Abstract
A critical factor in the maturation of influenza vaccine responses is the nearly inevitable binding of vaccine antigens by exiting anti-influenza IgGs. These antigen-IgG immune complexes direct the response to immunization by modulating cellular processes that determine antibody and T-cell repertoires: maturation of dendritic cells, processing and presentation of antigens to T cells, trafficking of antigens to the germinal center, and selection of B cells for antibody production. By focusing on the recent advances in the study of the immunomodulatory processes mediated by IgG immune complexes upon influenza vaccination, we discuss a pathway that is critical for modulating the breadth and potency of anti-HA antibody responses and has previously led to the development of strategies to improve influenza vaccine efficacy.
This year marks the 100-year anniversary of the 1918 influenza pandemic, one of the deadliest natural disasters in the history of mankind, accounting for 100 million deaths and infecting over half billion of the global population. Although pandemic influenza outbreaks occur on a periodic basis (the most recent being the 2009 H1N1 pandemic), every year seasonal influenza epidemics cause hundreds of thousands of deaths and account for over 5 million cases of severe illness worldwide, having a tremendous socioeconomic impact on global health. For over half a century, vaccination has been the main approach for the prevention of influenza outbreaks; however, licensed influenza vaccines commonly provide sub-optimal protection (typically ranging from as low as 10% to 60%), as they largely elicit strain-specific immunity against circulating influenza strains, necessitating annual reformulation to provide adequate protection. More importantly, conventional influenza vaccines provide little or no protection against antigenically drifted strains, which have the capacity to cause pandemic outbreaks with devastating effects on global public health. Intensive research efforts over the past recent years focusing on influenza immune evasion mechanisms and the immune responses elicited against influenza have led to exciting new findings that could guide strategies for the optimization of the influenza vaccine efficacy to elicit universal protection against diverse influenza strains that would minimize morbidity and mortality caused by seasonal influenza and prevent potential pandemic outbreaks in the future. Indeed, these studies have renewed optimism in the field and made the development of a universal influenza vaccine a more realistic prospect.
By focusing on the study of B-cell responses against influenza, a number of key immune determinants of antibody-mediated immunity against influenza have been identified. For example, recent epidemiologic studies on the immune responses against influenza revealed that circulating influenza strains that are dominant during childhood shape immunological memory and impact future responses against influenza during adulthood [1], supporting a clear role for pre-existing influenza immunity in modulating the magnitude and quality of the antibody responses against future antigenic encounters [2–5]. Additionally, systematic characterization of the B-cell responses against influenza resulted in the discovery of panels of monoclonal antibodies (mAbs) that specifically recognize influenza hemagglutinin (HA) and neuraminidase (NA) proteins and exhibit broadly protective activity against diverse influenza strains [6–11]. Indeed, the isolation and pre-clinical evaluation of anti-influenza antibodies capable of neutralizing a broad range of influenza viruses –with some even recognizing both group 1 and group 2 hemagglutinins (HAs) – has led not only to the development of novel mediators that could potentially be used for the prevention or treatment of pandemic influenza infections, but also provided evidence on the capacity of the human immune system to elicit specific IgG responses to target highly conserved viral epitopes [6–11]. These studies have, in turn, provided useful insights into the functional properties and immunogenicity of influenza antigens, leading to the identification and characterization of highly conserved epitopes that have guided the design of novel influenza immunogens to elicit immune responses with broadly protective activity against diverse influenza strains [12–15]. These findings clearly illustrate that the in-depth study of the capacity of anti-influenza antibodies to specifically recognize highly conserved epitopes on HA and NA could lead to the development of novel vaccination strategies to elicit broadly protective responses. However, in addition to the study of the Fab-mediated antigenic recognition of broadly protective anti-influenza IgG antibodies, improved influenza vaccine efficacy could be achieved through the systematic characterization of the effector activities mediated through the Fc domain of antibodies elicited upon influenza infection.
IgG Fc domain effector functions
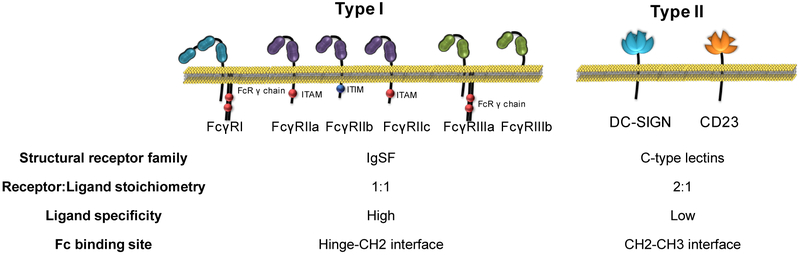
The protective activity of an IgG molecule is mediated through its two functional domains: (i) the Fab domain that facilitates highly specific antigenic recognition and (ii) the Fc domain that contributes to the IgG effector activity through specific interactions with Fcγ receptors (FcγRs) expressed by several leukocyte types [16]. FcγRs comprise a family of immunoreceptors and are broadly divided into two main types: Type I and II, with each type having unique structural and functional characteristics [17](Figure 1). Upon crosslinking by the Fc domains of IgG immune complexes, FcγRs trigger signaling events through their intracellular signaling motifs, inducing diverse immunomodulatory processes that readily influence the functional activity of effector leukocytes and consequently several aspects of the innate and adaptive immune response [17]. For example, ITAM (immunoreceptor tyrosine-based activation motif)-containing, Type I FcγRs induce the activation of signaling pathways with pro-inflammatory biological consequences, including cellular activation, antibody-dependent cellular cytotoxicity (ADCC), phagocytosis, as well as expression and release of inflammatory cytokines and chemokines. These activities are counterbalanced by the inhibitory Type I FcγR, FcγRIIb, which limits ITAM-mediated signaling in effector leukocytes [17]. Likewise, engagement of Type II FcγRs by the IgG Fc domain has pleiotropic immunomodulatory effects. For example, DC-SIGN engagement on regulatory macrophages leads to the induction of Th2-polarizing immunity that suppresses Th1 and Th17 responses and limits IgG-mediated inflammation through upregulation of FcγRIIb expression on myeloid effector leukocytes [18,19]. On the other hand, engagement by IgG immune complexes of the other Type II FcγR, CD23 on B-cells modulates FcγRIIb expression in an autocrine manner, influencing B-cell selection and the development of high-affinity IgG responses [20].
Figure 1: Structure and properties of Type I and Type II FcγRs. FcγRs are divided into two main types: Type I and II.
Despite their common property of interacting with the Fc domain of IgG antibodies, FcγR types present distinct structural and functional differences and have differential capacity to induce diverse immunomodulatory consequences that affect several aspects of immunity.
Given the capacity of Type I and Type II FcγRs to activate diverse immunomodulatory pathways upon engagement, Fc-FcγR interactions are dynamically regulated through specific modulation of the Fc domain structure, either in the primary amino acid sequence (IgG subclasses) or in the Fc-associated glycan composition [17,20,21]. Such differences in the IgG subclass and Fc domain glycan structure contribute to substantial Fc domain heterogeneity and it is estimated that over 103 Fc domain variants exist, each with differential FcγR affinity and immunomodulatory potential. For example, IgG glycan variants lacking the branching fucose residue (afucosylated) exhibit improved cytotoxic activity compared to their fucosylated counterparts through enhanced capacity to interact with and activate FcγRIIIa-expressing effector leukocytes [17,22,23]. Likewise, the presence of terminal sialic acid residues at the Fc-associated glycan structure determines the binding specificity of the Fc domain for Type I and Type II FcγRs [17]. Upon sialylation, the IgG Fc domain acquire the capacity to interact with Type II FcγRs (DC-SIGN and CD23), thereby inducing immunomodulatory activity with a profound impact on immune responses [24–27].
A series of recent studies have provided novel insights into the mechanisms by which Fc-FcγR interactions contribute to the antiviral activity of protective anti-influenza IgGs [20,28]. Systematic comparison of the in vivo protective activity of a panel of anti-influenza mAbs with differential neutralizing potency and breadth revealed that strain-specific, neutralizing mAbs directed against the globular head of the influenza HA confer protective activity without a requirement for FcγR engagement [29–31]. In contrast, broadly protective mAbs that target highly conserved influenza epitopes rely on interactions with activating Type I FcγRs to mediate antiviral activity in vivo [29–33]. These findings clearly highlight that the broad antiviral activity of these IgG antibodies is achieved not only through the targeting of specific, highly conserved epitopes on HA, but also through their capacity to engage and activate distinct FcγR pathways to confer protective effector functions. In addition to contributing to the antiviral activity of protective IgG antibodies by modulating the functional activity of innate effector leukocytes, Fc-FcγR interactions influence several aspects of adaptive immune responses, including antigen presentation, dendritic cell maturation, IgG affinity maturation, as well as B-cell selection and plasma cell survival [17,20,34–36]. These functions are regulated through the specific modulation of the Fc domain structure, which determines the affinity of the Fc domain for the various FcγR types. Analysis of the IgG subclass distribution and Fc glycan composition of antigen-specific IgGs elicited upon influenza vaccination in humans revealed that specific Fc glycoforms with differential FcγR binding affinity become enriched at different time points following vaccination [20]. Although a number of previous studies have also reported that the Fc domain structure is dynamically regulated in health and disease [20,21,37–41], analysis of the influenza vaccine-elicited IgG responses revealed that the observed heterogeneity in the Fc domain structure has significant biological consequences in shaping adaptive immune responses against influenza, as the abundance of specific Fc glycoforms correlated with the affinity and breadth of vaccine-elicited IgG responses, thereby predicting vaccine efficacy [20,28]. These findings are discussed in detail in the next section and have been instrumental for the rational design and selection of novel influenza immunogens to elicit broadly protective anti-influenza immunity through modulation of the activity of specific FcγR pathways.
IgG immune complex immunogens
The role of immune complexes (ICs) in the ontogeny of adaptive immune responses is of particular relevance in the context of influenza immunity. This is because a majority of individuals who receive the seasonal influenza vaccine have serum IgGs that will bind to influenza antigens upon vaccination. Vaccine ICs, in turn, engage FcγRs on immune cells, triggering cellular processes that can promote maturation of high affinity antibody responses, such as promoting the maturation of dendritic cells, enhanced processing and presentation of antigens by antigen presenting cells to T cells, increasing trafficking of antigens to the germinal center (in the form of ICs), and modulated selection of B cells [20,42–46]. Which immune cells will be engaged by an IC, and the effector functions that will be triggered depends entirely on the composition of IgGs within the IC. For example, anti-HA Fab specificity, IgG binding density, and the Fc domain repertoire (IgG subclasses and Fc glycoforms) all impact Fc-FcγR interactions [18,20,29,47].
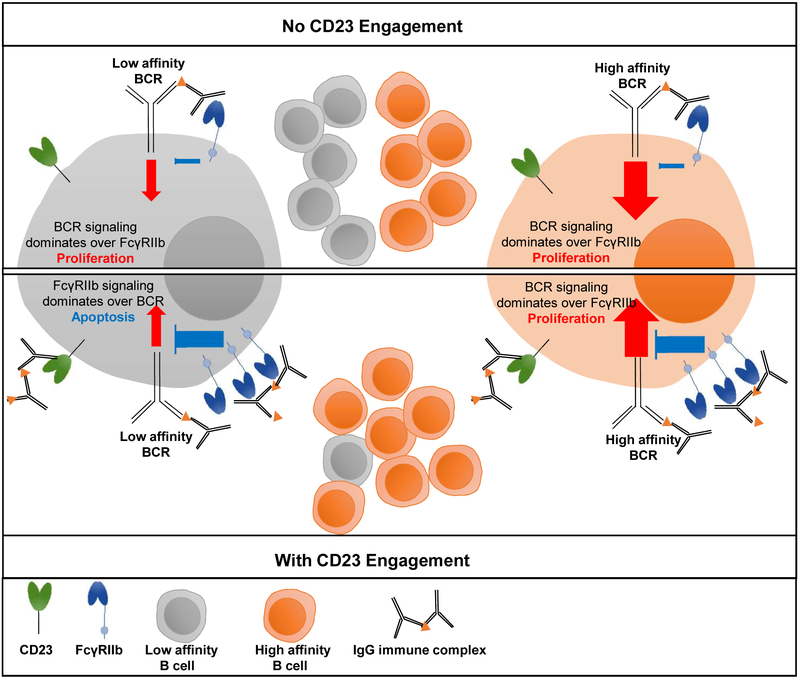
Recent studies have identified a specific determinant - sialylated Fc glycoforms - within HA ICs that trigger maturation of antibody responses with increased anti-HA affinity. This increased affinity, in turn, confers increased potency and breadth of protective activity against distinct influenza strains. This discovery was made through characterization of the natural anti-HA Fc domain repertoire after seasonal vaccination in adults. It was observed that baseline levels of anti-HA IgG Fc sialylation correlated with the quality of response (affinity and hemagglutination inhibition titer) to the seasonal influenza virus vaccine [20,48]. This finding suggested a feedback mechanism for regulation of B cell selection after vaccination by sialylated ICs. Subsequent in vitro studies showed that B cells incubated with sialylated HA immune complexes increased expression of the inhibitory FcγRIIb. This finding was intriguing as FcγRIIb is known to play a major role in fixing the threshold for B cell survival based on the affinity of the B cell receptor (BCR)(Figure 2). The expression of the inhibitory Type I FcγRIIb is nearly always coupled to expression of activating Type I FcγRs, which ensures balanced signaling and specificity of cellular maturation and effector functions [49,50]. B cells represent an important exception to this rule as they express FcγRIIb throughout development, without expression of activating FcγRs. Rather than moderating the activity of activating FcγRs, FcγRIIb on B cells balances activating signaling that is triggered by antigen binding to the BCR. Signaling through FcγRIIb increases the requirement for activating signaling through BCR to enable B cell survival; thus, increasing the expression of FcγRIIb results in the selection of cells with BCR of higher affinity for the antigen.
Figure 2: Overview of the coordinated activity of Type I (FcγRIIb) and Type II (CD23) FcγRs in the regulation of B cell activation and selection.
Development of high-affinity IgG responses is determined by the activity of the CD23-FcγRIIb pathway. Engagement of CD23 by sialylated IgG immune complexes upregulates FcγRIIb expression on B cells, which in turn raises the threshold for the B-cell receptor (BCR)-mediated signaling and B-cell selection. Upon CD23 engagement, only B cells with high-affinity BCRs are selected due to the higher levels of FcγRIIb[20,28].
A key variable in the regulation of B cell activation is the expression level of FcγRIIb, which changes during the development of B cells, but is also inducible. Without FcγRIIb expression, or with low expression or signaling, B cells lack appropriate activation thresholds and produce low-affinity IgGs. Poor FcγRIIb expression is also linked to autoimmune antibody production in mice and in humans [50–53]. Because FcγRIIb is a critical determinant of B cell selection, regulation of its expression over time is essential. The findings described above, that sialylated ICs triggered upregulation of B cell FcγRIIb, revealed a mechanism for coupling B cell FcγRIIb expression and signaling with the presence of antigen [20,28]. Further experiments revealed that expression of the B cell Type II FcγR, CD23, was required for upregulation of FcγRIIb by sialylated ICs [20]. Thus, anti-HA IgGs with sialylated Fc domains form ICs upon vaccination. These ICs signal through CD23 to trigger elevated FcγRIIb expression on B cells. Increased B cell FcγRIIb, in turn, results in the selection of higher affinity HA-specific B cells (Figure 2). While the primary purpose of the work described above was to investigate whether regulated changes in the Fc domain repertoire of antigen-specific IgGs might modulate the maturation of vaccine responses, the experiments ultimately revealed a mechanism that can be leveraged to increase the breadth and potency of the anti-HA response [20,28].
Concluding Remarks
An important area for further investigation is the role that different adjuvants can play in increasing the breadth of protection conferred by influenza vaccines – both seasonal vaccines and experimental universal influenza virus vaccines [54]. Studies showing the critical role of activating FcγRs in heterologous influenza immunity in vivo suggest that skewing the influenza vaccine antibody response away from IgG2 could significantly improve the breadth and potency of elicited IgGs [29,30,32]. This could potentially be done using adjuvants that have Th1-polarizing activity, such as those that trigger pattern-recognition receptors. In-depth understanding of the mechanisms that determine Fc domain heterogeneity and regulate the immunomodulatory activity of FcγR pathways could have important implications for the development of novel vaccination strategies that would elicit broadly protective immunity with maximal effector function.
Highlights.
IgG immune complexes are generated upon influenza vaccination
Interactions of the IgG Fc domain with FcγRs induce immunomodulatory functions
Influenza vaccine efficacy is modulated by the activity of specific FcγR pathways
Engineered immune complex immunogens elicit responses with improved breadth
Acknowledgments
We acknowledge support from the Rockefeller University. Research reported in this publication was supported in part by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health (U19AI111825, U19AI109946). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The authors have no conflicting financial interests.
References
- 1.Gostic KM, Ambrose M, Worobey M, Lloyd-Smith JO: Potent protection against H5N1 and H7N9 influenza via childhood hemagglutinin imprinting. Science 2016, 354:722–726. [DOI] [PMC free article] [PubMed] [Google Scholar]; ** Statistical modeling analysis supporting the concept of HA immune imprinting. This study revealed that the individual’s first IAV infection confers lifelong protection against severe disease from novel hemagglutinin (HA) subtypes in the same phylogenetic group.
- 2.Andrews SF, Huang Y, Kaur K, Popova LI, Ho IY, Pauli NT, Henry Dunand CJ, Taylor WM, Lim S, Huang M, et al. : Immune history profoundly affects broadly protective B cell responses to influenza. Sci Transl Med 2015, 7:316ra192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kaur K, Zheng NY, Smith K, Huang M, Li L, Pauli NT, Henry Dunand CJ, Lee JH, Morrissey M, Wu Y, et al. : High Affinity Antibodies against Influenza Characterize the Plasmablast Response in SLE Patients After Vaccination. PLoS One 2015, 10:e0125618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Henry Dunand CJ, Leon PE, Kaur K, Tan GS, Zheng NY, Andrews S, Huang M, Qu X, Huang Y, Salgado-Ferrer M, et al. : Preexisting human antibodies neutralize recently emerged H7N9 influenza strains. J Clin Invest 2015, 125:1255–1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Andrews SF, Kaur K, Pauli NT, Huang M, Huang Y, Wilson PC: High preexisting serological antibody levels correlate with diversification of the influenza vaccine response. J Virol 2015, 89:3308–3317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Corti D, Voss J, Gamblin SJ, Codoni G, Macagno A, Jarrossay D, Vachieri SG, Pinna D, Minola A, Vanzetta F, et al. : A neutralizing antibody selected from plasma cells that binds to group 1 and group 2 influenza A hemagglutinins. Science 2011, 333:850–856. [DOI] [PubMed] [Google Scholar]
- 7.Ekiert DC, Bhabha G, Elsliger MA, Friesen RH, Jongeneelen M, Throsby M, Goudsmit J, Wilson IA: Antibody recognition of a highly conserved influenza virus epitope. Science 2009, 324:246–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ekiert DC, Friesen RH, Bhabha G, Kwaks T, Jongeneelen M, Yu W, Ophorst C, Cox F, Korse HJ, Brandenburg B, et al. : A highly conserved neutralizing epitope on group 2 influenza A viruses. Science 2011, 333:843–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sui J, Hwang WC, Perez S, Wei G, Aird D, Chen LM, Santelli E, Stec B, Cadwell G, Ali M, et al. : Structural and functional bases for broad-spectrum neutralization of avian and human influenza A viruses. Nat Struct Mol Biol 2009, 16:265–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wrammert J, Koutsonanos D, Li GM, Edupuganti S, Sui J, Morrissey M, McCausland M, Skountzou I, Hornig M, Lipkin WI, et al. : Broadly cross-reactive antibodies dominate the human B cell response against 2009 pandemic H1N1 influenza virus infection. J Exp Med 2011, 208:181–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen YQ, Wohlbold TJ, Zheng NY, Huang M, Huang Y, Neu KE, Lee J, Wan H, Rojas KT, Kirkpatrick E, et al. : Influenza Infection in Humans Induces Broadly Cross-Reactive and Protective Neuraminidase-Reactive Antibodies. Cell 2018, 173:417–429.e410. [DOI] [PMC free article] [PubMed] [Google Scholar]; ** Comparative analysis of immune responses upon influenza infection and vaccination revealed that infection induces anti-neuraminidase antibody immunity with broadly protective activity.
- 12.Krammer F, Hai R, Yondola M, Tan GS, Leyva-Grado VH, Ryder AB, Miller MS, Rose JK, Palese P, García-Sastre A, et al. : Assessment of influenza virus hemagglutinin stalk-based immunity in ferrets. J Virol 2014, 88:3432–3442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Krammer F: Emerging influenza viruses and the prospect of a universal influenza virus vaccine. Biotechnol J 2015, 10:690–701. [DOI] [PubMed] [Google Scholar]
- 14.Nachbagauer R, Kinzler D, Choi A, Hirsh A, Beaulieu E, Lecrenier N, Innis BL, Palese P, Mallett CP, Krammer F: A chimeric haemagglutinin-based influenza split virion vaccine adjuvanted with AS03 induces protective stalk-reactive antibodies in mice. NPJ Vaccines 2016, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]; * Pre-clinical evaluation study on the immunogenicity of engineered chimeric HA immunogens aiming to skew humoral immune responses towards conserved HA stalk epitopes.
- 15.Nachbagauer R, Liu WC, Choi A, Wohlbold TJ, Atlas T, Rajendran M, Solórzano A, Berlanda-Scorza F, García-Sastre A, Palese P, et al. : A universal influenza virus vaccine candidate confers protection against pandemic H1N1 infection in preclinical ferret studies. NPJ Vaccines 2017, 2:26. [DOI] [PMC free article] [PubMed] [Google Scholar]; ** Follow-up study demonstrating that the chimeric HA-based vaccination regimen induced higher stalk antibody titers than the seasonal vaccine, supporting further development of a universal influenza vaccine candidate built on the chimeric HA technology platform.
- 16.Bournazos S, DiLillo DJ, Ravetch JV: The role of Fc-FcγR interactions in IgG-mediated microbial neutralization. J Exp Med 2015, 212:1361–1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bournazos S, Wang TT, Dahan R, Maamary J, Ravetch JV: Signaling by Antibodies: Recent Progress. Annu Rev Immunol 2017, 35:285–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Anthony RM, Kobayashi T, Wermeling F, Ravetch JV: Intravenous gammaglobulin suppresses inflammation through a novel T(H)2 pathway. Nature 2011, 475:110–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fiebiger BM, Maamary J, Pincetic A, Ravetch JV: Protection in antibody- and T cell-mediated autoimmune diseases by antiinflammatory IgG Fcs requires type II FcRs. Proc Natl Acad Sci U S A 2015, 112:E2385–2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang TT, Maamary J, Tan GS, Bournazos S, Davis CW, Krammer F, Schlesinger SJ, Palese P, Ahmed R, Ravetch JV: Anti-HA Glycoforms Drive B Cell Affinity Selection and Determine Influenza Vaccine Efficacy. Cell 2015, 162:160–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang TT, Sewatanon J, Memoli MJ, Wrammert J, Bournazos S, Bhaumik SK, Pinsky BA, Chokephaibulkit K, Onlamoon N, Pattanapanyasat K, et al. : IgG antibodies to dengue enhanced for FcγRIIIA binding determine disease severity. Science 2017, 355:395–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ferrara C, Grau S, Jäger C, Sondermann P, Brünker P, Waldhauer I, Hennig M, Ruf A, Rufer AC, Stihle M, et al. : Unique carbohydrate-carbohydrate interactions are required for high affinity binding between FcgammaRIII and antibodies lacking core fucose. Proc Natl Acad Sci U S A 2011, 108:12669–12674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li T, DiLillo DJ, Bournazos S, Giddens JP, Ravetch JV, Wang LX: Modulating IgG effector function by Fc glycan engineering. Proc Natl Acad Sci U S A 2017, 114:3485–3490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Abboud N, Chow SK, Saylor C, Janda A, Ravetch JV, Scharff MD, Casadevall A: A requirement for FcgammaR in antibody-mediated bacterial toxin neutralization. J Exp Med 2010, 207:2395–2405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Anthony RM, Wermeling F, Karlsson MC, Ravetch JV: Identification of a receptor required for the anti-inflammatory activity of IVIG. Proc Natl Acad Sci U S A 2008, 105:19571–19578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pincetic A, Bournazos S, DiLillo DJ, Maamary J, Wang TT, Dahan R, Fiebiger BM, Ravetch JV: Type I and type II Fc receptors regulate innate and adaptive immunity. Nat Immunol 2014, 15:707–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sondermann P, Pincetic A, Maamary J, Lammens K, Ravetch JV: General mechanism for modulating immunoglobulin effector function. Proc Natl Acad Sci U S A 2013, 110:9868–9872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maamary J, Wang TT, Tan GS, Palese P, Ravetch JV: Increasing the breadth and potency of response to the seasonal influenza virus vaccine by immune complex immunization. Proc Natl Acad Sci U S A 2017, 114:10172–10177. [DOI] [PMC free article] [PubMed] [Google Scholar]; ** Evaluation of engineered IgG immune complex-based immunogens revealed that modulation of the activity of specific FcγR pathways enhances the breadth of protective immunity induced by seasonal influenza vaccination.
- 29.DiLillo DJ, Tan GS, Palese P, Ravetch JV: Broadly neutralizing hemagglutinin stalk-specific antibodies require FcgammaR interactions for protection against influenza virus in vivo. Nat Med 2014, 20:143–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.DiLillo DJ, Palese P, Wilson PC, Ravetch JV: Broadly neutralizing anti-influenza antibodies require Fc receptor engagement for in vivo protection. J Clin Invest 2016, 126:605–610. [DOI] [PMC free article] [PubMed] [Google Scholar]; * Assessment of the in vivo protective activity of a panel of anti-HA and NA mAbs demonstrated that the breadth of protection is dependent upon engagement of activating FcγRs on effector leukocytes.
- 31.He W, Tan GS, Mullarkey CE, Lee AJ, Lam MM, Krammer F, Henry C, Wilson PC, Ashkar AA, Palese P, et al. : Epitope specificity plays a critical role in regulating antibody-dependent cell-mediated cytotoxicity against influenza A virus. Proc Natl Acad Sci U S A 2016, 113:11931–11936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Leon PE, He W, Mullarkey CE, Bailey MJ, Miller MS, Krammer F, Palese P, Tan GS: Optimal activation of Fc-mediated effector functions by influenza virus hemagglutinin antibodies requires two points of contact Proc Natl Acad Sci U S A. 2016, In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mullarkey CE, Bailey MJ, Golubeva DA, Tan GS, Nachbagauer R, He W, Novakowski KE, Bowdish DM, Miller MS, Palese P: Broadly Neutralizing Hemagglutinin Stalk-Specific Antibodies Induce Potent Phagocytosis of Immune Complexes by Neutrophils in an Fc-Dependent Manner. MBio 2016, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bournazos S, Ravetch JV: Fcγ receptor pathways during active and passive immunization. Immunol Rev 2015, 268:88–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bolland S, Ravetch JV: Spontaneous autoimmune disease in Fc(gamma)RIIB-deficient mice results from strain-specific epistasis. Immunity 2000, 13:277–285. [DOI] [PubMed] [Google Scholar]
- 36.DiLillo DJ, Ravetch JV: Differential Fc-Receptor Engagement Drives an Anti-tumor Vaccinal Effect. Cell 2015, 161:1035–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.de Man YA, Dolhain RJ, Hazes JM: Disease activity or remission of rheumatoid arthritis before, during and following pregnancy. Curr Opin Rheumatol 2014, 26:329–333. [DOI] [PubMed] [Google Scholar]
- 38.Gornik I, Maravić G, Dumić J, Flögel M, Lauc G: Fucosylation of IgG heavy chains is increased in rheumatoid arthritis. Clin Biochem 1999, 32:605–608. [DOI] [PubMed] [Google Scholar]
- 39.Kaneko Y, Nimmerjahn F, Ravetch JV: Anti-inflammatory activity of immunoglobulin G resulting from Fc sialylation. Science 2006, 313:670–673. [DOI] [PubMed] [Google Scholar]
- 40.Ohmi Y, Ise W, Harazono A, Takakura D, Fukuyama H, Baba Y, Narazaki M, Shoda H, Takahashi N, Ohkawa Y, et al. : Sialylation converts arthritogenic IgG into inhibitors of collagen-induced arthritis. Nat Commun 2016, 7:11205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Scherer HU, van der Woude D, Ioan-Facsinay A, el Bannoudi H, Trouw LA, Wang J, Häupl T, Burmester GR, Deelder AM, Huizinga TW, et al. : Glycan profiling of anti-citrullinated protein antibodies isolated from human serum and synovial fluid. Arthritis Rheum 2010, 62:1620–1629. [DOI] [PubMed] [Google Scholar]
- 42.Regnault A, Lankar D, Lacabanne V, Rodriguez A, Théry C, Rescigno M, Saito T, Verbeek S, Bonnerot C, Ricciardi-Castagnoli P, et al. : Fcgamma receptor-mediated induction of dendritic cell maturation and major histocompatibility complex class I-restricted antigen presentation after immune complex internalization. J Exp Med 1999, 189:371–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Getahun A, Dahlstrom J, Wernersson S, Heyman B: IgG2a-mediated enhancement of antibody and T cell responses and its relation to inhibitory and activating Fc gamma receptors. J Immunol 2004, 172:5269–5276. [DOI] [PubMed] [Google Scholar]
- 44.de Jong JM, Schuurhuis DH, Ioan-Facsinay A, Welling MM, Camps MG, van der Voort EI, Huizinga TW, Ossendorp F, Verbeek JS, Toes RE: Dendritic cells, but not macrophages or B cells, activate major histocompatibility complex class II-restricted CD4+ T cells upon immune-complex uptake in vivo. Immunology 2006, 119:499–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ding Z, Dahlin JS, Xu H, Heyman B: IgE-mediated enhancement of CD4(+) T cell responses requires antigen presentation by CD8alpha(−) conventional dendritic cells. Sci Rep 2016, 6:28290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hjelm F, Karlsson MC, Heyman B: A novel B cell-mediated transport of IgE-immune complexes to the follicle of the spleen. J Immunol 2008, 180:6604–6610. [DOI] [PubMed] [Google Scholar]
- 47.Nimmerjahn F, Ravetch JV: Divergent immunoglobulin g subclass activity through selective Fc receptor binding. Science 2005, 310:1510–1512. [DOI] [PubMed] [Google Scholar]
- 48.Selman MH, de Jong SE, Soonawala D, Kroon FP, Adegnika AA, Deelder AM, Hokke CH, Yazdanbakhsh M, Wuhrer M: Changes in antigen-specific IgG1 Fc N-glycosylation upon influenza and tetanus vaccination. Mol Cell Proteomics 2012, 11:M111.014563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Clynes R, Maizes JS, Guinamard R, Ono M, Takai T, Ravetch JV: Modulation of immune complex-induced inflammation in vivo by the coordinate expression of activation and inhibitory Fc receptors. J Exp Med 1999, 189:179–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.McGaha TL, Sorrentino B, Ravetch JV: Restoration of tolerance in lupus by targeted inhibitory receptor expression. Science 2005, 307:590–593. [DOI] [PubMed] [Google Scholar]
- 51.Fukuyama H, Nimmerjahn F, Ravetch JV: The inhibitory Fcgamma receptor modulates autoimmunity by limiting the accumulation of immunoglobulin G+ anti-DNA plasma cells. Nat Immunol 2005, 6:99–106. [DOI] [PubMed] [Google Scholar]
- 52.Su K, Wu J, Edberg JC, Li X, Ferguson P, Cooper GS, Langefeld CD, Kimberly RP: A promoter haplotype of the immunoreceptor tyrosine-based inhibitory motif-bearing FcgammaRIIb alters receptor expression and associates with autoimmunity. I. Regulatory FCGR2B polymorphisms and their association with systemic lupus erythematosus. J Immunol 2004, 172:7186–7191. [DOI] [PubMed] [Google Scholar]
- 53.Mackay M, Stanevsky A, Wang T, Aranow C, Li M, Koenig S, Ravetch JV, Diamond B: Selective dysregulation of the FcgammaIIB receptor on memory B cells in SLE. J Exp Med 2006, 203:2157–2164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Goff PH, Eggink D, Seibert CW, Hai R, Martínez-Gil L, Krammer F, Palese P: Adjuvants and immunization strategies to induce influenza virus hemagglutinin stalk antibodies. PLoS One 2013, 8:e79194. [DOI] [PMC free article] [PubMed] [Google Scholar]