is one of the most critical threats that face the global poultry industry. Chickens are susceptible to nine Eimeria species that belong to phylum Apicomplexa [45]. Understanding coccidiosis epidemiology and developing control and preventive measures rely on gaining knowledge about the distribution and structure of E. tenella [46].
Our results indicated that the used litter amendments produced high ability to destroy E. tenella oocyst directly from the 1st week in superphosphate-treated litter, to reduce E. tenella oocyst count with cessation of sporulation and achieving zero count by the 4th week in meta-bisulfide-treated litter, or to reduce E. tenella oocyst count without cessation of sporulation and achieving zero count by the 4th week in charcoal-treated litter. The used chemical amendments (superphosphate, meta-bisulfide, and charcoal) were able to modify litter abiotic conditions (pH and moisture content) creating harsh conditions for Eimeria oocyst to complete its development and sporulation in poultry litter. Results agreed with those of Fetterer et al. [47] who revealed that using 300 µg/ml aqueous concentrations of metam sodium (sodium N-methyldithiocarbamate) for 24 h was able to prevent the sporulation and significantly reduce the viability of E. tenella, Eimeria acervulina, and Eimeria maxima oocyst in poultry litter. Sahoo et al. [48] found that treating litter with SBTL was able to reduce Eimeria oocyst count to a little extent compared to ATL and control, although, of the numerical values, differences between the two amendments were statistically non-significant.
The results also agreed with Samaha et al. [49], who revealed a higher in vitro efficiency for ammonium hydroxide 5 and 10% and phenol 10% (99% reduction in count) compared to Eco-Bio (quaternary ammonium and glutaraldehyde combination) and ZixVirox (peracetic acid and hydrogen peroxide combination) against E. tenella oocyst under the influence of abiotic conditions as temperature, pH, and presence of organic matter. Meanwhile, Mesa et al. [50] observed no reduction in E. maxima count in poultry litter when covered with plastic canvas for 8 days.
Conclusion
Litter amendments as meta-bisulfide, superphosphate, and to little extent charcoal were able to mutate and modify neutral pH and high moisture percentage of litter, which were considered optimum conditions for bacterial survival, protozoal maturation, and sporulation. Application of litter amendments as meta-bisulfide or superphosphate in poultry farms with regular reapplication is indispensable managemental and preventive measure for maintaining the mutated abiotic conditions which reduce bacterial survival and inhibit protozoal maturation, thus maintaining a healthy flock.
Authors’ Contributions
ESS designed the experimental design and participated in the preparation, execution of the experiment, and writing the manuscript. NHS assisted in laboratory work and participated in the parasitological examination and in writing the manuscript. EMA participated in laboratory work, in the parasitological examination, and in writing the manuscript. All authors read and approved the final manuscript.
Acknowledgment
The authors’ sincere gratitude should be presented to Prof. Dr. M.A.A. Sobeih for his guidance and help in the execution of the work. Furthermore, we are grateful for Dr. Wafaa A. Abdelfatah, Animal Health Research Institute, Ismailia, for her understanding and supplying Eimeria oocyst freely. The current study received no specific grant from any funding agency and was funded by the authors.
Competing Interests
The authors declare that they have no competing interests.
References
- 1.Gençoğlan S, Gençoğlan C. The effect of the litter materials on broiler chickens welfare and performance. Turk. J. Agric. Food Sci. Technol. 2017;5(12):1660–1667. [Google Scholar]
- 2.Bjedov S, Žikić D, Perić L, ĐukićStojčić M, Milošević N. Effect of different litter treatments on production performance of broiler chickens. Biotechnol. Anim. Husbandry. 2013;29:625–630. [Google Scholar]
- 3.Dunlop M.W, Blackall P.J, Stuetz R.M. Odour emissions from poultry litter –A review litter properties, odour formation and odour emissions from porous materials. J. Environ. Manage. 2016;177:306–319. doi: 10.1016/j.jenvman.2016.04.009. [DOI] [PubMed] [Google Scholar]
- 4.Ritz C.W, Fairchild B.D, Lacy M.P. Litter Quality and Broiler Performance. Bulletin, Athens: Extension Poultry Scientists. UGA extension; 2014. p. 1267. [Google Scholar]
- 5.Blaustein R.A, Pachepsky Y.A, Shelton D.R, Hill R.L. Release and removal of microorganisms from land-deposited animal waste and animal manures: A review of data and models. J. Environm. Q. 2015;44(5):1338–1354. doi: 10.2134/jeq2015.02.0077. [DOI] [PubMed] [Google Scholar]
- 6.Lawal J.R, Gulani I.A, Ali A.M, Bello A.M, Abadam F.A, Mustapha M, Dauda J, Adamu L, Biu A.A. Dry season prevalence of avian Coccidia infection in domesticated chicken (Gallus domesticus) in Jere council, Borno state, Nigeria. J. Anim. Sci. Vet. Med. 2016;1:67–73. [Google Scholar]
- 7.Gadelhaq S.M, Arafa W.M, Aboelhadid S.M. Molecular characterization of Eimeria species naturally infecting Egyptian Baladi chickens. Iran J. Parasitol. 2015;10(1):87–95. [PMC free article] [PubMed] [Google Scholar]
- 8.Lawal J.R, Jajere S.M, Ibrahim U.I, Geidam Y.A, Gulani I.A, Musa G, Ibekwe B.U. Prevalence of coccidiosis among village and exotic breed of chickens in Maiduguri, Nigeria. Vet. World. 2016;9(6):653–659. doi: 10.14202/vetworld.2016.653-659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.El-Sayed N.M, Oda S.S, Tohamy H.G, El-Manakhly M.E.S. Pathologic study on the enterohepatic affections in chickens at Alexandria Province, Egypt. Adv. Anim. Vet. Sci. 2017;5(1):30–38. [Google Scholar]
- 10.Atapattu N.S.B.M, Lakmal L.G.E, Perera P.W.A. Effects of two litter amendments on air NH3levels in broiler closed-houses. Asian Aust. J. Anim. Sci. 2017;30(10):1500–1506. doi: 10.5713/ajas.16.0873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee K.W, Kim D.K, Lillehoj H.S, Jang S.I, Lee S.H. Immune modulation by Bacillus subtilis -based direct-fed microbial in commercial broiler chickens. Anim. Feed Sci. Technol. 2015;200:76–85. [Google Scholar]
- 12.Herigstad B, Hamilton M, Heersink J. How to optimize the drop plate method for enumerating bacteria. J. Microbiol. Meth. 2001;44(2):121–129. doi: 10.1016/s0167-7012(00)00241-4. [DOI] [PubMed] [Google Scholar]
- 13.National Research Council (NRC) Nutrient Requirements for Poultry. 9th Revised Edition, 1994. New York: National Research Council; 1994. [Google Scholar]
- 14.Kim S.K, Lee J.H. Biofilm modeling systems. Korean J. Microbiol. 2016;52(2):125–139. [Google Scholar]
- 15.Murray P.R, Rosenthal K.S, Pfaller M.A. Medical Microbiology. 8th ed. Philadelphia, PA, USA: Elsevier Health Sciences; 2015. [Google Scholar]
- 16.Sangster L, Blake D.P, Robinson G, Hopkins T.C, Sa R.C.C, Cunningham A.A, Chalmers R.M, Lawson B. Detection and molecular characterization of Cryptosporidium parvum in British European hedgehogs (Erinaceus europaeus) Vet. Parasitol. 2016;217:39–44. doi: 10.1016/j.vetpar.2015.12.006. [DOI] [PubMed] [Google Scholar]
- 17.Ghoneim N.H, Hassanain M.A, Hamza D.A, Shaapan R.M, Draz S.H. Prevalence and molecular epidemiology of Cryptosporidium infection in calves and hospitalized children in Egypt. Res. J. Parasitol. 2017;12:19–26. [Google Scholar]
- 18.Zajac A.Z, Conboy G.A. Veterinary Clinical Parasitology. 8th ed. Hoboken: Wiley Blackwell; 2012. pp. 8–11. [Google Scholar]
- 19.SPSS, Inc. SPSS for Windows. Author, Chicago: Computer Software; 2001. [Google Scholar]
- 20.SAS Institute, Inc. SAS Course Notes on Mixed Model Analysis Using SAS System. Cary, NC: SAS Institute, Inc; 2002. [Google Scholar]
- 21.Levesque R. SPSS Programming and Data Management: A Guide for SPSS and SAS®Users. 4th ed. Chicago IL: SPSS Inc; 2007. [Google Scholar]
- 22.Kim J, Diao J, Shepherd M.W, Jr, Singh R, Heringa S.D, Gong C, Jiang X. Validating thermal inactivation of Salmonella spp. in fresh and aged chicken litter. Appl. Environ. Microbiol. 2012;78:1302–1307. doi: 10.1128/AEM.06671-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bolan N.S, Szogi A.A, Chuasavathi T, Seshadri B, Rothrock M.J, Jr, Panneerselvam P. Uses and management of poultry litter. World's Poult. Sci. J. 2010;66:673–698. [Google Scholar]
- 24.Soliman E.S, Sobeih M.A.A, Ahmed Z.A, Hussein M.M, Abdel-Latiff H, Moneim A.A. Seasonal epidemiological surveillance on bacterial and fungal pathogens in broiler farms in Egypt. Int. J. Poult. Sci. 2009;8(8):720–727. [Google Scholar]
- 25.Barker K.J, Purswell J.L, Davis J.D, Parker H.M, Kidd M.T, McDaniel C.D, Kiess A.S. Distribution of bacteria at different poultry litter depths. Int. Jpn. Poult. Sci. 2010;9:10–13. [Google Scholar]
- 26.Chen Z, Jiang X. Microbial safety of chicken litter or chicken litter-based organic fertilizers: A review. Agriculture. 2014;4(1):1–29. [Google Scholar]
- 27.Sheffield C.L, Crippen T.L, Beier R.C. Multi-microbial compounds eliminate or reduce Salmonella Typhimuriumfrom one-third of poultry litter samples within 8 days. Res. J. Poult. Sci. 2018;11(1):5–8. [Google Scholar]
- 28.DaíPra M.A, Correa E.K, Roll V.B, Xavier E.G, Lopes D.C.N, Lourenço F.F, Zanusso J.T, Roll A.P. Use of virgin lime for the control of Salmonella spp and Clostridium spp in aviary beds. Ciência Rural. 2009;39:1189–1194. [Google Scholar]
- 29.Stringfellow K, Caldwell D, Lee J, Byrd A, Carey J, Kessler K, Mcreynolds J, Bell A, Stipanovic R, Farnell M. Pasteurization of chicken litter with steam and quicklime to reduce Salmonella Typhimurium. J. Appl. Poult. Res. 2010;19:380–386. doi: 10.3382/japr.2009-00097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wilkinson K.G, Tee E, Tomkins R.B, Hepworth G, Premier R. Effect of heating and aging of poultry litter on the persistence of enteric bacteria. Poult. Sci. 2011;90:10–18. doi: 10.3382/ps.2010-01023. [DOI] [PubMed] [Google Scholar]
- 31.Jacob F.G, Baracho M.S, Nääs I.A, Salgado D.A, Souza R. Incidence of pod dermatitis in broiler reared under two types of environment. Braz. J. Poult. Sci. 2015;18(2):247–254. [Google Scholar]
- 32.McWard G.W, Taylor D.R. Acidified clay litter amendment. J. Appl. Poult. Res. 2000;9:518–529. [Google Scholar]
- 33.Medeiros R, Santos B.J.M, Freitas M, Silva O.A, Alves F.F, Ferreira E. Addition of chemical additives and the effect of moisture in the volatilization of ammonia in poultry litter. Ciência Rural. 2008;38(8):2321–2326. [Google Scholar]
- 34.Sahoo S.P, Kaur D, Sethi A.P.S, Sharma A, Dwivedi P.N, Sandhu B.S. Use of acidified litter for broiler production in winter season. J. Anim. Res. 2015;5(2):283–288. [Google Scholar]
- 35.Schneider A.F, De Almeida D.S, Yuri F.M, Zimmermann O.F, Gerber M.W, Gewehr C.E. Natural zeolites in diet or litter of broilers. Br. Poult. Sci. 2016;57(2):257–263. doi: 10.1080/00071668.2016.1150962. [DOI] [PubMed] [Google Scholar]
- 36.Soliman E.S, Hassan R.A. Evaluation of superphosphate and meta-bisulfide efficiency in litter treatment on productive performance and immunity of broilers exposed to ammonia stress. Adv. Anim. Vet. Sci. 2017;5(6):253–259. [Google Scholar]
- 37.Vital M, Hammes F, Egli T. Escherichia coli O157 can grow in natural freshwater at low carbon concentrations. Environ. Microbiol. 2008;10(9):2387–2396. doi: 10.1111/j.1462-2920.2008.01664.x. [DOI] [PubMed] [Google Scholar]
- 38.Van Elsas J.D, Semenov A.V, Costa R, Trevors J.T. Survival of Escherichia coli in the environment: Fundamental and public health aspects. ISME J. 2011;5:173–183. doi: 10.1038/ismej.2010.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Soliman E.S, Taha E.G, Sobieh M.A.A, Reddy P.G. The influence of ambient environmental conditions on the survival of Salmonella enteric serovar Typhimurium in poultry litter. Int. J. Poult. Sci. 2009;8(9):848–852. [Google Scholar]
- 40.Bennett D.S, Higgins S.E, Moore R, Beltran R, Caldwell D, Byrd J.A, Hargis B.M. Effects of lime on Salmonella enteritidis survival in vitro. J. Appl. Poult. Res. 2003;12:65–68. [Google Scholar]
- 41.Bennett D.S, Higgins S.E, Moore R, Byrd J.A, Beltran R, Corsiglia C, Caldwell D, Hargis B.M. Effect of addition of hydrated lime to litter on recovery of selected bacteria and poult performance. J. Appl. Poult. Res. 2005;14:721–727. [Google Scholar]
- 42.Line J.E, Bailey J.S. Effect of on-farm litter acidification treatments on Campylobacter and Salmonella populations in commercial broiler houses in Northeast Georgia. Poult. Sci. 2006;85:1529–1534. doi: 10.1093/ps/85.9.1529. [DOI] [PubMed] [Google Scholar]
- 43.Vicente J.L, Higgins S.E, Hargis B.M, Tellez G. Effect of poultry guard litter amendment on horizontal transmission of Salmonella enteritidis in broiler chicks. Int. J. Poult. Sci. 2007;6:314–317. [Google Scholar]
- 44.Lopes M, Roll V.F.B, Leite F.L, Dai Prá M.A, Xavier E.G, Heres T, Valente B.S. Quicklime treatment and stirring of different poultry litter substrates for reducing pathogenic bacteria counts. Poult. Sci. 2013;92:638–644. doi: 10.3382/ps.2012-02700. [DOI] [PubMed] [Google Scholar]
- 45.Adewole S.O. The efficacy of drugs in the treatment of coccidiosis in chicken in selected poultries. Acad. Res. Int. 2012;2(1):20–24. [Google Scholar]
- 46.Blake D.P, Clark E.L, Macdonald S.E, Thenmozhi V, Kundu K, Garg R, Jatau I.D, Ayoade S, Kawahara F, Moftah A, Reid A.J, Adebambo A.O, Zapata R.A, Rao A.S.S, Thangaraj K, Banerjee P.S, Dhinakar-Raj G, Raman M, Tomley F.M. Population, genetic, and antigenic diversity of the apicomplexan Eimeria tenella and their relevance to vaccine development. Proc. Natl. Acad. Sci U. S. A. 2015;112(38):E5343–E5350. doi: 10.1073/pnas.1506468112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fetterer R.H, Jenkins M.C, Miska K.B, Cain G.D. Metam sodium reduces viability and infectivity of Eimeria oocyst. J. Parasitolol. 2010;96(3):632–637. doi: 10.1645/GE-2345.1. [DOI] [PubMed] [Google Scholar]
- 48.Sahoo S.P, Kaur D, Sethi A.P.S, Sharma A, Chandra M, Chandrahas A.S. Effect of chemically amended litter on litter quality and broiler performance in winter. J. Appl. Anim. Res. 2017;45(1):533–537. [Google Scholar]
- 49.Samaha H.A, Haggag Y.N, Nossair M.A, Habib H.M. Assessment efficiency of some chemical disinfectants commonly used against Coccidia in poultry farms. Alexandria J. Vet. Sci. 2013;39(1):82–90. [Google Scholar]
- 50.Mesa D, Lourenco M, Souza A, Bueno A, Pereira A, Sfeir M, Santine E. Influence of covering reused broiler litter with plastic canvas on litter characteristics and bacteriology and the subsequent immunity and microbiology of broilers. Braz. J. Poult. Sci. 2016;18(4):563–572. [Google Scholar]


