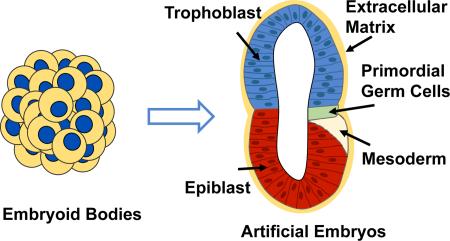
Abstract
Birth defects are a common occurrence in the United States and worldwide. Currently, evaluation of potential developmental toxicants (i.e., teratogens) relies heavily on animal-based models which do not always adequately mimic human development. In order to address this, researchers are developing in vitro human-based models which utilize human pluripotent stem cells (hPSCs) to assess the teratogenic potential of chemical substances. The field of human developmental toxicity assays includes a variety of platforms including monolayer, micropattern, embryoid body, and 3D organoid cultures. In this review, we will overview the field of human teratogenic assays, detail the most recent advances, and discuss current limitations and future perspectives.
Keywords: Development, Cell Microenvironment, Teratogens, Drug Screening Platforms, Microcontact Printing, Whole Embryo Cultures
Table of Contents Entry
Novel human pluripotent stem cell based assays for developmental toxicity screening
Introduction
In the United States, approximately one in 25 infants are affected by congenital abnormalities, with these birth defects accounting for roughly 20% of infant deaths annually.1 The adverse effects of environmental factors account for an estimated 10 – 15% of birth defects.2 These environmental factors are referred to as teratogens, which are defined as any chemical, drug, infection or factor that interferes with normal embryonic development, but show little or no toxicity in adults. Despite the prevalence of these potentially avoidable birth defects, barriers still remain in teratogen identification and assessment.
The Food and Drug Administration (FDA) previously categorized drugs into five different categories as they relate to teratogenic potential (A, B, C, D and X) with A and B indicating no or minimal risk, C signifying undetermined risk, D demonstrating small, moderate, or high risk, but with potential benefits that may outweigh the risks, and X having shown definitive fetal abnormalities in animal models or humans.3 This classification system was discontinued in 2015 in favor of requiring changes in prescription drug labeling, wherein sections on effects on pregnancy, lactation, and reproductive potential are to be added and updated as information becomes available.4 However, pregnancy risk assessment itself is not required for drug approval. This potentially results in up to 80% of approved prescriptions drugs having unknown effects on pregnancy; approximately 40% of drugs were categorized as Category C (undetermined risk) and an additional 40% had no category listed.3 Ultimately, physicians and patients have to rely on limited safety information in order to make decisions regarding medication usage during pregnancy.
Existing regulatory guidelines from the FDA and the Organization for Economic Cooperation and Development (OECD) recommend the use of animal models for teratogen testing.5,6 Nevertheless, animal models are both labor intensive and costly to use, thus leading researchers to explore alternatives. Early efforts included developing whole embryo culture (WEC) for rodents and utilizing embryos of aquatic animals such as zebrafish.7–9 WEC systems are highly advantageous because they can be easily manipulated and assessed for specific developmental and teratogenic endpoints.10 Although a considerably lower number of animals are required, the system still requires the use of rodents.10 Similarly, the zebrafish embryo model has been extensively used as zebrafish are easy to raise and maintain, imaging-compatible due to their transparency, and straightforward to genetically modify.9,11,12 While these systems are an improvement over previous animal models, moving toward human in vitro models would likely allow for more representative developmental outcomes. Currently, human cell-based assays are being developed and optimized in order to move away from these animal models. The use of human pluripotent stem cells (hPSCs) has allowed for innovation in the area of toxicity assays, resulting in more accurate high-throughput assays.
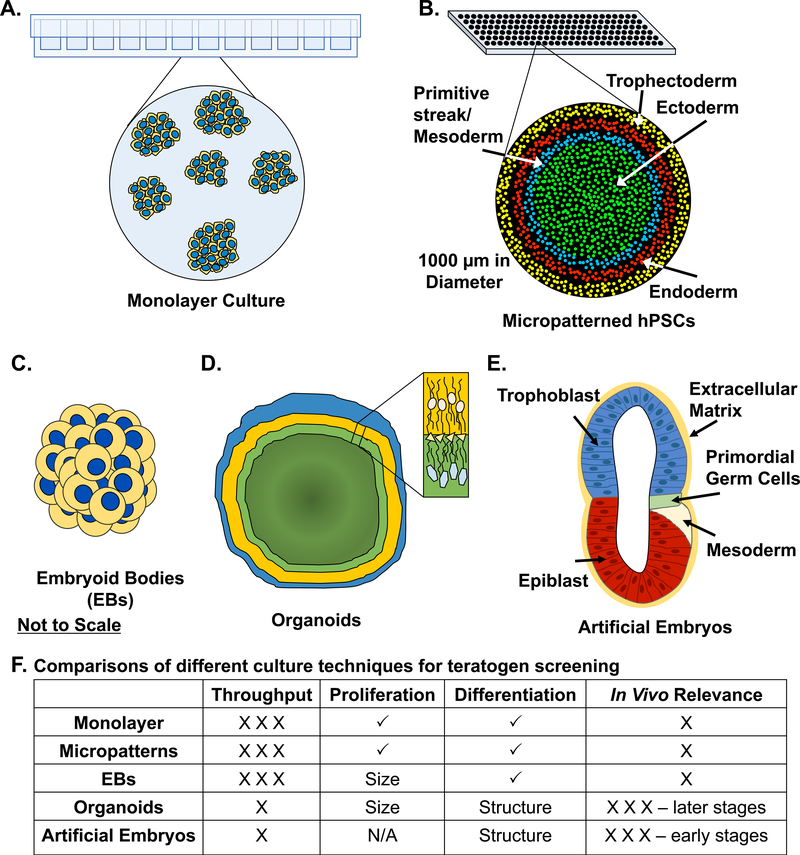
In this review, we will provide an overview of recent and influential studies focusing on human-based in vitro models for assessing developmental toxicity. There are a variety of platforms currently being evaluated, ranging from human embryonic stem cell (hESC) monolayer to micropattern cultures to embryoid bodies to 3D organoids (Figure 1). We will introduce the field of human teratogenic assays, explore the most recent advances, and discuss current limitations.
Figure 1.
Schematics of culture techniques used for developmental toxicity screening. A) Monolayer culture of human pluripotent stem cells (hPSCs) commonly performed in multi-well plates. B) Microcontact printing paired with immunostaining to visualize hPSC differentiation. C) Embryoid body culture. D) Organoid culture allowing for distinct structural cell arrangements. E) Artificial embryo displaying proper cell localization and organization. F) Table compares the above culture techniques; X to XXX– defines low to high ability for throughput or low to high level of in vivo relevance; check mark (√) indicates the ability to measure the condition directly. Graphics drawn not to scale. N/A: no data available.
2. Stem Cell Monolayer-based Toxicity Assays
Stem cells possess unique properties that qualify them for self-renewal, proliferation, migration, and differentiation in a progressive sequence of states. Failure to follow these sequences due to exposure to pharmaceutical drugs, toxicants, and chemical compounds can severely affect the formation of a complex organism, leading to birth defects. Recent advances in drug screening and discovery consist of high-throughput screening (HTS) of chemical libraries using human in vitro models such as hESCs and induced pluripotent stem cells (iPSCs).13–16 In this section, several seminal studies on culturing monolayers of stem cells as tools to assess toxicity at various stages in development including stem cell renewal, germ layer differentiation, and terminal differentiation will be described in detail.
2.1. Screening Assays that Detect hESCs Self-renewal and Pluripotency
Some studies have demonstrated the impact of adapting hESCs to HTS assays by focusing on germ layer differentiation. For example, Desbordes et al. introduced the first HTS study that identified marketed drugs and natural compounds which promoted short-term self-renewal and directed early lineage differentiation of hESCs.17 In their study, H1 and H9 cells were plated onto Matrigel-coated 384-well plates at high densities, and exposed to 2,880 compounds for over a week.17 In addition, they performed immunocytochemistry for important pluripotency markers (Oct4, Nanog, and Sox2) and differentiation markers (Pax6 for neuroectoderm; SMA for mesoderm; Sox17 for endoderm differentiation).17 Several compounds such as theanine, sinomenine, gatifloxacin, and flurbiprofen were found to significantly promote hESCs self-renewal in a dose-dependent manner (0 – 100 μM).17 Conversely, exposure to chemicals such as tretinoin, selegiline, cymarin, and sarmentogenin (0 – 100 μM) significantly upregulated genes that drove hESC differentiation.17
HTS assays on hESCs have also detected changes in cell survival, pluripotency, and proliferation as well as the morphology of stem cell colonies. For instance, Barbaric et al. developed an image-based, high-content phenotype screening assay that measured the percentage of cells within a colony that express TRA-1–60, a pluripotency marker that is downregulated during differentiation.18 The assay was used to screen 1,040 diverse compounds (960 compounds from marketed drugs and 80 compounds from a kinase library). Shef4 hESCs were plated in 96-well plates, and treated with compounds for 5 days before the high-content screen.18 The cells were analyzed for TRA-1–60 expression as well as the number of cells, colony geometry and intensity of nuclear staining.18 The results showed that steroids (e.g., betamethasone, dexamethasone, prednisolone, and 6-α methylprednisolone) at high dosages (100 μM) significantly promoted hESC differentiation with high expression levels of mesoderm and trophoblast markers, while reducing TRA-1–60, SSEA3, and Oct4 expression levels.18 An increased survival rate of different hESC cell lines (H7S14 and Shef4 to Sheft7) was attributed to regulation of activities of various kinases through treatment with 25 μM of Y-27362, HA1077, HA1004, or H-89, or through treatment with 100 μM pinacidil, an ATP-sensitive potassium channel agonist.18 One of the strengths of this assay was its potential to assess several effects of a compound on the phenotype of hESCs colonies in an automated and reproducible manner.
2.2. Screening Platforms to Detect Terminal Differentiation of hESCs
Other screening methods have focused on detecting substances that affect specific differentiation such as cardiovascular differentiation. Many studies use hPSCs-derived cardiomyocytes for toxicity screening,19,20 but only a few have looked into the possible effects of chemical substances on cardiac malformations of an embryo. Kameoka et al. developed a teratogenic assay utilizing 3-day directed differentiation of hESC monolayers with reduction in nuclear translocation of SOX17 as an endpoint.6 This rapid differentiation assay allowed for a HTS with 15 environmental toxicants and 71 synthesized drugs from Hoffmann-La Roche.6 The assay was able to predict the risk of teratogenicity of the compounds tested with high accuracy values indicating sensitivity and specificity.6 Despite the potential of this assay, additional work will be required to enhance the throughput by automating image analysis.
Teratogen screening for neural development is another important field of research, as the developing central nervous system is one of the most susceptible targets of toxicity due to the complex and highly orchestrated nature of its development.21–23 For example, a study by Stummann et al. found that methylmercury downregulated genes associated with neuronal precursor formation, but showed no effects on markers associated with later stages of neuronal differentiation.24 Interestingly, Colleoni et al. designed an in vitro neuronal teratogenicity test by inducing hESC embryoid bodies into neuronal rosettes on 2D Matrigel-coated plates to model neural plate and tube development.25 Neural rosettes are structures with similar properties to the neural plate and can recapitulate the formation and closure of the neural tube as well as the emergence of the neural crest.26,27 Human ESCs were exposed to different concentrations (2 nM - 2 μM) of retinoic acid (RA) during neuronal rosette formation for one week, then evaluated via changes in morphology and gene expression of genes relevant to neural development such as homeobox family genes (e.g., HoxA1, HoxA3, HoxB1, and HoxB4) as well as FoxA2, FoxC1 and Otx2. The authors found that these neuronal rosettes responded to RA in a similar concentration-dependent manner as those in the developing neural tube in vivo.25 Although only one teratogen was explored, this neural rosette model has the potential to be used for teratogenic screening specific to neural development at a large scale for other pharmacological treatments.
Besides genomics, metabolomics of hESCs have also been evaluated as possible endpoints for teratogenicity. West et al. dosed WA09 cells with 26 drugs of known teratogenicity, and performed mass spectrometry analysis to measure changes in abundance levels of small molecules in response to drug dosing.28 With statistical analyses, the authors were able to select specific mass features as a predictive biomarker for teratogenicity. They showed a correlation between teratogenicity and changes in the ratio of the metabolite arginine to asymmetric dimethylarginine levels.28 This finding was further examined with two blinded studies. Based on these promising results, the authors proposed that combining hESCs culture and metabolomics can be used as a reliable and predictive model for detecting toxins during development.28
Despite the use of in vitro human models with similar properties to in vivo conditions, several limitations exist with current screening platforms. The limited number of compounds tested do not definitively demonstrate the high-throughput or toxicity prediction capabilities of these platforms. HTS platforms are a valuable tool, but presently only recapitulate individual complex stem cell organization events which do not reflect in vivo developmental processes that occur simultaneously. Additionally, the development of new screening assays using hPSCs is challenging due to difficulties in plating, survival, and maintenance of pluripotency of these cells on 2D substrates. Finally, validation of screening results with established assays, such as those done with animal models, is urgently needed to determine consistencies and differences in order to further enhance our understanding in the field.
3. Teratogen Assays Using hESC Derived Embryoid Bodies
In addition to monolayer based hESC culture, hESC models for tetragon screening have also utilized embryoid bodies (EBs). EBs are a useful model due to their ability to differentiate into the three germ layers spontaneously.29 Additionally, the advent of technologies allowing for consistency in larger-scale production30 increases their suitability as a platform for teratogen evaluation. There are several different methods for EB formation, including but not limited to: suspension culture, hanging drop culture, and methylcellulose culture.30 Suspension culture methods in low-adherence vessels (e.g., round-bottom 96-well plates) have been utilized to create EBs of consistent size, which is essential for high-throughput applications.30,31
3.1. Embryoid Body Models for Developmental Toxicity
Past works have used hESC derived EBs in conjunction with known-teratogens as a model for developing and evaluating toxicity endpoints. Key gene expression markers were identified using two well-known developmental toxicants, 5-Fluorouracil (0 – 25μM) and RA (0 – 300 μM).32 Several of these crucial marker genes included genes for early differentiation (Oct4, hTert, and Dusp6) and cardiac differentiation (GATA-4 and Brachyury).32 Another study demonstrated that toxic insults could be observed in EBs as early as seven days following treatment with busulfan (IC50 = 0.38 μg/mL) or hydroxyurea (IC50 = 2.33 μg/mL), two strongly embryo-toxic compounds.33 Similar responses were observed among weakly teratogenic compounds, but only at much higher doses (caffeine: IC50 = 81.6 μg/mL; indomethacin: IC50 = 38.9 μg/mL).33 Subsequent analysis of gene expression patterns indicated significant down-regulation of markers representative for all three germ layers following exposure to both weakly and strongly embryo-toxic compounds.33 This assay was also used as a model to study the teratogenic effects of arsenic.34 Following arsenic treatment (IC50 = 5.99×103 μg/mL) a considerable number of genes representing the three germ layers were found to be significantly downregulated.34 In addition, genes associated with cell cycle regulation experienced a significant decrease while apoptotic gene expression increased.34 Recent efforts by Flamier et al. have resulted in a standardized human EB culture system through magnetic bead selection of highly pluripotent hESCs and aggregation of hESCs into uniformly sized EBs.35 To evaluate the sensitivity and accuracy of their EB systems, the authors studied three different compounds of varying teratogenicity: caffeine (a weak teratogen, 0.26 mM), penicillin-G (a moderate teratogen, 2.8 mM) and valproic acid (VPA, a strong teratogen, 1 mM) in three culture conditions (single EBs, pools of EBs, and EBs on Matrigel).35 Both single EBs and pools of EBs, but not EBs on Matrigel, were able to distinguish between the compounds, demonstrating key differences in EB growth and shape in different compounds. For the pools of EBs, differences in mesoderm and ectoderm gene expression were obtained between compounds.35 EBs have shown the capacity to be a useful tool for evaluating early embryonic toxicity and standardization of EB platforms would allow for high-throughput assays to be developed. However, current endpoints are often limited to a handful of pluripotency and developmental genes which restricts the ability to create predictive models.
3.2. Advancing Endpoints Using –Omic Based Approaches
In order to improve the accuracy and predictivity of teratogenic assays, -omic based approaches have been explored as alternative methodologies in developing more precise endpoints. The rationale of these approaches stems from the fact that thousands of genes are distinctly expressed over the course of differentiation.36 The -omic based approaches allow for more complete detection of mRNA or protein markers which are expressed at a select period in time.36 Transcriptomic approaches have been used to assess specific transcriptional responses to various teratogens in differentiating hESCs. Low concentrations (1 nM) of cytosine arabinoside (Ara-C) were found to induce ectodermal markers in EBs, while mesodermal markers were inhibited.36 Subsequent gene ontology indicated these changes potentially led to the dysregulation of processes related to neuronal differentiation, mesoderm development, and axonal guidance.36 A similar approach by Mayshar et al. showed that ethanol exposure (0.5%) led to an increase in endodermal differentiation markers.37 Additionally, RA (1 μM) led to misregulation in the neural development pathway, while thalidomide (10 μg/mL) had an adverse effect on both the aforementioned processes.37 Combinatorial -omic approaches have also been trialed with success. Meganathan et al. utilized both transcriptomic and proteomic approaches to study the effects of thalidomide (0.01 mM - 70 mM) on EBs.38 Proteomic analysis of thalidomide treated 14 day EBs indicated a loss of POU5F1 regulatory proteins and an overexpression of proteins involved in neuronal development.38 The use of –omic approaches has allowed for a more complete look into the influence of teratogens on both gene and protein expression in EB assays. Future studies will benefit from data sets that offer a broad spectrum of assessment, allowing for optimization and standardization of prospective teratogenic assays.
4. Geometric Confinements for Teratogen Screening
Engineered micropatterns, or microscale geometric confinements, are highly efficient biomedical tools to control and reproduce complex and dynamic in vitro cell niches to understand fundamentals of cell proliferation, migration, differentiation, and tissue-like morphogenesis.31,39 Micropatterns are usually fabricated using photolithography.40 Polydimethylsiloxane (PDMS) stamps are then prepared, which consist of patterns of different geometries.40 These geometries are transferred to 2D substrates for cell attachments, allowing for tight control of cell-cell and cell-extracellular matrix interactions and better recapitulation of in vivo development.40,41
Specific lineage differentiation has been the endpoint of several micro-contact printing screening assays. For instance, Nazareth et al. developed a high-throughput assay that patterned hPSCs colonies into standard 96-well plates to characterize single-cell protein expression.42 The use of a micro-contact printing (μCP) system allows for the control of the size, shape, and spacing of heterogeneous colonies of hPSCs. Immunofluorescence co-staining of Oct4 and Sox2 in the cells permitted discrimination of several stem cell fates. Through the screening of 27 developmental signaling factors, several signaling inhibitors were identified for pluripotency (e.g., TGFβ1, FGF2, IGF1, Noggin), neuroectoderm (e.g., SB431542, PD0325901, LDN-193189), primitive streak formation (e.g., Activin A, and BMP4), and extraembryonic cells (e.g., BMP4, CHIR99021, SB203580).42 This approach allowed for exploration of multiple developmental events within complex stem cell microenvironments in a comprehensive manner. A follow-up study from the same group used the μCP system to measure the effects of 400 small-molecule kinase inhibitors on hPSC fates.43 Their results suggested that mammalian target of rapamycin (mTOR) inhibitors had a strong effect on inducing the mesendoderm differentiation of hPSCs alone, and therefore enhancing the formation of blood progenitor cells.43 In this study the yield and purity output in terms of mesendoderm differentiation of hPSCs were determined for each of the inhibitors at various concentrations.43 At high mTOR concentrations (1 μM), low levels of pluripotency and definitive endoderm markers were reported, while mesendoderm differentiation markers were significantly higher by almost 2-fold. The μCP system enabled rapid responses within a robust high-throughput screening, which reasonably discriminated complex and highly regulated early developmental processes.
Xing and colleagues used a similar method for human teratogen detection by patterning hPSCs onto circular Matrigel islands (diameter of 1 mm) with a PDMS stencil.44 The cells were induced with BMP4, Activin A, and FGF2 to direct mesoendoderm differentiation, transition into epithelial-mesenchymal cells, and prompt migration on the micropatterned geometries via the micropatterned human pluripotent stem cell test (μP-hPST).44 In this study, a few known teratogens and non-teratogens were tested and classified based on their ability to disrupt the differentiation of the mesoendoderm layer.44 The results from this patterned screening platform were compared with in vivo animal and human data as well as the mouse embryonic stem cell test (mEST).44 One of the highlights of this study was its sensitivity to morphological changes in the patterned hPSCs in response to the dosage-dependency of known teratogens. A follow up study from the same group used the same μP-hPST platform to expose hPSCs and adult dermal fibroblast cells to 30 pharmaceutical compounds to determine their effects on mesoendoderm differentiation.45 In this study, authors simplified the method of classification via a two-step teratogen classification assay, and results were within the FDA guidelines for pregnancy classification of drugs.45 Their screening results were compared to in vivo teratogenicity results, and generated 97% accuracy to classify the compounds, with 100% specificity and 93% sensitivity.45 Unlike the previously mentioned patterned platforms, this μP-hPST assay allowed for spatial and temporal control of the mesoendoderm formation process despite the low number of compounds so far examined and classified.
These micropatterned screens allowed for rapid and robust responses compared to non-patterned-based screening platforms since these introduced not only exogenous regulators, but also endogenous physical cues into the system. Thus, complex microenvironment events such as specific lineage differentiation and control over heterogeneous populations of hPSC colonies were observed and quantified at the microscale level. However, a plethora of new studies are urgently needed to overcome the limitations of these systems such as the inability to control and measure the effects of chemical gradients onto patterned cells. Other micropatterned platforms designed to recapitulate specific developmental events have the potential to further study the effects of teratogens on hPSCs. For instance, some of these platforms have mimicked the formation of the primitive streak and differentiation of the germ layers. Warmflash et al. introduced a method to culture hESCs on circular geometries (1000 μm in diameter), which spatially patterned differentiated cells in a self-organized manner under prolonged BMP4 treatments.46 This method not only allowed observation of the different germ layers, but also a trophoblast-like cell layer, similarly to the outermost layer in the embryo.46 A followed-up study used the same circular micropatterns as Warmflash and colleagues to generate regionalized cell identities that collectively contributed to the process of gastrulation.47 In this study, mESCs were patterned onto circular islands (1000 μm in diameter), and supplemented with various signaling factors (e.g., FGF, Activin A, BMP4, and Wnt3a) to induce different cell fates and developmental regions (e.g., anterior- and posterior-epiblast, primitive streak, definitive endoderm, extraembryonic mesoderm and embryonic mesoderm).47 Both micropatterned platforms have the potential to study the effects of teratogens with a robust and quantitative micropatterning system that recapitulated several developmental events and regions at the microscale level.
5. Developmentally Relevant 3D Organoid and Embryo Based Toxicity Assays
The use of 2D and various micro-technologies has allowed for great insights into the field of embryogenesis and specifically for the creation of assays to evaluate the toxicity of compounds. However, these systems often do not accurately reflect conditions in vivo. It is difficult to precisely model human development in 3D in a human-specific and ethical manner, which has led to the creation of 3D organ-specific models, referred to as organoids. There are several comprehensive reviews available which summarize the wide variety of organoid systems available and their current uses in the field.48–50 The majority of organoid systems focus on evaluating toxicity as it relates to a single adult organ tissue48 or more recently several organ tissues,51,52 but assessing teratogenic effects will require a developmentally relevant model that focuses on tissue formation. Here we will concentrate on the use of 3D organoid systems that have shown or show the capacity to be utilized in teratogenic screening, but we will also touch on recent advances in embryo culture, specifically advances in human embryo culture and the development of artificial embryos.
5.1. Neural Organoids in Evaluating Developmental Toxicity
Defects in the central nervous system are common birth defects.53 The recent Zika virus outbreak has spurred research in the field of neural organoids,54 due to the increase in microcephaly in the infants of exposed mothers.55 Several works utilize neural organoids to examine the effect of the Zika virus on neural development through the evaluation of organoid size,56–58 apoptosis markers,56–58 and relevant immunostaining,56,58 as well as transcriptional analysis to evaluate the similarity of the organoids to in vivo neural tissue.56,58 Additionally, human iPSCs were shown to be able to generate other types of neural organoids such as motor and glutamatergic neuron-specific organoids.59 Current limitations primarily arise from inconsistencies in the organoids themselves and a lack of standards for evaluation. Schwartz et al. addresses consistency in neural organoids through an innovative system that combines a polyethylene glycol (PEG) hydrogel platform with developmentally-relevant timed addition of hESC derived cell types important to neural development.60 More recently, work by the same group further demonstrated the reproducibility of these neural organoids over the course of 47 days of culture.61 These constructs initially consist of PEG hydrogels seeded with neural progenitor cells which are allowed to grow for several days.60,61 Vascular cells are then added at day 9 and microglia and macrophage precursors added at day 13 to mimic recruitment of blood vessels and microglia.60,61 This method led to self-assembly into 3D neural constructs which showed distinct similarities to in vivo neural development.60,61 Ultimately, the goal was to create consistent organoids in order to assess the neural organoids’ potential as a toxicity assay.60 Two hundred and forty constructs were treated with 34 toxic and 26 nontoxic compounds and then assessed using RNA sequencing.60 This data was then used to create a predictive model and tested in an unbiased blinded trial of ten chemicals.60 The predictive model was able to correctly classify nine out of the ten compounds, with one being a false positive.60 This work illustrates the feasibility of creating predictive models for evaluating neural toxicity. However, additional work; for example, including a larger number of compounds to add to the predictive capacity, will need to be done to optimization future predictive models.60 Creating standards is important for producing viable neural toxic assays, but it is equally important to continue to validate the constructs’ ability to recapitulate neural development and function.
5.2. Heart, Kidney and Retinal Organoid Platforms for Teratogen Assays
Neural organoids have seen the most recent advancement with regards to becoming developmentally relevant, however there has also been progress in heart, kidney and retinal organoids. Mills et al. derived cardiomyocytes from hPSCs and, utilizing photolithography and PDMS, fabricated a 96-well functional screening device for cardiac organoids.62 They were able to condense cardiac cells into 1 mm in length cardiac organoids between two elastic posts.62 This platform allowed for the high-throughput screening of these organoids for evaluation of contractile forces and for endpoint whole-mount immunostaining to visualize markers of interest (e.g. MLC2v, Ki-67, and α-actinin).62 They discovered through use of this screening method that mimicking the in vivo metabolic switch to fatty acid oxidation using palmitate treatment facilitated metabolic, transcriptional, and cell cycle maturation and thus maturation of the cardiac organoids.62 Similar work by Devarasetty et al. utilized cardiac organoids to examine the influence of various drugs (i.e. 100 μM isoprotenenol, 500 nM epinephrine, 1 μM quinidine, 100 nM astemizole, and 100 nM ricin A) on beat rate.63 The prevalence of birth defects that are heart-related53 makes having platforms that are able to examine function and relevant protein expression especially important for evaluating developmental toxicity.
While not as prevalent as neural and heart defects, birth defects in other organs and organ systems do occur and platforms to test teratogenic effects will need to be developed. Currently, there are a few organoid systems which are developmentally relevant and could be used to analyze potential teratogenic compounds. Morizane et al. derived nephron progenitor cells (NPCs) from hPSCs using a chemically defined differentiation protocol that mimicked in vivo metanephric kidney development.64 Spontaneous morphogenesis of 2D cultured NPCs into 3D PAX8+LHX1+ nephron structures occurred with low frequency, so the authors evaluated which stage of NPC differentiation, when re-plated in 3D suspension culture, would result in the greatest formation of kidney organoids.64 3D suspension culture of cells, cultured in 2D until day 9, resulted in organoids with nephron-like structures which exhibited features characteristic of the in vivo nephron.64 Additionally, NPC markers were absent from the kidney organoids after day 21 of culture, indicating mature structures.64 Disruption of kidney organoid structure was examined both for addition of the Notch signaling inhibitor, DAPT (10 μM), during organoid differentiation and for a common toxicant, gentamicin (5 mg/mL), after 21 days, resulting in suppression of proximal tubule formation and KIM-1 expression, a biomarker for proximal tubule injury, respectively.64 These experiments illustrate how this kidney organoid model can be used for evaluation of toxicants on both kidney maturation and adult kidney function. Efficient retinogenesis has also recently been achieved through the differentiation of both mouse and human PSCs into retinal organoids.65 Völkner et al. examined the gene expression of individual organoids showing reproducible generation of retinal organoids.65 Additionally, they were able to induce enrichment of cone or rod photoreceptors based on the timing of Notch signaling inhibition.65 These results indicate significant potential for the evaluation of teratogens in relation to eye defects.
The use of organoids to evaluate developmental toxicants specifically is a relatively new area, but progress is being made to increase consistency, standardize protocols, and ensure developmental relevance. 3D organoid culture systems allow for the evaluation of later stages of development permitting researches to better mimic organ development. Future works will likely continue to elucidate the accuracy of these organoid systems in mimicking organ development, increase consistency in organoid formation, standardize for high-throughput applications and start to incorporate multiple organoids in evaluations.
5.3. Advances in Embryo Culture and Artificial Embryos
The study of teratogenic compounds is typically thought of in terms of the effects on organ systems, however the most detrimental effects are often seen when exposure occurs during the first trimester. A system that can be utilized to study early embryogenesis is imperative in this situation. As discussed previously, EBs are currently used to study germ layer formation, however they do not adequately replicate all early embryonic events. Recent ground-breaking work by Shahbazi et al. has shown the capability to culture human embryos up to the 14-day guideline.66 Human amniogenesis was first observed with hPSCs in 3D biomaterial.67 While exciting for the field, it does bring up ethical questions regarding the use of human embryos. Doing toxicity studies with human embryos would often require a large number of embryos to be used and the current 14-day rule would not allow for long-term study. However, recent work by Harrison et al. could potentially alleviate some of these problems, while the current study uses mouse embryonic stem cells and extraembryonic cells,68 it seems likely future work will examine the feasibility of using human lineage-based cells. The authors were able to combine mESCs and extraembryonic trophoblast stem cells (TSCs) in a 3D Matrigel scaffold to create artificial embryos (ETS-embryos) that mimic mouse embryos.68 They were able to observe the formation of the pro-amniotic cavity as well as characteristic embryo architecture, correct patterning of the embryonic compartment and specification of a small cluster of primordial germ-like cells at the embryonic and extraembryonic boundary.68 Artificial embryos are an exciting new area that would allow for increased embryo uniformity, since the genetic material would be the same, and have the potential to create a large number of embryos for study at the same time. Both of these features would be useful for high-throughput assays. However, there are ethical concerns regarding transitioning from mouse to human stem cell types since it will be difficult to ascertain if artificial embryos will be viable. Limitations for this method are currently related to the yield of ETS-embryos, with only 22% of structures containing both ESCs and TSCs, however they attained 88 useable ETS-embryos.68 Mouse artificial ETS-embryos show great potential for investigating the effects of teratogenic compounds on early embryogenesis. Further work in the field will be necessary to address limitations and produce feasible assays for studying possible teratogens.
Conclusions
Teratogen evaluation still relies primarily on the use of in vivo animal-based assays which are limited in their capacity to mimic human development. Recently there have been advances in human in vitro models that can recapitulate development at different stages. Human embryonic stem cell monolayer culture, micropatterning and embryoid body cultures were some of the first teratogenic assays to be developed. These assays allowed scientists to revolutionize the field permitting the evaluation of self-renewal, pluripotency, proliferation, migration and terminal differentiation in a human model. However, these models typically replicate only early embryogenesis, so therefore the most recent work has focused on simulating organ development through the use of organoid cultures. Currently the use of organoid cultures for teratogen evaluation is limited, but advances in mimicking the organ development of the brain, kidneys, heart and eyes indicate their use is not far off. Additionally, advances in human embryo culture and the advent of artificial embryos may allow for a model which more exactly mimics in vivo development, but their use is not without ethical concern. While human teratogen assays have come a long way, there is still significant room for improvement. Ultimately, the goal of these models is to create an assay which is accurate, high-throughput, consistent, and uniform. It is unlikely that a single assay alone will ever allow for the complete evaluation of a compound’s teratogenic capacity, but improving and developing assays which look at a variety of stages in development is paramount to accurate classification.
Insight, Innovation, Integration.
Development of in vitro human models for embryonic toxicity assessment is imperative to accurately recapitulating human development and reducing animal usage. This review addresses the topic of developmental toxicity assessment through discussion of biologically relevant models and endpoints (e.g., gene expression, protein production, cell proliferation) as well as various advances in technologies which can increase throughput and ensure accuracy, consistency, and uniformity. Additionally, we review studies focusing on generating predictive comparative models based on characteristic features of endpoints with compounds of varying teratogenicity using large-scale screening.
Acknowledgements
This work was supported by the National Institutes of Health (OD/NICHD DP2HD083961 & 3DP2HD083961–01S1), National Science Foundation (CAREER CMMI-1254656), and American Heart Association (13SDG17230047). Leo Q. Wan is a Pew Scholar in Biomedical Sciences (PEW 00026185), supported by the Pew Charitable Trusts.
Footnotes
Conflicts of Interest
There are no conflicts to declare.
References:
- 1.Mai CT et al. Population-based birth defects data in the United States, 2008 to 2012: Presentation of state-specific data and descriptive brief on variability of prevalence. Birth Defects Res A Clin Mol Teratol 103, 972–993, doi: 10.1002/bdra.23461 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gilbert-Barness E Teratogenic causes of malformations. Ann Clin Lab Sci 40, 99–114 (2010). [PubMed] [Google Scholar]
- 3.Marcus DA & Bain PA in Effective Migraine Treatment in Pregnant and Lactating Women: A Practical Guide 29–45 (Humana Press, 2009). [Google Scholar]
- 4.U. S. Food and Drug Administration. Pregnancy and Lactation Labeling (Drugs) Final Rule, <https://www.fda.gov/Drugs/DevelopmentApprovalProcess/DevelopmentResources/Labeling/ucm093307.htm> (2014). [Google Scholar]
- 5.Bailey GP, Wise LD, Buschmann J, Hurtt M & Fisher JE Pre-and postnatal developmental toxicity study design for pharmaceuticals. Birth Defects Research Part B: Developmental and Reproductive Toxicology 86, 437–445 (2009). [DOI] [PubMed] [Google Scholar]
- 6.Kameoka S, Babiarz J, Kolaja K & Chiao E A High-Throughput Screen for Teratogens Using Human Pluripotent Stem Cells. Toxicological Sciences 137, 76–90, doi: 10.1093/toxsci/kft239 (2014). [DOI] [PubMed] [Google Scholar]
- 7.New D Whole‐embryo culture and the study of mammalian embryos during organogenesis. Biological Reviews 53, 81–122 (1978). [DOI] [PubMed] [Google Scholar]
- 8.Sadler T, Horton W & Warner C Whole embryo culture: a screening technique for teratogens? Teratogenesis, carcinogenesis, and mutagenesis 2, 243–253 (1982). [DOI] [PubMed] [Google Scholar]
- 9.Streisinger G, Walker C, Dower N, Knauber D & Singer F Production of clones of homozygous diploid zebra fish (Brachydanio rerio). Nature 291, 293 (1981). [DOI] [PubMed] [Google Scholar]
- 10.Zhang C, Ball J, Panzica-Kelly J & Augustine-Rauch K In Vitro Developmental Toxicology Screens: A Report on the Progress of the Methodology and Future Applications. Chemical Research in Toxicology 29, 534–544, doi: 10.1021/acs.chemrestox.5b00458 (2016). [DOI] [PubMed] [Google Scholar]
- 11.Kimmel CB & Warga RM Cell lineage and developmental potential of cells in the zebrafish embryo. Trends in Genetics 4, 68–74 (1988). [DOI] [PubMed] [Google Scholar]
- 12.Panzica-Kelly JM, Zhang CX & Augustine-Rauch K in Developmental Toxicology: Methods and Protocols (eds Harris Craig & Hansen M. Jason) 25–50 (Humana Press, 2012). [DOI] [PubMed] [Google Scholar]
- 13.Saxe JP et al. A phenotypic small-molecule screen identifies an orphan ligand-receptor pair that regulates neural stem cell differentiation. Chemistry & biology 14, 1019–1030, doi: 10.1016/j.chembiol.2007.07.016 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu X, Ding S, Ding Q, Gray NS & Schultz PG Small molecules that induce cardiomyogenesis in embryonic stem cells. Journal of the American Chemical Society 126, 1590–1591, doi: 10.1021/ja038950i (2004). [DOI] [PubMed] [Google Scholar]
- 15.Jincho Y et al. Identification of genes aberrantly expressed in mouse embryonic stem cell-cloned blastocysts. Biology of reproduction 78, 568–576, doi: 10.1095/biolreprod.107.064634 (2008). [DOI] [PubMed] [Google Scholar]
- 16.Liu S, Yin N & Faiola F Prospects and Frontiers of Stem Cell Toxicology. Stem Cells Dev 26, 1528–1539, doi: 10.1089/scd.2017.0150 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Desbordes SC et al. High-throughput screening assay for the identification of compounds regulating self-renewal and differentiation in human embryonic stem cells. Cell Stem Cell 2, 602–612, doi: 10.1016/j.stem.2008.05.010 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barbaric I et al. Novel regulators of stem cell fates identified by a multivariate phenotype screen of small compounds on human embryonic stem cell colonies. Stem Cell Res 5, 104–119, doi: 10.1016/j.scr.2010.04.006 (2010). [DOI] [PubMed] [Google Scholar]
- 19.Guo L et al. Estimating the risk of drug-induced proarrhythmia using human induced pluripotent stem cell-derived cardiomyocytes. Toxicological sciences : an official journal of the Society of Toxicology 123, 281–289, doi: 10.1093/toxsci/kfr158 (2011). [DOI] [PubMed] [Google Scholar]
- 20.Sirenko O et al. Assessment of beating parameters in human induced pluripotent stem cells enables quantitative in vitro screening for cardiotoxicity. Toxicol Appl Pharmacol 273, 500–507, doi: 10.1016/j.taap.2013.09.017 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Van Thriel C et al. Translating neurobehavioural endpoints of developmental neurotoxicity tests into in vitro assays and readouts. Neurotoxicology 33, 911–924 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kadereit S, Zimmer B, van Thriel C, Hengstler JG & Leist M Compound selection for in vitro modeling of developmental neurotoxicity. Front Biosci 17, 2442–2460 (2012). [DOI] [PubMed] [Google Scholar]
- 23.Song L, Wang K, Li Y & Yang Y Nanotopography promoted neuronal differentiation of human induced pluripotent stem cells. Colloids Surf B Biointerfaces 148, 49–58, doi: 10.1016/j.colsurfb.2016.08.041 (2016). [DOI] [PubMed] [Google Scholar]
- 24.Stummann TC, Hareng L & Bremer S Hazard assessment of methylmercury toxicity to neuronal induction in embryogenesis using human embryonic stem cells. Toxicology 257, 117–126, doi: 10.1016/j.tox.2008.12.018 (2009). [DOI] [PubMed] [Google Scholar]
- 25.Colleoni S et al. Development of a neural teratogenicity test based on human embryonic stem cells: response to retinoic acid exposure. Toxicological sciences : an official journal of the Society of Toxicology 124, 370–377, doi: 10.1093/toxsci/kfr245 (2011). [DOI] [PubMed] [Google Scholar]
- 26.Elkabetz Y et al. Human ES cell-derived neural rosettes reveal a functionally distinct early neural stem cell stage. Genes & development 22, 152–165 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lazzari G et al. Direct derivation of neural rosettes from cloned bovine blastocysts: a model of early neurulation events and neural crest specification in vitro. Stem Cells 24, 2514–2521 (2006). [DOI] [PubMed] [Google Scholar]
- 28.West PR, Weir AM, Smith AM, Donley EL & Cezar GG Predicting human developmental toxicity of pharmaceuticals using human embryonic stem cells and metabolomics. Toxicol Appl Pharmacol 247, 18–27, doi: 10.1016/j.taap.2010.05.007 (2010). [DOI] [PubMed] [Google Scholar]
- 29.Itskovitz-Eldor J et al. Differentiation of human embryonic stem cells into embryoid bodies compromising the three embryonic germ layers. Molecular medicine 6, 88 (2000). [PMC free article] [PubMed] [Google Scholar]
- 30.Kurosawa H Methods for inducing embryoid body formation: in vitro differentiation system of embryonic stem cells. J Biosci Bioeng 103, 389–398, doi: 10.1263/jbb.103.389 (2007). [DOI] [PubMed] [Google Scholar]
- 31.Kinney MA, Hookway TA, Wang Y & McDevitt TC Engineering three-dimensional stem cell morphogenesis for the development of tissue models and scalable regenerative therapeutics. Ann Biomed Eng 42, 352–367, doi: 10.1007/s10439-013-0953-9 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Adler S, Pellizzer C, Hareng L, Hartung T & Bremer S First steps in establishing a developmental toxicity test method based on human embryonic stem cells. Toxicology in vitro 22, 200–211 (2008). [DOI] [PubMed] [Google Scholar]
- 33.Mehta A, Konala VBR, Khanna A & Majumdar AS Assessment of drug induced developmental toxicity using human embryonic stem cells. Cell Biology International 32, 1412–1424, doi: 10.1016/j.cellbi.2008.08.012 (2008). [DOI] [PubMed] [Google Scholar]
- 34.Flora SJS & Mehta A Monoisoamyl dimercaptosuccinic acid abrogates arsenic-induced developmental toxicity in human embryonic stem cell-derived embryoid bodies: Comparison with in vivo studies. Biochemical Pharmacology 78, 1340–1349, doi: 10.1016/j.bcp.2009.07.003 (2009). [DOI] [PubMed] [Google Scholar]
- 35.Flamier A, Singh S & Rasmussen TP A standardized human embryoid body platform for the detection and analysis of teratogens. PLoS One 12, e0171101, doi: 10.1371/journal.pone.0171101 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jagtap S et al. Cytosine arabinoside induces ectoderm and inhibits mesoderm expression in human embryonic stem cells during multilineage differentiation. British Journal of Pharmacology 162, 1743–1756, doi: 10.1111/j.1476-5381.2010.01197.x (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mayshar Y, Yanuka O & Benvenisty N Teratogen screening using transcriptome profiling of differentiating human embryonic stem cells. Journal of Cellular and Molecular Medicine 15, 1393–1401, doi: 10.1111/j.1582-4934.2010.01105.x (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Meganathan K et al. Identification of Thalidomide-Specific Transcriptomics and Proteomics Signatures during Differentiation of Human Embryonic Stem Cells. PLoS ONE 7, 1–15, doi: 10.1371/journal.pone.0044228 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thery M Micropatterning as a tool to decipher cell morphogenesis and functions. Journal of cell science 123, 4201–4213, doi: 10.1242/jcs.075150 (2010). [DOI] [PubMed] [Google Scholar]
- 40.Kolind K, Leong KW, Besenbacher F & Foss M Guidance of stem cell fate on 2D patterned surfaces. Biomaterials 33, 6626–6633, doi: 10.1016/j.biomaterials.2012.05.070 (2012). [DOI] [PubMed] [Google Scholar]
- 41.Joshi R, Thakuri PS, Buchanan JC, Li J & Tavana H Microprinted Stem Cell Niches Reveal Compounding Effect of Colony Size on Stromal Cells-Mediated Neural Differentiation. Adv Healthc Mater 7, doi: 10.1002/adhm.201700832 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nazareth EJ et al. High-throughput fingerprinting of human pluripotent stem cell fate responses and lineage bias. Nature methods 10, 1225–1231, doi: 10.1038/nmeth.2684 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nazareth EJP, Rahman N, Yin T & Zandstra PW A Multi-Lineage Screen Reveals mTORC1 Inhibition Enhances Human Pluripotent Stem Cell Mesendoderm and Blood Progenitor Production. Stem Cell Reports 6, 679–691, doi: 10.1016/j.stemcr.2016.04.003 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xing J, Toh YC, Xu S & Yu H A method for human teratogen detection by geometrically confined cell differentiation and migration. Sci Rep 5, 10038, doi: 10.1038/srep10038 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xing J et al. In Vitro Micropatterned Human Pluripotent Stem Cell Test (microP-hPST) for Morphometric-Based Teratogen Screening. Sci Rep 7, 8491, doi: 10.1038/s41598-017-09178-1 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Warmflash A, Sorre B, Etoc F, Siggia ED & Brivanlou AH A method to recapitulate early embryonic spatial patterning in human embryonic stem cells. Nature methods 11, 847–854, doi: 10.1038/nmeth.3016 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Morgani SM, Metzger JJ, Nichols J, Siggia ED & Hadjantonakis AK Micropattern differentiation of mouse pluripotent stem cells recapitulates embryo regionalized cell fate patterning. Elife 7, doi: 10.7554/eLife.32839 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fang Y & Eglen RM Three-Dimensional Cell Cultures in Drug Discovery and Development. SLAS Discov 22, 456–472, doi: 10.1177/1087057117696795 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Astashkina A & Grainger DW Critical analysis of 3-D organoid in vitro cell culture models for high-throughput drug candidate toxicity assessments. Adv Drug Deliv Rev 69–70, 1–18, doi: 10.1016/j.addr.2014.02.008 (2014). [DOI] [PubMed] [Google Scholar]
- 50.Ranga A, Gjorevski N & Lutolf MP Drug discovery through stem cell-based organoid models. Adv. Drug Deliv. Rev 69, 19–28, doi: 10.1016/j.addr.2014.02.006 (2014). [DOI] [PubMed] [Google Scholar]
- 51.Materne EM et al. A multi-organ chip co-culture of neurospheres and liver equivalents for long-term substance testing. J Biotechnol 205, 36–46, doi: 10.1016/j.jbiotec.2015.02.002 (2015). [DOI] [PubMed] [Google Scholar]
- 52.Skardal A et al. Multi-tissue interactions in an integrated three-tissue organ-on-a-chip platform. Sci Rep 7, 8837, doi: 10.1038/s41598-017-08879-x (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Parker SE et al. Updated National Birth Prevalence estimates for selected birth defects in the United States, 2004–2006. Birth Defects Res A Clin Mol Teratol 88, 1008–1016, doi: 10.1002/bdra.20735 (2010). [DOI] [PubMed] [Google Scholar]
- 54.Hartley BJ & Brennand KJ Neural organoids for disease phenotyping, drug screening and developmental biology studies. Neurochem Int 106, 85–93, doi: 10.1016/j.neuint.2016.10.004 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Reynolds MR et al. Vital Signs: Update on Zika Virus-Associated Birth Defects and Evaluation of All U.S. Infants with Congenital Zika Virus Exposure - U.S. Zika Pregnancy Registry, 2016. MMWR Morb Mortal Wkly Rep 66, 366–373, doi: 10.15585/mmwr.mm6613e1 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dang J et al. Zika Virus Depletes Neural Progenitors in Human Cerebral Organoids through Activation of the Innate Immune Receptor TLR3. Cell Stem Cell 19, 258–265, doi: 10.1016/j.stem.2016.04.014 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Garcez PP et al. Zika virus impairs growth in human neurospheres and brain organoids. Science 352, 816–818, doi: 10.1126/science.aaf6116 (2016). [DOI] [PubMed] [Google Scholar]
- 58.Watanabe M et al. Self-Organized Cerebral Organoids with Human-Specific Features Predict Effective Drugs to Combat Zika Virus Infection. Cell Rep 21, 517–532, doi: 10.1016/j.celrep.2017.09.047 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yan Y et al. Neural patterning of human induced pluripotent stem cells in 3-D cultures for studying biomolecule-directed differential cellular responses. Acta Biomater 42, 114–126, doi: 10.1016/j.actbio.2016.06.027 (2016). [DOI] [PubMed] [Google Scholar]
- 60.Schwartz MP et al. Human pluripotent stem cell-derived neural constructs for predicting neural toxicity. Proc Natl Acad Sci U S A 112, 12516–12521, doi: 10.1073/pnas.1516645112 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Barry C et al. Uniform neural tissue models produced on synthetic hydrogels using standard culture techniques. Exp Biol Med (Maywood) 242, 1679–1689, doi: 10.1177/1535370217715028 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mills RJ et al. Functional screening in human cardiac organoids reveals a metabolic mechanism for cardiomyocyte cell cycle arrest. Proc Natl Acad Sci U S A 114, E8372–E8381, doi: 10.1073/pnas.1707316114 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Devarasetty M et al. Optical Tracking and Digital Quantification of Beating Behavior in Bioengineered Human Cardiac Organoids. Biosensors (Basel) 7, doi: 10.3390/bios7030024 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Morizane R et al. Nephron organoids derived from human pluripotent stem cells model kidney development and injury. Nat Biotechnol 33, 1193–1200, doi: 10.1038/nbt.3392 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Volkner M et al. Retinal Organoids from Pluripotent Stem Cells Efficiently Recapitulate Retinogenesis. Stem Cell Reports 6, 525–538, doi: 10.1016/j.stemcr.2016.03.001 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shahbazi MN et al. Self-organization of the human embryo in the absence of maternal tissues. Nat Cell Biol 18, 700–708, doi: 10.1038/ncb3347 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shao Y et al. Self-organized amniogenesis by human pluripotent stem cells in a biomimetic implantation-like niche. Nature materials 16, 419–425, doi: 10.1038/nmat4829 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Harrison SE, Sozen B, Christodoulou N, Kyprianou C & Zernicka-Goetz M Assembly of embryonic and extraembryonic stem cells to mimic embryogenesis in vitro. Science 356, doi: 10.1126/science.aal1810 (2017). [DOI] [PubMed] [Google Scholar]