Abstract
Background
Primary progressive (PP) multiple sclerosis (MS) is considered a clinically distinct entity from the spectrum of relapsing-remitting (RR) forms of the disease.
Objective
To compare the presence of brain and spinal cord lesions between PP and RR subjects.
Methods
We studied people with PPMS [n = 40, 17 (42.5%) men, age 50.7 ± 7.7 years, disease duration 10.1 ± 7.4 years, Expanded Disability Status Scale (EDSS) score 4.6 ± 2.1] and RRMS [n = 40, 12 (30%) men, age 47.9 ± 4.2, disease duration 13.7 ± 5.9, EDSS 1.7 ± 1.3]. MRI of the brain and full spinal cord at 1.5T was analyzed to define patients having: 1. brain only, 2. spinal cord only, or 3. brain and spinal cord MS lesions.
Results
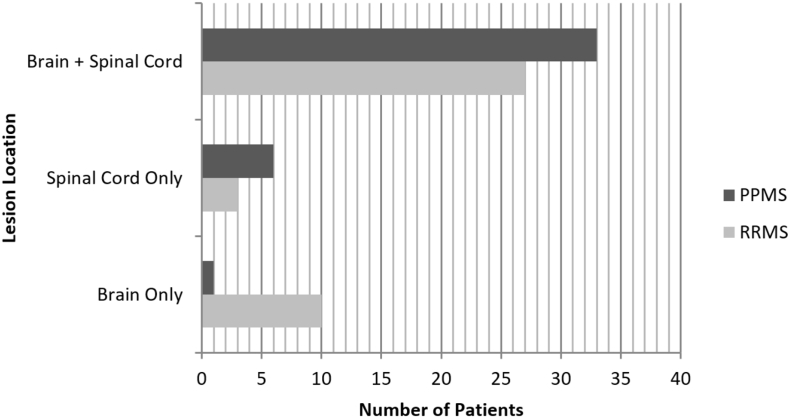
Lesions in the brain only were less common in PP (n = 1, 2.5% of people) than RR (n = 10, 25%) (Fisher's exact p = 0.007). Lesions in the spinal cord only (PP: n = 6, 15%, RR: n = 3, 7.5%, p = 0.481) or brain plus spinal cord (PP: n = 33, 83%, RR: n = 27, 68%, p = 0.196) were similar between groups. PP had higher EDSS and timed 25-ft walk (Wilcoxon tests, both p < 0.001), higher age (t-test p = 0.049), lower disease duration (t-test, p = 0.02), and a similar sex ratio (Fisher's exact p = 0.352) vs. RR.
Conclusions
We report a topographic difference in MRI lesion involvement between PPMS and RRMS. Lesions restricted to the brain are more common in RRMS. These findings provide support to the notion that PP may have features distinctive from the RR spectrum of the disease. Longitudinal comparisons and quantitative MRI analysis would be necessary to confirm and extend these results.
Keywords: Multiple sclerosis, MRI, Brain, Spinal cord, Primary progressive, Lesions
Highlights
-
•
The brain vs. spinal cord lesion distribution differs between PP and RR MS subjects.
-
•
The PP group was more likely to have lesions restricted to the spinal cord.
-
•
The RR group was more likely to have lesions restricted to the brain.
1. Introduction
Primary progressive (PP) multiple sclerosis (MS) is widely recognized as a unique form of MS that can be clinically distinguished from the relapsing-remitting (RR) spectrum of the disease [1]. While the underlying biologic basis for this distinction is not known, patients with PPMS show a range of differences such as a relative resistance to T-cell directed disease-modifying therapies (DMTs), a worse prognosis, a higher representation of men, and an older age of onset compared than those with RRMS [[2], [3], [4]].
There are reported MRI differences in most studies comparing patients with PPMS to those with relapsing forms of the disease [3]. Cerebral T2 hyperintense lesions may be smaller, less in number, and develop at a lower rate in PPMS [5]. Patients with PPMS may also develop diffuse T2 hyperintense lesions in the spinal cord [6]; moreover, atrophy of the spinal cord is more common in PPMS [3]. However, the demonstration of unique MRI features in PPMS has not been consistent across all studies [2]. The goal of the present study was to compare the presence of brain versus spinal cord MRI-defined MS lesions between people with PPMS and RRMS.
2. Methods
2.1. Subjects and MRI acquisition
Subject demographic and clinical information is summarized in the Table 1.
Table 1.
Subject demographic and clinical characteristics.
| Primary progressive MS | Relapsing-remitting MS | p-value | |
|---|---|---|---|
| Number of subjects | n = 40 | n = 40 | |
| Age (years) | 50.7 ± 7.7 | 47.9 ± 4.2 | 0.049† |
| Range | 35.8–64.5 | 40.6–54.8 | |
| Men, number of subjects (%) | 17 (42.5%) | 12 (30%) | 0.352⁎ |
| Women, number of subjects (%) | 23 (57.5%) | 28 (70%) | |
| Disease duration (years)^ | 10.1 ± 7.4 | 13.7 ± 5.9 | 0.020† |
| Range | 1.10–36.0 | 1.3–28.6 | |
| Expanded Disability Status Scale score | 4.6 ± 2.1 | 1.7 ± 1.3 | 0.001‡ |
| Range | 0–8.5 | 0–6.0 | |
| Timed 25-ft walk (seconds)* | 8.4 ± 5.5 | 4.8 ± 1.1 | 0.001‡ |
| Range | 4.0–25.4 | 3.6–9.2 |
Key: data expressed as mean ± standard deviation; ^time from first symptoms; *five people with primary progressive multiple sclerosis (MS) were unable to walk. †t-test; ‡Wilcoxon test; ⁎Fisher's exact test.
The PP group was identified from a previously-reported cohort [7], from which we enrolled those with the following inclusion criteria: 1) age <65 years, 2) the availability of a suitable quality single time point 1.5T MRI scan that included the brain, cervical spinal cord, and thoracic spinal cord, 3) acquisition of MRI on one of our institution's fleet of General Electric (Milwaukee, WI) scanners (to assure consistency in the data). Once the PP MRI cohort (n = 40) was defined, we randomly selected the same number of the oldest RR people from our previous studies who had comparable MRI acquisitions available [8,9]. All individuals were part of the Comprehensive Longitudinal Investigation of MS at the Brigham and Women's Hospital and Partners MS Center (CLIMB study) [10]. Subjects had scoring of Expanded Disability Status Scale (EDSS) [11] and timed 25-ft walk (T25FW) [12] as part of the CLIMB study, performed by an MS specialist neurologist at our institution within 3 months of MRI. All people had an established MS diagnosis by the International Panel criteria [13].
2.2. MRI analysis
MRI scans were analyzed by an experienced observer for the presence of characteristic MS lesions using brain fluid-attenuated inversion-recovery (FLAIR) axial and spinal cord T2-weighted axial and sagittal sequences. Brain lesions that were non-specific (e.g. <3 mm in diameter, punctate, or linear) were excluded based on our previous work [9]. Spinal cord proton-density or short-tau inversion-recovery sequences were not performed. Because spinal cord lesions are typically not seen in the normal population [14,15], all spinal cord lesions were presumed to be MS-related. Individuals were categorized as having: 1. brain only, 2. spinal cord only, or 3. brain and spinal cord lesions.
2.3. Statistical analysis
The demographic and clinical characteristics of the two groups were compared using two sample t-tests for continuous variables (age and disease duration), Fisher's exact tests for binary and categorical variable (sex), and Wilcoxon rank sum tests for ordinal or skewed variables (EDSS, T25FW). A p-value <0.05 was considered statistically significant.
3. Results
As shown in the table, the PP group had more advanced physical disability by the EDSS and T25FW (Wilcoxon tests, both p < 0.001) and higher age (t-test p = 0.049), but lower disease duration (t-test, p = 0.02), and a similar proportion of men (Fisher's exact p = 0.352) vs. the RR group. We further explored a breakdown in characteristics among the three PP and RR MRI subgroups (i.e., brain lesions only, brain+spinal cord lesions, or spinal cord lesions only). The “brain lesions only” PP subgroup (n = 1) was lower in disease duration and higher in physical disability vs. the other two PP MRI subgroups. The two remaining PP MRI subgroups were similar in terms of disease duration and disability. There were no major differences among the RR MRI subgroups, except for slightly higher EDSS score in the “spinal cord lesions only” subgroup. This would suggest that there was no obvious explanation for why patients had lesions restricted to specific regions. This is not formally compared or presented in detail due to small sample sizes within subgroups.
MRI findings are shown in Fig. 1, Fig. 2.
Fig. 1.
Lesion location according to patient group. Lesion locations of the primary progressive (PP) and relapsing-remitting (RR) multiple sclerosis (MS) cohorts are shown. Lesions in the brain-only were less common in PP (n = 1, 2.5% of people) than RR (n = 10, 25%) (Fisher's exact p = 0.007). The presence of lesions in the spinal cord-only (PP: n = 6, 15%, RR: n = 3, 7.5%, p = 0.481) or brain plus spinal cord lesions (PP: n = 33, 83%, RR: n = 27, 68%, p = 0.196) was similar between groups.
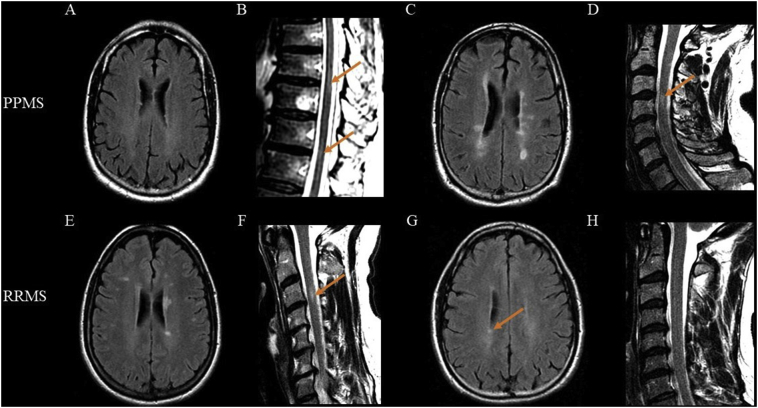
Fig. 2.
Examples of MRI findings in primary progressive and relapsing-remitting multiple sclerosis groups. Representative MRI scans at 1.5T of four cases - two each from the primary progressive (PP) and relapsing-remitting (RR) groups. Brain scans are T2-weighted fluid-attenuated inversion-recovery and spinal scans are T2-weighted fast spin-echo. (A–B): Sixty-one year-old man with PP multiple sclerosis (MS) with no brain lesion (A) but the presence of thoracic spinal cord lesions (B, orange arrows). (C–D): Fifty-two year-old man with PPMS with the presence of both brain (C, numerous lesions) and cervical spinal cord lesions (D, orange arrow). (E–F): Forty-three year-old woman with RRMS with brain (E, numerous lesions) plus a cervical spinal cord lesion (F, orange arrow). (G–H): Forty-one year-old man with RRMS with brain involvement (G, orange arrow shows a lesion) but not spinal cord lesions.
4. Discussion
Our cross-sectional study demonstrated a topographic difference in MRI lesion involvement between PP and RR forms of MS. While both brain and spinal cord involvement was common in both group, the PP group was more likely to have lesions restricted to the spinal cord and the RR group was more likely to have lesions restricted to the brain. The PP group showed other differences with the RR group, being significantly older, with higher physical disability, despite lower disease duration. These findings underscore the complementary information obtained by both brain and spinal cord MRI in the evaluation of possible PPMS; in particular, clinicians should be aware that normal brain imaging may be seen, with lesions restricted to the spinal cord, and the converse may occur in RRMS.
Previous work has shown unique topographic features regarding brain and spinal cord involvement in patients with PPMS. Similar to our results, other studies have shown a higher lesion load in the spinal cord lesion involvement in subjects with PPMS, relative to RRMS [16,17]. Additional observations have described diffuse high T2 signal in the spinal cord which is characteristic of PPMS, and is much less common in RRMS [6,18]. A clinical-MRI dissociation between physical disability and brain lesions is particularly common in PPMS [19,20]; conversely, the presence of spinal cord lesions is helpful to overcome this clinical-MRI paradox [20] and increases the sensitivity in the initial diagnosis of PPMS [18].
Spinal cord involvement in PPMS is particularly relevant to clinical status. Several studies have shown that spinal cord lesion burden correlates highly with ambulatory dysfunction and predicts worsening neurologic disability [21]. In addition to the relevance of spinal cord lesions, spinal cord atrophy, particularly of the upper cervical cord, is highly correlated with physical disability [5,22]. Taken together, these previous findings combined with the present results underscore the importance of spinal cord imaging in the diagnosis and monitoring of people with PPMS.
One study stands in contrast to our findings [16] in which subjects with PPMS had more brain MRI lesions than subjects with RRMS. Two noteworthy aspects of this study may explain the differences from the present study. First, the study was conducted from a Japanese cohort. Second, the subjects with PPMS had a high preponderance of cerebellar lesions. Thus, we could speculate that ethnicity may introduce heterogeneity in the disease characteristics of patients with PPMS. Our findings would seemingly be most applicable to an American/Western population.
Our results are line with previous observations that unique underlying pathobiologic factors may contribute to the development of PP vs. relapsing forms of MS and a propensity for spinal cord involvement [[23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36]]. In support of the notion that PP represents a unique form of the disease, which may be thought of a different from the RR spectrum, Bramow and colleagues [23] showed that PPMS is characterized histologically as having a robust regulatory and reparative mechanism protecting the brain's white matter, while the spinal cord tends to be vulnerable to impaired repair and recurrent demyelination; this was in distinction to findings from patients on the relapsing disease spectrum. Furthermore, Pagani and colleagues [24] noted that brain atrophy topographic distribution differed among MS phenotypes; cortical atrophy was characteristic of the PP group, while central/ventricular atrophy was typical in RRMS. The two subtypes of MS have somewhat different responses to immunotherapy, with two T-cell targeted therapies proven effective in RRMS failing to halt disease progression in PPMS in large phase III studies [26,27]. However, a B-cell targeted therapy, ocrelizumab, is the first therapy shown to benefit both RR and PP forms of the disease [28]. Numerous clinical features are known to differ between the two subtypes of the disease [25], such as the older age and higher rate of affected men in PP, vs. the RR spectrum [3,7,[29], [30], [31]]. Additional work from Koch and colleagues [32] have shown the subjects with PPMS and a positive family history of MS were affected at an earlier age than non-familial PP cases, while the RR spectrum showed no such relationship. Our findings may also reflect genetically determined differences between these two forms of MS. Sombekke and colleagues [33] showed, in a mixed MS sample including both RR and PP individuals, that carriers of the HLA-DRB*1501 gene were linked to a propensity for a higher spinal cord lesion burden than noncarriers; although the relationship to specific MS subtype was not reported. Furthermore, the APOE gene status [34], a variety of other differentially expressed genes [35], and serum antibodies [36] may differ between PP and RR groups.
There are several limitations of our study, which should be considered in future studies. The groups were age-matched to account for the normal aging effect on brain lesions that might have affected the diagnostic sensitivity of the study. A much younger group of RR patients than the PP patients might have overestimated the preponderance of brain lesions in the latter. However, matching the RR age led to their longer disease duration than both typical RR subjects and the selected PP group. This was not pre-planned in the study design, and may have underestimated the PP patients' cord selectivity, given that longer disease duration RR patients would be more likely to have spinal cord involvement than earlier stage RR patients. Our spinal cord imaging may have been limited by lower sensitivity with the reliance on available routine clinical images. With higher resolution research-dedicated protocols and other more sensitive sequences, such as short time inversion recovery (STIR), we may have been able to capture more lesions [37,38]. In addition, the MRI scans were performed at 1.5T; however, with the growing use of 3T scanners for routine MS care and 7T scanners for research investigations, and their potentially higher lesion sensitivity in the brain and spinal cord [15,39], it would be of interest to extend our results to higher field strengths. In addition, an investigation of quantitative lesion data and the anatomical distribution/topography of lesions [40] would provide further insight into structural differences between the groups. We also did not consider brain and spinal cord atrophy, which would have provided an additional assessment of region- and compartment-specific disease effects [22,41].
Acknowledgments
Acknowledgments
This work was presented in preliminary form at the 2015 annual meeting of the European Committee on Treatment and Research in Multiple Sclerosis (ECTRIMS), Barcelona, Spain; and at the 2016 annual meeting of the American Academy of Neurology, Vancouver, Canada. We thank Dr. Fariha Khalid for technical assistance.
Disclosure statement
Dr. Dastagir received research support from Novartis. Dr. Healy received research support from Merck-Serono, Genzyme, and Novartis. Dr. Chitnis received consulting fees from Biogen, Merck-Serono, Alexion and received research support from Merck-Serono and Novartis. Dr. Weiner has received consulting fees from Biogen, Nasvax, Novartis, Merck-Serono, and Teva Neurosciences, and has received grant support from Merck-Serono and Sanofi-Genzyme. Dr. Bakshi has received consulting fees from EMD Serono, Genentech, Guerbet, Sanofi-Genzyme, and Shire and research support from EMD Serono and Sanofi-Genzyme. The other authors have nothing to disclose.
Funding
None.
References
- 1.Katz-Sand I.B., Lublin F.D. Diagnosis and differential diagnosis of multiple sclerosis. Continuum (Minneap. Minn.) 2013;19:922–943. doi: 10.1212/01.CON.0000433290.15468.21. [DOI] [PubMed] [Google Scholar]
- 2.Kuchling J., Ramien C., Bozin I. Identical lesion morphology in primary progressive and relapsing-remitting MS--an ultrahigh field MRI study. Mult Scler. 2014;20:1866–1871. doi: 10.1177/1352458514531084. [DOI] [PubMed] [Google Scholar]
- 3.Miller D.H., Leary S.M. Primary-progressive multiple sclerosis. Lancet Neurol. 2007;6:903–912. doi: 10.1016/S1474-4422(07)70243-0. [DOI] [PubMed] [Google Scholar]
- 4.Comi G. Disease-modifying treatments for progressive multiple sclerosis. Mult Scler. 2013;19:1428–1436. doi: 10.1177/1352458513502572. [DOI] [PubMed] [Google Scholar]
- 5.Ingle G.T., Sastre-Garriga J., Miller D.H. Is inflammation important in early PPMS? A longitudinal MRI study. J Neurol Neurosurg Psychiatry. 2005;76:1255–1258. doi: 10.1136/jnnp.2004.036590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kearney H., Miller D.H., Ciccarelli O. Spinal cord MRI in multiple sclerosis, prognostic and clinical value. Nat Rev Neurol. 2015;11:327–338. doi: 10.1038/nrneurol.2015.80. [DOI] [PubMed] [Google Scholar]
- 7.Raghavan K., Healy B.C., Carruthers R.L. Progression rates and sample size estimates for PPMS based on CLIMB study population. Mult Scler. 2015;21:180–188. doi: 10.1177/1352458514541976. [DOI] [PubMed] [Google Scholar]
- 8.Bakshi R., Neema M., Healy B.C. Predicting clinical progression in multiple sclerosis with the magnetic resonance disease severity scale. Arch Neurol. 2008;65:1449–1453. doi: 10.1001/archneur.65.11.1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Neema M., Guss Z.D., Stankiewicz J.M. Normal findings on brain fluid-attenuated inversion recovery MR images at 3T. AJNR Am J Neuroradiol. 2009:911–916. doi: 10.3174/ajnr.A1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gauthier S.A., Glanz B.I., Mandel M. A model for the comprehensive investigation of a chronic autoimmune disease: the multiple sclerosis CLIMB study. Autoimmun Rev. 2006;5:532–536. doi: 10.1016/j.autrev.2006.02.012. [DOI] [PubMed] [Google Scholar]
- 11.Kurtzke J.F. Rating neurologic impairment in multiple sclerosis an expanded disability status scale (EDSS) Neurology. 1983;33:1444–1452. doi: 10.1212/wnl.33.11.1444. [DOI] [PubMed] [Google Scholar]
- 12.Fischer J.S., Rudick R.A., Cutter G.R. Multiple sclerosis functional composite measure (MSFC): an integrated approach to MS clinical outcome assessment. National MS Society Clinical Outcomes Assessment Task Force. Mult Scler. 1999;5:244–250. doi: 10.1177/135245859900500409. [DOI] [PubMed] [Google Scholar]
- 13.Polman C.H., Reingold S.C., Banwell B. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol. 2011;69:292–302. doi: 10.1002/ana.22366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stankiewicz J.M., Neema M., Alsop D.C. Spinal cord lesions and clinical status in multiple sclerosis: a 1.5 T and 3 T MRI study. J Neurol Sci. 2009;279:99–105. doi: 10.1016/j.jns.2008.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stankiewicz J.M., Glanz B.I., Healy B.C. Brain MRI lesion load at 1.5T and 3T versus clinical status in multiple sclerosis. J Neuroimaging. 2011;21:e50–e56. doi: 10.1111/j.1552-6569.2009.00449.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kira J., Tobimatsu S., Goto I. Primary progressive versus relapsing remitting multiple sclerosis in Japanese patients: a combined clinical, magnetic resonance imaging and multimodality evoked potential study. J Neurol Sci. 1993;117:179–185. doi: 10.1016/0022-510x(93)90171-t. [DOI] [PubMed] [Google Scholar]
- 17.Filippi M., Bozzali M., Horsfield M.A. A conventional and magnetization transfer MRI study of the cervical cord in patients with MS. Neurology. 2000;54:207–213. doi: 10.1212/wnl.54.1.207. [DOI] [PubMed] [Google Scholar]
- 18.Bot J.C., Barkohf F. Spinal-cord MRI in multiple sclerosis: conventional and nonconventional MR techniques. Neuroimaging Clin N Am. 2009;19:81–99. doi: 10.1016/j.nic.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 19.Nijeholt G.J., van Walderveen A.A., Castelijns J.A. Brain and spinal cord abnormalities in multiple sclerosis. Correlation between MRI parameters, clinical subtypes and symptoms. Brain. 1998;121:687–697. doi: 10.1093/brain/121.4.687. [DOI] [PubMed] [Google Scholar]
- 20.Healy B.C., Buckle G.J., Ali E.N. Chracterizing clinical and MRI dissociation in patients with multiple sclerosis. J Neuroimaging. 2017;27:481–485. doi: 10.1111/jon.12433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lukas C., Knol D.L., Sombekke M.H. Cervical spinal cord volume loss is related to clinical disability progression in multiple sclerosis. J Neurol Neurosurg Psychiatry. 2015;86:410–418. doi: 10.1136/jnnp-2014-308021. [DOI] [PubMed] [Google Scholar]
- 22.Cohen A.B., Neema M., Arora A. The relationships among MRI-defined spinal cord involvement, brain involvement, and disability in multiple sclerosis. J Neuroimaging. 2012;22:122–128. doi: 10.1111/j.1552-6569.2011.00589.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bramow S., Frischer J.M., Lassmann H. Demyelination versus remyelination in progressive multiple sclerosis. Brain. 2010;133:2983–2998. doi: 10.1093/brain/awq250. [DOI] [PubMed] [Google Scholar]
- 24.Pagani E., Rocca M.A., Gallo A. Regional brain atrophy evolves differently in patients with multiple sclerosis according to clinical phenotype. AJNR Am J Neuroradiol. 2005;26:341–346. [PMC free article] [PubMed] [Google Scholar]
- 25.Lublin F.D., Reingold S.C., Cohen J.A. Defining the clinical course of multiple sclerosis: the 2013 revisions. Neurology. 2014;83:278–286. doi: 10.1212/WNL.0000000000000560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lublin F., Miller D.H., Freedman M.S. Oral fingolimod in primary progressive multiple sclerosis (INFORMS): a phase 3, randomised, double-blind, placebo-controlled trial. Lancet. 2016;387:1075–1084. doi: 10.1016/S0140-6736(15)01314-8. [DOI] [PubMed] [Google Scholar]
- 27.Wolinsky J.S., Narayana P.A., O'Connor P. Glatiramer acetate in primary progressive multiple sclerosis: results of a multinational, multicenter, double-blind, placebo-controlled trial. Ann Neurol. 2007;61:14–24. doi: 10.1002/ana.21079. [DOI] [PubMed] [Google Scholar]
- 28.Mulero P., Midaglia L., Motalban X. Ocrelizumab: a new milestone in multiple sclerosis therapy. Adv. Neurol. Disord. 2018;11 doi: 10.1177/1756286418773025. (1756286418773025) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cottrell D.A., Kremenchutzky M., Rice G.P. The natural history of multiple sclerosis: a geographically based study. 5. The clinical features and natural history of primary progressive multiple sclerosis. Brain. 1999;122:625–639. doi: 10.1093/brain/122.4.625. [DOI] [PubMed] [Google Scholar]
- 30.Thompsom A.J., Polman C.H., Milller D.H. Primary progressive multiple sclerosis. Brain. 1997;120:1085–1096. doi: 10.1093/brain/120.6.1085. [DOI] [PubMed] [Google Scholar]
- 31.Correale J., Gaitlan M.I. Ysaraelit, et al. progressive multiple sclerosis: from pathogenic mechanism to treatment. Brain. 2017;140:527–546. doi: 10.1093/brain/aww258. [DOI] [PubMed] [Google Scholar]
- 32.Koch M., Uyttenboogaart M., Heerings M. Progression in familial and nonfamilial MS. Mult Scler. 2008;14:300–306. doi: 10.1177/1352458507084269. [DOI] [PubMed] [Google Scholar]
- 33.Sombekke M.H., Lukas C., Crusius J.B. HLA-DRB1*1501 and spinal cord magnetic resonance imaging lesions in multiple sclerosis. Arch Neurol. 2009;66:1531–1536. doi: 10.1001/archneurol.2009.278. [DOI] [PubMed] [Google Scholar]
- 34.Losonczi E., Bencsik K., Fricska Nagy Z. APOE epsilon status in Hungarian patients with primary progressive multiple sclerosis. Swiss Med Wkly. 2010;w13119:140. doi: 10.4414/smw.2010.13119. [DOI] [PubMed] [Google Scholar]
- 35.Koch M.W., Ilnytskyy Y., Golubov A. Global transcriptome profiling of mild relapsing-remitting versus primary progressive multiple sclerosis. Eur J Neurol. 2018;25:651–658. doi: 10.1111/ene.13565. [DOI] [PubMed] [Google Scholar]
- 36.Lucchinetti C., Bruck W. The pathology of primary progressive multiple sclerosis. Mult Scler. 2004;10(Suppl. 1):S23–S30. doi: 10.1191/1352458504ms1027oa. [DOI] [PubMed] [Google Scholar]
- 37.Tummala S., Singhal T., Oomen V. Spinal cord MRI as an adjunct to the brain in defining “no evidence of disease activity” in multiple sclerosis. Int. J. MS Care. 2017;19:158–164. doi: 10.7224/1537-2073.2016-068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Campi A., Pontesilli S., Gerevini S. Comparison of MRI pulse sequences for investigation of lesions of the cervical spinal cord. Neuroradiology. 2000;42:669–675. doi: 10.1007/s002340000368. [DOI] [PubMed] [Google Scholar]
- 39.Dula A.N., Pawate S., Dortch R.D. Magnetic resonance imaging of the cervical spinal cord in multiple sclerosis at 7T. Mult Scler. 2016;22 doi: 10.1177/1352458515591070. (320–238) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Di Perri C., Battaglini M., Stromillo M.L. Voxel-based assessment of differences in damage and distribution of white matter lesions between patients with primary progressive and relapsing-remitting multiple sclerosis. Arch Neurol. 2008;65:236–243. doi: 10.1001/archneurol.2007.51. [DOI] [PubMed] [Google Scholar]
- 41.Sharma J., Sanfilipo M., Benedict R.H. Whole-brain atrophy in multiple sclerosis measured by automated verses semiautomated MR imaging segmentation. AJNR Am J Neuroradiol. 2004;25:985–996. [PMC free article] [PubMed] [Google Scholar]