Abstract
AIM
To study the influence of different doses of tacrolimus (FK506) on gut microbiota after liver transplantation (LT) in rats.
METHODS
Specific pathogen-free Brown Norway (BN) rats and Lewis rats were separated into five groups: (1) Tolerance group (BN-BN LT, n = 8); (2) rejection group (Lewis-BN LT, n = 8); (3) high dosage FK506 (FK506-H) group (Lewis-BN LT, n = 8); (4) middle dosage FK506 (FK506-M) group (Lewis-BN LT, n = 8); and (5) low dosage FK506 (FK506-L) group (Lewis-BN LT, n = 8). FK506 was administered to recipients at a dose of 1.0 mg/kg, 0.5 mg/kg, and 0.1 mg/kg body weight for 29 d after LT to the FK506-H, FK506-M, and FK506-L groups, respectively. On the 30th day after LT, all rats were sampled and euthanized. Blood samples were harvested for liver function and plasma endotoxin testing. Hepatic graft and ileocecal tissues were collected for histopathology observation. Ileocecal contents were used for DNA extraction, Real-time quantitative polymerase chain reaction (RT-PCR) and digital processing of denaturing gradient gel electrophoresis (DGGE) profiles and analysis.
RESULTS
Compared to the FK506-H and FK506-L groups, FK506-M was optimal for maintaining immunosuppression and inducing normal graft function; the FK506-M maintained gut barrier integrity and low plasma endotoxin levels; furthermore, DGGE results showed that FK506-M induced stable gut microbiota. Diversity analysis indicated that FK506-M increased species richness and rare species abundance, and cluster analysis confirmed the stable gut microbiota induced by FK506-M. Phylogenetic tree analysis identified crucial bacteria associated with FK506-M; seven of the nine bacteria that were decreased corresponded to Bacteroidetes, while increased bacteria were of the Bifidobacterium species. FK506-M increased Faecalibacterium prausnitzii and Bifidobacterium spp. and decreased Bacteroides-Prevotella and Enterobacteriaceae, as assessed by RT-PCR, which confirmed the crucial bacterial alterations identified through DGGE.
CONCLUSION
Compared to the low or high dosage of FK506, an optimal dosage of FK506 induced immunosuppression, normal graft function and stable gut microbiota following LT in rats. The stable gut microbiota presented increased probiotics and decreased potential pathogenic endotoxin-producing bacteria. These findings provide a novel strategy based on gut microbiota for immunosuppressive dosage assessment for recipients following LT.
Keywords: Liver transplantation, Graft function, Gut microbiota, Immunosuppressor, Tacrolimus, Rejection, Denaturing gradient gel electrophoresis
Core tip: This is the first study to illustrate the effects of different dosages of Tacrolimus (FK506) on gut microbiota following liver transplantation (LT) and indicates that an optimal dosage of FK506 induces effective immunosupression, good graft function and stable gut microbiota after LT in rats. Based on the relationship between gut microbiota and the immunosuppressive dosage in this study, we can not only illustrate precise changes of gut microbiota given by different dosages of FK-506 following LT, but we also provide a novel monitoring strategy based on changes in gut microbiota for immunosuppressive dosage assessment in patients following LT.
INTRODUCTION
Liver transplantation (LT) is considered the only definitive therapy for end-stage liver diseases, including liver cirrhosis, liver failure and hepatocellular carcinoma[1,2]. Currently, the 1-year survival rate in patients following LT has reached 90% and the 5-year survival has reached 75%[3]. However, rejection after LT remains the major cause of hepatic graft dysfunction and is a life-threatening complication[4]. Treatment with inadequate immunosuppression might lead to a higher risk of rejection and even graft dysfunction, whereas excessive immunosuppression has been closely associated with a greater incidence of infection, sepsis, drug toxicity, cancer, chronic graft dysfunction, renal dysfunction, and even increased mortality[5-7], with bacterial infection being the most prevalent type of infection[8]. Thus, the optimal dosage of immunosuppressive medication is crucial for the prevention of rejection, lessening of immunosuppression toxicity and treatment of patients following LT[9].
The gut microbiota has been considered the most important micro-ecosystem that possesses a symbiotic relationship within the body[10-12]. The gut microbiota plays a crucial role in the development of fatty liver disease[13], liver cirrhosis[14], hepatocellular carcinoma[15], liver ischemic injury[16] and liver graft rejection[17]. Our previous study indicated that the healing of hepatic injury could ameliorate gut barrier function and promote gut microbial restoration following LT in rats[16]. We also found that graft rejection after LT could induce gut microbial alterations, thereby inducing subsequent gut barrier dysfunction, which presented a decrease of fecal secretory immunoglobulin A (sIgA) and an increase in blood endotoxin and tumor necrosis factor-α. RT-PCR results showed that the genus Faecalibacterium prausnitzii and Lactobacillus were decreased, whereas Clostridium bolteae was increased, which might in turn aggravate hepatic rejection[18].
Nevertheless, the influence of immunosuppressive medication on gut microbiota following LT remains unclear, and the association between the dosage of immunosuppressants and gut microbial alterations requires urgent elucidation. In this study, we studied the influence of different dosages of FK506 on hepatic graft function and gut microbiota following LT in rats. Furthermore, we identified the gut microbial profile and crucial bacterial community constituents using Denaturing gradient gel electrophoresis (DGGE), and further verified the alterations of dominant gut bacterial populations with RT-PCR.
MATERIALS AND METHODS
Animals
Specific pathogen-free (SPF) male inbred Brown Norway (BN) and Lewis rats (weight 220-250 g, age 12-15 wk) were purchased from Beijing Vital River Laboratories (Beijing, China). They were housed in a clean-level animal house located at the First Affiliated Hospital, School of Medicine, Zhejiang University. All rats were housed at 22-24 °C in 12 h light/dark cycles and fed sterilized standard rat chow and water.
Experimental design and protocol
The study animals were divided into five groups as follows: (1) Tolerance group (BN-BN LT, n = 8); (2) rejection group (Lewis-BN LT, n = 8); (3) high dosage FK506 (FK506-H) group (Lewis-BN LT, n = 8); (4) middle dosage FK506 (FK506-M) group (Lewis-BN LT, n = 8); (5) low dosage FK506 (FK506-L) group (Lewis-BN LT, n = 8). In the Tolerance group, both donors and recipients were BN rats; in the other four groups, both donors were Lewis and recipients were BN rats. FK506 was administered to recipients at a dose of 1.0, 0.5, and 0.1 mg/kg weight for 1 mo after LT to the FK506-H, FK506-M, and FK506-L groups, respectively. To mimic the clinical application, FK506 was administrated via abdominal subcutaneous injection once every 12 h for 7 d after LT, and then via intragastric administration, once per day for the following 8-29 d. The sustained-release FK506 was administered intragastrically to maintain a consistent dosage effect for 24 h.
The study was conducted in accordance with the ‘‘Guide for the Care and Use of Laboratory Animals’’ published by the National Institutes of Health (NIH publication 86-23, revised 1985). The experimental protocol was approved by the Animal Care and Use Committee of the First Affiliated Hospital, School of Medicine, Zhejiang University.
LT surgical procedures and sample collection
The LT surgery was performed according to our previous methods[16,19], with slight modifications. The anesthesia was performed by intraperitoneal injection of Ketamine Hydrochloride (100 mg/kg) and Atropine (1 mg/kg) (Shanghai No. 1 Biochemical and Pharmaceutical, China), and then ether was inhaled to maintain anesthesia[6]. All recipients were revived shortly after the procedure and no further treatment was administered.
All rats were sampled on the 30th day after LT. The abdominal aorta was punctured, and blood samples were harvested for liver function and plasma endotoxin testing. Hepatic graft and ileum tissues near the ileocecus were collected for morphological observation. Ileocecal contents were harvested and stored at -80 °C for gut microbial analysis. All rats were then euthanized via an overdose of anesthetic.
Liver function and plasma endotoxin testing
Plasma alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels were detected using an automatic biochemical analyzer (Hitachi 7600, Tokyo, Japan).Plasma endotoxin level was determined using a colorimetric Limulus Test (Shanghai Yihua Medical Technology Co., Ltd, China) in accordance with the manufacturer’s instructions.
Histopathology and transmission electron microscopy evaluation
The hepatic graft and an ileum tissue sample taken 3 cm from the ileocecus were fixed in 40 g/L neutral formaldehyde and embedded in paraffin, cut into 4-μm slices, stained with hematoxylin and eosin (HE), and then analyzed under light microscopy. At the same time, approximately 1 mm3 of the same samples were fixed in a 2.5% glutaraldehyde solution and prepared in accordance with the standard technical procedures for transmission electron microscopy (TEM), as previously described[20]. Hepatic and ileal ultrastructures were analyzed at the Imaging Facility of Core Facilities, Zhejiang University School of Medicine. Histopathology and TEM evaluation were investigated by a pathologist blinded to the treatment group.
DNA extraction of ileocecal contents
DNA extraction of a frozen aliquot of each ileocecal fecal sample was performed as in our previous studies[16]. DNA concentration was measured by NanoDrop (Thermo Scientific), and DNA integrity was verified using agarose gel electrophoresis.
Real-time quantitative PCR
The primers used for quantitative RT-PCR (RT-qPCR) are listed in Supplementary Table 1. All oligonucleotide primers were synthesized by TAKARA (Dalian, China). RT-qPCR was performed as reported previously[21]. The copy number of 16s rDNA operons per microliter of crude DNA template was determined by comparing serially diluted plasmid DNA standards running on the same plate.
DGGE profiling
The V3 variable region of 16S rDNA was amplified using a hot-start touchdown protocol with specific primers for the conserved regions of 16S rDNA. DGGE was performed using the D-Code universal mutation detection system apparatus (Bio-Rad, Hercules, CA, United States) with 16 cm × 18 cm × 1.5 mm gels according to the manufacturer’s protocol. On each DGGE gel, standard references in the middle and at each end were used for digital gel normalization and comparison of gels.
Digital processing of DGGE profiles
DGGE profiles were digitally processed using BioNumerics software version 6.01 (Applied Maths, St-Martens-Latem, Belgium) in a multistep procedure following the manufacturer’s instructions. The parameters for band-class allocation followed those of our previous study[16]. Quantitative information of a given band was calculated using the Gel-Pro analyzer 4 software (Media Cybernetics, United States). Diversity was calculated using Shannon’s diversity, Simpson index, species richness, Chao-1, Fisher alpha, and Menhinick index with Past software (http://folk.uio.no/ohammer/past/)[21]. Cluster analysis of the DGGE was conducted with an unweighted pair-group method with arithmetic means (UPGMA) based on the Dice similarity coefficient (band-based). Multidimensional scaling (MDS) and principal components analysis (PCA) were utilized following the instructions of the BioNumerics software.
Sequencing of DGGE bands
Specific DGGE bands of interest were excised and sequenced. Positive clones were verified and sequenced using Sanger’s method on an ABI 3730 automated sequencing system (Invitrogen, Shanghai, China). Homology searches of the GenBank DNA database were performed using the BLAST tool. Reference sequences of phylogenetic neighbor species (up to 97% similarity) were included to construct a phylogenetic tree with MEGA 5.0 program based on the neighbor-joining method[15].
Statistical analysis
Continuous variables were summarized as mean ± standard deviation (SD). One-way variance analysis followed by Dunn’s multiple comparison tests was utilized to evaluate statistical differences among different groups. Student t-test was used to examine parametric data between the two groups. Statistical analyses were performed using SPSS version 19.0 for Windows (SPSS Inc., Chicago, IL, United States). A P-value of less than 0.05 was considered statistically significant.
RESULTS
A middle dosage of FK506 is optimal for maintaining liver transplant immunosuppression
An overdose or insufficient dosage of FK506 may lead to graft dysfunction, thus we first observed survival of all recipients. All rats following LT survived except one LT recipient in the FK506-H group that died of severe abdomen infection, which suggested an over-suppression of the immune status, thereby inducing infection and increasing mortality.
To illustrate the influence of different dosages of FK506 on hepatic grafts, we observed graft morphology. Under light microscopy (Figure 1A), hepatic grafts in the tolerance group presented a regular structure with well-arranged hepatocyte cords. In the rejection group, graft histology showed no obvious hepatocyte cords, accumulation of numerous red blood cells, and marked hepatocyte necrosis. However, FK506-M treatment preserved an approximately normal hepatic structure without hepatic acute rejection. Notably, hepatic graft resulted in hepatocyte cord disruption, widened sinusoids, and middle rejection injury in the FK506-L dosage group, suggesting an insufficient suppression of the immune status, thereby inducing rejection. Thus, compared to the FK506-H and FK506-L groups, FK506-M was the optimal dosage for maintaining immunosuppression in rats following LT.
Figure 1.
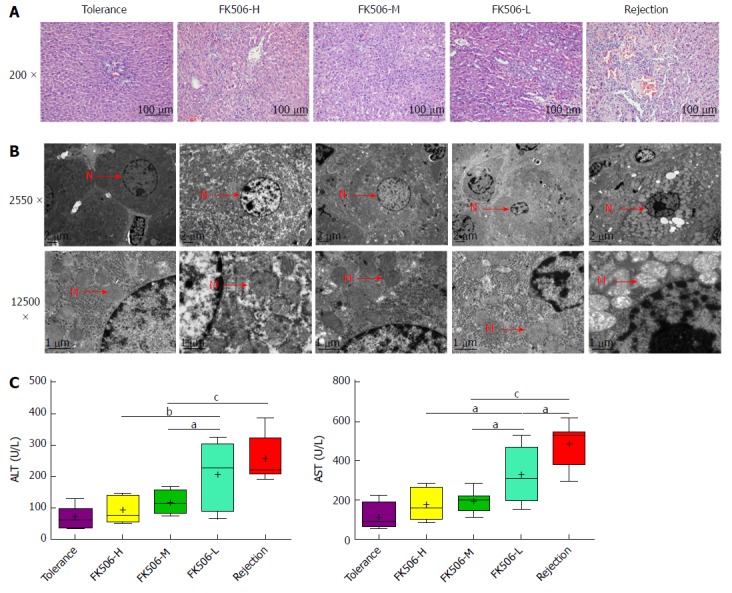
Middle dosage of FK506 is optimal for maintaining immunosuppression after liver transplantation. A: Representative image of the hepatic graft pathological structure stained with hematoxylin and eosin (HE, 200 ×); B: Representative hepatocyte ultrastructure obtained via transmission electron microscopy (TEM, 2550 ×, 12500 ×); C: Assessment of hepatic graft function by plasma levels of alanine aminotransferase (ALT) and aspartate aminotransferase (AST) in different treatment groups. N: Cell nucleus; M: Mitochondria. aP < 0.05; bP < 0.01; cP < 0.001.
We next observed hepatic graft ultrastructure with TEM (Figure 1B). In the tolerance group, a normal hepatocyte structure with intact mitochondria and endoplasmic reticulum was observed. Hepatic graft presented significant karyopyknosis, marked mitochondrial vacuolar degeneration, and organelle breakdown in the rejection group. Nevertheless, FK506-M treatment noticeably improved hepatocyte structure, mitochondria morphology, and other organelle structures. Notably, hepatocyte apoptosis and mild organelle damage were noted in the FK506-L group. Plasma ALT and AST levels reflected graft function. Compared with the rejection group, plasma ALT and AST were significantly decreased in the FK506-M treatment group (both P < 0.001). In addition, plasma ALT and AST were reduced in the FK506-M group versus the FK506-L group (both P < 0.05). There was no statistically significant difference between the tolerance and FK506-M-treated groups (Figure 1C).
An optimal dosage of FK506 keeps the gut barrier intact and maintains low endotoxin levels following LT
The liver-gut circulation and the gut-liver axis are closely associated with gut and liver function following LT. Thus, we next observed the ileal mucosa ultrastructure using TEM (Figure 2A). In the tolerance group, intestinal epithelial cells presented a normal morphology with many homogenously distributed microvilli and integrated tight junctions. In contrast, microvilli loss, tight junction damage, and bacterial invasion in epithelial cells were observed in the rejection group. However, FK506-M treatment significantly improved intestinal epithelial structure and kept microvilli and tight junctions intact. Notably, epithelial cell damage, microvilli disruption, and wider lateral spaces between neighboring cells were observed in the FK506-L treatment group.
Figure 2.
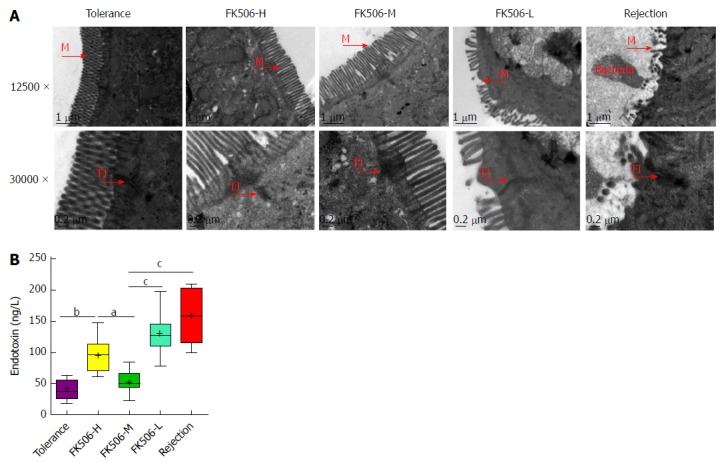
An optimal dosage of FK506 maintained the gut barrier and resulted in low endotoxin levels after liver transplantation. A: Representative intestinal mucosal ultrastructure shown by transmission electron microscopy for the different treatment groups (12500 ×, 30000 ×); B: Levels of plasma endotoxins in the different groups. M: Microvilli; TJ: Tight junction. aP < 0.05; bP < 0.01; cP < 0.001.
Plasma endotoxin reflects gut barrier function. Compared with the tolerance and FK506-M groups, plasma endotoxin was significantly increased in the FK506-H group (P < 0.01 and 0.05, respectively), suggesting that over-suppression of immune status might lead to higher endotoxin levels. Meanwhile, plasma endotoxins were remarkably elevated in the rejection and FK506-L groups compared to the tolerance group (both P < 0.001). However, FK506-M administration significantly decreased endotoxin levels compared to the rejection and FK506-L groups (both P < 0.001) (Figure 2B).
An optimal dosage of FK506 induces a stable gut microbiota as determined by DGGE
Endotoxins are the product of common gram-negative bacteria and can initiate various pathophysiological cascades[22]. Plasma endotoxin changes are mainly derived from changes in the gut microbiota. Based on the DGGE method, we analyzed the distribution of the ileocecal bacterial community (Figure 3). To characterize the DGGE profiles, we utilized the Dice coefficient and UPGMA as a cluster method to indicate band similarity. These profiles formed two primary clusters. The left cluster mainly consisted of samples from the tolerance group, in which total similarity was 80.1%, with the similarity among lanes ranging from 80.1% to 91.1%. The other cluster contained samples mainly from the rejection group and the different doses of the FK506 groups. Importantly, the similarity among lanes ranged from 82.4% to 96.7% in the rejection group and from 82.7% to 95.4% in the FK506-M treatment group, suggesting a significant uniqueness and stability of the gut microbiota.
Figure 3.
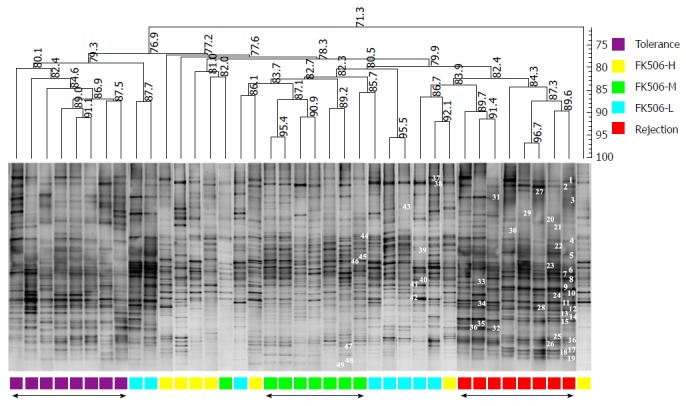
Optimal dosage of FK506 induced a stable gut microbiota as determined using denaturing gradient gel electrophoresis. Based on the DGGE method, the composition and distribution of ileocecal bacterial communities from samples was obtained in rats following LT. The Dice similarity coefficient (band-based) and unweighted pair-group method with arithmetic means (UPGMA) as a cluster method were utilized to analyze the similarity of each band in the different groups. Metric scale denotes the degree of similarity. Each band represents a bacterial clone. Band numbers indicate the position of bands excised for sequence analyses. DGGE: Denaturing gradient gel electrophoresis.
In contrast, the DGGE lanes from the FK506-H and FK506-L treatment groups presented an inordinate distribution and an evident difference. The similarity of these lanes was relatively low and could not be clustered together. These results based on the DGGE profile indicated that an optimal dosage of FK506 induced a unique and stable gut microbiota in rats following LT.
Diversity analysis and cluster analysis of gut microbiota using DGGE profiles
Gut microbial diversity and species richness are important indexes used to evaluate gut microbial balance and stability. Thus, we calculated the gray amount of each band in each lane of the DGGE profiles with Gel-Pro analyzer to compare microbial diversity. There were no statistical differences in the Shannon and Simpson indexes among the five groups (Figure 4A). Compared to the tolerance group, species richness was significantly decreased in the FK506-H, FK506-L, and rejection groups (all P < 0.05). In contrast, FK506-M treatment markedly increased species richness compared to the FK506-H, FK506-L, and rejection groups (P < 0.01, 0.05 and 0.001, respectively) (Figure 4A).
Figure 4.
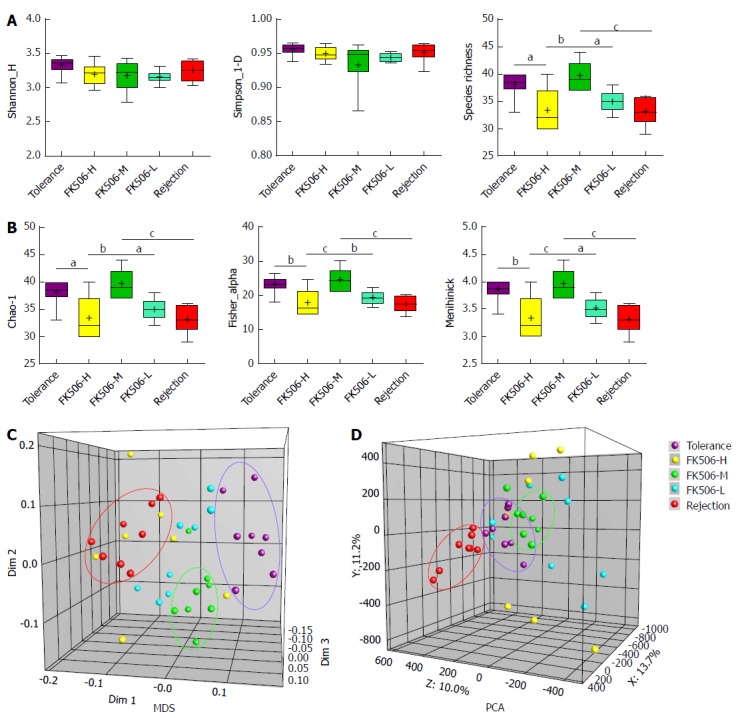
Diversity analysis and cluster analysis of denaturing gradient gel electrophoresis profiles based on Dice’s coefficient and the unweighted pair-group method with arithmetic means method. A: Based on amount of gray area of each band of each lane in the denaturing gradient gel electrophoresis (DGGE) profiles quantified using the Gel-Pro analyzer, the gut microbial diversity and species richness were assessed with the Shannon index and Simpson index in the different groups; B: The abundance and distribution of the rare species were estimated using the Chao-1 index, Fisher alpha index, and Menhinick index in the different groups; C: Multidimensional scaling (MDS) analysis of each sample of the DGGE profiles. The plot is an optimized three-dimensional representation of the similarity matrix obtained from BioNumerics software. The Euclidean distance between the two points reflects similarity; D: Principal components analysis (PCA) of fecal microbiota based on DGGE fingerprinting. The plot is orientated to maximize the variation among lanes along the first three principal components (with contributions of 13.7, 11.2 and 10.0, respectively) obtained from BioNumerics software. aP < 0.05; bP < 0.01; cP < 0.001.
We also analyzed the abundance of rare species, estimated using the Chao-1, Fisher alpha, and Menhinick indexes. Compared with the tolerance group, Chao-1, Fisher alpha and Menhinick indexes were remarkably reduced in the FK506-H, FK506-L, and rejection groups (all P < 0.05). However, FK506-M treatment significantly increased the abundance of rare species in contrast to the FK506-H, FK506-L, and rejection groups (all P < 0.05) (Figure 4B).
Furthermore, based on DGGE profiles, we conducted MDS and PCA analysis. The Euclidean distance between two points indicates the similarity between the two. Gut microbial structures from samples of the tolerance, FK506-M, and rejection groups were respectively clustered together, and showed separation from each other using MDS analysis (Dim 1, Dim 2, and Dim 3) (Figure 4C), suggesting a relatively unique and stable gut microbiota. PCA is an alternative approach to visualizing relationships among lanes using lane data (band classes). Our PCA analysis also gave similar results based on PCA axes X/Y/Z (13.7%, 11.2%, and 10.0%, respectively) (Figure 4D).
Phylogenetic tree analysis of sequences based on DGGE profiles
Of the 49 PCR-DGGE bands analyzed in the study, 48 band classes were selected. To identify crucial bacterial populations induced by FK506-M treatment, we compared band intensity between the FK506-M and the rejection groups. Of the 41 band classes selected, 28 showed slight variation in band intensity between the two groups. In contrast to the rejection group, the intensities of nine band classes [18.2 (band 1), 19.9 (band 2), 61.0 (band 9), 72.9 (band 15), 81.0 (band 16), 82.0 (band 26), 85.3 (band 17), 86.5 (band 18), and 88.2 (band 19)] were significantly decreased (Figure 5A), while four band classes [33.7 (band 20), 41.8 (band 39), 56.8 (band 41), and 82.7 (band 47)] were increased in the FK506-M treated group (Figure 5B).
Figure 5.
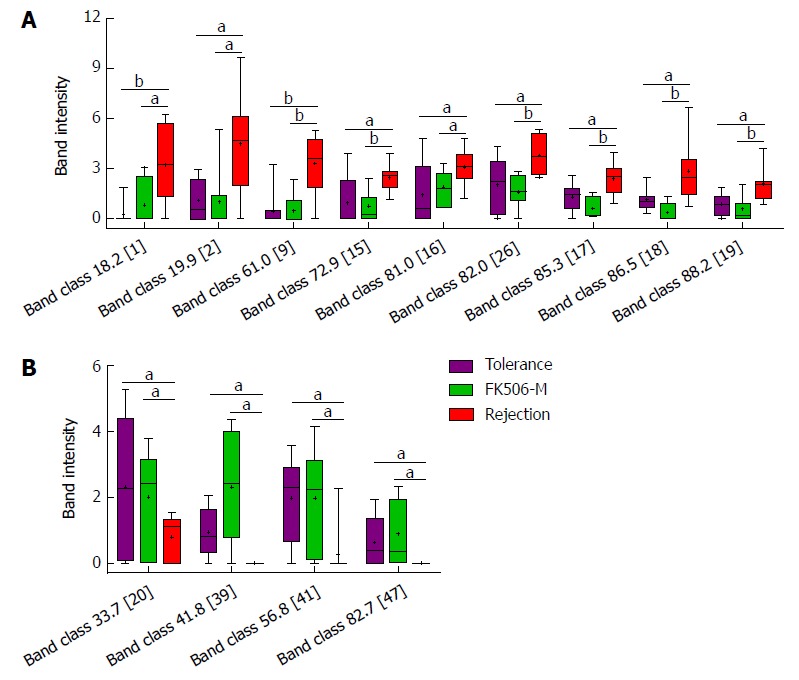
Identification of crucial bands of gut microbial changes induced by middle dosage tacrolimus treatment. A: Compared to the rejection group, the intensities of nine band classes including 18.2 (band 1), 19.9 (band 2), 61.0 (band 9), 72.9 (band 15), 81.0 (band 16), 82.0 (band 26), 85.3 (band 17), 86.5 (band 18), and 88.2 (band 19), were significantly decreased in the FK506-M-treated and tolerance groups; B: Compared with the rejection group, four band classes, including 33.7 (band 20), 41.8 (band 39), 56.8 (band 41), and 82.7 (band 47), showed increased intensities in the FK506-M-treated and tolerance groups. aP < 0.05, bP < 0.01.
To determine the bacterial phylogenetic relationships and identify key bacteria involved in the microbial changes induced by FK506-M, the phylogenetic tree of sequences derived from DGGE bands was analyzed using the MEGA 5 software (Figure 6). Almost all the matched bacteria of the 48 band classes were assigned to three phyla: Bacteroidetes (50.0%), Firmicutes (39.6%), and Gammaproteobacteria (10.4%). The closest matched bacteria shown on the phylogenetic tree corresponded to 13 crucial band classes associated with FK506-M treatment. Details of the 13 crucial band classes are presented in Supplementary Table 2. In these crucial bacteria associated with FK506-M treatment, 7 of the 9 decreased band classes were matched to Bacteroidetes bacterium, belonging to phylum Bacteroidetes, but the increased band classes were matched to the Bifidobacterium species.
Figure 6.
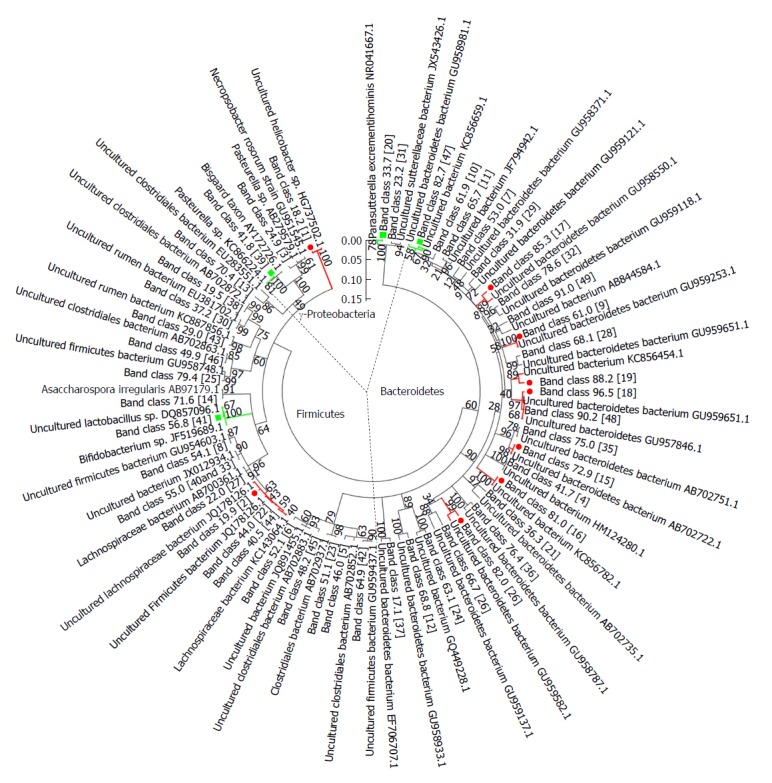
Phylogenetic tree analysis of sequences in the denaturing gradient gel electrophoresis profiles. Phylogenetic tree analysis of sequences from denaturing gradient gel electrophoresis (DGGE) profiles using the neighbor-joining method was conducted using MEGA 5 software. Almost all matched bacteria of the 48 DGGE band classes were assigned to three phyla: Bacteroidetes (50.0%), Firmicutes (39.6%), and Gammaproteobacteria (10.4%). The fragment sequences were defined according to their positions in gels using the band-matching tool with BioNumerics software version 6.01 (Applied Maths). The numbers in the brackets were consistent with the numbers shown in DGGE profiling. Nine band classes (labeled with a red dot) were decreased and four band classes (labeled with the green square) were higher in the FK506-M group than the rejection group. The plot was obtained using MEGA5 software (http://en.wikipedia.org/wiki/MEGA,_Molecular_Evolutionary_Genetics_Analysis).
Quantitative verification of the predominant bacterial community in fecal microbiota using RT-qPCR
To verify crucial bacteria alterations induced by FK506-M treatment, we further analyzed dominant bacterial populations using RT-qPCR (Figure 7). Compared with the tolerance group, Faecalibacterium prausnitzii and Bifidobacterium spp. were significantly decreased, whereas the Bacteroides-Prevotella group and Enterobacteriaceae were increased in the FK506-L treated and rejection groups (all P < 0.05). However, FK506-M treatment significantly increased Faecalibacterium prausnitzii and Bifidobacterium spp. and decreased the Bacteroides-Prevotella group and Enterobacteriaceae when compared to the FK506-L and rejection groups (all P < 0.05) (Figure 7A). These results essentially verified the changes observed in the levels of 13 crucial bacteria associated with FK506-M treatment based on the DGGE profiles.
Figure 7.
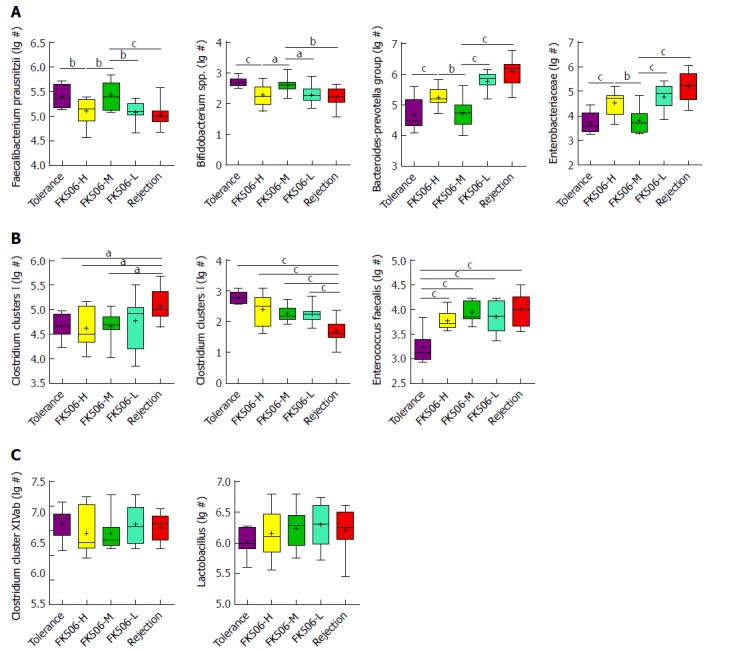
Quantitative verification of predominant bacterial community in fecal microbiota using RT-qPCR. Gut dominant bacterial populations were analyzed with RT-qPCR for the different groups. The dominant bacteria mainly included Faecalibacterium prausnitzii, Bifidobacterium spp., Bacteroides-Prevotella group, Enterobacteriaceae, Clostridium clusters I, Clostridium clusters XI, Enterococcus faecalis, Clostridium cluster XIVab, Lactobacillus. Statistical analysis was performed with one-way ANOVA. aP < 0.05, bP < 0.01, cP < 0.001. 1Log10 copies/g: log10 No. of 16S rDNA gene copies per gram feces (wet weight).
Moreover, in the rejection group, the Clostridium clusters I was elevated, whereas the Clostridium clusters XI was reduced, in contrast to the other groups (Figure 7B). Enterococcus faecalis was specifically decreased in the tolerance group (all P < 0.001). There were no statistical differences in the Clostridium cluster XIVab and Lactobacillus between the different groups (Figure 7C).
DISCUSSION
Liver transplantation is a life-saving technique for patients with end-stage liver disease. Appropriate dosage of immunosuppressive medications including FK506 could effectively prevent and treat allograft rejection in patients following LT. FK506 is a calcineurin inhibitor, which has become the most used immunosuppressant following LT[23]. Compared to cyclosporine, FK506 is more effective in reducing rejection, leading to better graft function and overall survival in LT patients[24]. Insufficient dosage of FK506 may lead to rejection and even graft dysfunction, while over dosage is closely associated with infection, increased complications, and even mortality. Unfortunately, FK506-related toxicity, mainly due to overload usage is frequent in LT patients[25].
In patients following LT, an optimal immunosuppressive therapy should balance the risk of infection, cancer, and drug toxicity caused by excessive immunosuppression, and the risk of rejection caused by an inadequately suppressed immune system[5]. To explore the optimal immunosuppressive dosage is very important for the long-term survival of the recipient and the liver graft. Jia et al[9] proved that compared to high FK506 blood concentration (10-15 ng/L) with adverse effects such as infection, early renal impairment, hepatocellular carcinoma recurrence, even death, reasonably low FK506 blood concentrations (5-10 ng/mL) by less oral dosage especially in the early phase, namely “Minimizing tacrolimus”, could prevent graft rejection, avoid related toxicity, protect the graft function, and promote long-term survival of the recipient. Moreover, Geng[26] proved that lower FK506 trough levels (< 5 ng/mL) in the late period of living donor LT are safe and improve the long-term outcomes.
Our previous studies indicated that bacterial community alterations in the gut were associated with liver cirrhosis[14], hepatic graft rejection[18], hepatic injury[19], and pancreatic carcinoma[27]. However, the influence of immunosuppressive medication on the gut microbiota and the association between the dosage of immunosuppressive drugs and alterations in gut microbiota have not been reported to date. In our study, all recipients survived following LT, except one recipient in the FK506-H dosage group that died of severe abdomen infection, suggesting an over-suppression of the immune status. Hepatic graft presented intermediate rejection injury in the FK506-L group, suggesting insufficient immunosuppression. In contrast, the FK506-M dosage maintained approximately normal hepatic structure and function. Survival results and graft morphology indicated that FK506-M treatment was optimal for maintaining immunosuppression. Meanwhile, the FK506-M dosage kept the gut barrier intact and maintained low levels of plasma endotoxin. Thus, the FK506-M dosage was considered optimal for maintaining immunosuppression in rats following LT.
Furthermore, we observed the influence of FK506 with different dosages on the gut microbiota. We further analyzed gut microbiota using a DGGE approach and found that the FK506-M dosage induced a unique and stable gut microbiota. MDS and PCA analysis validated the stable gut microbiota identified by DGGE. Phylogenetic tree analysis identified crucial bacteria associated with the FK506-M treatment. In addition, treatment with FK506-M significantly increased the growth of Faecalibacterium prausnitzii and Bifidobacterium spp. and decreased that of the Bacteroides-Prevotella group and Enterobacteriaceae as determined via RT-qPCR, which essentially verified the alterations in the population of crucial bacteria observed from the DGGE profiles.
Endotoxin, also known as lipopolysaccharide (LPS), is the main product of common gram-negative bacteria and is a crucial factor for the association of gut microbiota with liver inflammation[22]. In a pathogen-associated molecular pattern of microbiota-liver axis, LPS may possess the capacity to activate inflammation and initiate various pathophysiological cascades. High levels of LPS activate the NF-κB pathway, produce proinflammatory cytokines [tumor necrosis factor (TNF)-α, interleukin (IL)-6, and IL-1], and lead to liver inflammation and aggravation of hepatic oxidative damage[28]. In our study, plasma endotoxins were remarkably elevated in the rejection and FK506-L dosage groups, but the FK506-M dosage significantly decreased endotoxin levels. To explore the causes of the observed endotoxin changes, we analyzed gut microbial alterations and identified the crucial bacteria involved. Importantly, compared to the rejection and FK506-L dosage groups, RT-qPCR results verified that FK506-M treatment significantly increased Faecalibacterium prausnitzii and Bifidobacterium spp. and decreased the growth of the Bacteroides-Prevotella group and Enterobacteriaceae, which are the main common gram-negative bacteria producing LPS. Thus, we speculated that the optimal dosage of FK506 might lead to an increase in probiotics and a decrease in the potential pathogenic endotoxin-producing bacteria.
In patients following LT, blood concentrations of immunosuppressant and its stability are important. To mimic clinical application, FK506 was administered via abdominal subcutaneous injection for 7 d and then via intragastric administration during the subsequent 8-28 d after LT. In patients, routine monitoring of blood tacrolimus concentration (TC) was performed using the PRO-TracTMIITacrolimus Elisa Kit (Diasorin, United States), however, there is no kit for rat blood TC testing. According to our experimental experience, it may be due to immunological reasons, as the blood TC in rats can not be tested by the above kit used for humans. Fortunately, according to clinical practice of LT, different dosages of FK506 are positively correlated to different serum concentrations across a specific dosage range, meanwhile, intragastric dosages of FK506 were more feasible and easier to control the blood TC. In this experiment, the over-low dosage of FK506 led to a higher risk of rejection, whereas over-high dosage was closely associated with a greater incidence of infection, sepsis, and even increased mortality. Therefore, treatment with different dosages of FK506 instead of relying on the examination of blood TC was feasible and credible in rats after LT.
Gut microbiota alterations are closely associated with liver function in health and disease due to liver-gut circulation and the gut microbiota-liver axis[29]. Gut microbiota is involved in a variety of human liver diseases, such as alcoholic liver disease[30,31], non-alcoholic fatty liver disease[32], non-alcoholic steatohepatitis[33], hepatitis B infection[21], liver cirrhosis[14,34], and hepatocellular carcinoma[15,28,35]. Liver diseases always reflect changes due to alterations in intestinal permeability and gut microbial composition[29], while an imbalance in the gut microbiota in turn, has been reported to be an important contributor to the progression of liver disease or liver injury[36]. Notably, the improvement of hepatic injury might ameliorate gut barrier function and promote gut microbial restorations following LT, which might further benefit hepatic graft by positive feedback of the “gut-liver axis”[16,18]. Thus, the gut microbiota becomes an additional therapeutic target to improve hepatic injury after LT. In the present study, the optimal dosage of FK506 led to good hepatic graft function, kept the gut barrier intact, and induced a unique and stable gut microbiota, which further verify the effects of the “gut microbiota-liver” axis.
In conclusion, an optimal dosage of FK506 induces effective immunosuppression, normal graft function and stable gut microbiota after LT in rats. A stable gut microbiota resulted in increased probiotics, including Faecalibacterium prausnitzii and Bifidobacterium spp. and decreased potential pathogenic endotoxin-producing bacteria, such as the Bacteroides-Prevotella group and Enterobacteriaceae. These findings provide a novel strategy involving the use of the gut microbiota for the assessment of the dosage of immunosuppressive medications and its effects in recipients following LT.
ARTICLE HIGHLIGHTS
Research background
Liver transplantation (LT) is the only definitive therapy for end-stage liver diseases. The optimal dosage of immunosuppressive medication is crucial to prevent rejection, lessen the side effects of immunosuppressors (IS) and treatment of patients following LT. The gut microbiota plays a crucial role in the development of obesity, diabetes, liver diseases and so on. Nevertheless, the influence of IS on gut microbiota following LT remains unclear, the association between the dosage of IS and gut microbial alterations requires urgent elucidation.
Research motivation
Acute rejection is still a leading cause of hepatic graft dysfunction following LT. Each recipient of LT must take IS to prevent acute rejection, but the effect of IS on gut microbiota remains unclear. Tacrolimus (FK506) is the main IS following LT, so we studied the influence of different dosages of FK506 on gut microbiota after LT in rat.
Research objectives
In this study, we studied the influence of different dosages of FK506 on hepatic graft function and gut microbiota following LT in rats. Furthermore, we will identify the precise changes of the gut microbiota given by different doses of FK-506 and provide a new monitoring strategy for IS dosage assessment in recipients after LT.
Research methods
We performed LT experiments in rats by taking different dosage of FK506 for 29 d, and on the 30th day after LT, all rats were sampled and then euthanized. We studied the hepatic graft function using serum alanine aminotransferase and aspartate aminotransferase examination and morphology change using histopathology and transmission electron microscopy evaluation. We also identified the gut microbial profile and crucial bacterial community constituents using digital processing of denaturing gradient gel electrophoresis (DGGE), and further verified the alterations of dominant gut bacterial populations with RT-PCR.
Research results
Compared to the FK506-H and FK506-L groups, FK506-M was optimal for maintaining immunosuppression and induced normal graft function; the FK506-M maintained the integrity of gut barrier and low plasma endotoxin levels. Furthermore, FK506-M induced stable gut microbiota, increased species richness and rare species abundance by DGGE and Cluster analysis. The FK506-M increased Faecalibacterium prausnitzii and Bifidobacterium spp. and decreased Bacteroides-Prevotella and Enterobacteriaceae by Phylogenetic tree analysis and RT-PCR verification.
Research conclusions
An optimal dosage of FK506 induces effective immunosuppression, normal graft function and stable gut microbiota after LT in rat. A stable gut microbiota resulted in increased probiotics and decreased potential pathogenic endotoxin-producing bacteria. These findings provide a novel strategy involving the use of the gut microbiota for the assessment of the dosage of immunosuppressive medications and their effects on recipients following LT.
Research perspectives
This study is the first to illustrate the effects of FK506 with different dosages on gut microbiota following LT and indicates that an optimal dosage of FK506 induces effective immunosuppression, good graft function and stable gut microbiota after LT in rats. Based on the relationship between gut microbiota and immunosuppressive dosage in this study, we can not only illustrate precise changes of gut microbiota given by FK-506 with different dosages following LT, but we also provide a novel monitoring strategy based on changes in gut microbiota for IS assessment in recipients following LT. With the improvement of metagenomics and metabolomics techniques, the integrative study can further reveal how the gut microbiota participates in the side effects of IS on recipients and gut microbiota may be a target to reduce side effects of IS. In addition, in order to further study the mechanism of the involvement of gut microbiota on the side effects of IS on recipients, it is essential to do clinical research.
Footnotes
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
Institutional animal care and use committee statement: This study was reviewed and approved by the Research Ethics Committee of the First Affiliated Hospital, School of Medicine, Zhejiang University.
Conflict-of-interest statement: All authors declare no conflict of interest exists.
Data sharing statement: No additional data are available.
ARRIVE guidelines statement: The ARRIVE Guidelines have been adopted.
Peer-review started: June 21, 2018
First decision: July 18, 2018
Article in press: August 1, 2018
P- Reviewer: Cholongitas E, Venu RP S- Editor: Wang XJ L- Editor: Filipodia E- Editor: Bian YN
Contributor Information
Jian-Wen Jiang, Department of Hepatobiliary and Pancreatic Surgery, First Affiliated Hospital, School of Medicine, Zhejiang University, Key Laboratory of Combined Multi-organ Transplantation, Ministry of Public Health, Hangzhou 310003, Zhejiang Province, China; Collaborative Innovation Center for Diagnosis and Treatment of Infectious Diseases, Zhejiang University, Hangzhou 310003, Zhejiang Province, China; Health Management Center, First Affiliated Hospital, School of Medicine, Zhejiang University, Hangzhou 310003, Zhejiang Province, China.
Zhi-Gang Ren, Department of Hepatobiliary and Pancreatic Surgery, First Affiliated Hospital, School of Medicine, Zhejiang University, Key Laboratory of Combined Multi-organ Transplantation, Ministry of Public Health, Hangzhou 310003, Zhejiang Province, China; Collaborative Innovation Center for Diagnosis and Treatment of Infectious Diseases, Zhejiang University, Hangzhou 310003, Zhejiang Province, China; Department of Infectious Diseases, Precision Medicine Center, the First Affiliated Hospital of Zhengzhou University, Zhengzhou 450052, Henan Province, China.
Hai-Feng Lu, Collaborative Innovation Center for Diagnosis and Treatment of Infectious Diseases, Zhejiang University, Hangzhou 310003, Zhejiang Province, China; State Key Laboratory for Diagnosis and Treatment of Infectious Disease, First Affiliated Hospital, School of Medicine, Zhejiang University, Hangzhou 310003, Zhejiang Province, China.
Hua Zhang, Collaborative Innovation Center for Diagnosis and Treatment of Infectious Diseases, Zhejiang University, Hangzhou 310003, Zhejiang Province, China; State Key Laboratory for Diagnosis and Treatment of Infectious Disease, First Affiliated Hospital, School of Medicine, Zhejiang University, Hangzhou 310003, Zhejiang Province, China.
Ang Li, Collaborative Innovation Center for Diagnosis and Treatment of Infectious Diseases, Zhejiang University, Hangzhou 310003, Zhejiang Province, China; State Key Laboratory for Diagnosis and Treatment of Infectious Disease, First Affiliated Hospital, School of Medicine, Zhejiang University, Hangzhou 310003, Zhejiang Province, China; Department of Infectious Diseases, Precision Medicine Center, the First Affiliated Hospital of Zhengzhou University, Zhengzhou 450052, Henan Province, China.
Guang-Ying Cui, Collaborative Innovation Center for Diagnosis and Treatment of Infectious Diseases, Zhejiang University, Hangzhou 310003, Zhejiang Province, China; Department of Infectious Diseases, Precision Medicine Center, the First Affiliated Hospital of Zhengzhou University, Zhengzhou 450052, Henan Province, China.
Jun-Jun Jia, Department of Hepatobiliary and Pancreatic Surgery, First Affiliated Hospital, School of Medicine, Zhejiang University, Key Laboratory of Combined Multi-organ Transplantation, Ministry of Public Health, Hangzhou 310003, Zhejiang Province, China; Collaborative Innovation Center for Diagnosis and Treatment of Infectious Diseases, Zhejiang University, Hangzhou 310003, Zhejiang Province, China.
Hai-Yang Xie, Department of Hepatobiliary and Pancreatic Surgery, First Affiliated Hospital, School of Medicine, Zhejiang University, Key Laboratory of Combined Multi-organ Transplantation, Ministry of Public Health, Hangzhou 310003, Zhejiang Province, China; Collaborative Innovation Center for Diagnosis and Treatment of Infectious Diseases, Zhejiang University, Hangzhou 310003, Zhejiang Province, China.
Xin-Hua Chen, Department of Hepatobiliary and Pancreatic Surgery, First Affiliated Hospital, School of Medicine, Zhejiang University, Key Laboratory of Combined Multi-organ Transplantation, Ministry of Public Health, Hangzhou 310003, Zhejiang Province, China; Collaborative Innovation Center for Diagnosis and Treatment of Infectious Diseases, Zhejiang University, Hangzhou 310003, Zhejiang Province, China.
Yong He, Department of Hepatobiliary and Pancreatic Surgery, First Affiliated Hospital, School of Medicine, Zhejiang University, Key Laboratory of Combined Multi-organ Transplantation, Ministry of Public Health, Hangzhou 310003, Zhejiang Province, China.
Li Jiang, Department of Hepatobiliary and Pancreatic Surgery, First Affiliated Hospital, School of Medicine, Zhejiang University, Key Laboratory of Combined Multi-organ Transplantation, Ministry of Public Health, Hangzhou 310003, Zhejiang Province, China.
Lan-Juan Li, Collaborative Innovation Center for Diagnosis and Treatment of Infectious Diseases, Zhejiang University, Hangzhou 310003, Zhejiang Province, China; State Key Laboratory for Diagnosis and Treatment of Infectious Disease, First Affiliated Hospital, School of Medicine, Zhejiang University, Hangzhou 310003, Zhejiang Province, China. ljli@zju.edu.cn.
References
- 1.Hübscher SG. What is the long-term outcome of the liver allograft? J Hepatol. 2011;55:702–717. doi: 10.1016/j.jhep.2011.03.005. [DOI] [PubMed] [Google Scholar]
- 2.Sayegh MH, Carpenter CB. Transplantation 50 years later--progress, challenges, and promises. N Engl J Med. 2004;351:2761–2766. doi: 10.1056/NEJMon043418. [DOI] [PubMed] [Google Scholar]
- 3.Wiesner RH, Menon KV. Late hepatic allograft dysfunction. Liver Transpl. 2001;7:S60–S73. doi: 10.1053/jlts.2001.29094. [DOI] [PubMed] [Google Scholar]
- 4.Hu J, Wang Z, Tan CJ, Liao BY, Zhang X, Xu M, Dai Z, Qiu SJ, Huang XW, Sun J, et al. Plasma microRNA, a potential biomarker for acute rejection after liver transplantation. Transplantation. 2013;95:991–999. doi: 10.1097/TP.0b013e31828618d8. [DOI] [PubMed] [Google Scholar]
- 5.Ravaioli M, Neri F, Lazzarotto T, Bertuzzo VR, Di Gioia P, Stacchini G, Morelli MC, Ercolani G, Cescon M, Chiereghin A, et al. Immunosuppression Modifications Based on an Immune Response Assay: Results of a Randomized, Controlled Trial. Transplantation. 2015;99:1625–1632. doi: 10.1097/TP.0000000000000650. [DOI] [PubMed] [Google Scholar]
- 6.Jiang JW, Ren ZG, Cui GY, Zhang Z, Xie HY, Zhou L. Chronic bile duct hyperplasia is a chronic graft dysfunction following liver transplantation. World J Gastroenterol. 2012;18:1038–1047. doi: 10.3748/wjg.v18.i10.1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang W, Fung J. Limitations of current liver transplant immunosuppressive regimens: renal considerations. Hepatobiliary Pancreat Dis Int. 2017;16:27–32. doi: 10.1016/s1499-3872(16)60167-4. [DOI] [PubMed] [Google Scholar]
- 8.Humar A, Michaels M; AST ID Working Group on Infectious Disease Monitoring. American Society of Transplantation recommendations for screening, monitoring and reporting of infectious complications in immunosuppression trials in recipients of organ transplantation. Am J Transplant. 2006;6:262–274. doi: 10.1111/j.1600-6143.2005.01207.x. [DOI] [PubMed] [Google Scholar]
- 9.Jia JJ, Lin BY, He JJ, Geng L, Kadel D, Wang L, Yu DD, Shen T, Yang Z, Ye YF, et al. “Minimizing tacrolimus” strategy and long-term survival after liver transplantation. World J Gastroenterol. 2014;20:11363–11369. doi: 10.3748/wjg.v20.i32.11363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eckburg PB, Bik EM, Bernstein CN, Purdom E, Dethlefsen L, Sargent M, Gill SR, Nelson KE, Relman DA. Diversity of the human intestinal microbial flora. Science. 2005;308:1635–1638. doi: 10.1126/science.1110591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nenci A, Becker C, Wullaert A, Gareus R, van Loo G, Danese S, Huth M, Nikolaev A, Neufert C, Madison B, et al. Epithelial NEMO links innate immunity to chronic intestinal inflammation. Nature. 2007;446:557–561. doi: 10.1038/nature05698. [DOI] [PubMed] [Google Scholar]
- 12.Cani PD, Delzenne NM. The role of the gut microbiota in energy metabolism and metabolic disease. Curr Pharm Des. 2009;15:1546–1558. doi: 10.2174/138161209788168164. [DOI] [PubMed] [Google Scholar]
- 13.Shen F, Zheng RD, Sun XQ, Ding WJ, Wang XY, Fan JG. Gut microbiota dysbiosis in patients with non-alcoholic fatty liver disease. Hepatobiliary Pancreat Dis Int. 2017;16:375–381. doi: 10.1016/S1499-3872(17)60019-5. [DOI] [PubMed] [Google Scholar]
- 14.Chen Y, Yang F, Lu H, Wang B, Chen Y, Lei D, Wang Y, Zhu B, Li L. Characterization of fecal microbial communities in patients with liver cirrhosis. Hepatology. 2011;54:562–572. doi: 10.1002/hep.24423. [DOI] [PubMed] [Google Scholar]
- 15.Dapito DH, Mencin A, Gwak GY, Pradere JP, Jang MK, Mederacke I, Caviglia JM, Khiabanian H, Adeyemi A, Bataller R, et al. Promotion of hepatocellular carcinoma by the intestinal microbiota and TLR4. Cancer Cell. 2012;21:504–516. doi: 10.1016/j.ccr.2012.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ren Z, Cui G, Lu H, Chen X, Jiang J, Liu H, He Y, Ding S, Hu Z, Wang W, et al. Liver ischemic preconditioning (IPC) improves intestinal microbiota following liver transplantation in rats through 16s rDNA-based analysis of microbial structure shift. PLoS One. 2013;8:e75950. doi: 10.1371/journal.pone.0075950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xie Y, Luo Z, Li Z, Deng M, Liu H, Zhu B, Ruan B, Li L. Structural shifts of fecal microbial communities in rats with acute rejection after liver transplantation. Microb Ecol. 2012;64:546–554. doi: 10.1007/s00248-012-0030-1. [DOI] [PubMed] [Google Scholar]
- 18.Ren Z, Jiang J, Lu H, Chen X, He Y, Zhang H, Xie H, Wang W, Zheng S, Zhou L. Intestinal microbial variation may predict early acute rejection after liver transplantation in rats. Transplantation. 2014;98:844–852. doi: 10.1097/TP.0000000000000334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ren ZG, Liu H, Jiang JW, Jiang L, Chen H, Xie HY, Zhou L, Zheng SS. Protective effect of probiotics on intestinal barrier function in malnourished rats after liver transplantation. Hepatobiliary Pancreat Dis Int. 2011;10:489–496. doi: 10.1016/s1499-3872(11)60083-0. [DOI] [PubMed] [Google Scholar]
- 20.Ren Z, Chen X, Cui G, Yin S, Chen L, Jiang J, Hu Z, Xie H, Zheng S, Zhou L. Nanosecond pulsed electric field inhibits cancer growth followed by alteration in expressions of NF-κB and Wnt/β-catenin signaling molecules. PLoS One. 2013;8:e74322. doi: 10.1371/journal.pone.0074322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lu H, Wu Z, Xu W, Yang J, Chen Y, Li L. Intestinal microbiota was assessed in cirrhotic patients with hepatitis B virus infection. Intestinal microbiota of HBV cirrhotic patients. Microb Ecol. 2011;61:693–703. doi: 10.1007/s00248-010-9801-8. [DOI] [PubMed] [Google Scholar]
- 22.Lamping N, Dettmer R, Schröder NW, Pfeil D, Hallatschek W, Burger R, Schumann RR. LPS-binding protein protects mice from septic shock caused by LPS or gram-negative bacteria. J Clin Invest. 1998;101:2065–2071. doi: 10.1172/JCI2338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.US Multicenter FK506 Liver Study Group. A comparison of tacrolimus (FK 506) and cyclosporine for immunosuppression in liver transplantation. N Engl J Med. 1994;331:1110–1115. doi: 10.1056/NEJM199410273311702. [DOI] [PubMed] [Google Scholar]
- 24.Haddad EM, McAlister VC, Renouf E, Malthaner R, Kjaer MS, Gluud LL. Cyclosporin versus tacrolimus for liver transplanted patients. Cochrane Database Syst Rev. 2006:CD005161. doi: 10.1002/14651858.CD005161.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wiesner RH, Fung JJ. Present state of immunosuppressive therapy in liver transplant recipients. Liver Transpl. 2011;17 Suppl 3:S1–S9. doi: 10.1002/lt.22410. [DOI] [PubMed] [Google Scholar]
- 26.Geng L, Wang LD, Huang JJ, Shen T, Wang ZY, Lin BY, Ye YF, Zheng SS. Lower tacrolimus trough levels in the late period after living donor liver transplantation contribute to improvements in long-term clinical outcomes. Hepatobiliary Pancreat Dis Int. 2018;17:204–209. doi: 10.1016/j.hbpd.2018.05.001. [DOI] [PubMed] [Google Scholar]
- 27.Ren Z, Jiang J, Xie H, Li A, Lu H, Xu S, Zhou L, Zhang H, Cui G, Chen X, et al. Gut microbial profile analysis by MiSeq sequencing of pancreatic carcinoma patients in China. Oncotarget. 2017;8:95176–95191. doi: 10.18632/oncotarget.18820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Darnaud M, Faivre J, Moniaux N. Targeting gut flora to prevent progression of hepatocellular carcinoma. J Hepatol. 2013;58:385–387. doi: 10.1016/j.jhep.2012.08.019. [DOI] [PubMed] [Google Scholar]
- 29.Chassaing B, Etienne-Mesmin L, Gewirtz AT. Microbiota-liver axis in hepatic disease. Hepatology. 2014;59:328–339. doi: 10.1002/hep.26494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yan AW, Fouts DE, Brandl J, Stärkel P, Torralba M, Schott E, Tsukamoto H, Nelson KE, Brenner DA, Schnabl B. Enteric dysbiosis associated with a mouse model of alcoholic liver disease. Hepatology. 2011;53:96–105. doi: 10.1002/hep.24018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hartmann P, Chen P, Wang HJ, Wang L, McCole DF, Brandl K, Stärkel P, Belzer C, Hellerbrand C, Tsukamoto H, et al. Deficiency of intestinal mucin-2 ameliorates experimental alcoholic liver disease in mice. Hepatology. 2013;58:108–119. doi: 10.1002/hep.26321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Le Roy T, Llopis M, Lepage P, Bruneau A, Rabot S, Bevilacqua C, Martin P, Philippe C, Walker F, Bado A, et al. Intestinal microbiota determines development of non-alcoholic fatty liver disease in mice. Gut. 2013;62:1787–1794. doi: 10.1136/gutjnl-2012-303816. [DOI] [PubMed] [Google Scholar]
- 33.Zhu L, Baker SS, Gill C, Liu W, Alkhouri R, Baker RD, Gill SR. Characterization of gut microbiomes in nonalcoholic steatohepatitis (NASH) patients: a connection between endogenous alcohol and NASH. Hepatology. 2013;57:601–609. doi: 10.1002/hep.26093. [DOI] [PubMed] [Google Scholar]
- 34.Qin N, Yang F, Li A, Prifti E, Chen Y, Shao L, Guo J, Le Chatelier E, Yao J, Wu L, et al. Alterations of the human gut microbiome in liver cirrhosis. Nature. 2014;513:59–64. doi: 10.1038/nature13568. [DOI] [PubMed] [Google Scholar]
- 35.Zhang HL, Yu LX, Yang W, Tang L, Lin Y, Wu H, Zhai B, Tan YX, Shan L, Liu Q, et al. Profound impact of gut homeostasis on chemically-induced pro-tumorigenic inflammation and hepatocarcinogenesis in rats. J Hepatol. 2012;57:803–812. doi: 10.1016/j.jhep.2012.06.011. [DOI] [PubMed] [Google Scholar]
- 36.Schnabl B, Brenner DA. Interactions between the intestinal microbiome and liver diseases. Gastroenterology. 2014;146:1513–1524. doi: 10.1053/j.gastro.2014.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]


