Abstract
AIM
To clarify the underlying mechanism of formin-like 3 (FMNL3) in the promotion of colorectal carcinoma (CRC) cell invasion.
METHODS
The in vitro biological function analyses of FMNL3 were performed by gain- and loss-of function approaches. Changes in the F-actin cytoskeleton were detected by the technologies of phalloidin-TRITC labeling and confocal microscopy. The signaling pathway mediated by FMNL3 was explored by western blot, gelatin zymograph assay, co-immunoprecipitation (co-IP), immunofluorescence co-localization, and glutathione S-transferase (GST) pull-down assay.
RESULTS
The in vitro experimental results showed that FMNL3 significantly promoted the proliferation, invasion, and migration of CRC cells (P < 0.05 and P < 0.01). Moreover, FMNL3 regulated the remodeling of actin-based protrusions such as filopodia and lamellipodia in a RhoC-dependent manner. The western blot and gelatin zymograph assay results indicated that FMNL3 was involved in the RhoC/ focal adhesion kinase (FAK) pathway and acted as an effector of RhoC to activate the downstream signaling of p-FAK as well as p-MAPK and p-AKT. This resulted in the increased expression of matrix metalloproteinase 2 (MMP2), matrix metalloproteinase 9 (MMP9) and vascular endothelial growth factor (VEGF), and the subsequent promotion of CRC cell invasion. The results of TAE226, U0126 or Ly294002 treatment confirmed an essential role of FMNL3 in activation of the RhoC/FAK pathway and the subsequent promotion of CRC invasion. Co-IP, co-localization and GST pull-down assays showed the direct interaction of FMNL3 with RhoC in vivo and in vitro.
CONCLUSION
FMNL3 regulates the RhoC/FAK signaling pathway and RhoC-dependent remodeling of actin-based protrusions to promote CRC invasion.
Keywords: Formin-like 3, Colorectal carcinoma, Invasion, RhoC/FAK pathway, Actin assembly
Core tip: Formin-like 3 (FMNL3) belongs to the subfamily of diaphanous-related formins, which govern the actin-dependent processes, including cell motility and invasion. The increased expression of FMNL3 in colorectal carcinoma (CRC) was shown to contribute to metastasis and poor prognosis of patients in previous studies, however its regulatory mechanism remains unclear. This work reveals that FMNL3 plays a positive role in CRC cell proliferation, invasion and migration. Moreover, FMNL3 activates the RhoC/FAK signaling pathway, and also regulates RhoC-dependent remodeling of actin-based protrusion, such as filopodia and lamellipodia, to promote CRC cell invasion. FMNL3 can be applied as a promising specific biomarker for CRC progression and metastasis.
INTRODUCTION
Colorectal carcinoma (CRC) is the third most common diagnosis and second deadliest malignancy, and metastasis remains the major cause of mortality in patients with CRC[1,2]. Deregulated cell motility and invasion is a key initial step in metastasis[3]. Invasive cell migration involves movement through tissues, dynamic interactions with the extracellular matrix, rearrangements of cell-to-cell contacts and the cytoskeleton[4]. Further understanding of the underlying regulatory mechanisms may provide novel therapeutic regimes for reducing cancer cell dissemination, blocking metastatic progression, and prolonging life expectancy of patients with CRC.
Diaphanous-related formins (DRFs) are ubiquitously expressed proteins and known to govern cell shape, adhesion, and motility by remodeling the actin cytoskeleton[5-8]. The DRF protein contains a Rho-GTPase binding domain (GBD) in the NH2-terminus. Upon binding to a Rho-GTPase, the bound NH2-terminal diaphanous inhibitory domain is dissociated from the C-terminal diaphanous autoregulatory domain. This, in turn, results in the release of inactive DRF autoinhibition, and subsequently allows the formin homology 2 (FH2) domain to function as the regulator of actin assembly. Three members of the DRFs (DRF1-DRF3) were reported to be associated with invadopodia formation and the invasion of breast tumor cells[9]. As the largest family of Rho GTPase effectors, DRFs regulate cytoskeletal remodeling and cancer cell invasion downstream of Rho GTPase signaling. The DRF protein formin-like 2 (FMNL2) drives actin-based protrusion and migration downstream of CDC42 in melanoma cells[10], and drives the amoeboid invasive cell motility downstream of RhoC[3]. Positive feedback between Dia1, LARG, and RhoA regulates cancer cell morphology and invasion by affecting actin assembly[11].
Formin-like 3 (FMNL3), another novel member of the DRF family, has been recently identified[12]. Several studies have demonstrated the role of FMNL3 in cytoskeletal remodeling and cell migration. FMNL3 participates in filopodia assembly, microtubule acetylation and cell-cell adhesion[13-15], as well as induces protein N-myristoylation required for cellular morphological changes[16]. FMNL3 is also required for the polarized trafficking of podocalyxin to the early apical surface in vascular lumenogenesis, and is a crucial regulator of angiogenesis[17,18]. Recent studies have demonstrated upregulation of FMNL3 in cutaneous melanoma and nasopharyngeal cancer[19,20], as well as its promotion of cancer cell invasion and migration in nasopharyngeal, esophageal carcinoma and neuroblastoma[20-22]. Our previous study also indicated that increased expression of FMNL3 contributes to metastasis and poor prognosis in patients with CRC[23]. Although literature on FMNL3 expression and function in multiple tumors has been presented, the underlying molecular mechanism of FMNL3-promoting tumor progression and metastasis remains to be elucidated.
Hence, in this study we investigate the effects of FMNL3 on CRC cell proliferation, invasion and migration in vitro using gain- and loss-of-function approaches. Moreover, we reveal an essential role for FMNL3 in regulating the RhoC/FAK pathway and actin assembly dynamics, and the subsequent promotion of CRC invasion.
MATERIALS AND METHODS
Cell lines and reagents
All four CRC cell lines (LOVO, SW620, SW480 and HCT116) and the 293T cell line were purchased from Cell Bank of Chinese Academy of Sciences (Shanghai, China). The cell lines were cultured at 37 °C in a 50 mL/L CO2-humidified atmosphere with the appropriate medium according to the requirements of the Cell Bank. Anti-(p-) Pyk2 (proline-rich tyrosine kinase 2), anti-(p-) FAK, anti-(p-) MAPK (Mitogen activated protein kinase), anti-(p-) AKT and anti-RhoC antibodies were purchased from Cell Signaling Technology. Anti-flag, anti-VEGF (vascular endothelial growth factor) and anti-FMNL3 antibodies were obtained from Abbkine, Inc (Redlands, CA, United States) and Abnova (Taiwan, China), respectively. For inhibitor treatment, 1 μmol/L TAE226 (Selleck), 20 μmol/L U0126 (Selleck) or 20 μmol/L Ly294002 (Selleck) was added to the cultured cells for 48 h, respectively.
Construction of plasmids and transfection
Two groups of specific RNA interference sequences targeting the coding regions of FMNL3 and Pyk2 genes were designed as in the previous study[24,25]. The ones were separately cloned into the GV102 plasmid (Genechem Biotechnology, Shanghai, China) to construct FMNL3-silenced cell lines, named “FMNL3/shRNA1” and “FMNL3/shRNA2”. A scrambled shRNA, which has no homology with the mammalian mRNA sequences, was inserted into the GV102 vector and served as the control. The same method was used to construct the Pyk2-silenced cell lines, named “Pyk2/shRNA1” and “Pyk2/shRNA2”. To obtain an active mutant construct of RhoC-V14, the wild-type coding region of RhoC was amplified by polymerase chain reaction (PCR) and inserted into the expression plasmid pGEX-4T-1. The mutant construct was then generated with the KOD-Plus-Mutagenesis Kit (TOYOBO, Japan). The primers were designed as follows: 5’-GCTGCAATCCGAAAGAAGCTGGTGA-3’ or 5’ –TCAGAGAATGGGACAGCCCCTCCGA-3’. DNA was purified with a Mini plasmid Purification Kit (Qiagen, Japan) and digested with suitable restriction enzymes. DNA fragments were electrophoresed on 1% agarose to verify the insertion of sequences. Cells were plated into 6-well plates using 1 × 106 cells/well to grow overnight to 90% confluence, and transiently transfected with 3 μg of plasmid using 2 μL Lipofectamine™ 2000 (Invitrogen, United States) according to the instructions. Cells were incubated for 48 h until they were ready for further assays.
Establishment of cell lines stably expressing FMNL3
Commercialization of the viral particles that express the coding region of the FMNL3 gene, fused EGFP and three flag genes were purchased from GeneCopoeia, Inc (Guangzhou, China). The FMNL3 gene was amplified by PCR and then inserted into the plasmid pcDNA3 (Invitrogen, Forster City, CA, United States). The primers used were as follows: forward 5’-TCCGATTCATTCTTAC-3’, reverse 5’-CCGCCTCAACTCTGCTATT-3’. The PCR conditions were as follows: 95°C for 3 min, followed by 35 cycles of amplification (94 °C for 30 s, 55 °C for 40 s, 72°C for 2 min). The fragment was inserted into the pGC-FU-EGFP-3FLAG lentiviral vector. The FMNL3 overexpression vector was transfected into lentiviral packaging 293T cells. The culture supernatant containing viral particles was harvested 48h after transfection of 293T cells. The day before the infection of viral particles, CRC cells were seeded into 24-well plates using 1 × 104 cells/well. The next day, 2 × 1012 TU/L of viral supernatant containing 5 μg/mL of polybrene was added to the cells. After 72 h, 2.5 mg/L puromycin (Sigma, United States) was added to the culture for screening. On approximately day 14, puromycin-resistant cell pools were established by selection. Following amplification culture, real-time PCR and Western blot were performed to validate the upregulation of FMNL3.
MTT assay
Cells were inoculated into 96-well plates (1 × 102 cells/well) with 100 ul/well medium and cultured for 5 d. Every 24 h, MTT (20 μL, 5 mg/mL; Promega) was added to the cells to incubate for 4 h until purple precipitates were visible. Precipitates were then dissolved with 150 μL of DMSO. The absorbance value of each well was measured with a microplate reader set at 570 nm. The experiment was repeated three times and the average value was calculated.
In vitro invasive assay
The in vitro invasive ability was tested by Boyden chamber assay. The invasion chamber was equipped with 8 μm pores in polyethylene terephthalate membrane coated with matrigel (BD Biosciences, Foster City, CA, United States). First, 1.5 × 105 tumor cells in serum-free RPMI 1640 medium were added to the upper chamber, and the RPMI 1640 with 10% fetal bovine serum was added to the lower chamber as the chemotactic factor. Each cell group was plated in three replicate wells. After incubation for 24 h, the noninvasive cells were gently removed with a cotton swab. Cells that invaded the membrane were fixed with methanol and stained with Giemsa. The number of invaded cells was counted under a light microscope in five random visual fields. The experiment was repeated three times and the average value was calculated.
In vitro scratch assay
The in vitro scratch assay is an easy, low-cost and well-developed method to measure cell migration in vitro[26]. Cells were seeded into a 24-well plate. When the cells were cultured to confluence, the cell monolayer was scraped in the form of a cross with a plastic pipette tip. Then the three “wound” areas were marked for orientation and photographed by a phase-contrast microscope both immediately and after 24 h of incubation. The experiment was repeated three times.
F-actin staining and observation
Cells were seeded into 14 mm Confocal Petri dishes and cultured for 24 h. The cells were then fixed with 40 g/L formaldehyde for 30 min, permeabilized by 0.1% Triton X-100 for 10 min, and then blocked with 1% BSA for 30 min, followed by incubation with 5 μg/mL rhodamine-conjugated phalloidin (Sigma, United States) for 1 h. After counter-staining with DAPI, F-actin images were acquired with an Olympus FV1000 confocal microscope (Olympus, Japan) using a 100 × oil immersion objective. The length of filopodia and the cells with broad lamellipodia were quantified as in the previous study[18,24]. The experiment was repeated three times and the average value was calculated.
Western blot assay
Cells were washed twice with cold phosphate-buffered saline (PBS) and lysed using ice Lysis buffer containing 0.1% protease inhibitors and 0.5% phenylmethanesulfonyl fluoride (Keygen, China). The proteins in the cells were quantified using the bicinchoninic acid method. Fifty micrograms of proteins were loaded onto 10% sodium dodecyl-polyacrylamide gel electrophoresis (SDS-PAGE). The proteins were then electro-transferred onto PVDF membranes (Millipore) and blocked in 5% nonfat dry milk in tris-buffered saline. Membranes were immunoblotted overnight at 4 °C with anti-FMNL3 antibody (Abnova), anti-RhoC, anti-Pyk2 (or -p-Pyk2), anti-MAPK (or -p-MAPK), anti-AKT (or -p-AKT) (Cell signaling technology), anti-VEGF or anti-GAPDH antibody (Abbkine), respectively, and followed by respective horseradish peroxidase-conjugated secondary antibodies (Abbkine). Signals were detected by BeyoECL Plus (Beyotime Biotechnology, China).
Gelatin zymograph assay
Cells were seeded into 6-well plates and incubated in serum-free medium for 48 h. The cell supernatant was then collected, and the protein concentration was quantified. The cell supernatant was mixed with 5 × SDS loading buffer followed by electrophoresis on 10% SDS-PAGE containing 0.1% gelatin at 4 °C. The gel was washed with the eluent (containing 2.5% Triton X-100, 50 mmol/L Tris-HCl, 5 mmol/L CaCl2, pH 7.6) for 80 min and rinsed (50 mmol/L Tris-HCl, 5 mmol/L CaCl2, pH7.6) for 40 min. The cells were then incubated in the reaction buffer (50 mmol/L Tris-HCl, 5 mmol/L CaCl2, 0.02% Brij-35, pH 7.6) at 37 °C for 42 h, stained with 0.05% coomassie brilliant blue for 3 h, and then destained with buffer containing 30% methanol and 10% acetic acid for 2 h. The image of each band was finally photographed.
Immunofluorescence co-localization assay
For fluorescence staining, cells were fixed with 40 g/L formaldehyde, permeabilized with 0.1% Triton X-100 in PBS, and blocked with 1% BSA in PBS for 30 min, followed by incubation overnight at 4 °C with both anti-flag and anti-RhoC antibodies. The cells were washed three times with PBS for 5 min, incubated with DyLight™ 488 conjugated Goat anti-Mouse IgG along with DyLight™ 549 conjugated Goat anti-Rabbit IgG for 30 min, and then nuclear stained with 1 mg/L 4, 6-diamidino-2-phenylindole (DAPI, Roche, Germany). The fluorescence images were acquired with an Olympus FV1000 confocal microscope (Olympus, Japan) using a 100 × oil immersion objective.
Co-immunoprecipitation assay
Cell lysates from the stably-expressing FMNL3-3 flag cells were prepared in lysis buffer (FNN0021, Life technologies). Dynabeads-Ab compound was prepared with rotation overnight at 4 °C (Dynabeads Protein G: 10004D, Life technologies; anti-flag antibody: Abbkine, Inc. Redlands, CA, United States; anti-RhoC antibody: Cell Signaling Technology). Dynabeads-mouse IgG (M30016, Ab-mart) was used as control. Then the co-incubated Dynabeads-Ab with cell lysates (adjusted the total protein concentration to 1 g/L before co-incubation, added 500 μL) was used to form Dynabeads-Ab-Ag compound. After this, 30 μL of 1 × SDS-PAGE loading buffer was added to the Dynabeads-Ab-Ag compound, and then boiled for western blot detection.
GST pull-down assay
The recombinant pGEX-4T-1-RhoC-V14 plasmids were transformed into colibacillus BL21 (DE3) and induced for expression by IPTG. SDS-PAGE was used for detection and analysis. Glutathione-Sepharose 4B (GE Hearthcare, Little Chalfont, United Kingdom) affinity chromatography was performed to purify GST-RhoC-V14 or GST protein according to the manufacturer’s instructions. The purified proteins were then incubated with 293T cell lysates for 2 h at 4 °C (293T cells were transfected with the FMNL3-EGFP-3FLAG fusion gene). The Glutathione-Sepharose 4B beads were then washed with ice-cold PBS, and then bound proteins were eluted and subjected to both electrophoresis and detection with the indicated antibodies.
Statistical analysis
In vitro studies and the quantity of filopodia and lamellipodia were tested using One-Way ANOVAs or t-tests. SPSS Statistics 17.0.1 software (SPSS, Chicago, IL, United States) was used for all statistical analyses. P < 0.05 was considered as statistically significant differences.
RESULTS
FMNL3 promotes CRC cell proliferation, invasion and migration in vitro
Our previous study showed lower expression of FMNL3 in low metastatic potential cell lines (HCT116, HT29, LS174T and SW480) than in high metastatic potential cell lines (LOVO and SW620)[23]. Hence, we chose LOVO and SW620 to construct stable FMNL3-knockdown cell lines, as well as HCT116 and SW480 for stable FMNL3-overexpressing cell lines (Figure 1 and Supplementary Figure 1A and B). Then, a series of in vitro assays were performed to detect the effect of FMNL3 expression or silencing on CRC cell proliferation, invasion and migration. MTT assays showed that forced expression of FMNL3 caused a significant increase in the proliferation rate of SW480 and HCT116 cells (Figure 1C). Overexpression of FMNL3 also markedly enhanced CRC cell invasion (Figure 1D) and migration (Figure 1E) by the Boyden chamber assay and scratch assays in vitro, respectively. In contrast, FMNL3-depletion showed the opposite effects (Supplementary Figure 1). These data suggest that FMNL3 promotes CRC cell proliferation, invasion and migration in vitro.
Figure 1.
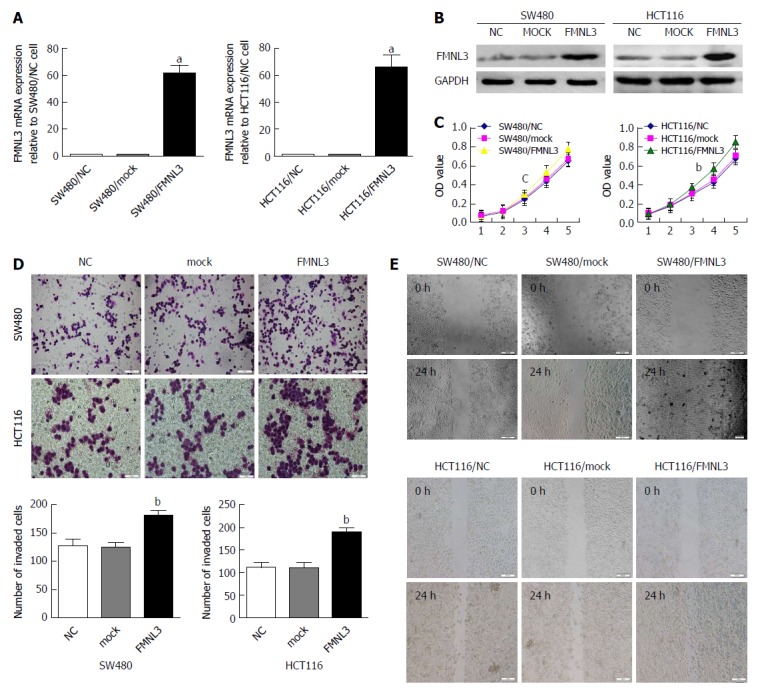
Forced expression of FMNL3 promotes colorectal carcinoma cell proliferation, invasion, and migration in vitro. A and B: Identification of FMNL3 expression in FMNL3-overexpressing cells by real-time quantitative PCR and western blot. C: Effects of FMNL3 overexpression on cell proliferation by MTT assay. D: Effects of FMNL3 overexpression on invasive abilities by Boyden chamber assay. Morphological comparison of cell penetration into the artificial basement membrane is shown. E: Effects of FMNL3 overexpression on migratory abilities by scratch assay in vitro. Scale bars represent 50 μm (cell invasion assay) or 100 μm (cell migration assay), respectively. aP < 0.001, bP < 0.01 and cP < 0.05 vs NC or mock group. Error bars indicate mean ± SD.
FMNL3 regulates the assembly of actin-based protrusions
Next, we observed the effects of FMNL3 overexpression or silencing on the actin cytoskeleton within filopodia and lamellipodia in CRC cells by analysing the rhodamine-phalloidin staining of F-actin. We found that the filopodia were remarkably more abundant and longer, however the lamellipodia were more narrow in FMNL3-overexpressing cells compared with mock cells (Figure 2A and B). On the contrary, the filopodia were fewer and shorter but the lamellipodia were wider in FMNL3-depleted cells compared with scrambled cells (Figure 2C). These results were consistent with the findings from other cells in previous studies[13,18,24], and indicated that FMNL3 plays an important role in regulating the assembly of actin-based protrusions.
Figure 2.
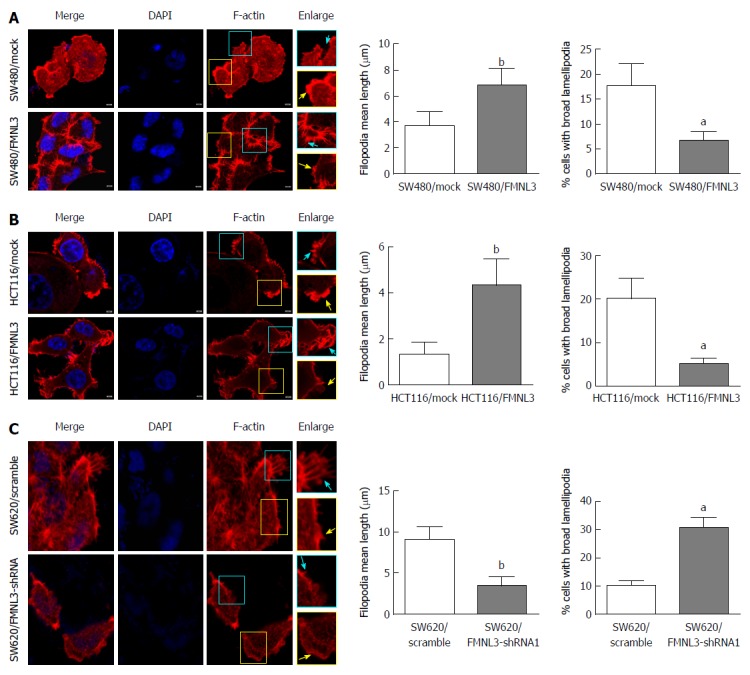
Effects of formin-like 3 overexpression (A and B) and depletion (C) on filopodia and lamellipodia in colorectal carcinoma cells. Cells are displayed using tritc-phalloidine (F-actin, Red) and DAPI (nuclear, blue) staining, and laser scanning confocal microscopy detection. Enlarged views of the boxed regions are shown on the right side of the figures. Blue arrows indicate filopodia, yellow arrows indicate lamellipodia. Scale bars represent 5 μm. aP < 0.001, and bP < 0.01 vs mock or scramble group. Error bars indicate mean ± SD. FMNL3: Formin-like 3.
FMNL3 plays an essential role in the RhoC/FAK pathway to promote CRC cell invasion
To further gain insight into the signaling pathways by which FMNL3 promotes invasive phenotypes, we prepared cell lysates from FMNL3-overexpressing cells, FMNL3-depleted cells and the corresponding control cells. As the invasive and metastatic abilities of tumor cells were often correlated with the product of matrix metalloproteinases (MMPs) and VEGF[27,28], we measured the expression of these proteins by gelatin zymograph assay and western blot, respectively. Forced expression of FMNL3 significantly caused up-regulation of MMP-2, MMP-9 and VEGF (Figure 3A), and vice versa when FMNL3 was suppressed (Figure 3B). Therefore, our results showed that the invasive phenotypes induced by FMNL3 in CRC cells were partly due to the improved expression of MMP-2, MMP-9 and VEGF.
Figure 3.
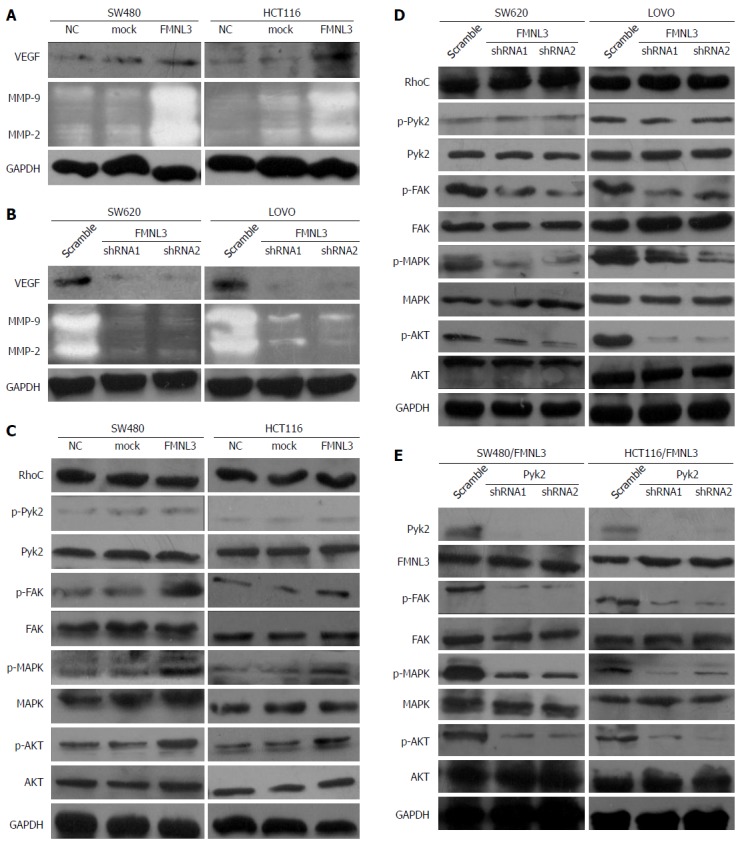
Formin-like 3 regulates the RhoC/FAK signaling pathway to promote colorectal carcinoma invasion. A and B: Analysis of VEGF, MMP-2 and MMP-9 expression in FMNL3-overexpressing or -depleted colorectal carcinoma cells by western blot and gelatin zymography experiments, respectively. C and D: Analysis of the effects of FMNL3 overexpression or depletion on the expression of RhoC, (p-)Pyk2, (p-)FAK, (p-)MAPK and (p-)AKT by western blot. E: Effects of Pyk2 silencing on the expression of FMNL3, (p-)FAK, (p-)MAPK and (p-)AKT in FMNL3-expressing cells by western blot. FMNL3: Formin-like 3; VEGF: vascular endothelial growth factor; MMP: matrix metalloprotein; Pyk2: Proline rich tyrosine kinase 2; FAK: Focal adhesion kinase; MAPK: Mitogen activated protein kinases; AKT: Protein kinase B.
The levels of MMP-2 and MMP-9 were regulated by phosphorylated MAPK and AKT[29,30], which were activated by RhoC[31-33] or in sequence activated by FAK, Pyk2 and RhoC[25]. Moreover, the expression of VEGF and MMP-9 were inhibited by the down-regulation of RhoC[28]. More importantly, FMNL3 acts as a downstream effector of RhoC[24]. We thus speculated that FMNL3 participates in a RhoC-dependent signaling pathway. To validate this speculation, the expression of p-MAPK, p-AKT, p-FAK, p-Pyk2 and RhoC in CRC cells was measured by western blot. As shown in Figure 3C, the overexpression of FMNL3 strongly increased the expression of p-MAPK, p-AKT, p-FAK, while FMNL3 silencing generated opposite results (Figure 3D). There were no significant differences in the total amounts of these proteins (Figure 3C and D). However, neither overexpression nor depletion of FMNL3 led to any changes in the expressions of p-Pyk2 and RhoC. These results suggested that FMNL3 may play an essential role in the RhoC signaling pathway, and act downstream of RhoC and p-pyk2 as well as upstream of p-FAK, p-MAPK and p-AKT. However, the inhibition of Pyk2 did not affect the expression of FMNL3, although it resulted in the downregulation of p-FAK, p-MAPK and p-AKT as expected (Figure 3E). This suggested that FMNL3 may not work downstream of p-Pyk2, but only downstream of RhoC. Indeed, when FMNL3/shRNA1 and RhoC genes were transfected simultaneously into SW480 and HCT116 cells, the RhoC-dependent upregulations of MMPs and VEGF were partly blocked by FMNL3 silencing (Figure 4A). These results confirmed the notion that FMNL3 acts as a downstream effector of RhoC. Thus, FMNL3 may act downstream of RhoC (but not downstream of RhoC and p-Pyk2) and upstream of p-FAK, p-MAPK and p-AKT.
Figure 4.
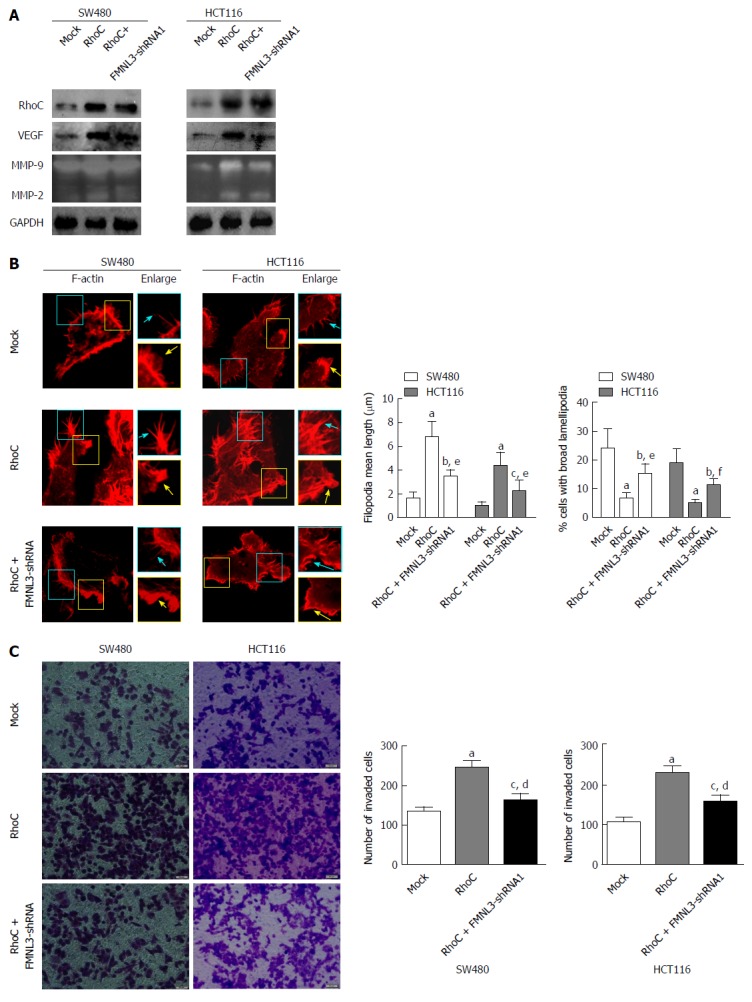
Formin-like 3 depletion blocks RhoC-dependent increases of matrix metalloproteins and vascular endothelial growth factor (A), assembly of actin-based protrusions (B), and invasion (C) in colorectal carcinoma cells. MMPs and VEGF were detected by gelatin zymography experiments and western blot. F-actin is displayed using tritc-phalloidine (Red) staining and laser scanning confocal microscopy detection. Enlarged views of the boxed regions are shown on the right side of the figures. Blue arrows indicate filopodia, yellow arrows indicate lamellipodia. Cell invasion was compared using the Boyden chamber assay. Scale bars represent 5 μm (F-actin) or 50 μm (cell invasion assay), respectively. aP < 0.001, bP < 0.01 and cP < 0.05 vs Mock group, dP < 0.001, eP < 0.01 and fP < 0.05 vs RhoC-overexpressing group. Error bars indicate mean ± SD. FMNL3: Formin-like 3; MMP: Matrix metalloprotein; VEGF: Vascular endothelial growth factor.
We also investigated the effects of the co-transfection of FMNL3/shRNA1 and RhoC genes on the actin cytoskeleton and invasive abilities of CRC cells. We found that the RhoC-dependent restriction of lamellipodial broadening, promotion of filopodia elongation and enhancement of cell invasion were partly inhibited by FMNL3 depletion (Figure 4B and C). These results suggested that FMNL3 regulates the assembly of actin-based protrusions and cell invasion of CRC in a RhoC-dependent manner.
To further validate the above data, we treated FMNL3-overexpressing cells with TAE226 (a FAK-specific inhibitor), U0126 (a MAPK/ERK-specific inhibitor) or Ly294002 (a PI3K/AKT-specific inhibitor) separately. Then the expressions of MMP2, MMP-9 and VEGF, as well as the difference of cell invasive ability, were measured using the same methods as above. We found that inhibition of FAK, MAPK/ERK or PI3K/AKT indeed significantly blocked the effects of FMNL3-induced increases in these three proteins and invasion in CRC cells (Figure 5). These results strongly confirmed the essential role of FMNL3 in the RhoC/FAK signaling pathway.
Figure 5.
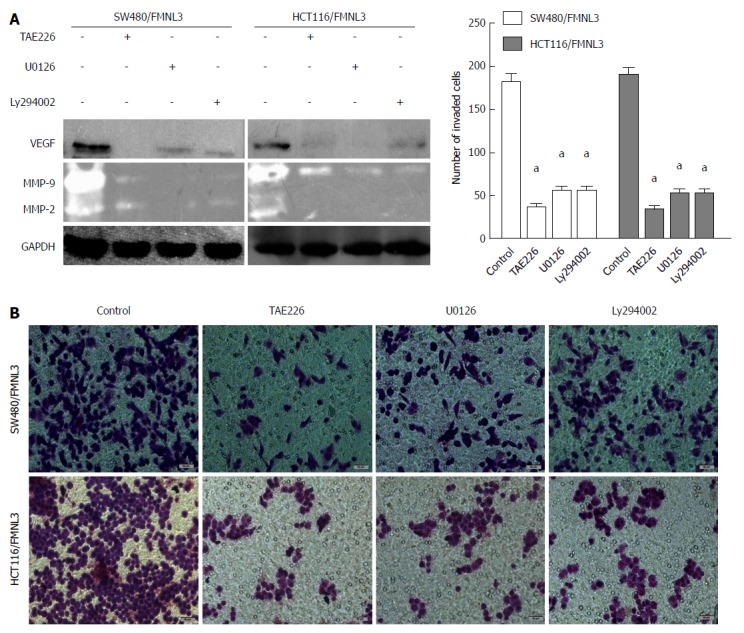
Effects of TAE226, U0126 and Ly294002 treatments, respectively, on the expression of matrix metalloproteins and vascular endothelial growth factor by gelatin zymography experiments and western blot (A), as well as cell invasion by Boyden chamber assay (B and C). Morphological comparison of cells penetrating into the artificial basement membrane is also shown. Scale bars represent 50 μm. aP < 0.001 vs control group. Error bars indicate mean ± SD. MMP: Matrix metalloprotein; VEGF: Vascular endothelial growth factor.
Taken together, FMNL3 regulates the RhoC/FAK signaling pathway and RhoC-dependent remodeling of actin-based protrusions to promote CRC invasion.
FMNL3 interacts directly with RhoC
Finally, we explored the partner of FMNL3 in the RhoC/FAK signaling pathway. Since FMNL3 belongs to the DRF subfamily and contains a GBD domain in the NH2-terminus, it provides the structural basis for the activation by Rho-GTPases via direct binding. The possibility of the interaction between FMNL3 and RhoC was therein tested by co-immunoprecipitation, immunofluorescence-based confocal microscopy and GST-pull down assays. Indeed, we found that FMNL3 and RhoC co-localized in the cytoplasm (Figure 6A), and immunoprecipitated with each other by one or the other antibody (Figure 6B). The results of GST pull-down assays confirmed the direct binding of FMNL3 to RhoC in vitro (Figure 6C). These results demonstrated that FMNL3 interacts directly with RhoC in vivo and in vitro, which were in accordance with the findings of the Vega FM group regarding the interaction between FMNL3 and RhoC in vitro[24].
Figure 6.
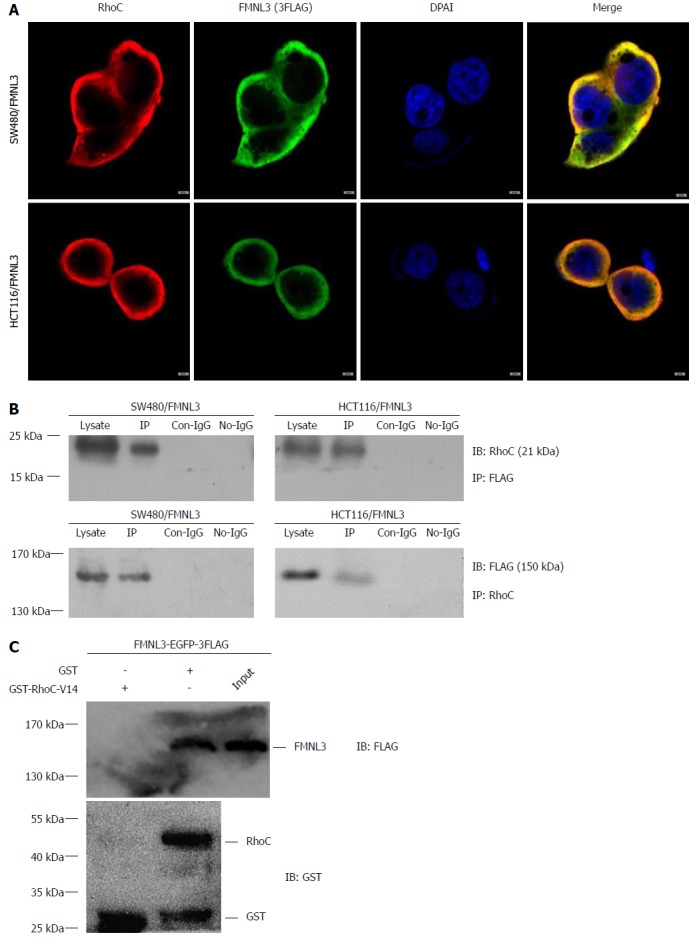
Interaction of formin-like 3 with RhoC. A: FMNL3 co-localizes with RhoC in the cytoplasm, detected by immunofluorescence staining and laser confocal microscope. B and C: FMNL3 interacts directly with RhoC in co-immunoprecipitation experiments and GST-pull down assays in vitro. Scale bars represent 5 μm. IP: Immunoprecipitation; GST: Glutathionine-S-transferase; FMNL3: Formin-like 3.
DISCUSSION
We have shown in previous studies that increased FMNL3 expression contributes to metastasis and poor prognosis in patients with CRC[23]. However, the underlying molecular mechanism remains unclear. In this study, we explored the possible signaling pathway responsible for CRC cell invasion and migration induced by FMNL3. We first determined the biological effects of FMNL3 on CRC cells in vitro. Our results showed the positive roles of FMNL3 in CRC cell proliferation, migration and invasion in vitro, which were inconsistent with the promotion of FMNL3 in tumor growth and metastasis in vivo found in our previous study[23]. Recent studies have also reported the relevant function of FMNL3 in tumor cell growth and proliferation[34]. Other DRF members were also involved in cell proliferation and division through cell cycle regulation[35] or microtubule stabilization in a cell type-selective manner[36]. Moreover, FMNL3 has been shown to promote cell invasion, migration and metastasis in various cell types[19-22], confirming our previous and present study results both in vivo and in vitro[23].
Previous studies have reported that the reorganization of the actin cytoskeleton is responsible for enhanced cell motility that is necessary for cancer cell invasion and metastasis[4]. As a Rho-GTPase-binding protein, DRF possesses conserved function in actin cytoskeletal dynamics exerted through the formin homology 2 (FH2) domain[37]. DRF contains a NH2-terminal GBD domain, where upon binding to a Rho-GTPase, the bound NH2-terminal diaphanous inhibitory domain dissociates from the COOH-terminal diaphanous autoregulatory domain. This, in turn, results in the release of inactive DRF autoinhibition and subsequently allows the FH2 domain to function as a direct regulator of actin polymerization[37]. DRFs are major actin filament nucleators, which can bundle linear actin filaments and generate membrane protrusions such as filopodia and lamellipodia[38,39]. Here, we found that FMNL3 overexpression promotes the elongation of filopodia and restricts the broadening of lamellipodia. Some researchers have also reported the assembly of filopodia and lamellipodia by FMNL3[13,22,24] and verified the structure of the FMNL3 FH2/actin complex-mediated actin nucleation and elongation[40].
Evidence has shown that DRFs regulate the assembly of actin-related structures and cancer cell invasion downstream of Rho GTPases[9-11,41]. Rho family GTPases, including RhoA, RhoB, RhoC, Rac and Cdc42, are key regulators of actin cytoskeletal dynamics associated with cell motility and invasion, and their expression and activation generally increase with tumor progression[42,43]. RhoC is the best-characterized Rho GTPase among them, and its overexpression has recently been shown to be closely linked with highly invasive and metastatic forms of many human cancers[44]. Recent studies have also reported that RhoC promotes polarized migration through FMNL3 by restricting the lamellipodia broadening in prostate cancer[24]. Our results also showed that FMNL3 could regulate the actin-based protrusions of filopodia and lamelipodia in a RhoC-dependent manner to accelerate CRC cell invasion. These results were consistent with the findings of the Higgs HN and Ridley AJ groups regarding the roles of FMNL3 in the regulation of filopodia and lamellipodia that contribute to the enhanced migratory and invasive potential in other cell types[13,18,24].
The molecular mechanism by which FMNL3 promotes tumor progression and metastasis is a fascinating subject. Evidence has shown that RhoC is closely related to the high invasion and metastasis of various types of human cancers[44]. RhoC induces tumor cell motility and invasion via MAPK- and PI3K/AKT-dependent pathways[25,31-33]. However, whether FMNL3 regulates the RhoC-dependent signaling pathway to promote CRC cell invasion and migration needs further investigation.
MMP-2, MMP-9 and VEGF are important effectors of the RhoC-dependent pathway[25,28,45]. Moreover, MMP-2 and MMP-9 are the two key proteases for tumor metastasis[27]. VEGF is one of the important angiogenetic factors required for tumor angiogenesis[46]. Our results provide evidence for the role of FMNL3 in activation of the two proteases and VEGF in CRC cells, suggesting a possible role of FMNL3 in RhoC-dependent pathway activation during CRC cell invasion.
Both MMP-2 and MMP-9 were shown to be activated by phosphorylated MAPK and AKT[29,30]. In addition, MAPK and AKT signaling pathways were activated by RhoC[31-33] or in sequence by FAK, Pyk2 and RhoC[25]. Moreover, RhoC expression levels are correlated with the expressions levels of VEGF and MMP9[28]. In addition, p-FAK regulates VEGFR2 transcription in angiogenesis[47]. Our study shows that FMNL3 induced the phosphorylation of FAK and subsequent phosphorylation of MAPK and AKT, resulting in the upregulation of MMP-2, MMP-9 and VEGF, and the subsequent promotion of enhanced CRC cell invasion. FAK is a protein tyrosine kinase that was first identified within the extracellular matrix and at integrin receptor cell adhesion sites, and is a key regulator of cell movement[48]. Resent studies showed increased expression of p-FAK in the nuclei of cells in laryngeal cancer and four digestive cancers, including colorectal cancer[49,50]. Nuclear FAK promotes cell proliferation and survival through enhanced P53 degradation[51], suggesting an association between p-FAK and abnormal cell proliferation. In addition, nuclear expression of p-FAK is also associated with poor prognosis in colorectal cancer[52]. In this study, we found that the phosphorylation of FAK (triggered by RhoC/FMNL3 signaling) induced the activation of MAPK and AKT and the subsequent upregulation of MMPs and VEGF, and contributed to the invasive potential of CRC cells. Of course, it may also be correlated with the nuclear location of p-FAK, which is triggered by RhoC/FMNL3 signaling. Other studies also presented the correlation of DRF members with MAPK, AKT and/or FAK, as well as Rho signaling[3,10,53,54]. The DRF FMNL2 enhanced CRC cell invasion via the MAPK/ERK and PI3K/AKT pathways[53], and regulated the invasive cell motility and migration downstream of RhoC and Cdc42[3,10]. Another DRF member, mDia1, acts downstream of RhoA and upstream of MAPK, ERK and FAK to induce intestinal epithelial cell migration[54].
Finally, we uncovered RhoC as the partner of FMNL3 in the RhoC/FAK signaling pathway by co-IPs, immunofluorescence co-localization experiments and GST pull-down assays. This finding was also supported by the structural basis of the GBD domain in the N-terminus of FMNL3, and has also been verified by the GST pull-down assay from the Ridley AJ group[24]. However, other groups reported the interaction of FMNL3 with Cdc42 or RhoJ, and proposed that FMNL3 is a downstream effector of Cdc42 or RhoJ to promote the filopodial outgrowth during endothelial lumen formation[17,55]. Unfortunately, there was a limitation to our present study, as there may be cross-talk between RhoC and other small GTPase-binding proteins such as Cdc42 and RhoJ during FMNL3-dependent CRC cell invasion. This thus needs further investigation.
In conclusion, FMNL3 plays a positive role in CRC cell proliferation, invasion and migration. In addition, FMNL3 activates the RhoC/FAK signaling pathway via its interaction with RhoC, and regulates RhoC-dependent remodeling of actin-based protrusions, such as filopodia and lamellipodia, to promote CRC cell invasion. FMNL3 can be applied as a promising specific biomarker for CRC progression and metastasis.
ARTICLE HIGHLIGHTS
Research background
Formin-like 3 (FMNL3) is a novel member of the diaphanous-related formins subfamily, which act as downstream effectors of Rho-GTPase signaling and regulate actin-dependent processes, such as cell motility and invasion. Increased expression of FMNL3 has been identified to contribute to metastasis and the poor prognosis of colorectal carcinoma (CRC). However, the exact molecular mechanism by which FMNL3 promotes CRC cell invasion and metastasis remains ambiguous. Therefore, elucidation of the underlying mechanism may help to block the metastatic progression and improve the survival rate of patients with CRC.
Research motivation
It is necessary to explore whether FMNL3 regulates Rho GTPase signaling to affect cytoskeletal organization and subsequent CRC cell invasion. Recent studies have demonstrated FMNL3 in reorganization of actin-dependent protrusions, such as filopodia and lamellipodia. The potential role of FMNL3 in tumor cell invasion and metastasis has also been reported in several tumor types. Moreover, FMNL3 acts as a downstream effector of RhoC to promote prostate cancer invasion by controlling lamellipodia. These findings give us a good lead for further study regarding the mechanism of FMNL3 regulation during CRC cell invasion and metastasis.
Research objectives
In this study, we investigate the effects of FMNL3 on CRC cell proliferation, invasion and migration in vitro by gain- and loss-of –function approaches. Moreover, we explore the role of FMNL3 in the RhoC-dependent signaling pathway and actin assembly dynamics, and the relation with CRC cell invasion. Our study provides significant insights into the signaling mechanism of FMNL3 during CRC invasion that may contribute to the future design of more effective metastasis-related therapies.
Research methods
Experiments using gene transfection or silencing were conducted to construct FMNL3 stably-expressed or –depleted cell lines to complete the following functional studies. A series of in vitro experiments, such as MTT, transwell chamber and scratch assays, were performed to explore the effects of FMNL3 on cell proliferation, invasion and migration. Rhodamine-conjugated phalloidin staining and confocal microscopy was used to display and observe F-actin dependent protrusions, such as filopodia and lamellipodia. Western blots and gelatin zymography assays were carried out to explore how the signaling pathway of FMNL3 is involved in CRC invasion. The key inhibitors of the RhoC/FAK pathway were used to treat CRC cells to verify the reliability of the signaling mechanism. In addition, the experiments involving immunofluorescence co-localization, co-immunoprecipitation and GST-pull downs were performed to unveil the partner of FMNL3 in the signaling pathway.
Research results
The results of in vitro experiments showed a positive role of FMNL3 in cell proliferation, invasion and migration of CRC. Rhodamine-conjugated phalloidin staining and confocal microscopy-based observations demonstrated that FMNL3 induced the elongation of filopodia, but inhibited the broadening of lamellipodia in a RhoC-dependent manner to enhance the invasive abilities of CRC cells. In addition, the results of western blots and gelatin zymography assays suggested that FMNL3 was involved in the RhoC/FAK signaling pathway and acted as an effector of RhoC to activate the downstream signaling of p-FAK as well as p-MAPK and p-AKT. This was followed by the increased expression of MMP-2, MMP-9 and VEGF, resulting in the promotion of CRC cell invasion. The results of inhibitor treatments confirmed the essential role of FMNL3 in the activation of the RhoC/FAK pathway and the subsequent promotion of CRC cell invasion. A direct interaction of FMNL3 with RhoC in vivo and in vitro was displayed by co-IP, co-localization and GST-pull down analyses.
Research conclusions
In conclusion, FMNL3 plays a positive role in CRC cell proliferation, invasion and migration. In addition, FMNL3 activates the RhoC/FAK signaling pathway via its interaction with RhoC. FMNL3 also regulates RhoC-dependent remodeling of actin-based protrusions, such as filopodia and lamellipodia, to promote CRC cell invasion. FMNL3 can be applied as a promising specific biomarker for CRC progression and metastasis.
Research perspectives
Our study illuminates the role and molecular mechanism of FMNL3 in the regulation of CRC invasion, and revealed RhoC’s involvement in the RhoC/FAK signaling pathway as the partner of FMNL3. Other groups reported the interaction of FMNL3 with Cdc42 or RhoJ, and proposed that FMNL3 acted as a downstream effector of Cdc42 or RhoJ to promote the filopodia outgrowth during endothelial lumen formation. There may be cross-talk between RhoC and other small GTPase proteins, such as Cdc42 and RhoJ, to coordinate the regulation of FMNL3-dependent CRC cell invasion. This, however, needs further investigation.
Footnotes
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
Institutional review board statement: The study was reviewed and approved by Ethical Committee of Jiangxi Provincial People’s Hospital.
Conflict-of-interest statement: The authors declare no conflicts of interest in the present study.
Data sharing statement: No additional data are available.
Peer-review started: May 7, 2018
First decision: May 23, 2018
Article in press: June 27, 2018
P- Reviewer: Abdel-Rahman WM, Jeong KY, Lin JM S- Editor: Gong ZM L- Editor: Filipodia E- Editor: Yin SY
Contributor Information
Yuan-Feng Zeng, Department of Pathology, Jiangxi Provincial People’s Hospital, Nanchang 330006, Jiangxi Province, China. zyf760928@163.com.
Yi-Sheng Xiao, Teaching and Researching Section of Morphology, College of Basic Medicine, Jiangxi University of Traditional Chinese Medicine, Nanchang 330004, Jiangxi Province, China.
Yong Liu, Department of Pathology, Jiangxi Provincial People’s Hospital, Nanchang 330006, Jiangxi Province, China.
Xiao-Jiang Luo, Department of General Surgery, Jiangxi Provincial People’s Hospital, Nanchang 330006, Jiangxi Province, China.
Li-Dan Wen, Clinical Medical Sciences Institute, Jiangxi Provincial People’s Hospital, Nanchang 330006, Jiangxi Province, China.
Qian Liu, Department of Pathology, Jiangxi Provincial People’s Hospital, Nanchang 330006, Jiangxi Province, China.
Min Chen, Department of Pathology, Jiangxi Provincial People’s Hospital, Nanchang 330006, Jiangxi Province, China.
References
- 1.Recio-Boiles A, Cagir B. Cancer, Colon. StatPearls [Internet] Treasure Island (FL): StatPearls Publishing; 2018. [Google Scholar]
- 2.Viganò L, Ferrero A, Lo Tesoriere R, Capussotti L. Liver surgery for colorectal metastases: results after 10 years of follow-up. Long-term survivors, late recurrences, and prognostic role of morbidity. Ann Surg Oncol. 2008;15:2458–2464. doi: 10.1245/s10434-008-9935-9. [DOI] [PubMed] [Google Scholar]
- 3.Kitzing TM, Wang Y, Pertz O, Copeland JW, Grosse R. Formin-like 2 drives amoeboid invasive cell motility downstream of RhoC. Oncogene. 2010;29:2441–2448. doi: 10.1038/onc.2009.515. [DOI] [PubMed] [Google Scholar]
- 4.Kedrin D, van Rheenen J, Hernandez L, Condeelis J, Segall JE. Cell motility and cytoskeletal regulation in invasion and metastasis. J Mammary Gland Biol Neoplasia. 2007;12:143–152. doi: 10.1007/s10911-007-9046-4. [DOI] [PubMed] [Google Scholar]
- 5.Faix J, Grosse R. Staying in shape with formins. Dev Cell. 2006;10:693–706. doi: 10.1016/j.devcel.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 6.Eisenmann KM, Harris ES, Kitchen SM, Holman HA, Higgs HN, Alberts AS. Dia-interacting protein modulates formin-mediated actin assembly at the cell cortex. Curr Biol. 2007;17:579–591. doi: 10.1016/j.cub.2007.03.024. [DOI] [PubMed] [Google Scholar]
- 7.Wodarz A, Näthke I. Cell polarity in development and cancer. Nat Cell Biol. 2007;9:1016–1024. doi: 10.1038/ncb433. [DOI] [PubMed] [Google Scholar]
- 8.Higashida C, Miyoshi T, Fujita A, Oceguera-Yanez F, Monypenny J, Andou Y, Narumiya S, Watanabe N. Actin polymerization-driven molecular movement of mDia1 in living cells. Science. 2004;303:2007–2010. doi: 10.1126/science.1093923. [DOI] [PubMed] [Google Scholar]
- 9.Lizárraga F, Poincloux R, Romao M, Montagnac G, Le Dez G, Bonne I, Rigaill G, Raposo G, Chavrier P. Diaphanous-related formins are required for invadopodia formation and invasion of breast tumor cells. Cancer Res. 2009;69:2792–2800. doi: 10.1158/0008-5472.CAN-08-3709. [DOI] [PubMed] [Google Scholar]
- 10.Block J, Breitsprecher D, Kühn S, Winterhoff M, Kage F, Geffers R, Duwe P, Rohn JL, Baum B, Brakebusch C, et al. FMNL2 drives actin-based protrusion and migration downstream of Cdc42. Curr Biol. 2012;22:1005–1012. doi: 10.1016/j.cub.2012.03.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kitzing TM, Sahadevan AS, Brandt DT, Knieling H, Hannemann S, Fackler OT, Grosshans J, Grosse R. Positive feedback between Dia1, LARG, and RhoA regulates cell morphology and invasion. Genes Dev. 2007;21:1478–1483. doi: 10.1101/gad.424807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Katoh M, Katoh M. Identification and characterization of human FMNL1, FMNL2 and FMNL3 genes in silico. Int J Oncol. 2003;22:1161–1168. [PubMed] [Google Scholar]
- 13.Harris ES, Gauvin TJ, Heimsath EG, Higgs HN. Assembly of filopodia by the formin FRL2 (FMNL3) Cytoskeleton (Hoboken) 2010;67:755–772. doi: 10.1002/cm.20485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thurston SF, Kulacz WA, Shaikh S, Lee JM, Copeland JW. The ability to induce microtubule acetylation is a general feature of formin proteins. PLoS One. 2012;7:e48041. doi: 10.1371/journal.pone.0048041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gauvin TJ, Young LE, Higgs HN. The formin FMNL3 assembles plasma membrane protrusions that participate in cell-cell adhesion. Mol Biol Cell. 2015;26:467–477. doi: 10.1091/mbc.E14-07-1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moriya K, Yamamoto T, Takamitsu E, Matsunaga Y, Kimoto M, Fukushige D, Kimoto C, Suzuki T, Utsumi T. Protein N-myristoylation is required for cellular morphological changes induced by two formin family proteins, FMNL2 and FMNL3. Biosci Biotechnol Biochem. 2012;76:1201–1209. doi: 10.1271/bbb.120069. [DOI] [PubMed] [Google Scholar]
- 17.Richards M, Hetheridge C, Mellor H. The Formin FMNL3 Controls Early Apical Specification in Endothelial Cells by Regulating the Polarized Trafficking of Podocalyxin. Curr Biol. 2015;25:2325–2331. doi: 10.1016/j.cub.2015.07.045. [DOI] [PubMed] [Google Scholar]
- 18.Hetheridge C, Scott AN, Swain RK, Copeland JW, Higgs HN, Bicknell R, Mellor H. The formin FMNL3 is a cytoskeletal regulator of angiogenesis. J Cell Sci. 2012;125:1420–1428. doi: 10.1242/jcs.091066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gardberg M, Heuser VD, Koskivuo I, Koivisto M, Carpén O. FMNL2/FMNL3 formins are linked with oncogenic pathways and predict melanoma outcome. J Pathol Clin Res. 2016;2:41–52. doi: 10.1002/cjp2.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu Y, Shen Z, Wang K, Ha Y, Lei H, Jia Y, Ding R, Wu D, Gan S, Li R, et al. High FMNL3 expression promotes nasopharyngeal carcinoma cell metastasis: role in TGF-β1-induced epithelia-to-mesenchymal transition. Sci Rep. 2017;7:42507. doi: 10.1038/srep42507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gao X, Wang X, Cai K, Wang W, Ju Q, Yang X, Wang H, Wu H. MicroRNA-127 is a tumor suppressor in human esophageal squamous cell carcinoma through the regulation of oncogene FMNL3. Eur J Pharmacol. 2016;791:603–610. doi: 10.1016/j.ejphar.2016.09.025. [DOI] [PubMed] [Google Scholar]
- 22.Lynch J, Meehan MH, Crean J, Copeland J, Stallings RL, Bray IM. Metastasis suppressor microRNA-335 targets the formin family of actin nucleators. PLoS One. 2013;8:e78428. doi: 10.1371/journal.pone.0078428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zeng YF, Xiao YS, Lu MZ, Luo XJ, Hu GZ, Deng KY, Wu XM, Xin HB. Increased expression of formin-like 3 contributes to metastasis and poor prognosis in colorectal carcinoma. Exp Mol Pathol. 2015;98:260–267. doi: 10.1016/j.yexmp.2015.03.008. [DOI] [PubMed] [Google Scholar]
- 24.Vega FM, Fruhwirth G, Ng T, Ridley AJ. RhoA and RhoC have distinct roles in migration and invasion by acting through different targets. J Cell Biol. 2011;193:655–665. doi: 10.1083/jcb.201011038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Iiizumi M, Bandyopadhyay S, Pai SK, Watabe M, Hirota S, Hosobe S, Tsukada T, Miura K, Saito K, Furuta E, et al. RhoC promotes metastasis via activation of the Pyk2 pathway in prostate cancer. Cancer Res. 2008;68:7613–7620. doi: 10.1158/0008-5472.CAN-07-6700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liang CC, Park AY, Guan JL. In vitro scratch assay: a convenient and inexpensive method for analysis of cell migration in vitro. Nat Protoc. 2007;2:329–333. doi: 10.1038/nprot.2007.30. [DOI] [PubMed] [Google Scholar]
- 27.Deryugina EI, Quigley JP. Matrix metalloproteinases and tumor metastasis. Cancer Metastasis Rev. 2006;25:9–34. doi: 10.1007/s10555-006-7886-9. [DOI] [PubMed] [Google Scholar]
- 28.Zhao Y, Zong ZH, Xu HM. RhoC expression level is correlated with the clinicopathological characteristics of ovarian cancer and the expression levels of ROCK-I, VEGF, and MMP9. Gynecol Oncol. 2010;116:563–571. doi: 10.1016/j.ygyno.2009.11.015. [DOI] [PubMed] [Google Scholar]
- 29.González MN, de Mello W, Butler-Browne GS, Silva-Barbosa SD, Mouly V, Savino W, Riederer I. HGF potentiates extracellular matrix-driven migration of human myoblasts: involvement of matrix metalloproteinases and MAPK/ERK pathway. Skelet Muscle. 2017;7:20. doi: 10.1186/s13395-017-0138-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lv C, Yang S, Chen X, Zhu X, Lin W, Wang L, Huang Z, Wang M, Tu G. MicroRNA-21 promotes bone mesenchymal stem cells migration in vitro by activating PI3K/Akt/MMPs pathway. J Clin Neurosci. 2017;46:156–162. doi: 10.1016/j.jocn.2017.07.040. [DOI] [PubMed] [Google Scholar]
- 31.van Golen KL, Bao LW, Pan Q, Miller FR, Wu ZF, Merajver SD. Mitogen activated protein kinase pathway is involved in RhoC GTPase induced motility, invasion and angiogenesis in inflammatory breast cancer. Clin Exp Metastasis. 2002;19:301–311. doi: 10.1023/a:1015518114931. [DOI] [PubMed] [Google Scholar]
- 32.Ruth MC, Xu Y, Maxwell IH, Ahn NG, Norris DA, Shellman YG. RhoC promotes human melanoma invasion in a PI3K/Akt-dependent pathway. J Invest Dermatol. 2006;126:862–868. doi: 10.1038/sj.jid.5700211. [DOI] [PubMed] [Google Scholar]
- 33.Yang H, Zhou J, Mi J, Ma K, Fan Y, Ning J, Wang C, Wei X, Zhao H, Li E. HOXD10 acts as a tumor-suppressive factor via inhibition of the RHOC/AKT/MAPK pathway in human cholangiocellular carcinoma. Oncol Rep. 2015;34:1681–1691. doi: 10.3892/or.2015.4194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Martín-Rufián M, Segura JA, Lobo C, Matés JM, Márquez J, Alonso FJ. Identification of genes downregulated in tumor cells expressing antisense glutaminase mRNA by differential display. Cancer Biol Ther. 2006;5:54–58. doi: 10.4161/cbt.5.1.2238. [DOI] [PubMed] [Google Scholar]
- 35.Carreira S, Goodall J, Denat L, Rodriguez M, Nuciforo P, Hoek KS, Testori A, Larue L, Goding CR. Mitf regulation of Dia1 controls melanoma proliferation and invasiveness. Genes Dev. 2006;20:3426–3439. doi: 10.1101/gad.406406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ju R, Cirone P, Lin S, Griesbach H, Slusarski DC, Crews CM. Activation of the planar cell polarity formin DAAM1 leads to inhibition of endothelial cell proliferation, migration, and angiogenesis. Proc Natl Acad Sci U S A. 2010;107:6906–6911. doi: 10.1073/pnas.1001075107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Goode BL, Eck MJ. Mechanism and function of formins in the control of actin assembly. Annu Rev Biochem. 2007;76:593–627. doi: 10.1146/annurev.biochem.75.103004.142647. [DOI] [PubMed] [Google Scholar]
- 38.Schirenbeck A, Bretschneider T, Arasada R, Schleicher M, Faix J. The Diaphanous-related formin dDia2 is required for the formation and maintenance of filopodia. Nat Cell Biol. 2005;7:619–625. doi: 10.1038/ncb1266. [DOI] [PubMed] [Google Scholar]
- 39.Yang C, Czech L, Gerboth S, Kojima S, Scita G, Svitkina T. Novel roles of formin mDia2 in lamellipodia and filopodia formation in motile cells. PLoS Biol. 2007;5:e317. doi: 10.1371/journal.pbio.0050317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thompson ME, Heimsath EG, Gauvin TJ, Higgs HN, Kull FJ. FMNL3 FH2-actin structure gives insight into formin-mediated actin nucleation and elongation. Nat Struct Mol Biol. 2013;20:111–118. doi: 10.1038/nsmb.2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pellegrin S, Mellor H. The Rho family GTPase Rif induces filopodia through mDia2. Curr Biol. 2005;15:129–133. doi: 10.1016/j.cub.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 42.Sahai E, Marshall CJ. RHO-GTPases and cancer. Nat Rev Cancer. 2002;2:133–142. doi: 10.1038/nrc725. [DOI] [PubMed] [Google Scholar]
- 43.Wheeler AP, Ridley AJ. Why three Rho proteins? RhoA, RhoB, RhoC, and cell motility. Exp Cell Res. 2004;301:43–49. doi: 10.1016/j.yexcr.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 44.Hakem A, Sanchez-Sweatman O, You-Ten A, Duncan G, Wakeham A, Khokha R, Mak TW. RhoC is dispensable for embryogenesis and tumor initiation but essential for metastasis. Genes Dev. 2005;19:1974–1979. doi: 10.1101/gad.1310805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yang H, Liang J, Zhou J, Mi J, Ma K, Fan Y, Ning J, Wang C, Wei X, Li E. Knockdown of RHOC by shRNA suppresses invasion and migration of cholangiocellular carcinoma cells via inhibition of MMP2, MMP3, MMP9 and epithelial-mesenchymal transition. Mol Med Rep. 2016;13:5255–5261. doi: 10.3892/mmr.2016.5170. [DOI] [PubMed] [Google Scholar]
- 46.Hori Y, Ito K, Hamamichi S, Ozawa Y, Matsui J, Umeda IO, Fujii H. Functional Characterization of VEGF- and FGF-induced Tumor Blood Vessel Models in Human Cancer Xenografts. Anticancer Res. 2017;37:6629–6638. doi: 10.21873/anticanres.12120. [DOI] [PubMed] [Google Scholar]
- 47.Sun S, Wu HJ, Guan JL. Nuclear FAK and its kinase activity regulate VEGFR2 transcription in angiogenesis of adult mice. Sci Rep. 2018;8:2550. doi: 10.1038/s41598-018-20930-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mitra SK, Hanson DA, Schlaepfer DD. Focal adhesion kinase: in command and control of cell motility. Nat Rev Mol Cell Biol. 2005;6:56–68. doi: 10.1038/nrm1549. [DOI] [PubMed] [Google Scholar]
- 49.Aronsohn MS, Brown HM, Hauptman G, Kornberg LJ. Expression of focal adhesion kinase and phosphorylated focal adhesion kinase in squamous cell carcinoma of the larynx. Laryngoscope. 2003;113:1944–1948. doi: 10.1097/00005537-200311000-00017. [DOI] [PubMed] [Google Scholar]
- 50.Murata T, Naomoto Y, Yamatsuji T, Okawa T, Shirakawa Y, Gunduz M, Nobuhisa T, Takaoka M, Sirmali M, Nakajima M, et al. Localization of FAK is related with colorectal carcinogenesis. Int J Oncol. 2008;32:791–796. [PubMed] [Google Scholar]
- 51.Lim ST, Chen XL, Lim Y, Hanson DA, Vo TT, Howerton K, Larocque N, Fisher SJ, Schlaepfer DD, Ilic D. Nuclear FAK promotes cell proliferation and survival through FERM-enhanced p53 degradation. Mol Cell. 2008;29:9–22. doi: 10.1016/j.molcel.2007.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Albasri A, Fadhil W, Scholefield JH, Durrant LG, Ilyas M. Nuclear expression of phosphorylated focal adhesion kinase is associated with poor prognosis in human colorectal cancer. Anticancer Res. 2014;34:3969–3974. [PubMed] [Google Scholar]
- 53.Liang L, Li X, Zhang X, Lv Z, He G, Zhao W, Ren X, Li Y, Bian X, Liao W, et al. MicroRNA-137, an HMGA1 target, suppresses colorectal cancer cell invasion and metastasis in mice by directly targeting FMNL2. Gastroenterology. 2013;144:624–635.e4. doi: 10.1053/j.gastro.2012.11.033. [DOI] [PubMed] [Google Scholar]
- 54.Chaturvedi LS, Marsh HM, Basson MD. Role of RhoA and its effectors ROCK and mDia1 in the modulation of deformation-induced FAK, ERK, p38, and MLC motogenic signals in human Caco-2 intestinal epithelial cells. Am J Physiol Cell Physiol. 2011;301:C1224–C1238. doi: 10.1152/ajpcell.00518.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wakayama Y, Fukuhara S, Ando K, Matsuda M, Mochizuki N. Cdc42 mediates Bmp-induced sprouting angiogenesis through Fmnl3-driven assembly of endothelial filopodia in zebrafish. Dev Cell. 2015;32:109–122. doi: 10.1016/j.devcel.2014.11.024. [DOI] [PubMed] [Google Scholar]


