Abstract
A more effective vaccine to control tuberculosis (TB), a major global public health problem, is urgently needed. Current vaccine candidates focus predominantly on eliciting cell-mediated immunity but other arms of the immune system also contribute to protection against TB. We review here recent studies that enhance our current knowledge of antibody-mediated functions against M. tuberculosis. These findings, which contribute to the increasing evidence that antibodies have a protective role against TB, include demonstrations that i) distinct human antibody Fc glycosylation patterns, found in latent M. tuberculosis infection but not in active TB, influence the efficacy of the host to control M. tuberculosis infection, ii) antibody isotype influences human antibody functions, and iii) that antibodies targeting M. tuberculosis surface antigens are protective. We discuss these findings in the context of TB vaccine development and highlight the need for further research on antibody-mediated immunity in M. tuberculosis infection.
Graphical abstract
Introduction
Active tuberculosis (TB) is a transmissible respiratory disease that is caused by uncontrolled Mycobacterium tuberculosis (Mtb) infection. It is one of the top 10 causes of death globally, and the leading cause of death from a single pathogen worldwide, surpassing HIV [1]. To control this major global public health problem, more effective vaccines are urgently needed [2]. Recent estimates suggest that a quarter of the world’s population, approximately 1.7 billion people, has asymptomatic controlled latent Mtb infection (LTBI) [3]. However, only ~10% of ostensibly healthy people develop TB during their lifetime [1]. The immune components preventing and controlling Mtb infection remain incompletely understood [4,5]. It has long been known that cell-mediated immunity (CMI) plays a pivotal role (reviewed in [6]), but there is increasing evidence that the innate immunity (reviewed in [7-9]), and other arms of the adaptive immune response (reviewed in [10,11]) contribute to protection against the disease. While it becomes increasingly apparent that all arms of the immune response and their interplay are important in the effective prevention of TB development, their detailed discussion is beyond the scope of this review. We focus here on the humoral immune response, and review recent studies providing further evidence for a role for antibodies (Abs) in protecting against Mtb infection. We discuss the relevance of these findings for TB vaccine development, highlight the need for further research on Ab-mediated immunity in Mtb infection, and discuss the challenges involved in such investigations.
The ideal TB vaccine would both prevent Mtb infection, and, in the already infected, the development of the disease (Figure 1A). While the Bacillus Calmette–Guerin (BCG) vaccine, the only TB vaccine in clinical use, prevents disseminated TB in young children, it has limited efficacy in preventing transmissible disease in adolescents and adults in its present version (reviewed in [12,13]). Current TB vaccine candidates predominantly target the enhancement of CMI [4,5,12-15]. However, the only recent large human TB vaccine trial targeting CMI (MVA85A) showed no enhanced protection [16]. This trial was run in infants and the efficacy of several MVA85A trails, currently being performed in adolescents or adults, may differ. The observation that elevated IgG titers to Ag85A were associated with a decreased risk for TB development in a post hoc analysis [17], and other data from the TB vaccine and pathogenesis fields discussed below, argue for a more unbiased approach to TB vaccine development [4,5] (Box 1 and 2).
Figure 1.
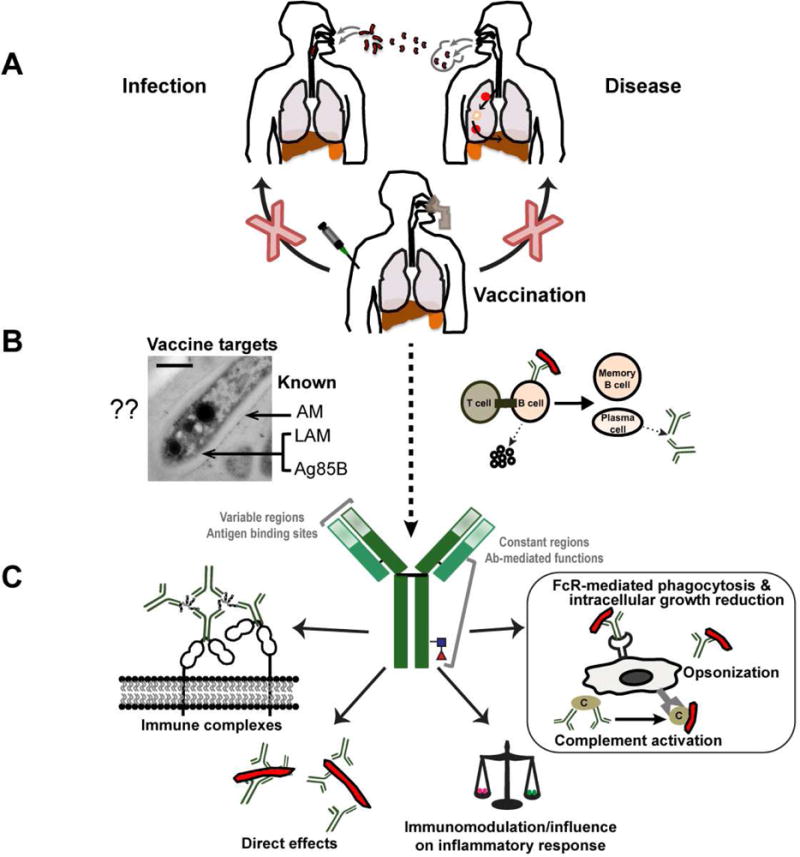
Conceptual view of Ab-mediated protection induced by a TB vaccine. (A) The ideal vaccine would prevent M. tuberculosis infection in the uninfected and development of disease in the already infected individual through mucosal airway and/or systemic vaccination. (B) Induction of protective M. tuberculosis antigen-specific antibody responses with potential enhancement of cell-mediated responses. (C) Illustration of several antibody-mediated functions against M. tuberculosis, including opsonization, FcR-mediated phagocytosis and intracellular growth reduction, influence on the host’s inflammatory response, direct effects on the M. tuberculosis physiology, and influence of immune complexes on the host and host cells.
Box 1. The need for an unbiased and holistic evaluation of the humoral immune responses to Mtb.
The tremendous heterogeneity of the humoral immune response to Mtb, even among individuals with apparently similar immune competency and Mtb infection states creates major challenges in delineating beneficial Ab responses in humans. Several factors, most of all the state of Mtb infection (controlled in LTBI versus uncontrolled in TB), immune competency (e.g. HIV uninfected versus HIV co-infected), and age (young children versus adults) have a major impact on Ab responses to Mtb. Other factors – prior exposure to environmental non-tuberculous mycobacteria, the infecting Mtb strain, and host genetics, to name just a few – are also likely influencing the repertoire of mycobacterial antigens eliciting Abs in humans. Mtb Ab responses must be analyzed in the context of these factors to avoid drawing misleading conclusions. Recent Positron Emission Tomography/Computed Tomography (PET/CT) studies demonstrate the diversity of Mtb infection even within a single individual (reviewed in [32]). Investigations of this type could provide new insights into additional causes for the heterogeneity of Ab responses. Nevertheless, the extrinsic and intrinsic factors affecting the tremendous heterogeneity of the humoral immune response to Mtb remain incompletely understood. Even less is known about the involvement and timing of immune responses at the local airway level. Studies with non-human primates suggest that early immunological events at the airway level could impact the outcome of Mtb infection [33,79]. Therefore, an unbiased and comprehensive profiling of mucosal airway Abs is essential to delineate Ab isotypes and antigens involved in controlling Mtb infection at the local level. This knowledge could inform mucosal airway vaccines. Overall, profiling of both systemic and mucosal airway Ab responses to Mtb in humans and non-human primates, along the continuum from uninfected but Mtb exposed to asymptomatic controlled latent infection to symptomatic uncontrolled disease, could lead to rationally designed studies with antigen-specific mAbs. Such studies are facilitated by the development of new tools that allow precise mapping of the specificity of Abs that bind to mycobacterial antigens [20,28,29,80], as well as by studies that allow the detailed analysis of Ab Fc structures and mediated FcyR functions [35]. Ultimately, the use of chemically defined reagents in form of mAbs would allow the rigorous evaluation of the specific Ab structure– function relationships and associated mechanisms of action that are needed to inform TB vaccine development strategies.
Box 2. Improving TB vaccine efficacy.
One of the longstanding problems in the TB field is defining what a protective response against Mtb is. Contrary to most other pathogens, an initial infection does not provide protection against reactivation and/or reinfection. Therefore, what we can learn from natural infection might not be enough to generate an effective vaccine against TB. It is clear that T-cell mediated immunity is critical to generate a protective response against TB, but, it is also clear that it is insufficient for high vaccine efficacy. Nevertheless, TB vaccine candidates currently in clinical development are immunologically similar in that they are mostly based on CMI and do not exploit other arms of the adaptive immune response. This is apparent by the failure to perform detailed investigations on Ab responses and functions after vaccination. Past and new studies, mostly with PS-conjugate vaccines, have demonstrated that targeting the mycobacterial cell surface with Abs induced through vaccination can be complementary to and synergistic with the induced cellular response (Figure 1B). These data highlight that the understanding of what a protective immune response against TB requires should incorporate the interplay of the different components of the immune system.
Knowledge of the epitopes and Ab constant region (Fc) structure–function relationships most relevant for protection against Mtb infection in humans would have important implications in TB vaccine development. However such information remains quite limited (reviewed in [18] and [19-24]; Figure 1B). The reasons are manifold and some have been discussed in detail in prior reviews [10,25]. They include: i) the conviction that extracellular Ab is less relevant for immunity to a predominantly intracellular pathogen like Mtb; ii) inconsistent results of passive transfer studies (many decades to a century ago) using horse and other animal immune sera in various Mtb infected animal models including rabbits and guinea pigs animals, as well as in humans with TB (reviewed in [26]); and iii) the highly heterogeneous and tremendously diverse Ab responses to Mtb antigens in humans and non-human primates [20,27-29]. The latter issue is influenced by immune competency, age and Mtb infection states spanning the continuum from primary to controlled and uncontrolled infection [28,30-33] (Box 1). Other contributing factors are likely the infecting organism and host genetics.
We know that Abs contribute to the defense against many intracellular pathogens, including Mtb, through various functions (reviewed in [10,34]). These include interactions with Fc-gamma receptors (FcyR; reviewed in [35]), the modulation of innate and adaptive immune responses (reviewed in [35,36]), and the more recently demonstrated direct effects on the physiology of intracellular pathogens while residing both outside and inside host cells [24,37,38] (Figure 1C). Furthermore, recent studies in humans and non-human primates suggest the importance of direct B cell involvement in the defense against Mtb infection, at both the systemic and local granuloma level [39-41]. Collectively, these data highlight the need for a thorough, detailed, and unbiased profiling and characterization of both systemic and mucosal Ab responses in Mtb infection states spanning the continuum from primary infection to controlled and uncontrolled infection (Box 1).
Human anti-mycobacterial Ab functions
Passive transfer studies, performed by independent groups mostly decades ago with murine monoclonal Abs (mAbs) against a handful of mycobacterial antigens, have shown variable protective efficacy in Mtb infected mice (reviewed [25]). However, little is known about the specific functions of these murine mAbs or about the protective efficacy and functions of antigen-specific human Abs. Recently published data provide compelling evidence for a functional role of human Abs to Mtb [19-23]. Importantly, Mtb infection-state-specific differences in IgG functions have been observed [21,23]. Using an unbiased approach for Ab profiling, Lu et al. provided evidence for protective in vitro functions of human polyclonal IgG in subjects with LTBI but not those with TB [21]. They demonstrated that the protective LTBI IgG functions, including Ab-dependent cellular phagocytosis and cytotoxicity, were associated with distinct glycosylation profiles in the IgG Fc region. Such observations are important because IgG Fc–Fc R interactions have implications for vaccine development strategies (reviewed in [35]). However, the study was limited by analyzing total IgG and IgG to purified protein derivative (PPD), which contains many denatured Mtb proteins (>100–200). Different preparations of PPD vary in the concentrations of these proteins and contain moderate and varying amounts of the mycobacterial cell wall glycolipid lipoarabinomannan (LAM) [42], thereby limiting any conclusions on antigen-specific Ab structures. On the other hand, Zimmermann et al. showed that anti-heparin-binding hemagglutinin (HBHA) IgA, but not IgG mAbs, generated from B cells isolated from healthy individuals exposed to Mtb, inhibit mycobacterial infection of epithelial cells in vitro [22]. This finding suggests that isotype might be another key variable in influencing Ab efficacy for specific host cells. These results could have relevance in airway mucosa but should be taken with caution because the beneficial properties of naturally occurring and induced human systemic anti-Mtb IgGs have been demonstrated with human macrophages [19-21].
In passive intraperitoneal transfer experiments with mice infected with aerosolized Mtb, Li et al. demonstrated in vivo protective efficacy of total human serum IgG [23]. Serum from some LTBI and Mtb-exposed asymptomatic healthcare workers (7/48) were protective; however, that from 12 TB patients was not. The protective effects were reversed by pre-absorbing IgG against heat-killed Mtb but not against soluble Mtb antigens, thereby suggesting that the protective Abs targeted the Mtb surface. Similar to Lu et al. [21], this study investigated total serum IgG, thus limiting conclusions about specific protective antigens or relevant antigen-specific Ab structures. Nevertheless, both studies demonstrate differences in functions and efficacy between Abs from Mtb exposed but uninfected and/or individuals with controlled LTBI compared to Abs from individuals with uncontrolled infection (TB). In line with infection state-specific differences, Joosten et al. recently described the impairment of general human B-cell functions (e.g., proliferation, cytokine production and activation) during TB disease and recent Mtb infection, which resolved following TB treatment [39]. They further found that the B-cell dysfunction also compromised cellular immunity [39]. Collectively, these findings provide evidence of the diversity of functions of Mtb-specific Abs and B cells found in different infection contexts. These include patients with advanced disease despite high levels of Abs to many Mtb antigens and individuals who successfully resist or control Mtb infection with lower levels of Abs.
Relevance of Abs targeting the mycobacterial surface
Abs to capsular and other surface polysaccharides (PS) are protective against several microbial pathogens, including those with intracellular location [10,34]. Some of our most successful vaccines are based on inducing Abs to capsular PS [43,44]. Mycobacteria have a capsule, an important virulence factor consisting largely of PS, proteins, and, to a smaller extent, glycolipids [45,46]. The major capsular PS are α-glucan and arabinomannan (AM), accounting for 70–80% and 10–20% of PS content, respectively [47,48]. The lipidated counterpart of AM (LAM) is a component of the mycobacterial cell wall and membrane, but not of the capsule [45,49]. The Mtb capsule has antiphagocytic properties [46] but surface glycans can also mediate adhesion and promote Mtb uptake and intracellular survival via direct interaction with mannose host cell receptors [45,50,51]. Thus, targeting mycobacterial surface glycans with Abs could interfere with Mtb virulence by preventing macrophage uptake through mannose receptors, and instead promoting Fc R-mediated phagocytosis and intracellular growth inhibition.
Little is known about the relevance of specific potentially protective Mtb antigens. Our groups are especially interested in Abs targeting AM. Although α-glucan is the major mycobacterial capsular PS, it has not generated as much interest as AM. Early studies showed that Mtb produces α-glucan during experimental infection in mice, in which Abs to this PS can be elicited [52]. However, humans infected with Mtb have low levels of Abs to α-glucan [53]. This is presumably because α-glucan is very similar in structure to glycogen and starch [52], staples of the human diet. Passive murine transfer studies have demonstrated that some, but not all, murine mAbs to AM and LAM have protective efficacy in Mtb infected mice [54,55]. Similar effects have not been seen with Abs recognizing the α-glucan. This, together with the low levels anti-a-glucan Abs in humans, could explain the lack research on protective effects of Abs recognizing this PS. On the other hand, Mtb-infected humans have high titres of anti-AM Abs; immunization with AM/LAM-protein conjugates has been shown to improve the outcome of Mtb infected mice ([24,56-58]; discussed in more detail below). These studies provide important in vivo evidence. However, they are limited in capturing the tremendous complexity and heterogeneity of protective Mtb epitopes, as well as infection-specific Ab functions and structure–function Ab-antigen relationships encountered in humans [18]. We, and others, have demonstrated that Abs to AM and LAM are elicited in human Mtb infection and through BCG vaccination [19,20,53,59-61], and that reactivity to both correlates strongly (p<0.001) [20,53]. Importantly, IgG titers to AM/LAM were significantly associated with enhanced mycobacterial opsonophagocytosis and intracellular macrophage growth inhibition [19,20]. Using glycan microarrays, we found highly heterogeneous IgG responses to AM OS fragments and a significant correlation of IgG reactivity to specific AM OS with enhanced mycobacterial phagocytosis ([20] and unpublished data). These data provide important insights because the field of glycoconjugate vaccine development, in part informed by the study of human sera, is moving towards OS-conjugates. Such vaccines have proven to be effective for several extracellular and intracellular pathogens, such as Streptococcus pneumoniae, Candida albicans, and Shigella [62-68].
Evidence of Ab efficacy in TB vaccine studies
In the past twenty years, vaccine development for many intracellular pathogens has been limited by the dogma that T cell-mediated immunity is the main contributor to the protective response. Vaccination with protein antigens will generate both Ab and T-cell specific responses. Consequently, evaluation of protective responses after vaccination with such antigens would necessarily need to include the evaluation of how both arms of the adaptive immune response contribute to the overall measured protective response. Passive transfer of vaccine-immune serum and adoptive transfer of T cells would complement such studies. However, negative results could lead to incorrect interpretations because of the potential interdependence of the two arms. This is particularly evident in TB vaccine research where most studies do not incorporate measurement of Ab responses after vaccination; those that do lack functional investigations of Abs, such as FcyR-mediated effects. Surprisingly, studies testing vaccine efficacy of TB subunit vaccines typically neither assess nor mention Ab responses. This includes those using some of the most immunogenic Mtb antigens capable of inducing marked Th1 responses, such as the Ag85b and the RD1-encoded antigens ESAT-6 and CFP-10 [5]. In a more general perspective of vaccine development against intracellular pathogens, recent studies on PS-conjugate vaccines demonstrate that both cellular and humoral immunity can act synergistically to orchestrate a more efficient protective response than those based on inducing responses to either arm alone [24,69].
Most of the knowledge generated in the context of Ab-mediated protection against Mtb through vaccination has been restricted to targeting Mtb surface PS antigens (Box 2). This is because PS are classical B cell antigens with the inability to stimulate T-cell responses. To generate B cell memory, PS antigens need to be conjugated to T-cell dependent antigens (i.e., proteins), triggering the required T-cell help leading to B cell maturation and the production of high affinity Abs [70]. In this context, both cell wall-associated LAM and capsular AM have attracted most of the attention. Conjugates including secreted AM [58] or delipidated LAM [56] have been linked to Ag85b or other non-mycobacterial proteins as carriers. These studies demonstrated significant Ab-based protection against Mtb. More recently, other conjugate formulations including synthetic LAM OS [71] or a baculovirus-conjugated mimotope vaccine [72] have also demonstrated substantial immunogenicity. Similarly, ssDNA aptamers were developed to suppress the immunomodulatory properties of LAM leading to control of bacterial replication in animal models [73,74]. Other Mtb cell surface components, such as phenolic glycolipids, have also been targeted with conjugate vaccines [75]. However, in none of these studies was evidence of a direct contribution of vaccine-induced Abs provided.
We have recently reported PS-conjugate vaccines prepared using Ag85b and capsular AM to induce Ab responses to the mycobacterial cell surface [24]. Our studies showed protection at the level of BCG. More importantly, this work demonstrated, through passive transfer studies of immune sera, the significant contribution of both AM- and Ag85b-binding Abs to controlling bacterial dissemination in mice. The enhanced capacity of AM-Ag85b-immunized mice to reduce bacterial dissemination to the spleen relative to mice immunized with Ag85b alone supports the beneficial additive effect of AM-binding Abs to the overall protective response. This study also demonstrated, for the first time, that one of the most immunogenic Mtb protein antigens, Ag85b, induces protective Abs. Adoptive T-cell transfer from both Ag85b- and AM-Ag85b-immunized mice led to a reduction in bacterial burden in both the lung and spleen, indicating that both arms of the adaptive immune response contribute to the protective properties of Ag85b [24] (Figure 1B). We further demonstrated that several Ab mechanisms can explain the contribution of vaccine-induced Abs to the overall protective response. Changes in the Mtb transcriptional response upon binding of AM-specific Abs was also demonstrated. This novel observation indicates that Abs could have a direct effect on Mtb by compromising its physiology [24]. Similar Ab effects on the intracellular pathogen Cryptococcus neoformans have been reported [38]. We also demonstrated that pretreatment of Mtb with AM-specific polyclonal murine serum enhanced opsonophagocytosis by macrophages [24], an observation consistent with results from our human studies with high anti-AM IgG titer polyclonal sera and human macrophages [20]. Importantly, we and others have shown that the opsonic entry of Mtb into phagocytic cells triggers a macrophage response leading to reduced bacterial survival via increased phagosome–lysosome fusion [20,76].
The role of vaccine-induced Abs against Mtb during an ongoing infection has been also explored. Generation of such data is important because the WHO estimates that the largest immediate impact of a TB vaccine would be in the prevention of disease in already infected individuals thus preventing transmission [2]. Two independent studies have shown the benefit of the therapeutic administration of immune sera in Mtb-infected SCID [77] or B-cell knockout mice [78]. The results of the former study should, however, be taken with caution as they were developed in the context of TB relapse after antibiotic treatment using a DBA/2 mouse strain that is known to be impaired in Ab development after chemotherapy. The later study clearly showed the amelioration of lung inflammation associated with reduced neutrophil infiltration and Th17 levels after passive transfer of murine BCG-immune serum. No mechanism of regulation of neutrophilic response was provided. However, this study encourages further explorations between humoral and innate immune responses to gain more insight into additional potential Ab-mediated mechanisms and the interactions between the various arms of the immune response. Such investigations would provide critical information for TB vaccine development.
Future perspectives
While the studies discussed here support protective efficacy of human polyclonal IgG in vitro and in vivo, they also indicate substantial complexity in structure–function relationships of Abs targeting Mtb. This complexity contributes to the challenges of proving protective Ab efficacy, particularly in humans with highly heterogeneous Ab responses to Mtb [18]. To decipher this complexity, and to determine structure–function relationships relevant for protection, more single Ag-specific studies with both human polyclonal and mAbs are needed (Box 1). The studies described above should also encourage the rethinking of TB vaccine design strategies, specifically the consideration that neither Ab- nor T cell-mediated immunity alone might suffice to generate an optimal protective response against Mtb infection. Based on what we have learned from conjugate vaccine studies, future strategies aiming to exploit this interaction should consider the optimization and development of second generation conjugate vaccines that incorporate specific glycan fragments and protein epitopes. We anticipate that vaccines harnessing both aspects of the immune response could have the highest impact in the prevention of TB. Another important area that has potentially tremendous implications for vaccine efficacy, especially for Ab-based vaccines targeting the Mtb surface, is the phenomenon of microbial antigenic variability of Mtb. This is expected to be particularly important for developing vaccines that target different strains in various TB endemic regions.
In conclusion, current evidence now indicates that it is critical for TB vaccine development to consider the humoral response in the experimental design of novel candidates. In addition, for those vaccine candidates in clinical trials with reagents capable of eliciting an Ab response, it is essential for the field to learn whether part of the protective response is due to Abs. The complexity of the Ab response to Mtb is currently being increasingly uncovered. However, there is sufficient evidence to demonstrate that Abs could make a difference in developing vaccines that protect against this tremendously successful human pathogen.
Highlights.
Human antibody functions against Mycobacterium tuberculosis differ among states of M. tuberculosis infection, from asymptomatic controlled latent infection (LTBI) to symptomatic uncontrolled infection (active tuberculosis)
Antibody functions against M. tuberculosis are influenced by isotypes and IgG Fc glycosylation structures
Induction of M. tuberculosis surface antigen-specific antibody responses can influence TB vaccine efficacy
Antibodies induced through vaccination may be complementary to and synergistic with the induced cellular response against M. tuberculosis
Acknowledgments
JMA and RP-R and their discussed work are supported in part by the National Institutes of Health (NIH)/National Institute of Allergy and Infectious Diseases [grant numbers AI127173 (JMA), AI117927 (JMA), and AI115091 (RP-R)] as well as by funds from Aeras and the Bill and Melinda Gates Foundation (JMA & RP-R). RP-R is further a ‘Ramon y Cajal’ fellow from the Spanish Ministry of Economy and Competitiveness, and funded by the Ministry of Economy and Competitiveness SAF2016-77433-R. Tingting Chen and Elise Ishida assisted in the graphic design of Figure 1.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.WHO. Global tuberculosis report 2017. Geneva, Switzerland: World Health Organization; 2017. [Google Scholar]
- 2.WHO. Tuberculosis vaccine development. Vol. 2018 Geneva, Switzerland: World Health Organization; 2018. [Google Scholar]
- 3.Houben RM, Dodd PJ. The Global Burden of Latent Tuberculosis Infection: A Re-estimation Using Mathematical Modelling. PLoS Med. 2016;13:e1002152. doi: 10.1371/journal.pmed.1002152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Izzo AA. Tuberculosis vaccines – perspectives from the NIH/NIAID Mycobacteria vaccine testing program. Curr Opin Immunol. 2017;47:78–84. doi: 10.1016/j.coi.2017.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cardona PJ. What We Have Learned and What We Have Missed in Tuberculosis Pathophysiology for a New Vaccine Design: Searching for the “Pink Swan”. Front Immunol. 2017;8:556. doi: 10.3389/fimmu.2017.00556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cooper AM. Cell-mediated immune responses in tuberculosis. Annu Rev Immunol. 2009;27:393–422. doi: 10.1146/annurev.immunol.021908.132703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gold MC, Napier RJ, Lewinsohn DM. MR1-restricted mucosal associated invariant T (MAIT) cells in the immune response to Mycobacterium tuberculosis. Immunol Rev. 2015;264:154–166. doi: 10.1111/imr.12271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mayer-Barber KD, Barber DL. Innate and Adaptive Cellular Immune Responses to Mycobacterium tuberculosis Infection. Cold Spring Harb Perspect Med. 2015;5 doi: 10.1101/cshperspect.a018424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schaible UE, Linnemann L, Redinger N, Patin EC, Dallenga T. Strategies to Improve Vaccine Efficacy against Tuberculosis by Targeting Innate Immunity. Front Immunol. 2017;8:1755. doi: 10.3389/fimmu.2017.01755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Achkar JM, Chan J, Casadevall A. B cells and antibodies in the defense against Mycobacterium tuberculosis infection. Immunol Rev. 2015;264:167–181. doi: 10.1111/imr.12276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jacobs AJ, Mongkolsapaya J, Screaton GR, McShane H, Wilkinson RJ. Antibodies and tuberculosis. Tuberculosis (Edinb) 2016;101:102–113. doi: 10.1016/j.tube.2016.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dockrell HM, Smith SG. What Have We Learnt about BCG Vaccination in the Last 20 Years? Front Immunol. 2017;8:1134. doi: 10.3389/fimmu.2017.01134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nieuwenhuizen NE, Kaufmann SHE. Next-Generation Vaccines Based on Bacille Calmette-Guerin. Front Immunol. 2018;9:121. doi: 10.3389/fimmu.2018.00121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Andersen P, Kaufmann SH. Novel vaccination strategies against tuberculosis. Cold Spring Harb Perspect Med. 2014;4 doi: 10.1101/cshperspect.a018523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhu B, Dockrell HM, Ottenhoff THM, Evans TG, Zhang Y. Tuberculosis vaccines: Opportunities and challenges. Respirology. 2018 doi: 10.1111/resp.13245. [DOI] [PubMed] [Google Scholar]
- 16.Tameris MD, Hatherill M, Landry BS, Scriba TJ, Snowden MA, Lockhart S, Shea JE, McClain JB, Hussey GD, Hanekom WA, et al. Safety and efficacy of MVA85A, a new tuberculosis vaccine, in infants previously vaccinated with BCG: a randomised, placebo-controlled phase 2b trial. Lancet. 2013 doi: 10.1016/S0140-6736(13)60177-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17*.Fletcher HA, Snowden MA, Landry B, Rida W, Satti I, Harris SA, Matsumiya M, Tanner R, O’Shea MK, Dheenadhayalan V, et al. T-cell activation is an immune correlate of risk in BCG vaccinated infants. Nat Commun. 2016;7:11290. doi: 10.1038/ncomms11290. In a post-hoc analysis of the MVA85 tuberculosis vaccine trial in infants, this study found that levels of IgG to antigen 85A were associated with a decreased risk for active tuberculosis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Casadevall A. Antibodies to Mycobacterium tuberculosis. N Engl J Med. 2017;376:283–285. doi: 10.1056/NEJMcibr1613268. [DOI] [PubMed] [Google Scholar]
- 19.de Valliere S, Abate G, Blazevic A, Heuertz RM, Hoft DF. Enhancement of innate and cell-mediated immunity by antimycobacterial antibodies. Infect Immun. 2005;73:6711–6720. doi: 10.1128/IAI.73.10.6711-6720.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20*.Chen T, Blanc C, Eder AZ, Prados-Rosales R, Souza AC, Kim RS, Glatman-Freedman A, Joe M, Bai Y, Lowary TL, et al. Association of Human Antibodies to Arabinomannan With Enhanced Mycobacterial Opsonophagocytosis and Intracellular Growth Reduction. J Infect Dis. 2016;214:300–310. doi: 10.1093/infdis/jiw141. Using oligosaccharide glycan arrays, this is the first study to show the heterogeneity of human antibody responses to mycobacterial surface oligosaccharides, and the association of antibody functions against mycobacteria with IgG recognition of specific oligosaccharide fragments. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21*.Lu LL, Chung AW, Rosebrock TR, Ghebremichael M, Yu WH, Grace PS, Schoen MK, Tafesse F, Martin C, Leung V, et al. A Functional Role for Antibodies in Tuberculosis. Cell. 2016;167:433–443 e414. doi: 10.1016/j.cell.2016.08.072. Using an unbiased antibody profiling approach, the authors of this study were the first to demonstrate differences in IgG functions in vitro according to the diverse M. tuberculosis infection-specific states. They found that these differences were associated with distinct glycosylation profiles in the IgG constant region. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22*.Zimmermann N, Thormann V, Hu B, Kohler AB, Imai-Matsushima A, Locht C, Arnett E, Schlesinger LS, Zoller T, Schurmann M, et al. Human isotype-dependent inhibitory antibody responses against Mycobacterium tuberculosis. EMBO Mol Med. 2016;8:1325–1339. doi: 10.15252/emmm.201606330. The authors of this study were the first to demonstrate the relevance of antibody isotype in M. tuberculosis infection of specific host cells. They showed that human IgA but not IgG mAbs, generated from B cells isolated from healthy individuals exposed to Mtb, could inhibit mycobacterial infection of epithelial cells in vitro. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23*.Li H, Wang XX, Wang B, Fu L, Liu G, Lu Y, Cao M, Huang H, Javid B. Latently and uninfected healthcare workers exposed to TB make protective antibodies against Mycobacterium tuberculosis. Proc Natl Acad Sci U S A. 2017;114:5023–5028. doi: 10.1073/pnas.1611776114. This study is the first to show the difference between in vivo protective efficacy of polyclonal IgG from LTBI and M. tuberculosis exposed but uninfected healthcare workers relative to TB patients in passive transfer experiments with M. tuberculosis infected mice. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24*.Prados-Rosales R, Carreno L, Cheng T, Blanc C, Weinrick B, Malek A, Lowary TL, Baena A, Joe M, Bai Y, et al. Enhanced control of Mycobacterium tuberculosis extrapulmonary dissemination in mice by an arabinomannan-protein conjugate vaccine. PLoS Pathog. 2017;13:e1006250. doi: 10.1371/journal.ppat.1006250. Through passive transfer of immune sera elicited by a mycobacterial polysaccharide-protein conjugate vaccine, the authors of this study demonstrated the significant contribution of both polysaccharide and protein-binding antibodies to controlling M. tuberculosis dissemination in mice. They further showed that both the elicited humoral and cell-mediated immunity contributed to the protective response against M. tuberculosis infection in vaccinated mice. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Achkar JM, Casadevall A. Antibody-mediated immunity against tuberculosis: implications for vaccine development. Cell Host Microbe. 2013;13:250–262. doi: 10.1016/j.chom.2013.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Glatman-Freedman A, Casadevall A. Serum therapy for tuberculosis revisited: reappraisal of the role of antibody-mediated immunity against Mycobacterium tuberculosis. Clin Microbiol Rev. 1998;11:514–532. doi: 10.1128/cmr.11.3.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kunnath-Velayudhan S, Davidow AL, Wang HY, Molina DM, Huynh VT, Salamon H, Pine R, Michel G, Perkins MD, Xiaowu L, et al. Proteome-scale antibody responses and outcome of Mycobacterium tuberculosis infection in nonhuman primates and in tuberculosis patients. J Infect Dis. 2012;206:697–705. doi: 10.1093/infdis/jis421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kunnath-Velayudhan S, Salamon H, Wang HY, Davidow AL, Molina DM, Huynh VT, Cirillo DM, Michel G, Talbot EA, Perkins MD, et al. Dynamic antibody responses to the Mycobacterium tuberculosis proteome. Proc Natl Acad Sci U S A. 2010;107:14703–14708. doi: 10.1073/pnas.1009080107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Song L, Wallstrom G, Yu X, Hopper M, Van Duine J, Steel J, Park J, Wiktor P, Kahn P, Brunner A, et al. Identification of Antibody Targets for Tuberculosis Serology using High-Density Nucleic Acid Programmable Protein Arrays. Mol Cell Proteomics. 2017;16:S277–S289. doi: 10.1074/mcp.M116.065953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Achkar JM, Jenny-Avital ER. Incipient and subclinical tuberculosis: defining early disease states in the context of host immune response. J Infect Dis. 2011;204(Suppl 4):S1179–1186. doi: 10.1093/infdis/jir451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Achkar JM, Ziegenbalg A. Antibody responses to mycobacterial antigens in children with tuberculosis: challenges and potential diagnostic value. Clin Vaccine Immunol. 2012;19:1898–1906. doi: 10.1128/CVI.00501-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cadena AM, Fortune SM, Flynn JL. Heterogeneity in tuberculosis. Nat Rev Immunol. 2017;17:691–702. doi: 10.1038/nri.2017.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Coleman MT, Maiello P, Tomko J, Frye LJ, Fillmore D, Janssen C, Klein E, Lin PL. Early Changes by 18Fluorodeoxyglucose Positron Emission Tomography Coregistered with Computed Tomography Predict Outcome after Mycobacterium tuberculosis Infection in Cynomolgus Macaques. Infect Immun. 2014;82:2400–2404. doi: 10.1128/IAI.01599-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Casadevall A, Pirofski LA. A reappraisal of humoral immunity based on mechanisms of antibody-mediated protection against intracellular pathogens. Adv Immunol. 2006;91:1–44. doi: 10.1016/S0065-2776(06)91001-3. [DOI] [PubMed] [Google Scholar]
- 35.Bournazos S, Ravetch JV. Fcgamma Receptor Function and the Design of Vaccination Strategies. Immunity. 2017;47:224–233. doi: 10.1016/j.immuni.2017.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Igietseme JU, Eko FO, He Q, Black CM. Antibody regulation of Tcell immunity: implications for vaccine strategies against intracellular pathogens. Expert Rev Vaccines. 2004;3:23–34. doi: 10.1586/14760584.3.1.23. [DOI] [PubMed] [Google Scholar]
- 37.Edelson BT, Unanue ER. Intracellular antibody neutralizes Listeria growth. Immunity. 2001;14:503–512. doi: 10.1016/s1074-7613(01)00139-x. [DOI] [PubMed] [Google Scholar]
- 38.McClelland EE, Nicola AM, Prados-Rosales R, Casadevall A. Ab binding alters gene expression in Cryptococcus neoformans and directly modulates fungal metabolism. J Clin Invest. 2010;120:1355–1361. doi: 10.1172/JCI38322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39*.Joosten SA, van Meijgaarden KE, Del Nonno F, Baiocchini A, Petrone L, Vanini V, Smits HH, Palmieri F, Goletti D, Ottenhoff TH. Patients with Tuberculosis Have a Dysfunctional Circulating B-Cell Compartment, Which Normalizes following Successful Treatment. PLoS Pathog. 2016;12:e1005687. doi: 10.1371/journal.ppat.1005687. The authors of this study are the first to show the impairment of general human B-cell functions during TB disease and recent Mtb infection, a dysfunction that also impacts cellular immunity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40*.Foreman TW, Mehra S, LoBato DN, Malek A, Alvarez X, Golden NA, Bucsan AN, Didier PJ, Doyle-Meyers LA, Russell-Lodrigue KE, et al. CD4+ T-cell-independent mechanisms suppress reactivation of latent tuberculosis in a macaque model of HIV coinfection. Proc Natl Acad Sci U S A. 2016;113:E5636–5644. doi: 10.1073/pnas.1611987113. Through co-infection of non-human primates with M. tuberculosis and simian immunodeficiency virus, the authors of this study demonstrated that expanded lung B-cell follicles correlated with protection from M. tuberculosis reactivation in a sub-population of dualy infected animals. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41*.Phuah J, Wong EA, Gideon HP, Maiello P, Coleman MT, Hendricks MR, Ruden R, Cirrincione LR, Chan J, Lin PL, et al. Effects of B Cell Depletion on Early Mycobacterium tuberculosis Infection in Cynomolgus Macaques. Infect Immun. 2016;84:1301–1311. doi: 10.1128/IAI.00083-16. Through B-cell depletion in M. tuberculosis-infected non-human primates the authors showed that B cells modulate the granulomatous response during actute M. tuberculosis infection. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cho YS, Dobos KM, Prenni J, Yang H, Hess A, Rosenkrands I, Andersen P, Ryoo SW, Bai GH, Brennan MJ, et al. Deciphering the proteome of the in vivo diagnostic reagent “purified protein derivative” from Mycobacterium tuberculosis. Proteomics. 2012;12:979–991. doi: 10.1002/pmic.201100544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Astronomo RD, Burton DR. Carbohydrate vaccines: developing sweet solutions to sticky situations? Nat Rev Drug Discov. 2010;9:308–324. doi: 10.1038/nrd3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ada G, Isaacs D. Carbohydrate-protein conjugate vaccines. Clin Microbiol Infect. 2003;9:79–85. doi: 10.1046/j.1469-0691.2003.00530.x. [DOI] [PubMed] [Google Scholar]
- 45.Daffe M, Etienne G. The capsule of Mycobacterium tuberculosis and its implications for pathogenicity. Tuber Lung Dis. 1999;79:153–169. doi: 10.1054/tuld.1998.0200. [DOI] [PubMed] [Google Scholar]
- 46.Stokes RW, Norris-Jones R, Brooks DE, Beveridge TJ, Doxsee D, Thorson LM. The glycan-rich outer layer of the cell wall of Mycobacterium tuberculosis acts as an antiphagocytic capsule limiting the association of the bacterium with macrophages. Infect Immun. 2004;72:5676–5686. doi: 10.1128/IAI.72.10.5676-5686.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lemassu A, Daffe M. Structural features of the exocellular polysaccharides of Mycobacterium tuberculosis. Biochem J. 1994;297(Pt 2):351–357. doi: 10.1042/bj2970351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ortalo-Magne A, Dupont MA, Lemassu A, Andersen AB, Gounon P, Daffe M. Molecular composition of the outermost capsular material of the tubercle bacillus. Microbiology. 1995;141(Pt 7):1609–1620. doi: 10.1099/13500872-141-7-1609. [DOI] [PubMed] [Google Scholar]
- 49.Vergne I, Gilleron M, Nigou J. Manipulation of the endocytic pathway and phagocyte functions by Mycobacterium tuberculosis lipoarabinomannan. Front Cell Infect Microbiol. 2014;4:187. doi: 10.3389/fcimb.2014.00187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Torrelles JB, Knaup R, Kolareth A, Slepushkina T, Kaufman TM, Kang P, Hill PJ, Brennan PJ, Chatterjee D, Belisle JT, et al. Identification of Mycobacterium tuberculosis clinical isolates with altered phagocytosis by human macrophages due to a truncated lipoarabinomannan. J Biol Chem. 2008;283:31417–31428. doi: 10.1074/jbc.M806350200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kang PB, Azad AK, Torrelles JB, Kaufman TM, Beharka A, Tibesar E, DesJardin LE, Schlesinger LS. The human macrophage mannose receptor directs Mycobacterium tuberculosis lipoarabinomannan-mediated phagosome biogenesis. J Exp Med. 2005;202:987–999. doi: 10.1084/jem.20051239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schwebach JR, Glatman-Freedman A, Gunther-Cummins L, Dai Z, Robbins JB, Schneerson R, Casadevall A. Glucan is a component of the Mycobacterium tuberculosis surface that is expressed in vitro and in vivo. Infect Immun. 2002;70:2566–2575. doi: 10.1128/IAI.70.5.2566-2575.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yu X, Prados-Rosales R, Jenny-Avital ER, Sosa K, Casadevall A, Achkar JM. Comparative evaluation of profiles of antibodies to mycobacterial capsular polysaccharides in tuberculosis patients and controls stratified by HIV status. Clin Vaccine Immunol. 2012;19:198–208. doi: 10.1128/CVI.05550-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Teitelbaum R, Glatman-Freedman A, Chen B, Robbins JB, Unanue E, Casadevall A, Bloom BR. A mAb recognizing a surface antigen of Mycobacterium tuberculosis enhances host survival. Proc Natl Acad Sci U S A. 1998;95:15688–15693. doi: 10.1073/pnas.95.26.15688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hamasur B, Haile M, Pawlowski A, Schroder U, Kallenius G, Svenson SB. A mycobacterial lipoarabinomannan specific monoclonal antibody and its F(ab′) fragment prolong survival of mice infected with Mycobacterium tuberculosis. Clin Exp Immunol. 2004;138:30–38. doi: 10.1111/j.1365-2249.2004.02593.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hamasur B, Kallenius G, Svenson SB. Synthesis and immunologic characterisation of Mycobacterium tuberculosis lipoarabinomannan specific oligosaccharide-protein conjugates. Vaccine. 1999;17:2853–2861. doi: 10.1016/s0264-410x(99)00124-3. [DOI] [PubMed] [Google Scholar]
- 57.Hamasur B, Haile M, Pawlowski A, Schroder U, Williams A, Hatch G, Hall G, Marsh P, Kallenius G, Svenson SB. Mycobacterium tuberculosis arabinomannan-protein conjugates protect against tuberculosis. Vaccine. 2003;21:4081–4093. doi: 10.1016/s0264-410x(03)00274-3. [DOI] [PubMed] [Google Scholar]
- 58.Glatman-Freedman A, Casadevall A, Dai Z, Jacobs WR, Jr, Li A, Morris SL, Navoa JA, Piperdi S, Robbins JB, Schneerson R, et al. Antigenic evidence of prevalence and diversity of Mycobacterium tuberculosis arabinomannan. J Clin Microbiol. 2004;42:3225–3231. doi: 10.1128/JCM.42.7.3225-3231.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Brown RM, Cruz O, Brennan M, Gennaro ML, Schlesinger L, Skeiky YA, Hoft DF. Lipoarabinomannan-reactive human secretory immunoglobulin A responses induced by mucosal bacille Calmette-Guerin vaccination. J Infect Dis. 2003;187:513–517. doi: 10.1086/368096. [DOI] [PubMed] [Google Scholar]
- 60.Keitel WA, Dai Z, Awe RW, Atmar RL, Morris S, Schneerson R, Robbins JB. Effects of infection and disease with Mycobacterium tuberculosis on serum antibody to glucan and arabinomannan: two surface polysaccharides of this pathogen. BMC Infect Dis. 2013;13:276. doi: 10.1186/1471-2334-13-276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hoft DF, Kemp EB, Marinaro M, Cruz O, Kiyono H, McGhee JR, Belisle JT, Milligan TW, Miller JP, Belshe RB. A double-blind, placebo-controlled study of Mycobacterium-specific human immune responses induced by intradermal bacille Calmette-Guerin vaccination. J Lab Clin Med. 1999;134:244–252. doi: 10.1016/s0022-2143(99)90204-4. [DOI] [PubMed] [Google Scholar]
- 62.Robbins JB, Kubler-Kielb J, Vinogradov E, Mocca C, Pozsgay V, Shiloach J, Schneerson R. Synthesis, characterization, and immunogenicity in mice of Shigella sonnei O-specific oligosaccharide-core-protein conjugates. Proc Natl Acad Sci U S A. 2009;106:7974–7978. doi: 10.1073/pnas.0900891106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kubler-Kielb J, Vinogradov E, Lagergard T, Ginzberg A, King JD, Preston A, Maskell DJ, Pozsgay V, Keith JM, Robbins JB, et al. Oligosaccharide conjugates of Bordetella pertussis and bronchiseptica induce bactericidal antibodies, an addition to pertussis vaccine. Proc Natl Acad Sci U S A. 2011;108:4087–4092. doi: 10.1073/pnas.1100782108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lipinski T, Wu X, Sadowska J, Kreiter E, Yasui Y, Cheriaparambil S, Rennie R, Bundle DR. A beta-mannan trisaccharide conjugate vaccine aids clearance of Candida albicans in immunocompromised rabbits. Vaccine. 2012;30:6263–6269. doi: 10.1016/j.vaccine.2012.08.010. [DOI] [PubMed] [Google Scholar]
- 65.Johnson MA, Bundle DR. Designing a new antifungal glycoconjugate vaccine. Chem Soc Rev. 2013;42:4327–4344. doi: 10.1039/c2cs35382b. [DOI] [PubMed] [Google Scholar]
- 66.Anish C, Schumann B, Pereira CL, Seeberger PH. Chemical biology approaches to designing defined carbohydrate vaccines. Chem Biol. 2014;21:38–50. doi: 10.1016/j.chembiol.2014.01.002. [DOI] [PubMed] [Google Scholar]
- 67.Broecker F, Martin CE, Wegner E, Mattner J, Baek JY, Pereira CL, Anish C, Seeberger PH. Synthetic Lipoteichoic Acid Glycans Are Potential Vaccine Candidates to Protect from Clostridium difficile Infections. Cell Chem Biol. 2016;23:1014–1022. doi: 10.1016/j.chembiol.2016.07.009. [DOI] [PubMed] [Google Scholar]
- 68.Emmadi M, Khan N, Lykke L, Reppe K, S GP, Lisboa MP, Wienhold SM, Witzenrath M, Pereira CL, Seeberger PH. A Streptococcus pneumoniae Type 2 Oligosaccharide Glycoconjugate Elicits Opsonic Antibodies and Is Protective in an Animal Model of Invasive Pneumococcal Disease. J Am Chem Soc. 2017;139:14783–14791. doi: 10.1021/jacs.7b07836. [DOI] [PubMed] [Google Scholar]
- 69.Scott AE, Burtnick MN, Stokes MG, Whelan AO, Williamson ED, Atkins TP, Prior JL, Brett PJ. Burkholderia pseudomallei capsular polysaccharide conjugates provide protection against acute melioidosis. Infect Immun. 2014;82:3206–3213. doi: 10.1128/IAI.01847-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pollard AJ, Perrett KP, Beverley PC. Maintaining protection against invasive bacteria with protein-polysaccharide conjugate vaccines. Nat Rev Immunol. 2009;9:213–220. doi: 10.1038/nri2494. [DOI] [PubMed] [Google Scholar]
- 71.Wang L, Feng S, An L, Gu G, Guo Z. Synthetic and Immunological Studies of Mycobacterial Lipoarabinomannan Oligosaccharides and Their Protein Conjugates. J Org Chem. 2015;80:10060–10075. doi: 10.1021/acs.joc.5b01686. [DOI] [PubMed] [Google Scholar]
- 72.Shin HJ, Franco LH, Nair VR, Collins AC, Shiloh MU. A baculovirus-conjugated mimotope vaccine targeting Mycobacterium tuberculosis lipoarabinomannan. PLoS One. 2017;12:e0185945. doi: 10.1371/journal.pone.0185945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pan Q, Wang Q, Sun X, Xia X, Wu S, Luo F, Zhang XL. Aptamer against mannose-capped lipoarabinomannan inhibits virulent Mycobacterium tuberculosis infection in mice and rhesus monkeys. Mol Ther. 2014;22:940–951. doi: 10.1038/mt.2014.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pan Q, Yan J, Liu Q, Yuan C, Zhang XL. A single-stranded DNA aptamer against mannose-capped lipoarabinomannan enhances anti-tuberculosis activity of macrophages through downregulation of lipid-sensing nuclear receptor peroxisome proliferator-activated receptor gamma expression. Microbiol Immunol. 2017;61:92–102. doi: 10.1111/1348-0421.12470. [DOI] [PubMed] [Google Scholar]
- 75.Meng X, Ji C, Su C, Shen D, Li Y, Dong P, Yuan D, Yang M, Bai S, Meng D, et al. Synthesis and immunogenicity of PG-tb1 monovalent glycoconjugate. Eur J Med Chem. 2017;134:140–146. doi: 10.1016/j.ejmech.2017.03.058. [DOI] [PubMed] [Google Scholar]
- 76.Kumar SK, Singh P, Sinha S. Naturally produced opsonizing antibodies restrict the survival of Mycobacterium tuberculosis in human macrophages by augmenting phagosome maturation. Open Biol. 2015;5:150171. doi: 10.1098/rsob.150171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Guirado E, Amat I, Gil O, Diaz J, Arcos V, Caceres N, Ausina V, Cardona PJ. Passive serum therapy with polyclonal antibodies against Mycobacterium tuberculosis protects against post-chemotherapy relapse of tuberculosis infection in SCID mice. Microbes Infect. 2006;8:1252–1259. doi: 10.1016/j.micinf.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 78.Kozakiewicz L, Chen Y, Xu J, Wang Y, Dunussi-Joannopoulos K, Ou Q, Flynn JL, Porcelli SA, Jacobs WR, Jr, Chan J. B cells regulate neutrophilia during Mycobacterium tuberculosis infection and BCG vaccination by modulating the interleukin-17 response. PLoS Pathog. 2013;9:e1003472. doi: 10.1371/journal.ppat.1003472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lin PL, Rodgers M, Smith L, Bigbee M, Myers A, Bigbee C, Chiosea I, Capuano SV, Fuhrman C, Klein E, et al. Quantitative comparison of active and latent tuberculosis in the cynomolgus macaque model. Infect Immun. 2009;77:4631–4642. doi: 10.1128/IAI.00592-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zheng RB, Jegouzo SAF, Joe M, Bai Y, Tran HA, Shen K, Saupe J, Xia L, Ahmed MF, Liu YH, et al. Insights into Interactions of Mycobacteria with the Host Innate Immune System from a Novel Array of Synthetic Mycobacterial Glycans. ACS Chem Biol. 2017;12:2990–3002. doi: 10.1021/acschembio.7b00797. [DOI] [PMC free article] [PubMed] [Google Scholar]


