Abstract
Background
Magnetic resonance imaging (MRI), including perfusion MRI with arterial spin labeling (ASL) and diffusion-weighted imaging (DWI), are applied in the periictal detection of circulatory and metabolic consequences associated with epilepsy. Although previous report revealed that prolonged ictal hyperperfusion on ASL can be firstly detected and cortical hyperintensity of cytotoxic edema on DWI secondarily obtained from an epileptically activated cortex, the hemodynamic state of the periictal hyperperfusion has not been fully demonstrated.
Methods: study-1
We retrospectively analyzed the relationship between seizure manifestations and the development of periictal MRI findings, in Case 1 with symptomatic partial epilepsy, who underwent repeated periictal ASL/DWI examination for three epileptic ictuses (one examination for each ictus). Study-2: We evaluated the hemodynamic state of periictal hyperperfusion with the ASL technique using a dual postlabeling delay (PLD) of 1.5 and 2.5 s in nine patients, according to the presence or absence of the localized epileptogenic lesion (EL) on conventional 3 T-MRI, who were divided into Group EL+ (six patients) and Group EL− (three patients).
Results
Study-1 confirmed that the stratified representation of the periictal MRI findings depends on the time interval between the ictal cessation and MRI examination in addition to the magnitude and duration of the epileptic activity. In Study-2, two types of periictal hyperperfusion were noted. In all six Group EL+ patients, periictal ASL findings showed “fast flow type”. Markedly increased ASL signals were noted at the epileptically activated cortex, having a tight topographical relationship with EL, on ASL with a PLD of 1.5 s, which is decreased on ASL with a PLD of 2.5 s. In all three Group EL− patients, periictal ASL findings showed “gradual flow type”, which is characterized by gradual signal increase of the epileptically activated cortex on ASL with a PLD of 1.5 and 2.5 s.
Conclusion
We confirmed that ASL hyperperfusion is superior to DWI in the periictal detection of epileptic events. ASL with dual PLD offers the ability to document two types of hemodynamics of periictal hyperperfusion.
Keywords: Arterial spin labeling, Cytotoxic edema, Diffusion-weighted image, Ictal hyperperfusion
Abbreviations: ASL, arterial spin labeling; DWI, diffusion-weighted imaging; MRI, magnetic resonance imaging; CBF, cerebral blood flow; ATT, arterial transit time; PLD, postlabeling delay; ATA, arterial transit artifact; CT, computed tomography; FLAIR, fluid attenuated inversion recovery; EEG, electroencephalography; EL, epileptogenic lesion
1. Introduction
Arterial spin labeling (ASL) is a completely non-invasive and repeatable perfusion magnetic resonance image that uses magnetically-labeled water in the blood as an endogenous tracer. Recent studies have demonstrated that combined use of a perfusion image with ASL and diffusion-weighted imaging (DWI) can provide valuable information on the circulatory and metabolic consequences associated with epilepsy during ictal [[1], [2], [3], [4], [5], [6], [7]] or periictal (or postictal) periods [2, [8], [9], [10], [11], [12]].
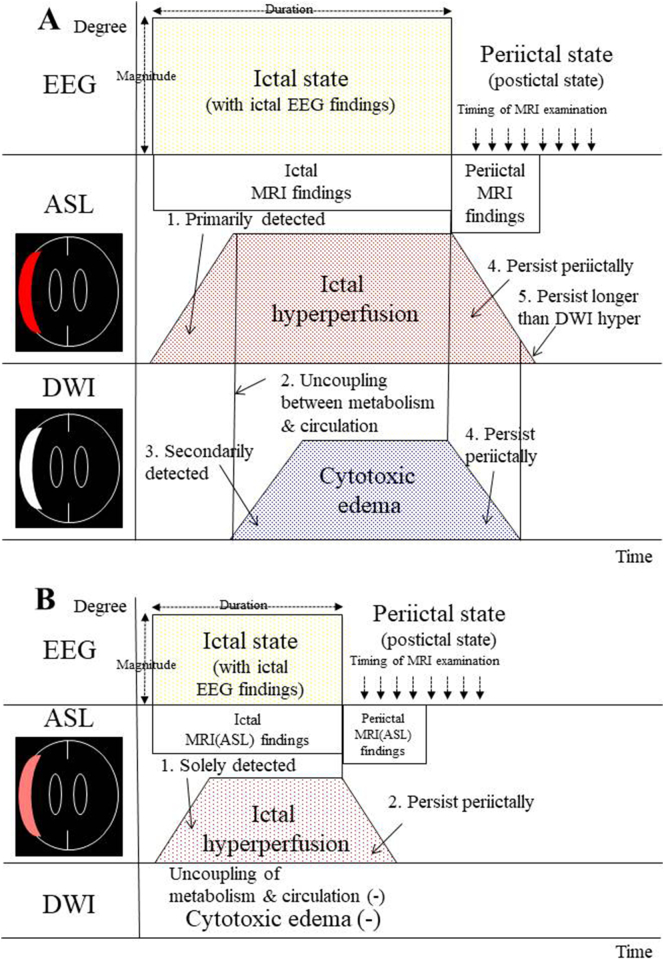
During ictal periods, the epileptogenic cortex is in an extreme electrophysiological state, with the activated cortex exhibiting increased glucose and oxygen usage, thereby causing compensatory regional hyperperfusion. This “ictal hyperperfusion” is primarily detected on ASL (Fig. 1A-1) [2, 5, 7, 8]. When this hyperperfusion is no longer sufficient to supply the hyperactive cortical area, with the induction of glutamate excitotoxity, pathophysiological changes leading to cytotoxic edema in epileptic cortical neurons can occur (Fig. 1A-2). The affected areas are secondarily detected as an abnormally high signal in the cortical lamina, designated “cortical hyperintensity” on DWI (Fig. 1A-3) [2, 5, 7, 8]. When the epileptic activities do not have enough intensity to induce the uncoupling between metabolism and circulation, no signs of cortical hyperintensity are found on DWI whereas ictal hyperperfusion is obtained on ASL (Fig. 1B-1) [2, 5]. Our previous reports [2, 5, 8] demonstrated that the development of the “ictal” magnetic resonance imaging (MRI) findings depends on the magnitude and duration of epileptic activity during ictal periods, although quantitative analysis of those are practically difficult in the neuroemergency of the field [2, 5].
Fig. 1.
Schematic drawing of the relationship between the development of electroencephalographic (EEG) findings and magnetic resonance imaging (MRI) findings (arterial spin labeling perfusion imaging (ASL) and diffusion-weighted imaging (DWI) findings) during ictal and periictal periods.
(A) During ictal states with ictal EEG findings, the epileptogenic cortex is in an extreme electrophysiological state, thereby causing compensatory “ictal hyperperfusion”. This phenomenon is primarily detected on ASL (1). When this hyperperfusion is no longer sufficient to supply the hyperactive cortical area, which induces the uncoupling between metabolism and circulation (2), cytotoxic edema in epileptic cortical neurons can occur. Consequently, the affected areas are secondarily detected as “cortical hyperintensity” on DWI (3). Such “ictal” MRI findings on ASL/DWI can persist during periictal periods, and are then designated “periictal” MRI findings (4). Postictally, increased ASL signals persist longer than DWI hyperintensities (5).
(B) When the epileptic activities do not have enough intensity to induce the uncoupling, cortical hyperintensity is not found on DWI whereas ictal hyperperfusion is solely obtained on ASL (1). Ictal hyperperfusion on ASL can persist during periictal periods (2), and are then considered periictal MRI (ASL) findings.
Such ictal MRI findings on ASL/DWI are reversible in most cases, however they can persist during periictal periods, and are then designated “periictal” MRI findings (Fig. 1A-4, B-2) [2, 5, 8, 9]. Furthermore, postictally, increased ASL signals persist longer than DWI hyperintensities (Fig. 1A-5) [2, 5, 8, 13, 14]. Thus, it is conceivable that ASL is superior to DWI in the periictal detection of epileptic events [2,5,9.10]. It is also naturally considered that the detection of the “periictal” MRI findings strongly depends on the time interval between the cessation of the ictus and MRI examination [2, 9, 10], in addition to the magnitude and duration of the epileptic activity. In Study-1 of the present study, we demonstrate the stratified representations of these periictal MRI findings in a patient with symptomatic partial epilepsy who underwent repeated periictal ASL and DWI examination for three epileptic ictuses (one examination for each ictus).
Although ictal or periictal hyperperfusion can be demonstrated with ASL, the precise hemodynamic state of ictal or periictal ASL hyperperfusion has not been not fully elucidated [2, 5, [7], [8], [9]]. One reason is that quantitative assessment of the CBF value is difficult with ASL because ASL is highly sensitive to the arrival time of the labeled blood in the tissue, which is defined as the arterial transit time (ATT) [[15], [16], [17], [18]]. The acquisition of ASL involves a time delay, the postlabeling delay (PLD), which is the time between inversion of the blood spins passing through the labeling plane in the neck and the time of image acquisition in any plane after tissue perfusion by the labeled blood. The fundamental tradeoff of ASL acquisition is that short PLDs do not allow complete delivery of labeled blood to the tissue, whereas long PLDs result in strong T1 decay and therefore reduced signal-to-noise ratio [15, 17, 18]. To resolve this tradeoff between allowing sufficient delay to visualize “ictal hyperperfusion” and maintaining adequate diagnostic quality, PLDs of 1.5–1.8 s have been traditionally used in previous reports, including ours [2, 5, [7], [8], [9], [10]].
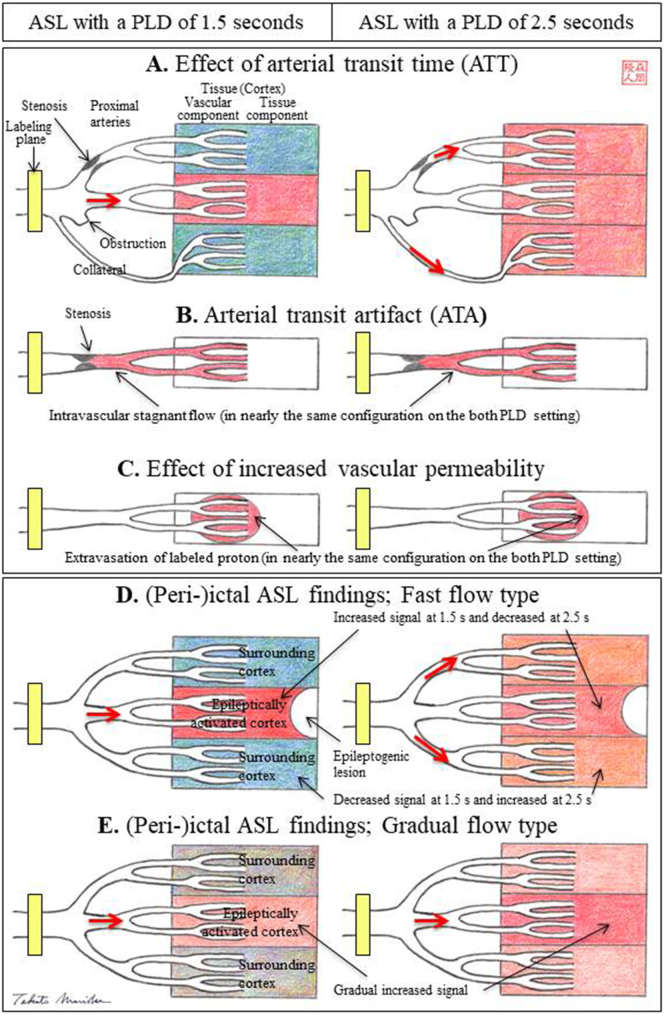
However, ASL measurements that employ a conventional single PLD of 1.5 s lead to underestimation of the CBF because the slowly streaming CBF could not be assessed. To make the most of its characteristics, we developed a simple ASL technique using dual PLD settings: we selected another PLD of 2.5 s in addition to the routinely used PLD of 1.5 s [15, 17, 18]. For example, in patients with steno-occlusive cerebrovascular disease, labeled blood that travels via stenotic proximal arteries or collateral pathways exhibits prolonged ATT. Thus, ASL measurements that employ a conventional single PLD of 1.5 s lead to underestimation of CBF, and the slowly streaming CBF could be assessed on ASL with a PLD of 2.5 s (Fig. 2A) [15, 17, 18].
Fig. 2.
Basic principle of ASL with dual postlabeling delay (PLD).
(A) The effect of arterial transit time (ATT). ASL is highly sensitive to the arrival time of the labeled blood in the tissue, which is defined as ATT. For example, in patients with steno-occlusive cerebrovascular disease, labeled blood that travels via stenotic proximal arteries or collateral pathways exhibits prolonged ATT. Thus, ASL measurements that employ a conventional single PLD of 1.5 s (ASL with a PLD of 1.5 s) lead to underestimation of the CBF, and the slowly streaming CBF could be assessed on ASL with a PLD of 2.5 s (ASL with a PLD of 2.5 s). (B) Arterial transit artifact (ATA). Apparent hyperperfusion is demonstrated in territories where intravascular magnetically labeled protons are stagnant, a condition termed “ATA”. ATAs appear in nearly the same configuration on both PLD settings. (C) Effect of increased vascular permeability. Extravasation of labeled protons into the tissue due to increased vascular permeability or opening of the blood brain barrier also appears in nearly the same configuration on the dual PLD setting.
Two types of periictal ASL findings with dual PLD.
(D) Fast flow type is characterized by markedly increased signals of the epileptically activated cortex on ASL with a PLD of 1.5 s, which is markedly decreased or mostly washed out on ASL with a PLD of 2.5 s. Signals of the surrounding or contralateral cortices are decreased on ASL with a PLD of 1.5 s, probably due to the steal phenomenon, which is mostly improved on ASL with a PLD of 2.5 s. There is a tight topographical relationship between the epileptogenic lesion and the epileptically activated cortex. (E) The gradual flow type is characterized by increased signals of the epileptically activated cortex on ASL with a PLD of 1.5 s, which is further increased on ASL with a PLD of 2.5 s.
Another characteristic of ASL with a single PLD is that apparent hyperperfusion is demonstrated in territories where intravascular magnetically labeled protons are stagnant, a condition termed ‘arterial transit artifact (ATA) [16]. In previous reports, we demonstrated the further utility of this dual PLD method in that it can facilitate the differentiation of ATAs from focal hyperperfusion. ATAs appear in nearly the same configuration on both PLD settings (Fig. 2B) [15, 18]. Extravasation of labeled protons due to increased vascular permeability or opening of the blood brain barrier [19] also appear in nearly the same configuration on dual PLD (Fig. 2C).
In previous reports, we demonstrated that ASL with dual PLD could easily assess not only hemodynamic states with steno-occlusive cerebrovascular disease [15, 17] but also the dynamic changes of giant internal carotid artery aneurysms and the associated hemodynamic state following reconstructive surgery [18]. In Study-2, we will demonstrate the hemodynamic state of periictal hyperperfusion by using ASL with dual PLD.
2. Methods
2.1. Subjects
From April 2014 to May 2017, 134 patients were admitted to Kyushu Rosai Hospital for control of epileptic ictus as neuroemergency cases. Most patients underwent the first emergent neuroradiological examinations such as computed tomographic (CT) scan or/and “conventional” MR examination, including T1/T2 weighted images, images with fluid attenuated inversion recovery (FLAIR) and DWI on arrival, with a subsequent routine electroencephalography (EEG) being scheduled within several hours. In 59 (44%) of 134 patients, ASL examinations were added at the first conventional MRI examination on arrival or second MRI examination, for clinical purposes, depending on the patients' conditions and the discretion of the attendant physicians.
2.1.1. Study-1
Among these patients, we retrospectively selected those who developed epileptic ictuses more than three times and underwent periictal ASL/DWI examinations on each of their ictuses. Case 1 with symptomatic partial epilepsy associated with old putaminal hemorrhage was the only patient who satisfied the requirement. In Case 1, we retrospectively analyzed the relationship between seizure manifestations and development of ASL/DWI findings on each of the three occasions of the epileptic ictus (Table 1).
Table 1.
Relationship between seizure manifestations and ASL/DWI and EEG findings of each ictus in Case 1.
| No. of ictus | Postope./Age (year) | Seizure |
MRI examination |
Subsequent EEG findings | |||
|---|---|---|---|---|---|---|---|
| Type | Approximate duration | Approximate timing | ASL findings | DWI findings | |||
| 1 | 1.5/66 | SGS | Several minutes | 3 h | − (Fig. 3D) | – | Slow wave on Lt. (Fig. 3E) |
| 2 | 2.5/67 | SGS | 30 min | 18 h | + (Fig. 3F) | – | Slow wave on Lt. with paroxysms on Lt. temporal (Fig. 3G) |
| 3 | 4.5/69 | SGS | 1 h | Within 1 h | ++ (Fig. 3H) | ++ (Fig. 3J) | Slow wave on Lt. with paroxysms on Lt. temporal (Fig. 3J) |
Abbreviations of this table: ASL, arterial spin labeling; DWI, diffusion weighted image; EEG, electroencephalography; MRI, magnetic resonance imaging; SGS, secondarily generalized tonic seizure; −, negative; +, positive; ++, strongly positive; Lt, left.
2.1.2. Study-2
We selected nine patients (Cases 2–10, six men, three women, mean age 76 years, range 46–87 years) who periictally underwent ASL with dual PLD (Table 2). According to the presence or absence of the localized epileptogenic lesion (EL) on conventional 3 T-MRI, nine patients were divided into two groups according to the presence (EL+) or absence (EL−) of the localized epileptogenic lesion (EL). Six patients (Cases 2–7) with the localized epileptogenic lesions belonged to Group EL+. On the basis of subsequently performed comprehensive examinations, they were diagnosed as having symptomatic partial epilepsies associated with the epileptogenic lesion including old contusion, old intracerebral hemorrhage, clipped ruptured aneurysm of the anterior communicating artery (through the interhemispheric approach), convexity meningioma and recurrent primary malignant lymphoma. Group EL− had three patients (Cases 8–10) without the epileptogenic lesion. Cases 8 and 9 were diagnosed as having situation-related seizure as acute symptomatic seizures. Possible causative factors in these two patients included the association of hyponatremia (Cases 8 and 9, 123 and 124 mEq/L, respectively), chronic alcoholism (Case 8), and non-ketotic hyperglycemia (Case 9, 264 mg/dl). Case 10 was diagnosed as having non-lesional elderly epilepsy because there was no cortical lesion whereas deep white matter rarefaction was noted. Informed consent was obtained from the patients or their families.
Table 2.
Clinical profile and periictal MRI and EEG findings of 9 patients of Study 2.
| Case No. | Age/Sex | Clinical diagnosis | Epileptogenic lesion | Seizure |
IV Medi-cation | Timing of MRI | ASL with dual PLD findings |
DWI findings | Subsequent EEG findings | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Type | Approximate duration | Epilepto-genic lesion | Epileptically activated area | Spreading area | ||||||||
| Group EL+ | ||||||||||||
| 2 | 46/M | Symptomatic partial epilepsy | Old contusion Bifrontal apicies | Generalized seizure | 1 h | DZP MDZ fPTH | 1 h | Hypo-signal | Bifrontal, Interhemispheric; Fast flow type | – | – | Generalized slow |
| 3 | 83/M | Symptomatic partial epilepsy | Old contusion Lt.fronto-parietal | Tonic seizure Rt. Face& Upper limb | 1 h intermittent | DZP fPTH | 2 days | Hypo-signal | Lt. fronto-parietal; Fast flow type | – | – | No paroxysms |
| 4 | 70/M | Symptomatic partial epilepsy | Old ICH Lt. frontal | Rt. Facial seizure | 3 days intermittent | DZP fPTH LEV | 1 day | Hypo-signal | Lt frontal; Fast flow type | – | – | No paroxysms |
| 5 | 87/F | Symptomatic partial epilepsy | Clipped Acom aneurysm | Generalized seizure | Several minutes | DZP fPTH LEV | 1 day | ? (Clip artifact) | Bifrontal, Interhemispheric; Fast flow type | – | – | Generalized slow |
| 6 | 76/M | Symptomatic partial epilepsy | Recurrent PML Rt. Frontal base | Generalized seizure | Several minutes (twice) | DZP LEV | Within 30 min | ? (Small lesion) | Rt. Frontal; Fast flow type | Generalized Predominantly Rt; Fast flow type | + (See text) | Not examined |
| 7 | 80/F | Symptomatic partial epilepsy | Convexity meningioma Rt. Frontal | Tonic seizure Lt. Face& Upper limb | Several minutes | DZP, fPTH, LEV | 1 day | Hyper-signal | Rt. Frontal; Fast flow type | Bifrontal, Interhemispheric; Fast flow type | – | Not examined |
| Group EL− | ||||||||||||
| 8 | 80/M | Situation-related seizure | -*Hyponatoremia Chronic alcoholism | Generalized seizure | Several minutes(3 times) | DZP, MDZ | 1 day | – | Bil. Frontal; Gradual flow type | – | – | Subclinical seizure activities at F4&C4 |
| 9 | 81/F | Situation-related seizure | -* Hyponatoremia Diabetus mellitus | Generalized seizure | Several minutes (twice) | DZP, fPTH | 2 days | – | Bil. Frontal; Gradual flow type | – | – | Not examined |
| 10 | 77/M | Non-lesional elderly epilepsy | -* | Generalized seizure | Several minutes (twice) | DZP, MDZ | 1 h | – | Lt. frontal base; Gradual flow type | Rt. Frontal, Interhemispheric; Fast flow type | – | Generalized slow+** |
Abbreviations of this table: MRI, magnetic resonance imaging; EEG, electroencephalography; ASL, arterial spin labeling; PLD, dual postlabeling delay; DWI, diffusion weighted image; IV, intravenous; EL, epilleptogenic lesion; M, male; F, female Lt, left; Rt, right; ICH, intracerebral hemorrhage; Acom, anterior communicating artery; PML, primary malignant lymphoma; −*, no epileptogenic lesion but with subcortical T2 prolonged lesion; DZP, diazepam; MDZ, midazolam; fPHT, fosphenytoin; LEV, levetiracetum; min, minutes; −, negative; +, positive; **, absence of α rhythm on Lt frontal region.
2.2. MRI and EEG
MRI was performed using a 3-T scanner (HDxt Signa; GE Healthcare, Milwaukee, WI) equipped with an 8-channel receive-only head coil for signal reception. The ASL was prepared using a 3-dimensional spiral fast-spin echo sequence with background suppression for perfusion imaging covering the entire brain, as previously described [5, 7, 8, 12, 15, 17, 18, 20]. A pulsed continuous protocol was employed. The acquisition parameters were as follows: 4 arms with 1004 points in each spiral arm, phase encoding in the z direction = 32, section thickness = 4 mm, time to repeat (TR) = 4728, and number of excitation (NEX) = 2. The labeling duration was 1.5 s. Two PLDs of 1.5 s (1.525 s) and 2.5 s (2.525 s) were chosen, as described elsewhere [15, 17, 18]. Acquisition times (minutes: seconds) of ASL with PLDs of 1.5 and 2.5 s were 1:44 and 1:59, respectively. Evaluation of the periictal MRI findings was based on visual inspection by two radiologists (A.H. and A.N.) who were blind to the clinical data. No differences in the radiologists' interpretations were noted on independent assessments (kappa = 1) [21].
Subsequent routine EEG recordings were obtained from an 18-channel digital EEG machine (Neurofax; Nihon-Kohden, Tokyo, Japan) with electrode placement according to the International EEG 10–20 system, as described previously [5, 7, 8, 12, 20]. Evaluation of the EEG findings was also based on visual inspection by two board certified electroencephalographers (T.M. and A.S.) who were blind to the clinical data. No differences in the electroencephalographers' interpretations were noted on independent assessments (kappa = 1) [21].
3. Results
3.1. Study-1 (case 1)
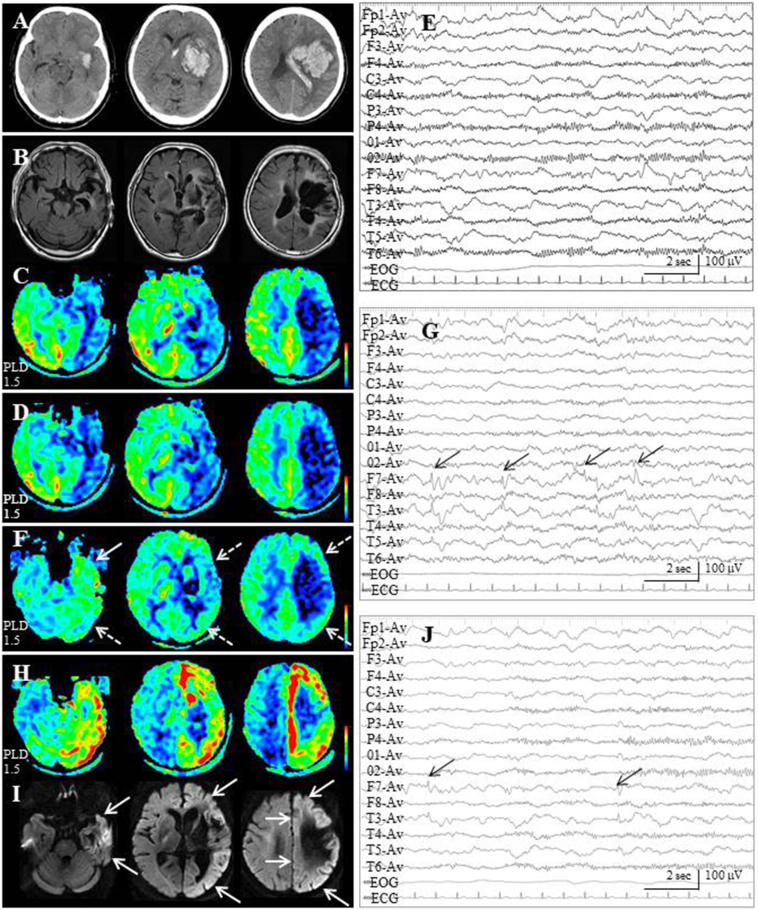
The relationship between seizure manifestations, development of ASL/DWI and EEG findings on each of the three occasions of epileptic ictus in Case 1 are described below and summarized in Table 1. A 64-year-old woman developed left putaminal hemorrhage (Fig. 3A) and subsequently underwent craniotomy and evacuation of the hematoma through the trans-cortical approach on the left frontal lobe. During the interictal state, at postoperative 1 year, FLAIR demonstrated huge old hematoma cavity in the left putamen, surrounded by gliosis, extending to the left fronto-parietal and temporal lobes (Fig. 3B). On ASL with PLD of 1.5 s, marked decreased ASL signals were noted in the extended area of the left hemisphere (Fig. 3C).
Fig. 3.
Case 1. (A) Computed tomographic scan at the onset of stroke reveals left huge putaminal hematoma with intraventricular hemorrhage. (B) At postoperative 1 year (interictal state), MR images with fluid attenuated inversion recovery (FLAIR) demonstrate a huge old hematoma cavity, surrounded by gliosis, in the left putamen extending to the left fronto-parietal and temporal lobes. (C) On ASL with a PLD of 1.5 s, markedly decreased ASL signals are noted in the extended area of the left hemisphere.
(D) At the first ictus, ASL with a PLD of 1.5 s, which is performed approximately 3 h after the secondary generalized seizure for 3 min, fails to reveal ictal hyperperfusion. (E) On subsequent EEG, continuous slow wave activities are noted on the left hemisphere, while breach rhythm appears on the right hemisphere due to left large craniotomy.
(F) ASL with a PLD of 1.5 s, which is performed approximately 18 h after the second epileptic ictus for 30 min, ASL signals in the left hemisphere except for old hematoma cavity (white dotted arrows), especially in the temporal lobe (white arrow), are increased compared with those of the interictal state. On the contrary, ASL signals on the right side, especially the right temporal lobe, are decreased. (G) Subsequent EEG shows continuous slow wave activities on the left hemisphere with interictal paroxysmal activities on the left temporal region (F7 & T3 of the International EEG 10–20 system, black arrows).
(H) ASL with a PLD of 1.5 s, which is performed within 1 h after the third epileptic ictus for 1 h, depicts a marked increase of ASL signals in the cortex of the left hemisphere, except for a surgical defect. (I) DWI shows cortical hyperintensity in the corresponding area to that of the ictal hyperperfusion on ASL (white arrows). (J) Subsequent EEG shows continuous slow wave activities on the left hemisphere with interictal paroxysmal activities on the left temporal region (black arrows).
At the first ictus, in postoperative 1.5 years, she developed generalized seizure for several minutes. Although MRI examination was performed approximately 3 h after the cessation of her seizure, both ASL and DWI findings were negative. ASL indicated decreased ASL signals in the left hemisphere (Fig. 3D), which were identical to the findings of the interictal state. Subsequent EEG showed slow wave activities on the left hemisphere (Fig. 3E).
At the second ictus, in postoperative 2.5 years, the duration of her seizure was approximately 30 min. MRI examination was performed approximately 18 h after the cessation of her seizure. Although DWI findings were negative, ASL signals in the left hemisphere except for the old hematoma cavity (Fig. 3F, white dotted arrows), especially in the temporal lobe (Fig. 3F, white arrow), were increased compared with those of the interictal state (Fig. 3F). To the contrary, ASL signals in the right side, especially on the right temporal lobe, were decreased compared with those of the interictal state (Fig. 3F). Subsequent EEG showed continuous slow wave activities on the left hemisphere with interictal paroxysmal activities on the left temporal region (F7 & T3 of International EEG 10–20 system, Fig. 3G, black arrows).
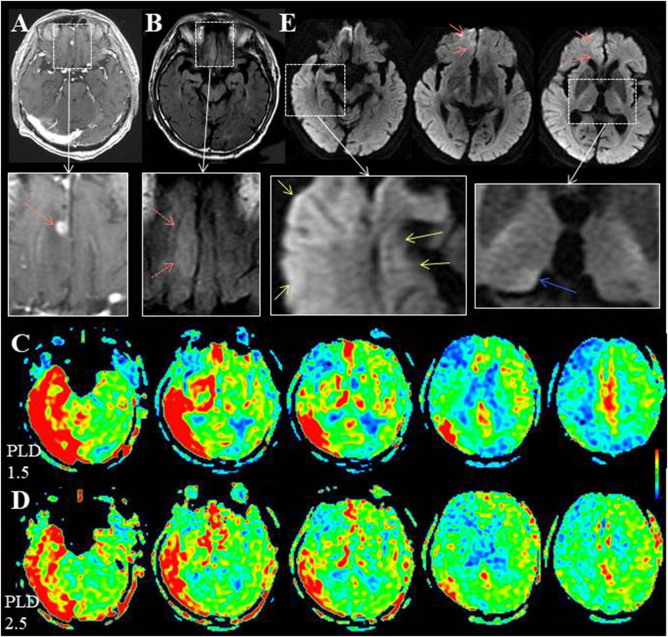
At the third ictus, in postoperative 4.5 years, the duration of her seizure was approximately 1 h. MRI examination was performed within 1 h of cessation of her seizure. Both of ASL and DWI findings were strongly positive. A marked increase of ASL signals at the cortex of the left hemisphere, except for a surgical defect, was noted (Fig. 3H). DWI showed cortical hyperintensity in the area corresponding to that of the periictal hyperperfusion on ASL (Fig. 3I, white arrows). Subsequent EEG showed continuous slow wave activities on the left hemisphere with interictal paroxysmal activities on the left temporal region (Fig. 3J, black arrows).
3.2. Study-2 (cases 2–10)
Clinical presentations and ASL/DWI and EEG findings of nine patients (Cases 2–10) who underwent the ASL examination with dual PLD setting are summarized in Table 2. The type and approximate duration of their seizures were various. The time interval between ictal cessation and MRI examination was also various: within 30 min in one case, 1 h in two, 1 day in four, 2 days in two.
3.2.1. Periictal ASL findings with dual PLD
In all cases, periictal ASL findings were obtained. Two types of periictal ASL findings with dual PLD was noted: One is “fast flow type” and the other is “gradual flow type”. Fast flow type is characterized by markedly increased signals of the epileptically activated cortex or cortices on ASL with a PLD of 1.5 s, which is markedly decreased or mostly washed out on ASL with a PLD of 2.5 s (Fig. 2D). Signals of the surrounding or contralateral cortices are decreased on ASL with a PLD of 1.5 s, which are mostly improved on ASL with a PLD of 2.5 s. Gradual flow type is characterized by increased signals of the epileptically activated cortex or cortices on ASL with a PLD of 1.5 s, which are further increased on ASL with a PLD of 2.5 s (Fig. 2E).
3.2.2. Periictal ASL findings with dual PLD in group EL+ patients
In all six patients of Group EL+, periictal ASL findings demonstrated fast flow type. Furthermore, there was a tight topographical relationship between the localization of the epileptogenic lesion and periictal ASL findings.
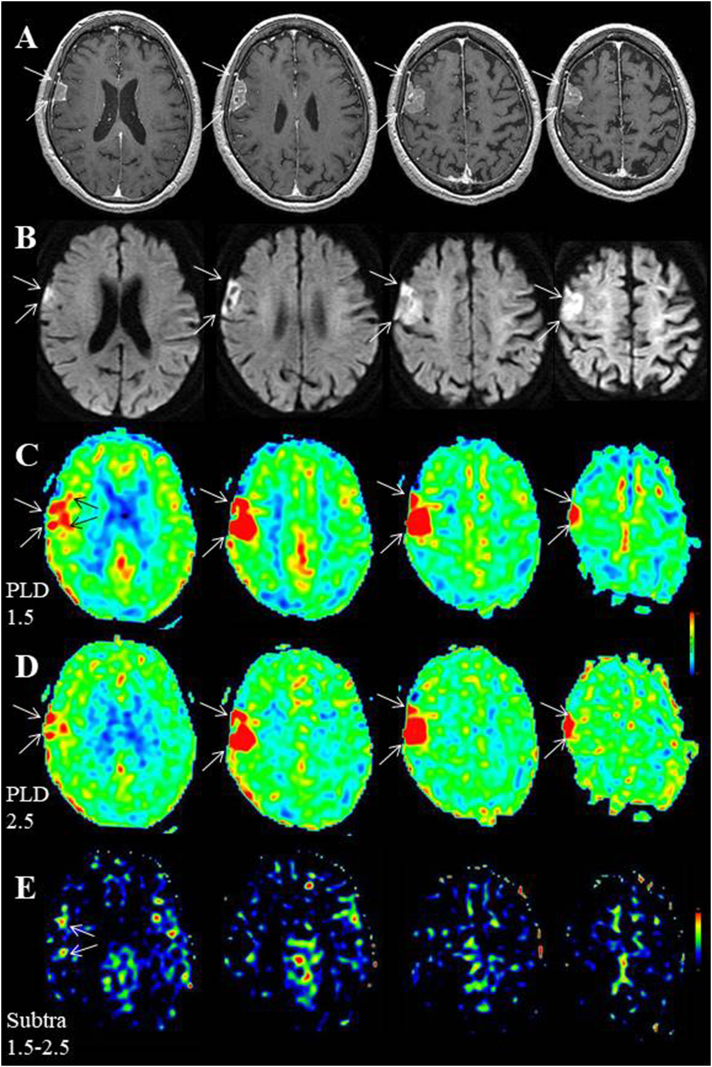
Case 2, who had posttraumatic epilepsy associated with old contusion in the bilateral frontal lobes (Fig. 4A, white arrows), was a representative case because the interictal ASL with dual PLD data were available. Interictally, EEG demonstrated slow wave activities on the bilateral frontal poles (Fp1 & Fp2, Fig. 4B) and ASL with dual PLD showed gradual visualization of the entire cortices except for the area of old contusion (Fig. 4C, white arrows, D). Periictal MR examination was performed 1 h after the secondarily generalized seizure, which was 1 h in duration. On ASL with a PLD of 1.5 s, marked increased signals were noted at the interhemispheric surface of the left frontal lobe, dorso-posterior to the contusional area (Fig. 4E). Because visualization of the surrounding anterior circulation territories was poor compared with that of the interictal state, the area of the increased ASL signals was striking. On ASL with a PLD of 2.5 s, the increased ASL signals were partly decreased and became inconspicuous with the increase of signals in the bilateral anterior circulation territories (Fig. 4F). Subsequent EEG demonstrated postictal generalized slow wave activities (Fig. 4G).
Fig. 4.
Case 2. (A) During the interictal state, FLAIR indicates old contusion areas, surrounded by gliosis, in the bilateral frontal lobes (white arrows). (B) Interictal EEG demonstrates continuous slow wave activities on bilateral frontal poles (Fp1 & Fp2). (C,D) Interictal ASL with dual PLD shows gradual visualization of the entire cortices, except for old contusional lesions (white arrows). (E) On periictal ASL with a PLD of 1.5 s, markedly increased signals are noted at the interhemispheric surface of the left frontal lobe, dorso-posterior to the contusional area. Visualization of the surrounding anterior circulation territories is poor compared with that of the interictal state. (F) On ASL with a PLD of 2.5 s, the increased ASL signals are partly decreased and became inconspicuous with the increase of signals in the bilateral anterior circulation territories. Visualization of the bilateral posterior circulation territories remains poor compared with that of the interictal state. (G) Subsequent EEG demonstrates postictal generalized slow wave activities.
Another representative case is Case 6, with small sized tumor (recurrent malignant lymphoma, Fig. 5A, red arrow) with perifocal edema (Fig. 5B, red dotted arrows) at the interhemispheric surface of the right frontal base; he was the sole case in whom periictal MRI findings were obtained both on ASL and DWI in the nine patients of Study-2. There were two secondarily generalized seizures running for several minutes each. MRI examination was performed within 30 min after withdrawal of the second seizure. On ASL with a PLD of 1.5 s, markedly increased signals were noted at the right temporo-occipital lobe, including the hippocampus, bilateral thalami, interhemispheric surfaces of the bilateral frontal lobes and left frontal convexity, as well as right frontal base, including the epileptogenic lesion (Fig. 5C). Visualization of the right fronto-parietal convexity is poor, compared with that of the left side. On ASL with a PLD of 2.5 s, the increased ASL signals are partly decreased (Fig. 5D). Signals of the right fronto-parietal convexity are partly improved. DWI shows cortical hyperintensity in the parts of the corresponding areas to those of the periictal ASL hyperperfusion, such as the interhemispheric surface of the right frontal base (Fig. 5E red arrows), the right temporal lobe, including the hippocampus (Fig. 5E, yellow arrows) and the right pulvinar of the thalamus (Fig. 5E, blue arrow). Unfortunately, subsequent EEG was not performed because the patient was transferred to another hospital for treatment of the recurrent tumor.
Fig. 5.
Case 6. (A,B) T1-weighted image with gadolinium enhancement and FLAIR show a small enhanced tumor (recurrent malignant lymphoma, red arrow on the enlarged view of A) with perifocal edema (red dotted arrows on the enlarged view of B) at the interhemispheric surface of the right frontal base. White dotted boxes indicate the extent of the enlarged view. (C) On ASL with a PLD of 1.5 s, markedly increased signals are noted at right temporo-occipital lobe, including the hippocampus, bilateral thalami and interhemispheric surfaces of the frontal lobes as well as right frontal base. Visualization of the right fronto-parietal convexity is poor compared with that of the left side. (D) On ASL with a PLD of 2.5 s, the increased ASL signals are partly decreased. Signals of the right fronto-parietal convexity are increased, although the visualization is still poor compared with that of the left side. (E) DWI shows hyperintensity of the right frontal base (red arrows), right temporal lobe, including right hippocampus (yellow arrows) and right pulvinar of the thalamus (blue arrow). Enlarged view of the right hippocampus shows hyperintensity with the hippocampal digitations. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Of the six patients, the epileptogenic lesion itself was demonstrated as hyposignal on ASL in three (Cases 2, 3, 4). In two patients, the epileptogenic lesion was unclear on ASL because of the clip artifact in Case 5 and small sized lesion in Case 6 (Fig. 5C, D). In Case 7, the epileptogenic lesion (convexity meningioma in the right frontal region, Fig. 6A, B white arrows) was demonstrated as a strong hypersignal area in nearly the same configuration on both PLD settings (Fig. 6C, D, white arrows). On ASL with a PLD of 1.5 s, increased signals were noted at the right frontal convexity, just ventral to the tumor (Fig. 6C, black arrows), which was washed out on ASL with a PLD of 2.5 s (Fig. 6D). To eliminate the increased ASL signals of the meningioma in the same configuration on the dual PLD setting, the data from each voxel of the ASL with a PLD of 1.5 s were subtracted from the data from the corresponding voxel of the ASL with a PLD of 2.5 s using the clinically-used volume analyzer SYNAPSE VINCENT (Fujifilm, Tokyo, Japan), based on the method reported by Lou et al. [22]. The remaining signal on the subtracted images was the increased ASL signals with PLDs of 1.5 s at the bilateral frontal interhemispheric surfaces and left frontal convexity, in addition to the right frontal convexity, just ventral to the tumor (Fig. 6E, white arrows).
Fig. 6.
Case 7. (A) T1-weighted images after administration of gadolinium show the convexity meningioma of the right frontal region. (B) On DWIs, the convexity meningioma is depicted as hyperintensity (white arrows), while cortical hyperintensity is not evident. (C,D) On ASL with both PLD settings, the meningioma is demonstrated as a hypersignal area in nearly the same configuration. On ASL with a PLD of 1.5 s, increased signals are noted at the right frontal convexity, just ventral to the tumor (C, black arrows), which is washed out on ASL with a PLD of 2.5 s. (E) The subtraction image (the data of the ASL with a PLD of 1.5 s was subtracted from the data of ASL with a PLD of 2.5 s) allows ASL signals in the same configuration on the dual PLD setting to be eliminated. Increased ASL signals were noted at the bilateral frontal interhemispheric surfaces and left frontal convexity, in addition to the right frontal convexity, just ventral to the tumor (white arrows).
As was demonstrated in Cases 6 and 7, increased signals at the remote area from the epileptogenic lesion were observed on ASL with a PLD of 1.5 s. In Case 6 with a recurrent tumor in the right frontal base, increased ASL signals were noted at the right temporo-occipital lobe, including the hippocampus, bilateral thalami, interhemispheric surfaces of the bilateral frontal lobes and left frontal convexity (Fig. 5C). In Case 7 with meningioma at the right frontal convexity, ASL signals were at the bilateral frontal interhemispheric surfaces and left frontal convexity at the propagated area from the primarily activated area (Fig. 6C). These increased signals were partly and mostly decreased on ASL with a PLD of 2.5 s, respectively (Figs. 5D, 6D), demonstrating fast flow type.
3.2.3. Periictal ASL findings with dual PLD in group EL− patients
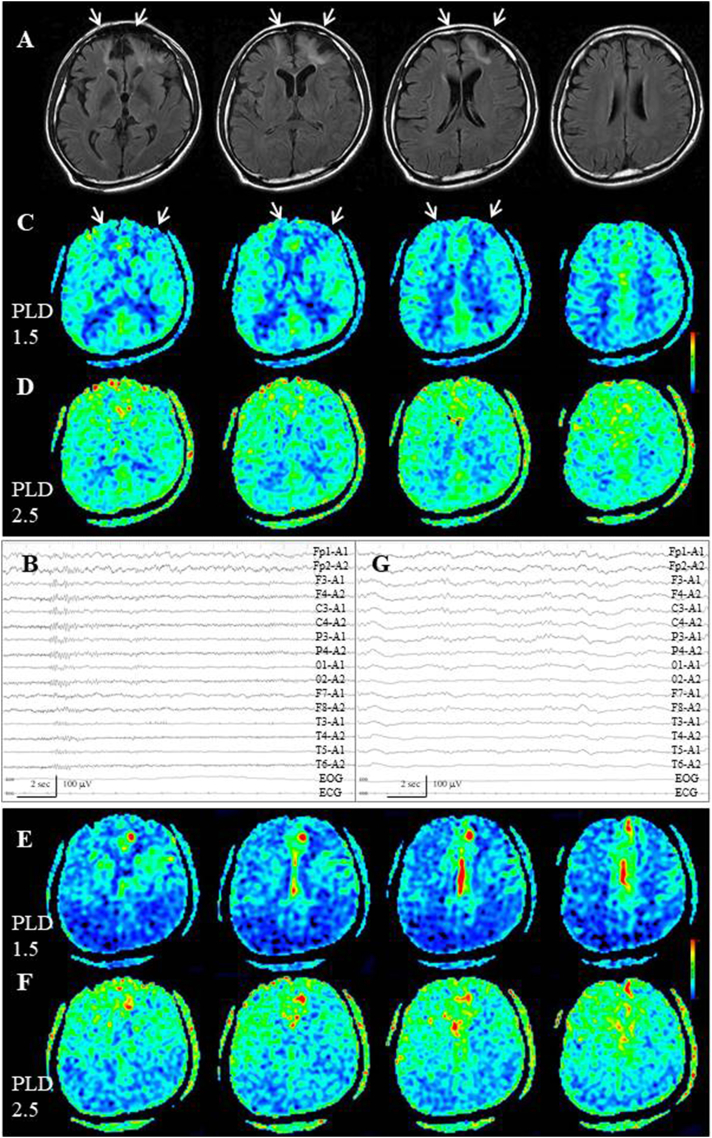
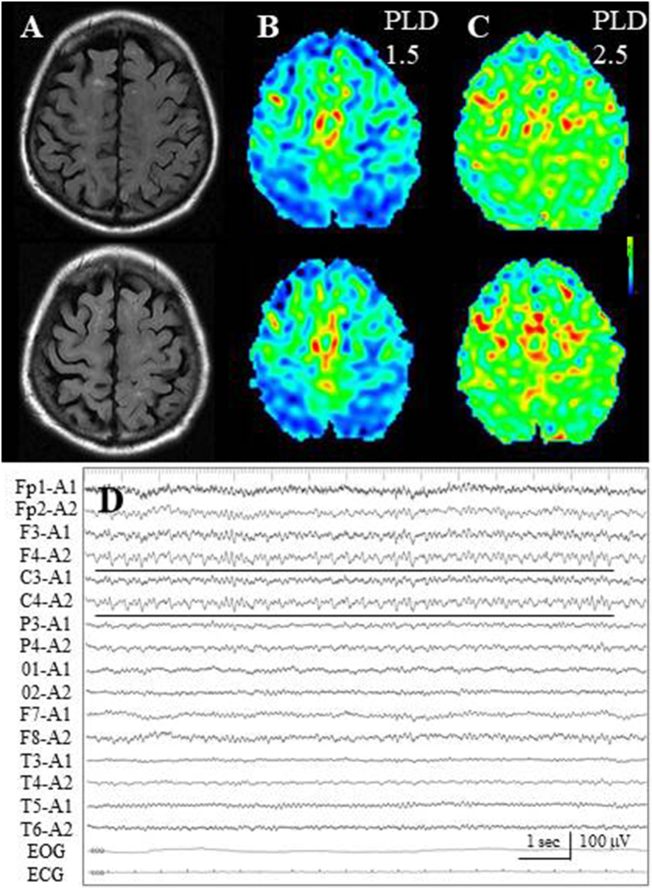
In all three Group EL-patients, periictal ASL findings demonstrated gradual flow type. In Cases 8 and 9, who developed situation-related seizure, a gradual signal increase of the bilateral frontal lobes was noted: an increase in signal was noted at the bilateral frontal lobe, especially on the interhemispheric surfaces, on ASL with a PLD of 1.5 s, although there was no epileptogenic lesion (Fig. 7A, B). Further increased signals were observed on ASL with a PLD of 2.5 s (Fig. 7C). In Case 8, subsequent EEG demonstrated subclinical seizure activities on the right fronto-central region (F4, C4, Fig. 7D), although periictal MRI and subsequent EEG examination was performed 1 day after the seizure withdrawal.
Fig. 7.
Case 8. (A) FLAIR images fail to reveal epileptogenic cortical lesion, while sporadic subcortical hyperintensities are noted. (B) On ASL with a PLD of 1.5 s, increased signals are noted on the bilateral frontal lobe, especially on the interhemispheric surfaces. (C) At ASL with a PLD 2.5 of seconds, further increased signals are observed. (D) Subsequent EEG demonstrates subclinical seizure activities on the right fronto-central region (black lines).
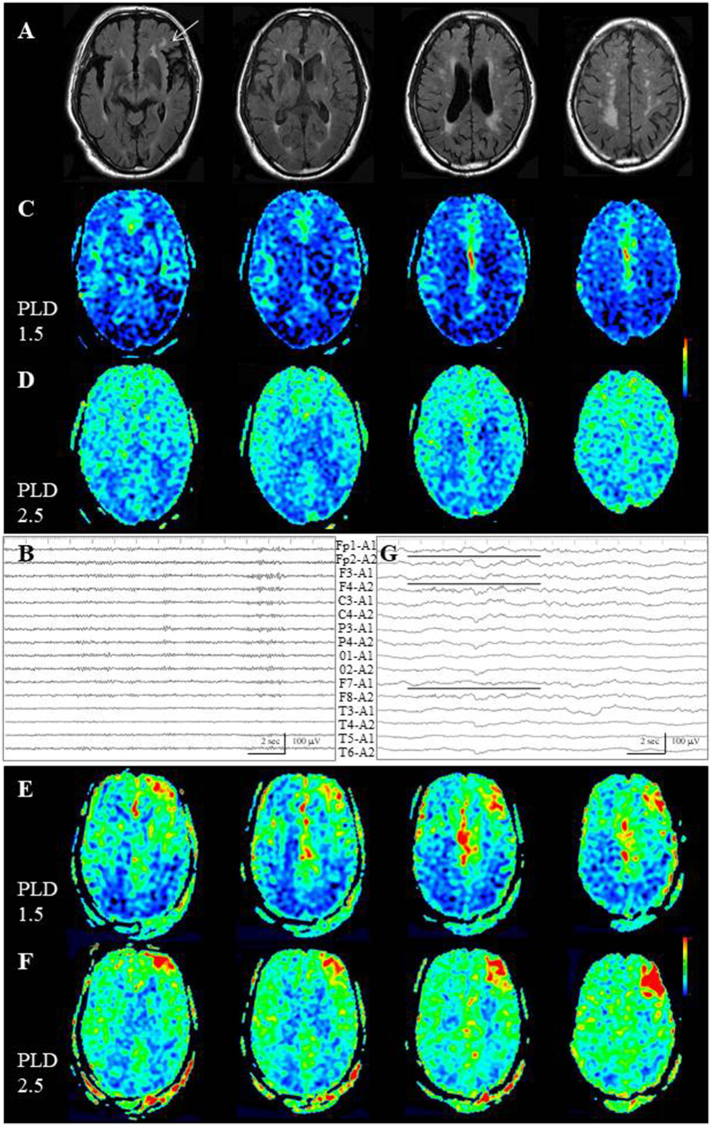
In Case 10, with non-lesional elderly (77-year-old) epilepsy, there was no cortical epileptogenic lesion, yet deep white matter rarefaction was noted, especially in the left frontal lobe (Fig. 8A, white arrow). Interictally, EEG failed to reveal a localized abnormality (Fig. 8B). ASL with dual PLD showed gradual visualization of the entire cortices (Fig. 8C, D). There were two generalized seizures running for several minutes each. MRI examination was performed 1 h after withdrawal of the second seizure. Periictally, on ASL with a PLD of 1.5 s, increased signals were noted at the convexity and interhemispheric surface of the left frontal lobe (Fig. 8E). On ASL with a PLD of 2.5 s, further increased signals were noted on the convexity, while the increased ASL signals of the interhemispheric area were mostly washed out (Fig. 8F). Because subsequent EEG depicted an absence of α rhythm on the left frontal lesion (Fig. 8G, black lines), as well as generalized slow wave activities, primarily epileptically activated cortex was estimated to the left frontal convexity and the interhemispheric area was thought to be the propagated area.
Fig. 8.
Case 10. (A) On FLAIR images, there is no epileptogenic cortical lesion, although deep white matter rarefaction is noted, especially on the left frontal lobe (white arrow). (B) Interictal EEG fails to reveal localized abnormalities. (C,D) Interictal ASL with dual PLD shows gradual visualization of the entire cortices. (E) On periictal ASL with a PLD of 1.5 s, increased signals are noted at the convexity and interhemispheric surface of the left frontal lobe. (F) On ASL with a PLD of 2.5 s, further increased signals are noted on the convexity, whereas the increased ASL signals are mostly washed out at the interhemispheric surface. (G) Subsequent EEG depicts generalized slow wave activities and the absence of α rhythm on the left frontal lesion (black lines).
4. Discussion
4.1. Development of periictal MRI findings
Case 1 in Study-1 is the first reported case to confirm that the stratified representation of the periictal MRI findings depends on the time interval between the ictal cessation and MRI examination in addition to the magnitude and duration of the epileptic activity within a same individual. At the first ictus, periictal MRI findings could not be obtained on either ASL or DWI because the MR examination was performed 3 h after the ictus for as short as 3 min in duration. While the second ictus of 30 min in duration was strong enough to induce prolonged increased ASL signals in the ipsilateral hemisphere to the epileptogenic lesion, the MRI examination was performed 18 h after the ictus. Furthermore, at the third ictus, prominent periictal MRI findings were obtained in the ipsilateral hemisphere on both ASL and DWI because the MRI examination was performed just after the ictus for as long as 1 h in duration.
In Study-2, only Case 5, in whom the MRI examination was performed within 30 min after the ictus, showed periictal MRI findings both on ASL and DWI. However, the major difference from the periictal MRI findings of Case 1 was that hyperintensity area on DWI was noted at the parts of the increased ASL signal areas, such as the left frontal base, temporal lobe, including the hippocampus, and thalamic pulvinar, where the uncoupling between the metabolism and circulation was thought to have occurred. The other eight patients, in whom MRI examination was performed 1 h to 2 days after the ictus, showed only periictal ASL findings. These findings again confirm the previous idea that ASL is superior to DWI in periictal detection of the circulatory consequences associated with epilepsy [2, 5].
In daily clinical practice, quantitative analysis of the magnitude and duration of epileptic activities are difficult [2, 5]. Evaluation of the time interval between the ictal cessation and MRI examination is also difficult without continuous EEG facilities [2, 5, 8, 23]. In most emergency hospitals in Japan, even routine EEG examination is unavailable outside working hours or on weekends, and the timing of EEG recording is often delayed [5, 8]. However, subsequent EEG should be performed to distinguish postictal from ictal periods, such as the evolution to non-convulsive status epilepticus (NCSE) (subtle status epilepticus) at the timing of the MRI examination [2, 5]. In fact, Case 8 underwent periictal MRI examination 1 day after seizure withdrawal, yet subsequent EEG demonstrated the subclinical seizure activities.
Another concern is that periictal MRI findings reveal only the circulatory consequences associated with epileptic activity. However, EEG reflects more directly the epileptic excitation. Thus, complementary use of MRI and subsequent EEG should be performed to reveal the pathophysiological states of the epileptic ictus [2, 5]. In Case 1, left hemispheric involvement was demonstrated on periictal MRI; however, subsequent EEG showed “localized” interictal paroxysmal activities on the left temporal region. It is therefore hard to localize the epileptogenic focus or area with periictal ASL findings only, although the cortical area involved in the spread of the epileptic activity in prolonged epilepsy can be clearly localized [5, 14].
4.1.1. Periictal ASL findings with dual PLD
It is conceivable that cytotoxic edema detected on DWI could persist even after the ictal cessation [5, 8]. However, it could not be properly explained why prolonged increased ASL signals were noted postictally. Ictal hyperperfusion is theoretically followed by postictal hypoperfusion [8, 24], although temporal evolution of regional ictal hyperperfusion in the postictal stage has not been clearly defined [8, 25]. One possible explanation is the effect of increased vascular permeability during the periictal period. Tanaka et al. [19] demonstrated that in postischemic hyperperfusion in rats, ASL overestimated CBF because of increased vascular permeability. However, the present ASL study with dual PLD could exclude this possibility because extravasation of labeled protons due to increased vascular permeability appears in nearly the same configuration on the dual PLD setting (Fig. 2C). In groups with and without epileptogenic lesion, ASL with dual PLD clearly demonstrated the hemodynamic state of prolonged ictal hyperperfusion during periictal periods.
4.1.2. Periictal ASL findings with dual PLD in Group EL+ patients
In all six patients with localized epileptogenic lesion, periictal ASL findings demonstrated fast flow type. On ASL with a PLD of 1.5 s, markedly increased signals of the epileptically activated cortex, which had a tight topographical relationship with the epileptogenic lesion, were observed. Because these increased ASL signals were decreased or mostly washed out on ASL with a PLD of 2.5 s, ATT is estimated to be fast. On the contrary, ASL signals of the surrounding or contralateral cortices were decreased on ASL with a PLD of 1.5 s, which were mostly improved on ASL with a PLD of 2.5 s. These phenomena resulted from the different ATT values [18], in addition to the different CBF values, between the primarily epileptically activated cortex and the surrounding cortex. This effect was probably caused by the steal phenomenon from the surrounding cortex to the epileptically activated cortex at 1.5 s and delayed anterograde flow at 2.5 s (Fig. 2D), as demonstrated in our previous reports (Fig. 2A) [15, 17, 18]. Periictal ASL with dual PLD at the area remote from the epileptogenic lesion, namely the propagated area, also demonstrated fast flow type.
The epileptogenic lesion itself was mostly demonstrated as a hyposignal on ASL because the epileptogenic lesions in these cases were the chronic stage of trauma or stroke. Only the epileptogenic lesion (convexity meningioma) of Case 7 was demonstrated as a hypersignal area both on ASL with dual PLD. The meningioma of this patient was enhanced with gadolinium, indicating that the tumor had rich vascular density without blood brain barrier. In our previous report [15, 18], we demonstrated the further utility of the dual PLD method in that it can facilitate the differentiation of ATAs or extravasation of labeled protons, which appear in nearly the same configuration on both PLD settings, from focal hyperperfusion (Fig. 2B, C). On ASL with a PLD of 1.5 s, periictal hyperperfusion was noted at the right frontal convexity, just ventral to the tumor, which was washed out on ASL with a PLD of 2.5 s. To eliminate the increased ASL signals of the meningioma in the same configuration on the dual PLD setting, the subtraction method was useful to demonstrate the propagated area at the bilateral frontal interhemispheric surfaces and left frontal convexity, in addition to the primarily activated area at the right frontal convexity, just ventral to the tumor.
4.1.3. Periictal ASL findings with dual PLD in Group EL− patients
In the three patients without epileptogenic lesion, Cases 8 and 9 were diagnosed having situation-related ‘de novo’ seizure of frontal origin, which was triggered by the conjunction of several factors such as drug withdrawal and hyponatremia [26, 27]. Although this seizure was acute symptomatic seizure, ictal hyperperfusion in the bilateral frontal lobes was reported by single photon emission computed tomography with 99mTc hexamethyl propylene amino oxime [27] and ASL [5]. In the present study, a gradual signal increase of the bilateral frontal lobes was noted: an increased signal was noted at the bilateral frontal lobe on ASL with a PLD of 1.5 s, although there was no epileptogenic lesion. Further increased signals were observed on ASL with a PLD of 2.5 s.
In Case 10 with non-lesional elderly epilepsy, there was no cortical lesion though deep white matter rarefaction was noted, especially in the left frontal lobe (Fig. 8A). Judging from the periictal EEG findings of this case, the primarily epileptically activated cortex was thought to be the left frontal convexity. In this case, the topographical relationship between deep white matter rarefaction and the increased ASL signal area on the left frontal lobe could not be totally denied, while there was a difference in the subcortex and cortex. There are no previous reports of epileptogenicity related to the subcortical rarefaction [28], yet it remains possible that the subcortical lesion was epileptogenic in Case 10. Although no absolute conclusion can be drawn from a single case, periictal hyperperfusion in non-lesional elderly epilepsy might show the hemodynamic state of gradual flow type, being different from that of symptomatic partial epilepsy. However, periictal hyperperfusion at the propagated area might show fast flow type, which is identical to that of symptomatic partial epilepsy.
4.1.4. Difference in hemodynamic state in Group EL+ and EL− patients
Although the exact reason there are different hemodynamic patterns between epilepsies with and without epileptogenic lesion remains unknown, one possible explanation is difference in the electromotive force of the epileptically activated area. The localized electromotive force generated by the epileptogenic lesion was strong enough to induce periictal hyperperfusion of fast flow type. In contrast, in a patient with situation-related seizure or non-lesional elderly epilepsy, the electromotive force is thought to be not so strong. Consequently, the PLD is the most significant parameter that contributes to accurate detection of periictal ASL hyperperfusion. In patients with epileptogenic lesion, 1.5 s is an adequate PLD. In contrast, a PLD of 2.5 s may be more useful in patients without epileptogenic lesion. Furthermore, ASL with dual PLD offers the ability to document two types of hemodynamics of periictal hyperperfusion.
4.2. Limitations
The present study has some limitations. First, the data analysis in our study was performed via visual inspection. However, it offers the advantage of not requiring special software or prolonged analysis time in the daily clinical practice. Second, because all the patients with epilepsy did not undergo ASL with dual PLD setting during the study period, it is impossible to calculate the diagnostic accuracy of this method. Third, as was described in the introduction, long PLDs such as 2.5 s result in strong T1 decay and therefore reduced signal-to-noise ratio [15, 17, 18]. However, the obtained ASL with a PLD of 2.5 s in this study was sufficient image to compare with the ASL with a PLD of 1.5 s. Finally, this is a retrospective study, with a small number of patients under a variety of treatment protocols.
5. Conclusion
Although further studies with more sophisticated methods, such as simultaneous examination of continuous EEG monitoring and ASL with dual PLD, and larger numbers of patients are required, our findings show that ASL with dual PLD offers the ability to document two types of hemodynamics of periictal hyperperfusion.
Grant support
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Acknowledgements
The authors thank Dr. Aya Hirata and Dr. Asako Nakanishi for interpreting the peri-ictal MRI findings and Ms. Miki Kishigami, Ms. Yoko Noichi and Ms. Emiko Amano for their valuable assistance in preparing the manuscript. The authors have no conflicts of interest to declare.
References
- 1.Altrichter S., Pendse N., Wissmeyer M. Arterial spin-labeling demonstrates ictal cortical hyperperfusion in epilepsy secondary to hemimegalencephaly. J. Neuroradiol. 2009;36:303–305. doi: 10.1016/j.neurad.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 2.Murao K., Morioka T., Shimogawa T. Various pathophysiological states of acute symptomatic seizures immediately after ischemic stroke, namely “onset seizures”, shown by complementary use of peri-ictal magnetic resonance imaging and electroencephalography. Neurol. Clin. Neurosci. 2017;5:169–177. [Google Scholar]
- 3.Nguyen D., Kapinia V., Seeck M. Ictal hyperperfusion demonstrated by arterial spin-labeling MRI in status epileptics. J. Neuroradiol. 2010;37:250–251. doi: 10.1016/j.neurad.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 4.Oishi M., Ishida G., Morii K. Ictal focal hyperperfusion demonstrated by arterial spin-labeling perfusion MRI in partial epilepsy status. Neuroradiology. 2012;54:653–656. doi: 10.1007/s00234-012-1027-7. [DOI] [PubMed] [Google Scholar]
- 5.Shimogawa T., Morioka T., Sayama T. The initial use of arterial spin labeling perfusion and diffusion-weighted magnetic resonance images in the diagnosis of nonconvulsive partial status epilepticus. Epilepsy Res. 2017;129:162–173. doi: 10.1016/j.eplepsyres.2016.12.008. [DOI] [PubMed] [Google Scholar]
- 6.Telado M., Munuera J., Salas-Puig X. Localization value of ictal arterial spin-labelled sequences in partial seizures. Epileptic Disord. 2011;13:336–339. doi: 10.1684/epd.2011.0445. [DOI] [PubMed] [Google Scholar]
- 7.Wakisaka K., Morioka T., Shimogawa T. Epileptic ictal hyperperfusion on arterial spin labeling perfusion and diffusion-weighted magnetic resonance images in posterior reversible encephalopathy syndrome. J. Stroke Cerebrovasc. Dis. 2016;25:228–237. doi: 10.1016/j.jstrokecerebrovasdis.2015.09.023. [DOI] [PubMed] [Google Scholar]
- 8.Kanazawa Y., Morioka T., Arakawa S. Nonconvulsive partial status epilepticus mimicking recurrent infarction revealed by diffusion-weighted and arterial spin labeling perfusion magnetic resonance images. J. Stroke Cerebrovasc. Dis. 2015;24:731–738. doi: 10.1016/j.jstrokecerebrovasdis.2014.09.026. [DOI] [PubMed] [Google Scholar]
- 9.Matsuura K., Maeda M., Okamoto K. Usefulness of arterial spin-labeling images in periictal state diagnosis of epilepsy. J. Neurol. Sci. 2015;359:424–429. doi: 10.1016/j.jns.2015.10.009. [DOI] [PubMed] [Google Scholar]
- 10.Miyaji Y., Yokoyama M., Kawabata Y. Arterial spin-labeling magnetic resonance imaging for diagnosis of late seizure after stroke. J. Neurol. Sci. 2014;339:87–90. doi: 10.1016/j.jns.2014.01.026. [DOI] [PubMed] [Google Scholar]
- 11.Pizzini F.B., Farace P., Manganotti P. Cerebral perfusion alterations in epileptic patients during peri-ictal and post-ictal phase: PASL vs DSC-MRI. Magn. Reson. Imaging. 2013;31:1001–1005. doi: 10.1016/j.mri.2013.03.023. [DOI] [PubMed] [Google Scholar]
- 12.Takahara K., Morioka T., Shimogawa T. Perfusion image with arterial spin labeling in acute encephalopathy with reduced subcortical diffusion following secondary generalized status epileptics. Epilepsy & Seizure. 2017;9:32–39. [Google Scholar]
- 13.Flacke S., Wullner U., Keller E. Reversible changes in echo planar perfusion- and diffuseion-weighted MRI in status epileptics. Neuroradiology. 2000;42:92–95. doi: 10.1007/s002340050021. [DOI] [PubMed] [Google Scholar]
- 14.Szabo K., Poepel A., Pohimann-Eden B. Diffusion weighted and perfusion MRI demonstrated parenchymal changes in complex partial status epileptics. Brain. 2005;128:1369–1376. doi: 10.1093/brain/awh454. [DOI] [PubMed] [Google Scholar]
- 15.Akiyama T., Morioka T., Shimogawa T. Arterial spin-labeling magnetic resonance imaging with dual post-labeling delay in internal carotid artery steno-occlusion: validation with digital subtraction angiography. J. Stroke Cerebrovasc. Dis. 2016;25:2099–2108. doi: 10.1016/j.jstrokecerebrovasdis.2016.06.005. [DOI] [PubMed] [Google Scholar]
- 16.Amukotuwa S.A., Caroline Y., Zaharchuk G. 3D pseudocontinuous arterial spin labeling in routine clinical practice: a review of clinically signify artifacts. J. Magn. Reson. Imaging. 2015;43:11–27. doi: 10.1002/jmri.24873. [DOI] [PubMed] [Google Scholar]
- 17.Haga S., Morioka T., Shimogawa T. Arterial spin labeling perfusion magnetic resonance image with dual postlabeling delay: a correlative study with acetazolamide loading 123I-Iodoanphetamine single-photon emission computed tomography. J. Stroke Cerebrovasc. Dis. 2016;25:1–6. doi: 10.1016/j.jstrokecerebrovasdis.2015.08.025. [DOI] [PubMed] [Google Scholar]
- 18.Shimogawa T., Morioka T., Akiyama T. Sequential changes of arterial spin-labeling perfusion MR images with dual postlabeling delay following reconstructive surgery for giant internal carotid artery aneurysm. Surg. Neurol. Int. 2017;8:222. doi: 10.4103/sni.sni_73_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tanaka Y., Nagaoka T., Nair G. Arterial spin labeling and dynamic susceptibility contrast CBF MRI in postischemic hyperperfusion, hypercapnia, and after mannitol injection. J. Cereb. Blood Flow Metab. 2011;31:1403–1411. doi: 10.1038/jcbfm.2010.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shimogawa T., Morioka T., Sayama T. Signal changes on magnetic resonance perfusion images with arterial spin labeling after carotid endarterectomy. Surg. Neurol. Int. 2016;7:S1031–S1040. doi: 10.4103/2152-7806.196322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McHugh M.L. Interratery reliability: the kappa statics. Biochem Med. 2012;22:276–282. [PMC free article] [PubMed] [Google Scholar]
- 22.Lou X., Ma X., Liebeskind D.S. Collateral perfusion using arterial spin labeling in symptom middle cerebral artery stenosis. J. Cereb. Blood Flow Metab. Jan 1 2017 doi: 10.1177/0271678X17725212. (271678X17725212, Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morioka T., Sayama T., Mukae N. Nonconvulsive status epileptics during perioperative period of cerebrovascular surgery. Neurol. Med. Chir. (Tokyo) 2011;51:171–179. doi: 10.2176/nmc.51.171. [DOI] [PubMed] [Google Scholar]
- 24.Farrell J.S., Colangeli R., Wolff M.D. Postictal hypoperfusion/hypoxia provides the foundation for a unified theory of seizure-induced brain abnormalities and behavioral dysfunction. Epilepsia. 2017;58:1493–1501. doi: 10.1111/epi.13827. [DOI] [PubMed] [Google Scholar]
- 25.Lee D.S., Lee S.K., Kim S.-K. Late postictal residual perfusion abnormality in epileptogenic zone found on 6-hour postictal SPECT. Neurology. 2000;55:835–841. doi: 10.1212/wnl.55.6.835. [DOI] [PubMed] [Google Scholar]
- 26.Thomas P., Beaumanoir A., Genton P. ‘De novo’ absence status of late: report of 11 cases. Neurology. 1992;42:104–110. doi: 10.1212/wnl.42.1.104. [DOI] [PubMed] [Google Scholar]
- 27.Thomas P., Zifkin B., Migneco O. Nonconvulsive status epileptics of frontal origin. Neurology. 1999;52:1174–1183. doi: 10.1212/wnl.52.6.1174. [DOI] [PubMed] [Google Scholar]
- 28.Ferlazo E., Gasparini S., Beghi E. Epilepsy in cerebrovascular diseases: review of experimental and clinical data with meta-nalysis of risk factors. Epilepsia. 2016;57:1205–1214. doi: 10.1111/epi.13448. [DOI] [PubMed] [Google Scholar]