Abstract
Manipulation of human T cell functioning by delivery of macromolecules such as DNA, RNA, or protein is limited, unless the human T cells have been stimulated or electropermeabilized. To achieve successful adaptation and survival of a grafted organ, the alloreactive T cells that induce graft rejection must be regulated. Corticosteroids, calcineurin inhibitors, and mTOR inhibitors, which are systemic immunosuppressants, are currently used for transplantation, with significant side effects. In this study, we demonstrated that a cell-permeable peptide (CPP), dNP2, could efficiently deliver proteins into human CD4 and CD8 T cells. We confirmed regulatory functioning of the cytoplasmic domain of CTLA-4 conjugated with dNP2 (dNP2-ctCTLA-4) in human T cell activation, proliferation, and chemokine receptor expression. We utilized a human skin allograft system in SCID/beige mice to examine whether dNP2-ctCTLA-4 could inhibit allograft rejection by controlling T cell responses. The grafted skin tissue inflammation, allogeneic T cell infiltration, and blood cytokine level was markedly reduced by dNP2-ctCTLA4, resulting in successful transplantation. In addition, it also inhibited T cell alloresponses against microvessels formed form Bcl-2-transduced human umbilical vein endothelial cells implanted into Balb/c Rag1−/−/IL-2Rγ−/− double knockout (DKO) mice, assessed as reduced T cell infiltration and granzyme B expression. These results collectively suggest that dNP2 peptide conjugation offers a valuable tool for delivering macromolecules like proteins into human T cells, and dNP2-ctCTLA-4 is a novel agent that shows potential in controlling human T cell responses to allow successful adaptation of grafted tissues.
Keywords: dNP2, ctCTLA-4, human T cell, alloresponse, transplantation, graft rejection
1. Introduction
Delivery of macromolecules into human T cells to manipulate their functioning has been accomplished mainly through viral gene delivery [1, 2] or electropermeabilization [3] of the cell membrane. These methods require stimulation of human T cells ex vivo to optimize delivery efficiency and viral vector-based gene transfer, as in vivo circumstances may have potential safety issues in certain clinical applications [4]. In such situations, cell-permeable peptides (CPPs) offer a potential alternative method, with the ability to deliver macromolecules such as DNAs, RNAs and proteins into intact target cells [5]. However, existing clinical examples of CPP use are limited to treatment of cancer [6], cardiovascular disease [7], myocardial infarction [8] and muscular dystrophy [9], which are not directly related to T cell response.
Acute allogeneic graft rejection is a model for understanding pathological processes mediated by adaptive immune responses involving T cells and/or antibodies and limits the effectiveness of organ transplantation, a potentially life-saving procedure for treating end-stage organ failure. Specifically, alloreactive human T cells act as inducers of inflammatory responses as well as effectors of direct cytotoxicity, two processes that underlie cell-mediated allograft rejection [10]. Combinations of small molecules, including cyclosporine A (CsA) [11], tacrolimus (FK506) [12], mTOR inhibitors [13], mycophenolate mofetil [14] and corticosteroids [15], are widely used to chronically suppress T cell-mediated rejection, and monoclonal antibodies (mAb) have also been used to specifically target extracellular CD3 [16], CD25 [17], and CD52 [18], depleting T cells as part of induction therapy. Although treatment with T cell-depleting mAbs can reduce graft rejection rates, the development of novel graft rejection therapeutics that do not deplete T cell populations is still needed because of toxicities and concern for increased infection risk [19].
The activation of naïve T cells typically requires a second signal, characteristically delivered through ligands on antigen-presenting cells that engage CD28. Once a T cell is activated, it will typically express cytotoxic lymphocyte antigen-4 (CTLA-4), which competes with CD28 for the same ligands and, being of higher affinity, limits further CD28 signaling. A fusion protein composed of a modified extracellular portion of CTLA-4 and the Fc region of human IgG (belatacept) has been studied in renal transplantation [20]. Like endogenously expressed CTLA-4, belatacept targets co-stimulatory molecules on antigen-presenting cells and prevents their interaction with CD28 expressed on resting T cells. However, this approach has three limitations. First, CTLA-4 is more than a competitor of CD28, signaling in its own right to deliver inhibitory signals to activated effector T cells; it was the first immune checkpoint molecule to be identified in this role [21, 22]. Notably, the signaling domain of CTLA-4 without its associated ligand has been reported to inhibit the secretion of IL-2 and activation of T cell receptor signaling molecules such as ZAP70, emphasizing the importance of the cytoplasmic domain signaling of CTLA-4 [23, 24]. Second, CTLA-4 is constitutively expressed in Foxp3+ regulatory T cells (Tregs), and enhances, rather than inhibits, Treg suppressive functions [25]. These observations suggest that delivering CTLA-4-mediated signals to T cells would have actions distinct from those of belatacept, inhibiting T effector cells while stimulating Tregs. Third, alloreactive memory T cells, which are abundant in adult humans and whose frequency better correlates with rejection than naïve T cells, can receive co-stimulation through other than CD28 and sometimes lack CD28 altogether. We previously evaluated dNP2-CPP, which enables intracellular delivery of the cytoplasmic domain of CTLA-4 (dNP2-ctCTLA-4) in murine T cells and ameliorated murine autoimmune encephalomyelitis by inhibiting T helper 1 cell (Th1) and T helper 17 cell (Th17) responses [26]. However, its clinical potential in humans is unclear and its therapeutic effects on primary human T cells must first be determined.
In the present study, we evaluated the protein delivery efficiency of dNP2 in primary human T cells without any stimulation or chemical/physical disruptions of the cell membrane, along with the functional ability of dNP2-ctCTLA-4 to control alloreactive human T cell responses to grafted tissues in vivo. We designed both human skin graft and human umbilical vascular endothelial cell (HUVEC) graft experiments in immune-deficient mice. We demonstrate that dNP2 delivers its cargo protein into primary human T cells effectively compared to TAT, and that dNP2-ctCTLA-4 remarkably regulates human T cell activation, cytokine production, proliferation, and infiltration both in vitro and in vivo, reducing effector responses of alloreactive T cells and resulting in successful protection of grafted human tissues.
2. Materials and Methods
Purification of recombinant proteins.
dNP2-ctCTLA-4 and dNP2-EGFP were purified using bacterial systems as previously described [26]. In brief, BL21 (DE3) Star pLysS were transformed with pRSET-b plasmids encoding the proteins. The colony was cultured in ampicillin-containing Luria-Bertani broth media at 37°C, and protein expression was induced by 1 mM isopropyl-β-D-thiogalactopyranoside (IPTG; Sigma-Aldrich). The 6-His-tagged proteins were purified via Ni-NTA (Qiagen) affinity chromatography in either native (dNP2EGFP) or denatured (dNP2-ctCTLA-4) condition and desalted using a PD-10 Sephadex G-25 column (GE Healthcare Life Sciences). To obtain highly purified proteins, an additional ion-exchange protein purification step was performed using SP Sepharose High Performance (GE Healthcare Life Sciences), followed by desalting on a PD-10 Sephadex G-25 column. Proteins were stored at −80°C and the concentrations were measured using the Bradford solution (Bio-Rad) right before the experiments.
Animals.
C.B-17 SCID/beige female mice and Rag1−/−IL-2rγnull (DKO) mice were used at about 8 weeks of age. All protocols involving animals were approved by the Yale Animal Care and Use committee. For skin graft experiments, the animals were housed individually in microisolator cages and fed autoclaved food and water. For HUVEC-collagen gel graft experiments, 4–5 animals were housed in microisolator cages and fed autoclaved food and water.
Cell lines and cell culture
EL4 (mouse lymphoma T cell line) and Jurkat (human lymphoma T cell line) cells were purchased from the American Type Culture Collection (ATCC) and cultured using Roswell Park Memorial Institute (RPMI) 1640 media (Corning) with 10% fetal bovine serum (FBS; Corning) and 1% penicillin/streptomycin antibiotics (HyClone). All cells were maintained in a 5% CO2 incubator at 37°C.
Human skin graft model.
SCID/beige mice were given two human skin grafts as previously described [27–29]. In brief, human skin was obtained from cadaveric donors through the Yale University Skin Bank under a protocol approved by the Yale Human Investigations Committee. Next, 0.5-mm-thick sheets were divided into 1-cm2 pieces, kept at 4°C in RPMI 1640 medium (Corning), and fixed onto similarly sized defects on the dorsum of SCID/beige recipients using staples. After ~4 weeks, 2 × 108 isolated human peripheral blood mononuclear cells from an allogeneic donor in 500 μl of PBS were transferred intraperitoneally. PBS or 50 μg of dNP2ctCTLA-4 were injected intraperitoneally every other day for 2 weeks. On day 14, the blood was collected through cardiac puncture and skin grafts were harvested and prepared as paraffin or frozen blocks.
HUVEC-collagen gel graft model.
DKO mice were given two HUVEC-collagen gel grafts as previously described [30]. In brief, HUVECs were isolated by collagenase treatment of human umbilical veins under a protocol approved by the Yale Human Investigation Committee and cultured in Medium 199 containing 20% fetal calf serum (both from Thermo Fisher Scientific), 50 μg/mL EC growth supplement, 100 μg/mL porcine intestinal heparin, 2 mM L-glutamine, 100 U/mL penicillin, and 100 μg/mL streptomycin (Corning). HUVECs were transfected with retroviral vectors expressing the caspase-resistant form of Bcl-2 in the presence of polybrene daily for up to 3 days. After that, HUVECs (3 × 106 cells) were suspended in 1 mL of a solution containing rat tail type 1 collagen (1.5 mg/mL; BD Biosciences), human plasma fibronectin (100 μg/mL; Sigma-Aldrich), 25 mM HEPES, 0.075% NaHCO3 (both from HyClone), and 10% fetal calf serum in M199 on ice. The pH was adjusted to 7.5 by 0.1 M NaOH. The cell suspension was pipetted into a 24-well plate and warmed to 37°C for 15 min to allow for polymerization of the collagen. For implantation into DKO mice, the gels were harvested and bisected 24 h after gel formation. Each gel segment was implanted subcutaneously into a bluntly dissected abdominal wall of the mouse. The wound was closed with staples. After 11 days, the staples were removed and 3 × 107 isolated human peripheral blood mononuclear cells from an allogeneic donor in 500 μl of PBS were transferred intraperitoneally. PBS or 50 μg of dNP2-ctCTLA-4 were injected intraperitoneally every other day for 3 weeks. At day 21, the blood was collected through cardiac puncture and HUVEC-collagen gel grafts were harvested and prepared as paraffin or frozen blocks.
Peripheral blood mononuclear cells isolation.
Human peripheral blood mononuclear cells (PBMCs) were obtained from healthy donors by leukapheresis under a protocol approved by the Institutional Review Board of Hanyang University or the Yale Human Investigation Committee. PBMCs were isolated by density gradient centrifugation using lymphocyte separation medium. The cells were stored in 10% DMSO in liquid nitrogen for further use, thawed, and washed before use.
Tissue histology.
The paraffin blocks were sectioned (5-μm-thick) for immunostaining. Antihuman CD45RO antibody was applied overnight at 4°C. The slides were incubated with biotinylated secondary antibody for 1 h and washed with PBS. Slides were then incubated for 1 h with ABC reagent and incubated with diaminobenzidine (DAB; Vector Lab., Inc.) peroxide substrate until the desired color developed, followed by hematoxylin staining. The slides were observed by bright field microscope. To quantify tissue infiltration of lymphocytes, the number of human CD45RO T cells within the cross-section of the tissues (skin grafts or HUVEC-collagen gel grafts) was measured using Image J 1.50i software.
Immunofluorescence.
The frozen blocks were sectioned (8-μm-thick) for immunostaining. PEconjugated anti-human CD3, PE-conjugated anti-human CD8, and FITC-conjugated anti-human CD4 antibodies (all from BD Biosciences) were applied overnight at 4°C. The slides were washed and stained with DAPI to evaluate the nuclei. The slides were observed by fluorescence microscopy. To quantify tissue infiltration of CD4 or CD8 T cells, the number of markerpositive cells for each subset within the cross-section of the tissues was measured using Image J 1.50i software. To stain live HUVECs in the gel tissues, paraffin blocks were sectioned (5-μmthick) and incubated with FITC-conjugated ULEX (Vector Lab., Inc.) overnight at 4°C. The slides were washed and stained with DAPI (Thermo Fisher Scientific) to evaluate the nuclei. The slides were observed by fluorescence microscopy.
Blood cell flow cytometry.
Collected blood samples from in vivo experiments were separated into serum and blood cells. The cells were washed with PBS and erythrocytes were lysed using Ack buffer. After erythrocyte lysis, the remaining lymphocytes were fixed using fixation buffer (BD Biosciences) overnight at 4°C. The cells were analyzed by flow cytometry after staining with a specific combination of fluorescently-labelled antibodies against CD45, CD4, CD8 (all from BD Biosciences), granzyme B, and Foxp3 (both from Thermo Fisher Scientific). For intracellular staining, a Foxp3 staining kit (Thermo Fisher Scientific) was used according to the manufacturer’s instructions.
Luminex assay.
Collected serum samples from in vivo experiments were analyzed to quantify cytokine and chemokine expression. A 19-plex luminex kit (R&D, Inc.) for analyzing the concentration of human IFN-γ, IL-17A, TNF-α, CCL2, CCL3, CCL4, CXCL9, CXCL10, CXCL11, IL-1ra, IL-1β, IL-1α, GM-CSF, VEGF, and osteopontin, was designed and used according to the manufacturer’s instructions. The samples were analyzed with a Bioplex 2000 (Bio-Rad).
PBMC or T cell functional analysis in vitro.
2.5 × 105 isolated human PBMCs per well were incubated in a 96-well round bottom plate. The wells were coated with anti-CD3 and anti-CD28 monoclonal antibodies (both from BD Biosciences) for 5 h at 37°C. PBS, dNP2-EGFP, or dNP2-ctCTLA-4 proteins were added to the culture medium at the start of cell incubation in all in vitro experiments. To analyze CD25 and CD69 expression, the cells were harvested after 12 h and washed with PBS. The activation markers were analyzed by flow cytometry after anti-CD4, anti-CD8, anti-CD25, and anti-CD69 antibody staining (all from BD Biosciences). To analyze CXCR3 expression, the cells were harvested after 48 h and stained with anti-CXCR3 antibody (BD Biosciences). Next, human CD4+ T cells or CD8+ T cells were isolated from total PBMCs by magnetic-activated cell sorting (MACS; Miltenyi Biotec) according to the manufacturer’s instructions. The sorted cells were labeled with efluor 670 cell proliferation dye (Thermo Fisher Scientific). Next, 2.5 × 105 labeled cells were incubated with plate-bound anti-CD3 and antiCD28 monoclonal antibodies for 5 days at 37°C. The proliferating cells were analyzed by flow cytometry and the supernatants were analyzed with IFN-γ, TNF-α, and IL-17A ELISA kits (all from Biolegend). CD8 T cells were stimulated with plate-bound anti-CD3 and anti-CD28 monoclonal antibodies (both from BD Biosciences) for 5 days at 37°C. Functional activity of CD8 T cells was analyzed by flow cytometry after anti-CD8 (BD Biosciences) and antigranzyme B (Thermo Fisher Scientific) intracellular antibody staining using a Foxp3 staining kit (Thermo Fisher Scientific).
Statistics.
Data were analyzed using one-, or two-way ANOVA with multiple comparison tests or two-tailed Student’s t-tests. P-values < 0.05 were considered significant. Statistical analysis was performed using Prism 6 (GraphPad Software, Inc.).
3. Results
3.1. dNP2 efficiently delivers protein into primary human T cells
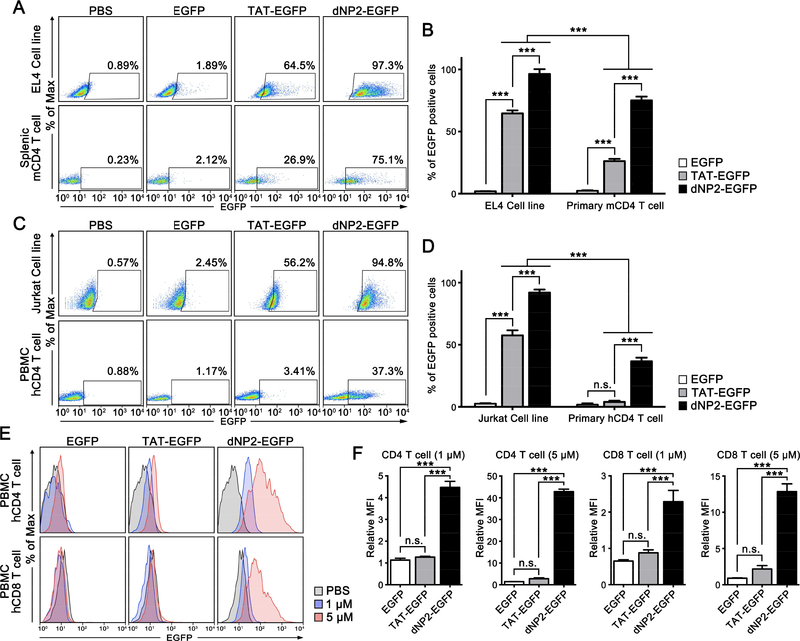
Previously, we described the cell-permeable peptide (CPP) dNP2, which has the ability to penetrate the blood-brain barrier with higher protein delivery efficiency than other control CPPs [26, 31]. We hypothesized that this superior CPP could deliver a functional protein into primary human T cells. To confirm this, we examined the efficiency of protein delivery of dNP2 in both murine and human cells at 5 μM. dNP2-CPP showed significant intracellular protein delivery in splenic CD4 T cells with much higher efficiency than TAT, while both of their delivery efficiencies in EL4 cancer cell lines were significantly higher than in primary cells (Figure 1A,B). A similar pattern was observed in human peripheral CD4 T cells and Jurkat cells (Figure 1C,D), which was consistent with a previous finding that a poly-arginine-based CPP was preferentially delivered into cancer cells due to highly expressing proteoglycans [32]. Various CPPs mediate proteoglycan interactions as their cellular entry mechanism [26]. Interestingly, protein delivery efficiency by TAT in human CD4 T cells was not significantly greater than the control (Figure 1C,D), although it did deliver significantly more protein into splenic mouse CD4 T cells than the control. Accordingly, dNP2 appears to be a better choice for human T cells than TAT. We evaluated dose dependency over a range of 0.1 to 20 μM of proteins in human PBMCs. At every dose, dNP2 showed significantly higher protein delivery efficiency than TAT (Figure S1). dNP2 was ~13.8 times higher in CD4 and ~5.33 times higher in CD8 at 5 μΜ (Figure 1E,F). In addition, there was no significant cytotoxicity in PBMCs at 0.1 to 20 μM of both dNP2 and TAT-conjugated proteins (Figure S2). These results suggest that dNP2-based protein delivery into human T cells would be an efficient methodology for modulating human T cell functioning.
Figure 1.
dNP2 delivers protein into primary human T cells with higher efficiency compared to TAT. (A-D) EL4 cells, mouse splenocytes, Jurkat cells, and human peripheral blood mononuclear cells (PBMCs) were incubated in the presence of 5 μM of EGFP, TAT-EGFP, dNP2-EGFP or PBS. After 1 h, the mouse splenocytes and human PBMCs were stained with anti-mouse CD4 or anti-human CD4 fluorescently-labelled antibodies. All cells were analyzed by flow cytometry. (E-F) PBS, 1 or 5 μM of EGFP, TAT-EGFP, or dNP2-EGFP were used to treat human PBMCs. After 1 h, the cells were stained with anti-human CD4 and anti-human CD8 fluorescently-labelled antibodies. The cells were analyzed by flow cytometry. Bar graphs are presented as the mean ± s.d. (B,D) Two-way ANOVA was used for statistical analysis and *** indicates p < 0.001, n.s. indicates non-significant. (F) One-way ANOVA was used for statistical analysis and *** indicates p<0.001. Relative MFI indicates mean fluorescence intensity (MFI) normalized with the MFI of PBS treated cells.
3.2. dNP2-ctCTLA-4 inhibits early T cell activation and chemokine receptor expression in vitro
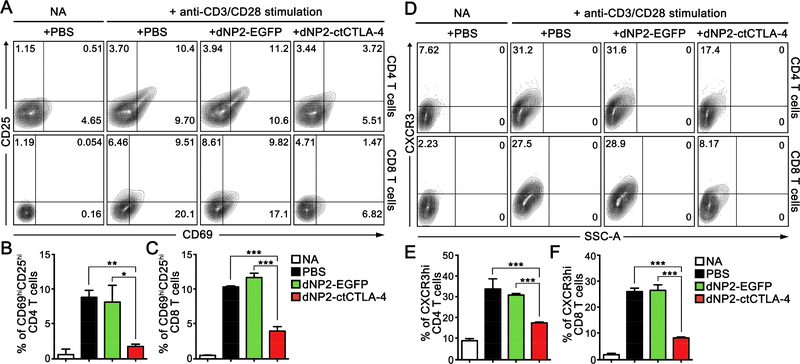
Previously, we described the ligand-independent cell-permeable recombinant protein CTLA-4 (dNP2-ctCTLA-4), which inhibits murine autoimmune encephalitis [26]. To determine the human T cell inhibitory function of dNP2-ctCTLA-4, we analyzed its effects in vitro. PBMCs were stimulated by anti-CD3 and anti-CD28 monoclonal antibodies for 12 h, followed by analysis of activation marker and cytokine production. Both CD4 and CD8 T cells treated with dNP2-ctCTLA-4 showed significantly lower levels of CD69 and CD25 expression (Figure 2A,B); IL-2 production in the media was also significantly reduced by dNP2-ctCTLA-4 compared to the PBS- or dNP2-EGFP-treated groups (Figure 2C), suggesting that dNP2ctCTLA-4 efficiently inhibits T cell activation. CXCR3 is a chemokine receptor on the T cell surface that is rapidly induced upon TcR stimulation, which may lead to cell infiltration into inflammatory regions. The surface expression level of CXCR3 was significantly induced in activated CD4 and CD8 T cells by anti-CD3 and CD28 antibodies at 48 h; however, dNP2ctCTLA-4 significantly reduced the expression of CXCR3 (Figure 2D–F), collectively suggesting that T cells with dNP2-ctCTLA-4 do not have effector and migratory ability to infiltrate the tissues.
Figure 2.
dNP2-ctCTLA-4 inhibits the expression of early-activation markers of human T cells. (A–C) Human peripheral blood mononuclear cells (PBMCs) were stimulated with anti-CD3 and anti-CD28 monoclonal antibodies for 12 h. (A,B) The cells were analyzed by flow cytometry after staining with anti-CD4, anti-CD8, anti-CD69, and anti-CD25 fluorescently-labelled antibodies. (C) IL-2 concentration in the supernatants of the culture medium was analyzed using an IL-2 ELISA kit. (D–F) Human PBMCs were stimulated with anti-CD3 and anti-CD28 monoclonal antibodies for 48 h. The cells were analyzed by flow cytometry after staining with anti-CD4, anti-CD8, and anti-CXCR3 fluorescently-labelled antibodies. n = 3 per group, and bar graphs are presented as the mean ± s.d. Student’s t-test was used and *** indicates p < 0.001.
3.3. dNP2-ctCTLA-4 inhibits mid-late T cell activation, proliferation, and cytokine production in vitro
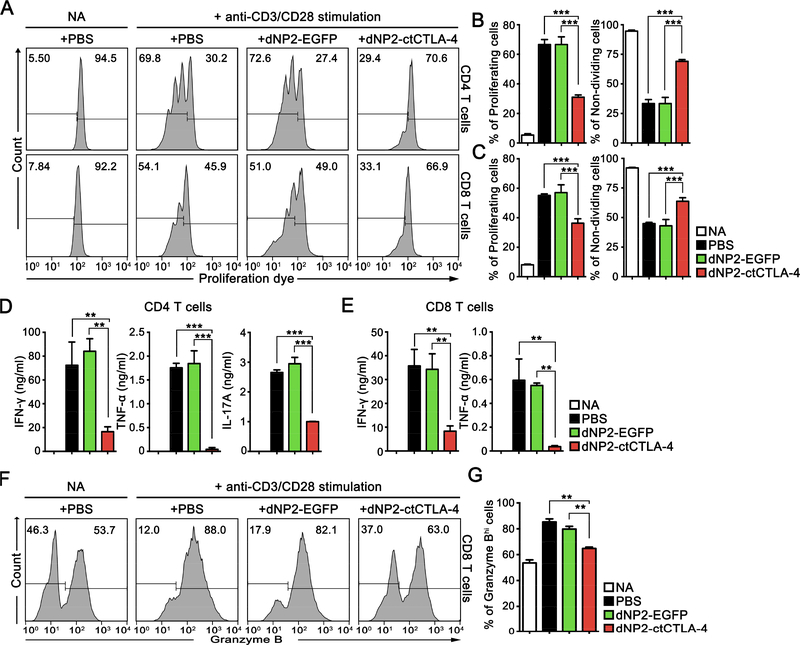
To more precisely analyze the changes in human T cells caused by dNP2-ctCTLA-4, we examined its effects on the proliferating moiety and effector molecule expression in activated human CD4 and CD8 T cells in vitro. We utilized MACS-sorted human CD4 and CD8 T cells from PBMCs for the experiments. The cells were stained with cell proliferation dye (efluor 670) and stimulated with anti-CD3 and CD28 antibodies in the presence of 1 μM dNP2ctCTLA-4 for 5 days. The number of cell divisions based on the peak division was analyzed, which revealed that dNP2-ctCTLA-4 significantly reduced the proliferation of both CD4 and CD8 T cells (Figure 3A–C, Figure S3). In addition, we confirmed that inflammatory cytokines, including IFN-γ, TNF-α, and IL-17A, were significantly reduced by dNP2-ctCTLA-4 compared to the PBS- or dNP2-EGFP-treated groups (Figure 3D,E). Because of the crucial roles CD8 T cell-mediated cytotoxicity plays in allograft rejection [33], we further examined the inhibitory effects of dNP2-ctCTLA-4 on granzyme B expression in CD8 T cells. Among all isolated CD8 T cells, approximately half were granzyme Bhi (GzmBhi) cells, while most produced GzmB after 5 days of stimulation with anti-CD3 and CD28 antibodies. Treatment with dNP2-ctCTLA-4 significantly reduced GzmB expression compared to the control groups, showing a nearly identical histogram pattern to the non-activation state (Figure 3F,G). Collectively, the results suggest that dNP2-ctCTLA-4 strongly inhibits both human CD4 and CD8 T cell proliferation and effector cytokine production, which may prevent allograft rejection.
Figure 3.
dNP2-ctCTLA-4 inhibits proliferation and production of effector molecules by human T cells. (A–C) Human CD4 T cells and CD8 T cells were isolated from PBMC by MACS and stimulated with anti-CD3 and anti-CD28 monoclonal antibodies for 5 days after staining with proliferation dye eFluor 670. The cells were analyzed by flow cytometry after staining with anti-CD4 fluorescently-labelled antibody. (D) Supernatant of the isolated CD4 T cell cultures was analyzed using IFN-γ, TNF-03B1, and IL-17A ELISA kits. (E) Supernatant of the isolated CD8 T cell cultures was analyzed using IFN-γ and TNF-α ELISA kits. (F,G) Cells were analyzed by flow cytometry after staining with anti-CD8 and anti-granzyme B fluorescently-labelled antibodies. n = 3 per groups, and bar graphs are presented as the mean ± s.d. Student’s t-test was used and ** indicates p < 0.01, *** indicates p < 0.001.
3.4. dNP2-ctCTLA-4 ameliorates human skin allograft rejection in humanized SCID/beige mice by inhibiting T cell infiltration
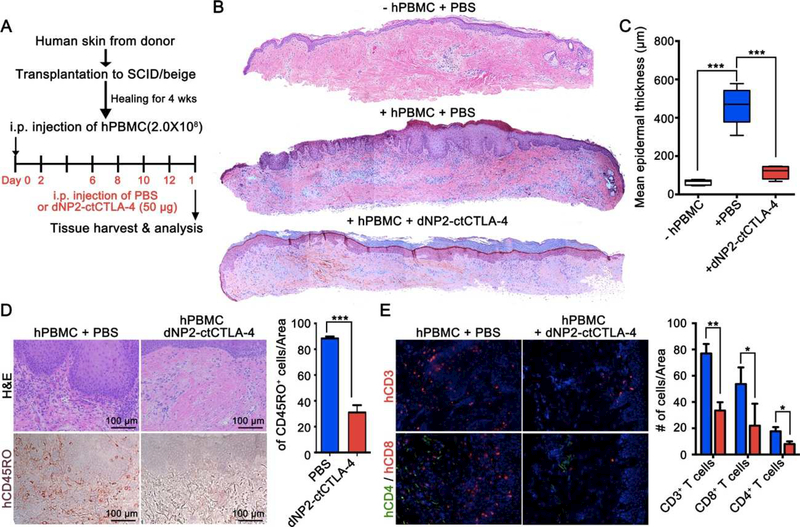
To investigate the availability of dNP2-ctCTLA-4 in controlling human T cell responses in vivo, we utilized a human skin graft model in SCID/beige mice [28, 34]. Split thickness human skin grafts, dermatomed from discarded tissues of unidentified donors that contain the papillary dermis and its superficial vascular plexus in addition to the epidermis, were transplanted onto the dorsa of a cohort of adult mice, 12–16 weeks of age. After 4–5 weeks, when the grafts were completely healed in by visual inspection, 2 × 108 human peripheral blood mononuclear cells (PBMCs) from another donor allogeneic to the skin donor were injected intraperitoneally into the animals. In this model, circulating effector memory T cells that directly recognize non-self class I and class II MHC molecules expressed on graft cells are recruited to the graft and mediate human microvessel destruction. For the next 2 weeks, we intraperitoneally injected 50 μg of dNP2-ctCTLA-4 or phosphate-buffered saline (PBS) into half of the mouse cohort every other day (Figure 4A). Upon harvest at 14 days, grafts analyzed by histology from mice that had received PBMCs and PBS showed thickening of the epidermis with elongated rete ridges, dermal and epidermal lymphocytic infiltration, and nuclei within the stratum corneum, while grafts from mice that had not received PBMCs showed no signs of inflammation. In contrast, grafts from dNP2-ctCTLA-4-treated mice showed dramatically reduced epidermal thickness and cell infiltration (Figure 4B,C and Figure S4A). By immunohistochemistry, significant human CD45RO+ lymphocyte infiltration was observed in PBS control skin grafts, while few skin-infiltrated lymphocytes were detected in the dNP2ctCTLA-4-treated group (Figure 4D). Infiltrated cells were mainly CD3+ T cells with a higher frequency of CD8+ T cells (red) than CD4+ T cells (green) in grafts from the control group (Figure 4E). In other words, dNP2-ctCTLA-4 treatment significantly reduced T cell infiltration. Importantly, circulating levels of human PBMCs in the blood were not significantly changed in the dNP2-ctCTLA-4-treated group compared to the control group (Figure S4B). These results suggest that dNP2-ctCTLA-4 can reduce human T cell infiltration into grafted skin tissues and significantly ameliorate graft inflammation.
Figure 4.
dNP2-ctCTLA-4 ameliorates skin allograft injury in human skin grafted humanized SCID/beige mice by inhibiting T cell infiltration. (A) Experimental scheme of human skin allograft model. (B) Skin grafts were harvested at day 14, and then prepared as paraffin blocks. The blocks were sectioned and stained with hematoxylin and eosin (H&E) solution. Whole sectioned tissues were observed by bright-field microscopy. (C) Mean epidermal thickness was measured using ImageJ 1.50i software. (D) Paraffin sections were stained with anti-human CD45RO antibody and detected using 3,3 -diaminobenzide (DAB) substrate after applying horseradish peroxidase (HRP)-linked secondary antibody. Hematoxylin was used for counter staining. CD45RO+ cells were counted using ImageJ 1.50i software. (E) Another part of the harvested skin grafts was prepared as frozen blocks and stained with anti-human CD3-PE or anti-human CD4-FITC and anti-human CD8-FITC antibodies. DAPI was used for nuclear staining. The slides were observed by fluorescence microscopy. Marker-positive cells were counted using ImageJ 1.50i software. n = 4 per group, and bar graphs are presented as the mean ± s.d. Student’s t-test was used and * indicates p < 0.05, ** indicates p < 0.01, *** indicates p < 0.001.
3.5. dNP2-ctCTLA-4 inhibits T cell-mediated alloresponses in HUVEC-collagen gel-grafted humanized mice
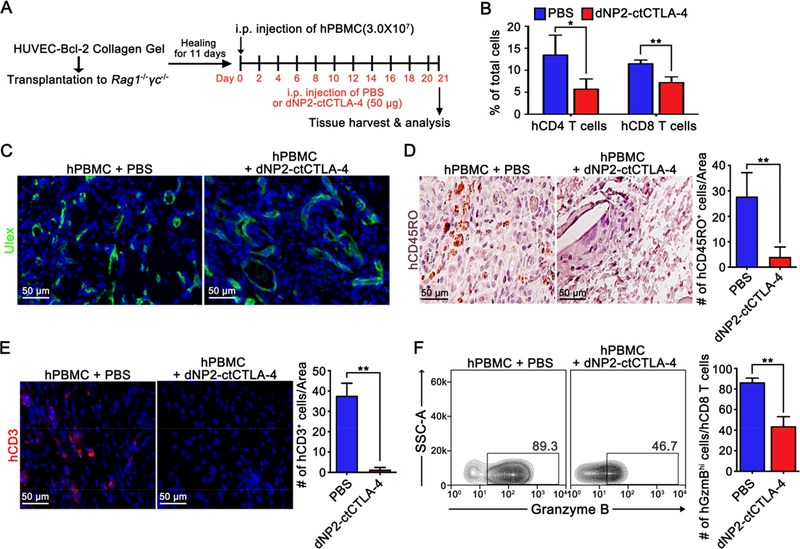
The human skin graft model involves potential roles for both antigen presentation and immune modulation by many different cell types, including resident leukocytes that could be affected by dNP2-ctCTLA-4. To more precisely delineate the effect on T cells, we employed a different graft model involving synthetic human microvessels formed by suspending Bcl-2 transduced HUVECs in a protein gel formed using rat tail type I collagen and human plasma fibronectin and then implanting such gels into the abdominal walls of immunodeficient mice. A previous study showed that Bcl-2-HUVEC-lined microvessels, which form spontaneously, anastomose with mouse microvessels so that they are perfused [30]. At this time point, adoptively transferred alloreactive T cells, primed against HUVECs from the same donor, will expand in the circulation and infiltrate these synthetic tissues whereas T cells primed against a different HUVEC donor do not. Unlike microvessels formed from untransduced HUVECs, the infiltrating T cells do not destroy synthetic microvessels formed from Bcl-2-transduced cells, allowing T cell infiltrates to accumulate [30]. We used this reductionist system to confirm that the effects of dNP2-ctCTLA-4 were being exerted on T cells. Specifically, we assembled HUVEC-collagen gel in vitro using Bcl-2-transfected cells and implanted the gels subcutaneously into RAG1 and IL2RG double knockout (DKO) mice. After 11 days, human PBMCs were injected into mice and treated with dNP2-ctCTLA-4 or PBS intraperitoneally for 3 weeks every other day (Figure 5A). In blood circulating cells, the proportion of CD4 and CD8 T cells was significantly reduced by dNP2-ctCTLA-4 treatment (Figure 5B), suggesting either inhibition of homeostatic proliferation, which was not seen in the skin graft experiments, or inhibition of antigen-specific expansion of T cells. In the HUVEC-collagen gel tissues, we observed similar levels of live endothelial cells in both groups by Ulex staining (Figure 5C). However, CD45RO+ or CD3+ human T cell infiltration in the gel tissue was significantly reduced by dNP2-ctCTLA-4 treatment (Figure 5D,E). In addition, circulating CD8 T cells in the peripheral blood of dNP2-ctCTLA-4-treated mice showed a significantly lower frequency of granzyme B expression than the control group (Figure 5F), indicating that dNP2-ctCTLA-4 inhibits both human CD4 and CD8 T cell activation and recruitment. Interestingly, we found that dNP2-ctCTLA-4 treatment significantly increased Foxp3+ regulatory CD4 T cells in the peripheral blood (Figure S5), which may down-regulate T cell-mediated alloresponses, consistent with the positive signaling role that CTLA-4 plays in this cell type.
Figure 5.
dNP2-ctCTLA-4 inhibits T cell-mediated immune response in HUVEC-collagen gelgrafted humanized mice. (A) Experimental scheme of HUVEC-collagen tissue allograft model. (B) At day 21, the blood of the mice was obtained through cardiac puncture and analyzed by flow cytometry after red blood cell lysis and stained with anti-human CD4 and CD8 fluorescently-labelled antibodies. (C) Collagen gel from the mice was harvested and prepared as paraffin blocks. The blocks were stained with anti-human CD45RO antibody and counter stained with hematoxylin. CD45RO+ cells were counted using ImageJ 1.50i software. (D) The paraffin blocks were stained using fluorescein-labeled Ulex Europaeus Agglutinin I (UEA I) and observed by fluorescence microscopy. (E) Another part of the tissues was prepared as frozen blocks and stained with anti-human CD3-PE antibody, and the nucleus was stained with DAPI. CD3+ cells were counted with ImageJ 1.50i software. (F–G) The lymphocytes in the blood were analyzed by flow cytometry after staining with anti-human CD8 and granzyme B or anti-human CD4 and Foxp3 fluorescently-labelled antibodies. n = 9; PBS group and n = 8; dNP2-ctCTLA-4 group, and bar graphs are presented as the mean ± s.d. Student’s t-test was used and * indicates p < 0.05, ** indicates p < 0.01.
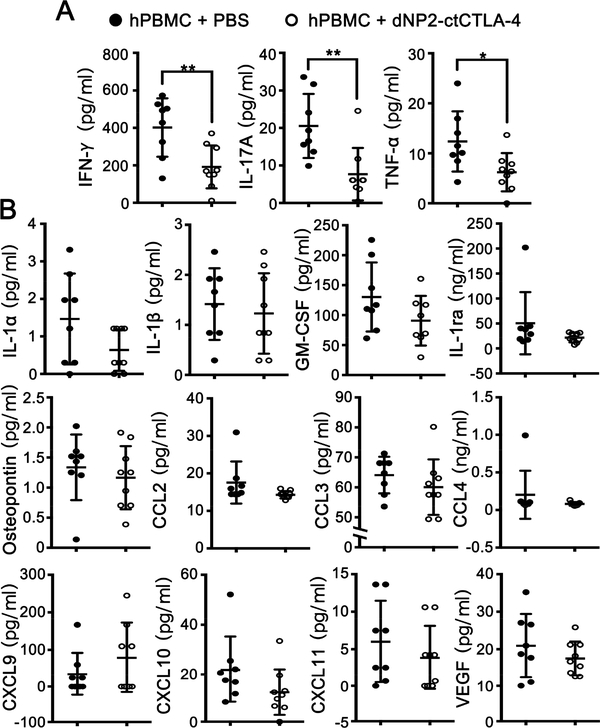
To further evaluate the in vivo mechanism of dNP2-ctCTLA-4 action to prevent graft rejection, we analyzed the serum of mice to investigate inflammatory cytokine and chemokine levels. dNP2-ctCTLA-4-treated mice showed significantly reduced levels of IFN-γ, IL-17A, and TNF-α, which are known to be secreted mainly by activated T cells in this model [35–37] (Figure 6A). Other cytokines and chemokines mainly produced by endothelial cells or APCs were not affected by dNP2-ctCTLA-4 treatment, such as IL-1α, IL-1β, GM-CSF, IL-1ra, osteopontin, CCL-2,−3,−4, CXCL-9, CXCL-10, CXCL-11, and VEGF (Figure 6B). To further confirm the possibility of dNP2-ctCTLA-4 function in other cells, we examined major histocompatibility complex (MHC) molecule expression on HUVEC cell surfaces, which is induced by in vitro cytokine stimulation, and found that there was no significant change (Figure S6). In addition, in vitro treatment of dNP2-ctCTLA-4 to FACS-sorted CD14 positive human primary monocytes and CD19 positive B cells did not alter mRNA expression of IL-6, IL-8, and IL-1β upon LPS stimulation (Figure S7). Taken together, these results suggest that prevention of human skin or HUVEC graft rejection by treatment with dNP2-ctCTLA-4 is mainly be due to successful modulation of allogeneic T cell activation.
Figure 6.
dNP2-ctCTLA-4 reduces T cell-related blood circulating cytokines in mice. (A–B) Serum from HUVEC-collagen tissue allografted mice was analyzed in a multi-plex luminex assay to analyze serum concentrations of IFN-γ, IL-17A, TNFα, CCL2, CCL3, CCL4, CXCL9, CXCL10, CXCL11, IL-1α, IL-1β, IL-1 receptor agonist (IL-1ra), GM-CSF, osteopontin, and VEGF. The graphs are presented as the mean ± s.d. with each individual values. Student’s t-test was used and * indicates p < 0.05, ** indicates p < 0.01.
4. Discussion
CPPs have been widely studied for delivery of macromolecules, including proteins, peptides, DNAs, and RNAs, into target cells [38, 39]. Despite a long history of use in the field, no Food and Drug Administration (FDA)-approved drugs utilizing CPPs are available yet, implying that the clinical use of CPPs may have limitations. In addition, no studies of human T cell manipulation by CPP application have been reported. Here, we suggest that dNP2 could be an optimal CPP for modulating human T cell functioning. We also reveal a regulatory function of the cytoplasmic domain of CTLA-4 without its ligand interaction in human T cells in vitro and in vivo. We demonstrate that dNP2-ctCTLA-4 could be used to regulate alloreactive T cells and promote successful transplantation.
Acute allograft rejection is primarily mediated by host T cells, which recognize graft antigens by various mechanisms [40]. Alloreactive naïve T cells in the recipient initially recognize graft antigens presented by graft dendritic cells (DC) which can migrate into the recipient’s draining lymph nodes (dLNs) or spleen. Alternatively, graft cells may shed exosomes or microparticles containing MHC molecules that can coat host DCs and similarly activate allorective naïve T cells, a process referred to as semi-direct recognition [41]. The initial response is dominated by the direct or semi-direct recognition of peptide-MHC antigens on donor or host DCs, respectively. At later times, indirect recognition of peptide antigens displayed on self-MHC molecules on recipient DCs can also contribute and perhaps dominate [42, 43]. By whichever mechanism they are activated, allorecognition triggers a large number of naïve T cells to differentiate into effector T cells that mediate graft rejection through inflammatory cytokine secretion and direct killing of grafted donor cells [44]. Central memory T cells can also be activated by these same mechanisms and in adult humans, alloreactive memory T cells arising from cross reactions to infectious microbes are equal in frequency to alloreactive naïve T cells [45]. In addition to naïve T cells and central memory T cells that become activated in dLNs or the spleen, circulating effector memory T cells can also directly recognize alloantigens, including MHC molecules expressed on graft vascular endothelial cells [46], bypassing the requirement for antigen presentation by DCs. Memory T cells, which are the major population of effector cells in acute cell-mediated graft rejection, are more difficult to suppress than naïve T cells and may account for most episodes of early graft rejection. A costimulation blockade by CTLA4-Ig, which is an extracellular domain of CTLA-4 combined with the Fc region of immunoglobulin, is less effective for these populations because they use many different costimulation pathways through OX-40, 4–1BB, or ICOS [35]. We previously generated the cytoplasmic domain of recombinant CTLA-4 conjugated to dNP2, which showed significant T cell regulatory functions without ligand interaction in a multiple sclerosis model. Here, we demonstrate that dNP2-ctCLTA-4 could regulate human T cell activation and effector functions in human skin or HUVEC graft models. Unlike CTLA-4-Ig, this strategy can possibly regulate memory T cells even if they receive costimulation though other ligands. dNP2-conjugated protein was delivered more significantly into memory or activated CD4 and CD8 T cells than naïve cells [31]. Thus, we examined dNP2-ctCTLA-4 in FACS-sorted CD45RA+CD45RO− human naïve T cells and CD45RA−CD45RO+ memory T cells, and found that it could significantly inhibit IFN-γ expression by memory T cells as well as naïve T cells upon TcR stimulation (Figure S8). We speculate that dNP2-ctCTLA-4 could regulate both human naïve and memory T cell responses in host versus graft inflammatory situations.
More recently, the role of Th17 cells has been evaluated in transplant rejection [47]. Clinically, transplant surgeries are accompanied by ischemia/reperfusion injury, which may result in the induction of IL-17-producing Th17 and IFN-γ/IL-17-producing Th1/17 cell differentiation [48]. These T cell subtypes are associated with chronic heart [49] and kidney rejection as well [50]. Previous reports have demonstrated that impairment of such T cell subtype-mediated immunity by micro-RNA knockout ameliorates chronic rejection in vivo [51]. These studies show that targeting of IL-17-producing cells and IFN-γ/IL-17-producing cells is critical for obtaining graft tolerance. In the present study, dNP2-ctCTLA-4 inhibited IFN-γ and IL-17 secretion in the blood and T cell infiltration in grafted tissue, suggesting that it might regulate critical effector T cell functions. In addition, our previous report revealed that IFN-γ- and IL-17-producing T cells in the spinal cord of EAE mice were inhibited by dNP2-ctCTLA-4 [26], supporting our hypothesis of possible inhibition of critical effector T cell subtypes.
After the early 1980s, the use of the calcineurin inhibitor cyclosporine enhanced patient and graft survival. Since then, various combinations of calcineurin inhibitors, anti-proliferative agents, and corticosteroids have been used as immune suppressive agents against graft rejection [15]. mTOR inhibitors have also been widely synergistically employed with calcineurin inhibitors to reduce graft rejection [52]. Because these small molecules may cause toxicity problems and act on unwanted cells, biological agents have recently been developed and used. The anti-CD3 monoclonal antibody (mAb) Muronomab-CD3 [53], anti-CD25 mAb Daclizumab [17], anti-CD52 mAb Alemtuzumab [54], and B-lymphocyte stimulator (BLyS)-inhibiting mAb Belimumab [55] have been well-studied and exhibit therapeutic effects. Although these antibody drugs show efficient therapeutic outcomes with low toxicities, they also have significant adverse effects such as increased risk of infections through their lymphocyte-depleting mechanisms of action [19]. Belatacept was approved by the U.S. Food and Drug Administration in 2011 for kidney transplantation. This fusion protein consists of the extracellular domain of CTLA-4 and Fc region of human IgG. The mechanism of reducing graft rejection by belatacept involves blockade of an interaction between APCs and T cells by binding to costimulatory molecules B7.1/B7.2 on APCs [56]. Due to the strong blockage of T cell activation by masking APC ligands of costimulation, urinary tract infection is reported as an adverse effect [57]. However, dNP2-ctCTLA-4, which consists of the intracellular domain of CTLA-4 and dNP2, directly inhibits T cell responses without altering APCs or endothelial cells. Moreover, belatacept appears to have inhibitory effects on generating regulatory T cells [58], which are critical for immune tolerance, while dNP2-ctCTLA-4 treatment significantly increased the frequency of circulating CD4+Foxp3+ regulatory T cells in vivo (Figure S5). Further study is needed to prove its action on regulatory T cells, but we speculate that its different mechanism of action would be advantageous over other immune modulators like belatacept.
CTLA-4 is a negative receptor on T cells that interacts with B7 molecules on APCs [21]. This interaction induces both co-stimulation blockade and negative signaling transduction simultaneously to inhibit T cell activation [22]. Interestingly, CTLA-4 can regulate T cell activation without ligand interactions. A B7 binding region mutant CTLA-4 inhibits T cell activation [23], and overexpression of ligand independent CTLA-4 (liCTLA-4), the exon 2deleted isoform of CTLA-4, significantly inhibits type 1 diabetes by impeding T cell activation and proliferation [24]. Functional motifs in the cytoplasmic domain of CTLA-4 inhibit T cell activation via tyrosine motifs, particularly the YVKM motif, which can bind to a number of important intracellular signaling proteins, including phosphatidylinositol (PI) 3-kinase [59] and tyrosine phosphatase SHP-2 [60]. Another motif, Box-1 like motif, can bind to Janus kinase 2 (Jak2) [61]. The cytoplasmic domain of CTLA-4 can bind to signal transducer and activator of transcription 5 [62], suggesting that CTLA-4 negatively regulates Jak-STAT signaling pathways through the cytoplasmic domain. The positive charge-rich region binds to protein kinase C-η (PKC-η) and transduces signals to regulate T cell activation [63]. In agreement with the findings for liCTLA-4 and its functional motifs, we previously demonstrated that intracellular delivery of the cytoplasmic domain of CTLA-4 protein conjugated to a cell-permeable peptide can ameliorate allergic airway inflammation [64, 65], collagen-induced arthritis [66], and autoimmune encephalomyelitis [26] by inhibiting Th1, Th2, and Th17 responses without ligand interaction. The cytoplasmic domain of CTLA-4 is evolutionally 100% conserved among numerous species including humans, mice, pigs, monkeys, horses, chickens, and whales. We found that dNP2-ctCTLA-4 protein strongly inhibited cytokine production and proliferation of both naïve and memory human CD4 and CD8 T cells. By utilizing the cell-permeable signaling domain of CTLA-4, this novel T cell immune modulatory peptide shows promise for human applications, although more research is clearly needed. Increasing in vivo specificity to T cells, half-life, and reducing production costs for recombinant CTLA-4 peptide or protein should be considered for further optimization.
5. Conclusion
In summary, we demonstrated that dNP2 is an optimal CPP for protein delivery into human T cells, and the cytoplasmic domain of recombinant CTLA-4 conjugated with dNP2 remarkably inhibits allogeneic human T cell responses such as activation, proliferation, inflammatory cytokine production, and infiltration, which reduces skin graft injury in vivo. This is the first report of successful manipulation of human T cell responses in vivo using a CPP-based application. Further studies of dNP2-ctCTLA-4 in clinical trials of human diseases are needed to facilitate CTLA-4 driven immune modulatory drug development.
Supplementary Material
Acknowledgements
This research was supported by the Bio & Medical Technology Development Program (NRF-2017M3A9C8027972) of the National Research Foundation (NRF) funded by the Korean government to J.-M. Choi; and the NIH grant R01-HL051014 to J.S. Pober.
Footnotes
Data Availability
The raw/processed data required to reproduce these findings cannot be shared at this time as the data also forms part of an ongoing study.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Rudoll T, Phillips K, Lee SW, Hull S, Gaspar O, Sucgang N, Gilboa E, Smith C, Highefficiency retroviral vector mediated gene transfer into human peripheral blood CD4+ T lymphocytes, Gene Ther 3(8) (1996) 695–705. [PubMed] [Google Scholar]
- [2].Cherkassky L, Morello A, Villena-Vargas J, Feng Y, Dimitrov DS, Jones DR, Sadelain M, Adusumilli PS, Human CAR T cells with cell-intrinsic PD-1 checkpoint blockade resist tumor-mediated inhibition, J Clin Invest 126(8) (2016) 3130–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Zhao Y, Zheng Z, Cohen CJ, Gattinoni L, Palmer DC, Restifo NP, Rosenberg SA, Morgan RA, High-efficiency transfection of primary human and mouse T lymphocytes using RNA electroporation, Mol Ther 13(1) (2006) 151–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Ferber D, Gene therapy. Safer and virus-free?, Science 294(5547) (2001) 1638–42. [DOI] [PubMed] [Google Scholar]
- [5].Guidotti G, Brambilla L, Rossi D, Cell-Penetrating Peptides: From Basic Research to Clinics, Trends Pharmacol Sci 38(4) (2017) 406–424. [DOI] [PubMed] [Google Scholar]
- [6].Warso MA, Richards JM, Mehta D, Christov K, Schaeffer C, Rae Bressler L, Yamada T, Majumdar D, Kennedy SA, Beattie CW, Das Gupta TK, A first-in-class, first-in-human, phase I trial of p28, a non-HDM2-mediated peptide inhibitor of p53 ubiquitination in patients with advanced solid tumours, Br J Cancer 108(5) (2013) 1061–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Abes R, Arzumanov AA, Moulton HM, Abes S, Ivanova GD, Iversen PL, Gait MJ, Lebleu B, Cell-penetrating-peptide-based delivery of oligonucleotides: an overview, Biochem Soc Trans 35(Pt 4) (2007) 775–9. [DOI] [PubMed] [Google Scholar]
- [8].Bates E, Bode C, Costa M, Gibson CM, Granger C, Green C, Grimes K, Harrington R, Huber K, Kleiman N, Mochly-Rosen D, Roe M, Sadowski Z, Solomon S, Widimsky P, Intracoronary KAI-9803 as an adjunct to primary percutaneous coronary intervention for acute ST-segment elevation myocardial infarction, Circulation 117(7) (2008) 886–96. [DOI] [PubMed] [Google Scholar]
- [9].Cirak S, Arechavala-Gomeza V, Guglieri M, Feng L, Torelli S, Anthony K, Abbs S, Garralda ME, Bourke J, Wells DJ, Dickson G, Wood MJ, Wilton SD, Straub V, Kole R, Shrewsbury SB, Sewry C, Morgan JE, Bushby K, Muntoni F, Exon skipping and dystrophin restoration in patients with Duchenne muscular dystrophy after systemic phosphorodiamidate morpholino oligomer treatment: an open-label, phase 2, dose-escalation study, Lancet 378(9791) (2011) 595–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Heeger PS, T-cell allorecognition and transplant rejection: a summary and update, American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons 3(5) (2003) 525–33. [DOI] [PubMed] [Google Scholar]
- [11].Colombo D, Ammirati E, Cyclosporine in transplantation - a history of converging timelines, Journal of biological regulators and homeostatic agents 25(4) (2011) 493–504. [PubMed] [Google Scholar]
- [12].Rath T, Tacrolimus in transplant rejection, Expert opinion on pharmacotherapy 14(1) (2013) 115–22. [DOI] [PubMed] [Google Scholar]
- [13].Pape L, Ahlenstiel T, mTOR inhibitors in pediatric kidney transplantation, Pediatric nephrology 29(7) (2014) 1119–29. [DOI] [PubMed] [Google Scholar]
- [14].Sollinger HW, Mycophenolate mofetil for the prevention of acute rejection in primary cadaveric renal allograft recipients. U.S. Renal Transplant Mycophenolate Mofetil Study Group, Transplantation 60(3) (1995) 225–32. [DOI] [PubMed] [Google Scholar]
- [15].Holt CD, Overview of Immunosuppressive Therapy in Solid Organ Transplantation, Anesthesiol Clin 35(3) (2017) 365–380. [DOI] [PubMed] [Google Scholar]
- [16].Cosimi AB, Burton RC, Colvin RB, Goldstein G, Delmonico FL, LaQuaglia MP, Tolkoff-Rubin N, Rubin RH, Herrin JT, Russell PS, Treatment of acute renal allograft rejection with OKT3 monoclonal antibody, Transplantation 32(6) (1981) 535–9. [DOI] [PubMed] [Google Scholar]
- [17].Vincenti F, Kirkman R, Light S, Bumgardner G, Pescovitz M, Halloran P, Neylan J, Wilkinson A, Ekberg H, Gaston R, Backman L, Burdick J, Interleukin-2-receptor blockade with daclizumab to prevent acute rejection in renal transplantation. Daclizumab Triple Therapy Study Group, N Engl J Med 338(3) (1998) 161–5. [DOI] [PubMed] [Google Scholar]
- [18].Basu A, Ramkumar M, Tan HP, Khan A, McCauley J, Marcos A, Fung JJ, Starzl TE, Shapiro R, Reversal of acute cellular rejection after renal transplantation with Campath-1H, Transplantation proceedings 37(2) (2005) 923–6. [DOI] [PubMed] [Google Scholar]
- [19].Mahmud N, Klipa D, Ahsan N, Antibody immunosuppressive therapy in solid-organ transplant: Part I, MAbs 2(2) (2010) 148–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Satyananda V, Shapiro R, Belatacept in kidney transplantation, Current opinion in organ transplantation 19(6) (2014) 573–7. [DOI] [PubMed] [Google Scholar]
- [21].Walunas TL, Lenschow DJ, Bakker CY, Linsley PS, Freeman GJ, Green JM, Thompson CB, Bluestone JA, CTLA-4 can function as a negative regulator of T cell activation, Immunity 1(5) (1994) 405–13. [DOI] [PubMed] [Google Scholar]
- [22].Krummel MF, Allison JP, CD28 and CTLA-4 have opposing effects on the response of T cells to stimulation, J Exp Med 182(2) (1995) 459–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Chikuma S, Abbas AK, Bluestone JA, B7-independent inhibition of T cells by CTLA-4, J Immunol 175(1) (2005) 177–81. [DOI] [PubMed] [Google Scholar]
- [24].Stumpf M, Zhou X, Bluestone JA, The B7-independent isoform of CTLA-4 functions to regulate autoimmune diabetes, J Immunol 190(3) (2013) 961–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Wing K, Onishi Y, Prieto-Martin P, Yamaguchi T, Miyara M, Fehervari Z, Nomura T, Sakaguchi S, CTLA-4 control over Foxp3+ regulatory T cell function, Science 322(5899) (2008) 271–5. [DOI] [PubMed] [Google Scholar]
- [26].Lim S, Kim WJ, Kim YH, Lee S, Koo JH, Lee JA, Yoon H, Kim DH, Park HJ, Kim HM, Lee HG, Yun Kim J, Lee JU, Hun Shin J, Kyun Kim L, Doh J, Kim H, Lee SK, Bothwell AL, Suh M, Choi JM, dNP2 is a blood-brain barrier-permeable peptide enabling ctCTLA-4 protein delivery to ameliorate experimental autoimmune encephalomyelitis, Nature communications 6 (2015) 8244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Pober JS, Bothwell AL, Lorber MI, McNiff JM, Schechner JS, Tellides G, Immunopathology of human T cell responses to skin, artery and endothelial cell grafts in the human peripheral blood lymphocyte/severe combined immunodeficient mouse, Springer Semin Immunopathol 25(2) (2003) 167–80. [DOI] [PubMed] [Google Scholar]
- [28].Murray AG, Petzelbauer P, Hughes CC, Costa J, Askenase P, Pober JS, Human T-cellmediated destruction of allogeneic dermal microvessels in a severe combined immunodeficient mouse, Proc Natl Acad Sci U S A 91(19) (1994) 9146–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Kirkiles-Smith NC, Mahboubi K, Plescia J, McNiff JM, Karras J, Schechner JS, Altieri DC, Pober JS, IL-11 protects human microvascular endothelium from alloinjury in vivo by induction of survivin expression, J Immunol 172(3) (2004) 1391–6. [DOI] [PubMed] [Google Scholar]
- [30].Zheng L, Gibson TF, Schechner JS, Pober JS, Bothwell AL, Bcl-2 transduction protects human endothelial cell synthetic microvessel grafts from allogeneic T cells in vivo, J Immunol 173(5) (2004) 3020–6. [DOI] [PubMed] [Google Scholar]
- [31].Lim S, Lee JA, Koo JH, Kang TG, Ha SJ, Choi JM, Cell Type Preference of a Novel Human Derived Cell-Permeable Peptide dNP2 and TAT in Murine Splenic Immune Cells, PloS one 11(5) (2016) e0155689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Nakase I, Konishi Y, Ueda M, Saji H, Futaki S, Accumulation of arginine-rich cellpenetrating peptides in tumors and the potential for anticancer drug delivery in vivo, J Control Release 159(2) (2012) 181–8. [DOI] [PubMed] [Google Scholar]
- [33].Kreisel D, Krupnick AS, Gelman AE, Engels FH, Popma SH, Krasinskas AM, Balsara KR, Szeto WY, Turka LA, Rosengard BR, Non-hematopoietic allograft cells directly activate CD8+ T cells and trigger acute rejection: an alternative mechanism of allorecognition, Nat Med 8(3) (2002) 233–9. [DOI] [PubMed] [Google Scholar]
- [34].Murray AG, Schechner JS, Epperson DE, Sultan P, McNiff JM, Hughes CC, Lorber MI, Askenase PW, Pober JS, Dermal microvascular injury in the human peripheral blood lymphocyte reconstituted-severe combined immunodeficient (HuPBL-SCID) mouse/skin allograft model is T cell mediated and inhibited by a combination of cyclosporine and rapamycin, Am J Pathol 153(2) (1998) 627–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Shiao SL, McNiff JM, Pober JS, Memory T cells and their costimulators in human allograft injury, J Immunol 175(8) (2005) 4886–96. [DOI] [PubMed] [Google Scholar]
- [36].Koh KP, Wang Y, Yi T, Shiao SL, Lorber MI, Sessa WC, Tellides G, Pober JS, T cell-mediated vascular dysfunction of human allografts results from IFN-gamma dysregulation of NO synthase, J Clin Invest 114(6) (2004) 846–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Jin W, Dong C, IL-17 cytokines in immunity and inflammation, Emerg Microbes Infect 2(9) (2013) e60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Mae M, Langel U, Cell-penetrating peptides as vectors for peptide, protein and oligonucleotide delivery, Curr Opin Pharmacol 6(5) (2006) 509–14. [DOI] [PubMed] [Google Scholar]
- [39].Huang YW, Lee HJ, Tolliver LM, Aronstam RS, Delivery of nucleic acids and nanomaterials by cell-penetrating peptides: opportunities and challenges, BioMed research international 2015 (2015) 834079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Benichou G, Yamada Y, Yun SH, Lin C, Fray M, Tocco G, Immune recognition and rejection of allogeneic skin grafts, Immunotherapy 3(6) (2011) 757–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Herrera OB, Golshayan D, Tibbott R, Salcido Ochoa F, James MJ, Marelli-Berg FM, Lechler RI, A novel pathway of alloantigen presentation by dendritic cells, J Immunol 173(8) (2004) 4828–37. [DOI] [PubMed] [Google Scholar]
- [42].Fangmann J, Dalchau R, Fabre JW, Rejection of skin allografts by indirect allorecognition of donor class I major histocompatibility complex peptides, Transplantation proceedings 25(1 Pt 1) (1993) 183–4. [PubMed] [Google Scholar]
- [43].Benichou G, Takizawa PA, Olson CA, McMillan M, Sercarz EE, Donor major histocompatibility complex (MHC) peptides are presented by recipient MHC molecules during graft rejection, J Exp Med 175(1) (1992) 305–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Boisgerault F, Anosova NG, Tam RC, Illigens BM, Fedoseyeva EV, Benichou G, Induction of T-cell response to cryptic MHC determinants during allograft rejection, Human immunology 61(12) (2000) 1352–62. [DOI] [PubMed] [Google Scholar]
- [45].Benichou G, Gonzalez B, Marino J, Ayasoufi K, Valujskikh A, Role of Memory T Cells in Allograft Rejection and Tolerance, Front Immunol 8 (2017) 170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Shiao SL, Kirkiles-Smith NC, Shepherd BR, McNiff JM, Carr EJ, Pober JS, Human effector memory CD4+ T cells directly recognize allogeneic endothelial cells in vitro and in vivo, J Immunol 179(7) (2007) 4397–404. [DOI] [PubMed] [Google Scholar]
- [47].Sullivan JA, Adams AB, Burlingham WJ, The emerging role of TH17 cells in organ transplantation, Transplantation 97(5) (2014) 483–9. [DOI] [PubMed] [Google Scholar]
- [48].Yoshida S, Haque A, Mizobuchi T, Iwata T, Chiyo M, Webb TJ, Baldridge LA, Heidler KM, Cummings OW, Fujisawa T, Blum JS, Brand DD, Wilkes DS, Anti-type V collagen lymphocytes that express IL-17 and IL-23 induce rejection pathology in fresh and well-healed lung transplants, American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons 6(4) (2006) 724–35. [DOI] [PubMed] [Google Scholar]
- [49].Vergani A, Tezza S, D’Addio F, Fotino C, Liu K, Niewczas M, Bassi R, Molano RD, Kleffel S, Petrelli A, Soleti A, Ammirati E, Frigerio M, Visner G, Grassi F, Ferrero ME, Corradi D, Abdi R, Ricordi C, Sayegh MH, Pileggi A, Fiorina P, Long-term heart transplant survival by targeting the ionotropic purinergic receptor P2X7, Circulation 127(4) (2013) 463–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Haynes LD, Jankowska-Gan E, Sheka A, Keller MR, Hernandez-Fuentes MP, Lechler RI, Seyfert-Margolis V, Turka LA, Newell KA, Burlingham WJ, Donor-specific indirect pathway analysis reveals a B-cell-independent signature which reflects outcomes in kidney transplant recipients, American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons 12(3) (2012) 640–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Zhang A, Wang K, Zhou C, Gan Z, Ma D, Ye P, Sun Y, Wu J, Huang X, Ren L, Deng P, Wu C, Yue Z, Ding X, Chen J, Xia J, Knockout of microRNA-155 ameliorates the Th1/Th17 immune response and tissue injury in chronic rejection, The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation 36(2) (2017) 175–184. [DOI] [PubMed] [Google Scholar]
- [52].Peddi VR, Wiseman A, Chavin K, Slakey D, Review of combination therapy with mTOR inhibitors and tacrolimus minimization after transplantation, Transplant Rev (Orlando) 27(4) (2013) 97–107. [DOI] [PubMed] [Google Scholar]
- [53].Aguero J, Almenar L, Martinez-Dolz L, Moro JA, Rueda J, Raso R, Chamorro C, Sanchez JM, Salvador A, Influence of immunosuppressive regimens on short-term morbidity and mortality in heart transplantation, Clinical transplantation 22(1) (2008) 98–106. [DOI] [PubMed] [Google Scholar]
- [54].Knechtle SJ, Pirsch JD, Fechner J JH, Becker BN, Friedl A, Colvin RB, Lebeck LK, Chin LT, Becker YT, Odorico JS, D’Alessandro AM, Kalayoglu M, Hamawy MM, Hu H, Bloom DD, Sollinger HW, Campath-1H induction plus rapamycin monotherapy for renal transplantation: results of a pilot study, American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons 3(6) (2003) 722–30. [DOI] [PubMed] [Google Scholar]
- [55].Galian JA, Mrowiec A, Muro M, Molecular targets on B-cells to prevent and treat antibody-mediated rejection in organ transplantation. Present and Future, Expert Opin Ther Targets 20(7) (2016) 859–67. [DOI] [PubMed] [Google Scholar]
- [56].Vincenti F, Larsen C, Durrbach A, Wekerle T, Nashan B, Blancho G, Lang P, Grinyo J, Halloran PF, Solez K, Hagerty D, Levy E, Zhou W, Natarajan K, Charpentier B, Belatacept Study G, Costimulation blockade with belatacept in renal transplantation, N Engl J Med 353(8) (2005) 770–81. [DOI] [PubMed] [Google Scholar]
- [57].Bassil N, Rostaing L, Mengelle C, Kallab S, Esposito L, Guitard J, CardeauDesangles I, Weclawiak H, Izopet J, Kamar N, Prospective monitoring of cytomegalovirus, Epstein-Barr virus, BK virus, and JC virus infections on belatacept therapy after a kidney transplant, Experimental and clinical transplantation : official journal of the Middle East Society for Organ Transplantation 12(3) (2014) 212–9. [PubMed] [Google Scholar]
- [58].Levitsky J, Miller J, Huang X, Chandrasekaran D, Chen L, Mathew JM, Inhibitory effects of belatacept on allospecific regulatory T-cell generation in humans, Transplantation 96(8) (2013) 689–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Schneider H, Prasad KV, Shoelson SE, Rudd CE, CTLA-4 binding to the lipid kinase phosphatidylinositol 3-kinase in T cells, J Exp Med 181(1) (1995) 351–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Marengere LE, Waterhouse P, Duncan GS, Mittrucker HW, Feng GS, Mak TW, Regulation of T cell receptor signaling by tyrosine phosphatase SYP association with CTLA-4, Science 272(5265) (1996) 1170–3. [DOI] [PubMed] [Google Scholar]
- [61].Chikuma S, Murakami M, Tanaka K, Uede T, Janus kinase 2 is associated with a box 1like motif and phosphorylates a critical tyrosine residue in the cytoplasmic region of cytotoxic T lymphocyte associated molecule-4, J Cell Biochem 78(2) (2000) 241–50. [PubMed] [Google Scholar]
- [62].Srahna M, Van Grunsven LA, Remacle JE, Vandenberghe P, CTLA-4 interacts with STAT5 and inhibits STAT5-mediated transcription, Immunology 117(3) (2006) 396–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Kong KF, Fu G, Zhang Y, Yokosuka T, Casas J, Canonigo-Balancio AJ, Becart S, Kim G, Yates JR 3rd, Kronenberg M, Saito T, Gascoigne NR, Altman A, Protein kinase C-eta controls CTLA-4-mediated regulatory T cell function, Nat Immunol 15(5) (2014) 465–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Choi JM, Ahn MH, Chae WJ, Jung YG, Park JC, Song HM, Kim YE, Shin JA, Park CS, Park JW, Park TK, Lee JH, Seo BF, Kim KD, Kim ES, Lee DH, Lee SK, Lee SK, Intranasal delivery of the cytoplasmic domain of CTLA-4 using a novel protein transduction domain prevents allergic inflammation, Nat Med 12(5) (2006) 574–9. [DOI] [PubMed] [Google Scholar]
- [65].Lim S, Ho Sohn J, Koo JH, Park JW, Choi JM, dNP2-ctCTLA-4 inhibits German cockroach extract-induced allergic airway inflammation and hyper-responsiveness via inhibition of Th2 responses, Exp Mol Med 49(8) (2017) e362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Choi JM, Kim SH, Shin JH, Gibson T, Yoon BS, Lee DH, Lee SK, Bothwell AL, Lim JS, Lee SK, Transduction of the cytoplasmic domain of CTLA-4 inhibits TcR-specific activation signals and prevents collagen-induced arthritis, Proc Natl Acad Sci U S A 105(50) (2008) 19875–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.