DEAR EDITOR
Hidradenitis suppurativa (HS), an inflammatory disorder of hair follicles in intertriginous areas, manifests as debilitating acute subcutaneous nodules with subsequent chronic sinus tract formation and scarring.1 Given the limited effectiveness of medical therapies, targeting the immunomodulatory mechanisms that mediate disease progression is of great interest.
Histological studies demonstrate macrophage infiltration in both acute and chronic HS lesions.2,3 Macrophage polarity is a vital regulator of inflammatory disease. Upon tissue extravasation, blood monocytes differentiate into tissue resident macrophages and depending on environmental cues polarize into M1/M2 subsets. M1 macrophages promote a pro-inflammatory environment in response to intracellular pathogens, IFNγ, TNFα, among other cytokines. Whereas M2 macrophages mediate wound healing, differentiation of fibroblasts to myofibroblasts, collagen deposition, attenuation of inflammation, and tissue fibrosis.4,5
Emerging data suggest that macrophage-mediated release of TNFα, IL-12, and IL-23 contribute to HS disease pathogenesis6–8 indicating a potential role for M1 macrophages in HS lesions. As the disease progresses, sinus tract formation and scarring predominate suggesting dysregulation of the homeostasis between fibroblast-mediated collagen deposition and extracellular matrix (ECM) degradation exemplifying a role for M2 macrophages in chronic HS lesions. CD163, a member of the cysteine-rich scavenger receptor family and M2 marker, plays a key role in mediating internalization of hemoglobin-haptoglobin complexes by macrophages9 and has been detected in the dermis of lesional HS tissue.10 CD163+ M2 macrophages also contribute to the aforementioned pro-fibrotic states via the release of the chemokine CCL18.5,11 We hypothesized that CD163+ macrophage-mediated CCL18 production stimulates excess collagen deposition in chronic fibrotic HS lesions. Thus, we evaluated the expression of CD163+ macrophages and CCL18 in chronic HS lesions compared to perilesional and normal tissue.
The study was approved by our institution’s Institutional Review Board. Normal, perilesional, and lesional HS tissues were obtained from surgical resections and from our institution’s tissue bank. Patients with Hurley stage II and III were considered to have chronic HS, i.e., sinus tracts and fibrotic tissue. Visual assessment at the time of surgery as well as clinical and histopathological diagnosis were validated for verification. Immunohistochemistry (IHC) was performed to determine human tissue resident macrophages (CD68) and M2 macrophages (CD163+) infiltration.12 Normal and lesional HS tissues were subjected to single-cell dissociation via the GentleMACs Octo Dissociator (Miltenyi Biotec, Inc.) and analyzed by flow cytometry to determine surface expression of CD68 (anti-human CD68-PE; 10μl/million cells) and CD163 (anti-human CD163-FITC; 10 μl/million cells) (Miltenyi Biotec, Inc.). We assessed CCL18 by immunofluorescence (IF) microscopy (PeproTech; 5 μg/ml). In addition, slides were analyzed by hematoxylin and eosin (H&E), Masson’s trichrome (MT), and Picrosirius Red (PSR) staining for histological and collagenofibrotic characterization.
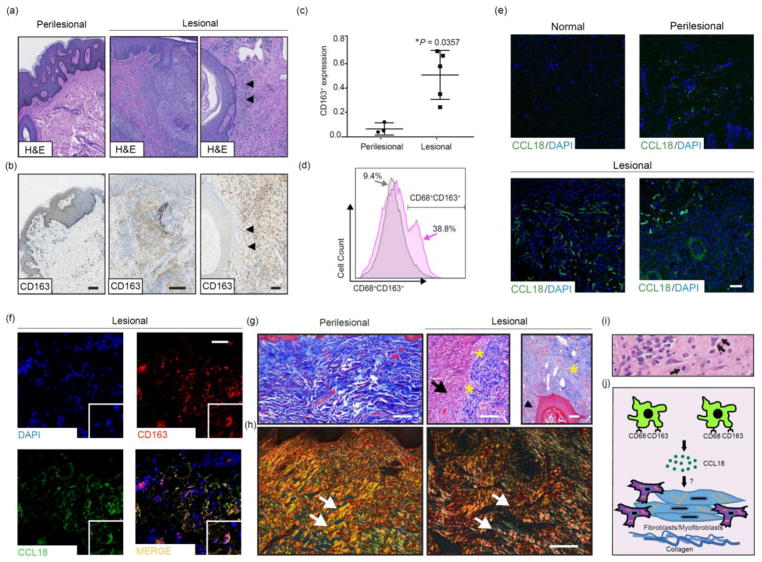
Microscopic analysis of perilesional and lesional HS tissue is consistent with known HS histopathological findings (Fig. 1a).2 CD163 immunohistochemistry demonstrated a significantly more abundant infiltrate of CD163+ cells, including myofibroblasts and CD163+ macrophages, in lesional HS tissue compared to perilesional (Fig. 1b). Notably, extensive infiltration of CD163+ macrophages surrounds hair follicles in lesional HS tissue (Figs. 1a and 1b, black arrowheads). Figure 1c shows elevated CD163 protein expression in lesional tissue regions compared to perilesional tissue regions (mean ± SD, p=0.0357, Mann-Whitney U test). To validate the CD163+ population, we generated single-cell suspensions from lesional tissue for comparison with normal tissue. In lesional tissue, flow cytometry revealed a 29.4% increase in CD68+CD163+ cells, suggesting polarization to the M2 phenotype in chronic HS (Fig. 1d). To further implicate a functional significance of CD163+ cells in chronic HS, there was an increased IF signal of the pro-fibrotic chemokine, CCL18 (Fig. 1e). As shown in Figure 1f, CD163 (red) co-localized with CCL18 (green), accompanied by collagen deposition (MT, Fig. 1g) in the dermis of lesional HS tissue compared to perilesional tissue. Areas of inflammation (Fig. 1g, black arrow), including surrounding hair follicles (Fig. 1g, black arrowhead), showed sparse immature thin collagen fibrils increasing towards the periphery of the inflammatory site, with scant macrophages and fibroblasts embedded within the collagen bundles (Fig. 1g, yellow asterisks). PSR staining further assessed collagen deposition, which showed areas of thin, immature collagen fibrils in lesional HS tissue. In comparison, perilesional tissue revealed thick, mature, and very dense collagen fibers (Fig. 1h, white arrows) similar to normal skin.13 Figure 1i illustrates a mixed inflammatory infiltrate, including macrophages (black arrow) and reactive myofibroblasts (black double arrows) in chronic lesional tissue.
Figure 1. Immunohistochemistry, flow cytometry, and immunofluorescence findings of chronic HS lesions.
(a) Perilesional and chronic lesional HS tissue sections demonstrate dermal infiltration of inflammatory cells, including macrophages in affected areas (n=15, H&E). (b) Immunohistochemistry demonstrates a CD163+ infiltrate in the dermis of in HS chronic lesional tissue (n=8, 20x, scale bar 100 μm outer panels; 10x, scale bar 250 μm middle panel), especially surrounding hair follicles (black arrowheads, 20x, scale bar 100 μm). (c) Quantification of CD163 protein expression in perilesional and lesional regions (mean ± SD, *p=0.0357, Mann-Whitney U test). (d) Flow cytometry demonstrates a 29.4% increase in CD68+C163+ cells in chronic HS lesions (n=3, pink) compared to normal control tissue (n=2, grey). Live single cells were used to set population gates followed by selecting CD68+ cells. (e) Indirect immunofluorescence for CCL18 in chronic HS lesions (n=5), normal control tissue (n=3) and perilesional tissue (n=3) (10x, scale bar 250 μm). (f) Double indirect immunofluorescence staining for CD163 (red), CCL18 (green), and DAPI for nuclei staining (blue). The images for CD163 and CCL18 were merged (yellow-gold) (n=5, 20x, scale bar 100 μm). (g) HS chronic lesional tissue (n=5) show inflammatory infiltrate (black arrow) adjacent to collagen (yellow asterisks, blue stained material) as well as near a hair follicle (black arrowhead) (10x, scale bar 250 μm, perilesional (n=5), Masson’s trichrome (MT)). (h) Picrosirius Red (PSR) staining shows more organized thick, mature, dense bundles of collagen in perilesional tissue (n=1) and thinner, less mature, loose collagen bundles in the chronic lesional HS tissue (n=2) (white arrows, 20x, scale bar 250 μm). (i) Mixed infiltrate including macrophages (black arrow) and reactive myofibroblasts (black double arrow) are present in chronic lesional tissue, 60x. (j) Schematic of proposed role for macrophages in chronic HS: CD68+CD163+ macrophage release of CCL18 stimulates fibroblast-mediated collagen deposition as seen clinically in chronic HS lesions.
In summary, we have demonstrated a potential CD163+ M2 macrophage-mediated CCL18 upregulation in HS and stimulation of fibroblast-mediated collagen production may play a role in HS disease progression to chronic lesions (Fig. 1j). The main limitation of this study is the small sample size. Furthermore, while reported as an M2 macrophage marker,9,12 CD163 may not distinctly identify M2 macrophages in inflammation,14 therefore, a more extensive determination of cellular mechanisms with broader analysis of cell surface markers and co-localization experiments could elucidate the inflammatory cell repertoire associated with discrete stages in the immunological evolution of HS progression. Studies of the in vivo contributions of M1 and M2 macrophage subsets based on HS stage/severity could identify the cross-talk between fibroblasts/myofibroblasts, ECM, and inflammatory cells, thus contributing to our understanding of the pathogenesis of HS and guiding more targeted treatment strategies.
Acknowledgments
Funding: Valeant Pharmaceuticals - This work was supported in part by a grant from the Valeant Pharmaceuticals, who had no involvement in the conception of the research idea, conduct of the experiments, and the writing of this manuscript.
We kindly thank Pierre Coulombe, PhD, Carmelo Carmona-Rivera, PhD, Karen Brown, PhD, Marc Edwards, PhD, Jonathan Reichner, PhD, Nate Archer, PhD, Robert Miller, Nora Viloria, and the JHUSOM Reference Histology and Immunopathology Cores.
Footnotes
Conflict of Interest: Dr. Ginette A. Okoye is a consultant for AbbVie.
References
- 1.Alavi A, Anooshirvani N, Kim WB, Coutts P, et al. Quality-of-life impairment in patients with hidradenitis suppurativa: a Canadian study. Am J Clin Dermatol. 2015;16:61–65. doi: 10.1007/s40257-014-0105-5. [DOI] [PubMed] [Google Scholar]
- 2.van der Zee HH, de Ruiter L, Boer J, et al. Alterations in leucocyte subsets and histomorphology in normal-appearing perilesional skin and early and chronic hidradenitis suppurativa lesions. Br J Dermatol. 2012;166:98–106. doi: 10.1111/j.1365-2133.2011.10643.x. [DOI] [PubMed] [Google Scholar]
- 3.Shah A, Alhusayen R, Amini-Nik S. The critical role of macrophages in the pathogenesis of hidradenitis suppurativa. Inflamm Res. 2017;66:931–945. doi: 10.1007/s00011-017-1074-y. [DOI] [PubMed] [Google Scholar]
- 4.Zarif JC, Hernandez JR, Verdone JE, et al. A phased strategy to differentiate human CD14+ monocytes into classically and alternatively activated macrophages and dendritic cells. Biotechniques. 2016;61:33–41. doi: 10.2144/000114435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Murray PJ, Allen JE, Biswas SK, et al. Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity. 2014;41:339–340. doi: 10.1016/j.immuni.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van der Zee HH, De Ruiter L, van den Broaecke DG, et al. Elevated levels of tumour necrosis factor (TNF)-α, interleukin (IL)-1β and IL-10 in hidradenitis suppurativa skin: a rationale for targeting TNF-α and IL-1β. Br J Dermatol. 2011;164:1292–1298. doi: 10.1111/j.1365-2133.2011.10254.x. [DOI] [PubMed] [Google Scholar]
- 7.Morgan B, Sweeney CM, Hughes R, et al. Hidradenitis suppurativa is characterized by dysregulation of the Th17:Treg cell axis, which is corrected by anti-TNF therapy. J Invest Dermatol. 2017;137:2389–2395. doi: 10.1016/j.jid.2017.05.033. [DOI] [PubMed] [Google Scholar]
- 8.Schlapbach C, Hänni T, Yawalkar N, Hunger RE. Expression of the IL-23/Th17 pathway in lesions of hidradenitis suppurativa. J Am Acad Dermatol. 2011;65:790–698. doi: 10.1016/j.jaad.2010.07.010. [DOI] [PubMed] [Google Scholar]
- 9.Schaer DJ, Schaer CA, Buehler PW, et al. CD163 is the macrophage scavenger receptor for native and chemically modified hemoglobins in the absence of haptoglobin. Blood. 2006;107:373–380. doi: 10.1182/blood-2005-03-1014. [DOI] [PubMed] [Google Scholar]
- 10.Schlapbach C, Yawalkar N, Hunger RE. Human beta-defensin-2 and psoriasin are overexpressed in lesions of acne inversa. J Am Acad Dermatol. 2009;61:58–65. doi: 10.1016/j.jaad.2008.12.033. [DOI] [PubMed] [Google Scholar]
- 11.Furukawa S, Moriyama M, Tanaka A, et al. Preferential M2 macrophages contribute to fibrosis in IgG4-related dacryoadenitis and sialoadenitis, so-called Mikulicz’s disease. Clin Immunol. 2015;156:9–18. doi: 10.1016/j.clim.2014.10.008. [DOI] [PubMed] [Google Scholar]
- 12.Williams DW, Engle EL, Shirk EN, et al. Splenic Damage during SIV Infection: Role of T-Cell Depletion and Macrophage Polarization and Infection. Am J Pathol. 2016;186:2068–2087. doi: 10.1016/j.ajpath.2016.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lattouf R, Younes R, Lutomski D, et al. Picrosirius Red Staining: A Useful Tool to Appraise Collagen Networks in Normal and Pathological Tissues. J Histochem Cytochem. 2014;62:751–780. doi: 10.1369/0022155414545787. [DOI] [PubMed] [Google Scholar]
- 14.Barros MH, Hauck F, Dreyer JH, et al. Macrophage Polarisation: an Immunohistochemical Approach for Identifying M1 and M2 macrophages. PLoS One. 2013;8:e80908. doi: 10.1371/journal.pone.0080908. [DOI] [PMC free article] [PubMed] [Google Scholar]