Abstract
Platelets play a key role in the pathogenesis of ventricular assist device (VAD) thrombosis; therefore, antiplatelet drugs are essential, both in the acute phase and in the long-term follow-up in VAD management. Aspirin is the most used agent and still remains the first-choice drug for lifelong administration after VAD implantation. Anticoagulant drugs are usually recommended, but with a wide range of efficacy targets. Dual antiplatelet therapy, targeting more than one pathway of platelet activation, has been used for patients developing a thrombotic event, despite an increased risk of bleeding complications. Although different strategies have been attempted, bleeding and thrombotic events remain frequent and there are no uniform strategies adopted for pharmacological management in the short and mid- or long-term follow up. The aim of this article is to provide an overview of the evidence from randomized clinical trials and observational studies with a focus on the pathophysiologic mechanisms underlying bleeding and thrombosis in VAD patients and the best antithrombotic regimens available.
Keywords: Assist device, Thrombosis, Bleeding, Antiplatelet therapy, Anticoagulation, Antithrombotic management
1. Introduction
Depending on the definition applied, the prevalence of heart failure (HF) is approximately 1–3% in the adult population, rising to 8–10% for subjects aged 75 years or older [1, 2]. In patients acutely hospitalized, HF is still associated with very poor medium-term prognosis, with all-cause death being approximately 20% and all-cause re-hospitalization rate up to 50% at 1-year follow up [3].
Permanent implantable ventricular assist devices (VADs) are a consolidated alternative to heart transplantation (HTx) for ineligible patients with end-stage HF (destination therapy). VADs are currently implanted also in patients on waiting list for HTx (as bridge to transplantation) or needing evaluation for candidacy in order to achieve end-organ recovery (bridge to candidacy) [1].
VADs can be categorized based on their mechanism of blood propulsion. First generation VADs were pulsatile devices mimicking the natural heart flow dynamic, whereas second- and third-generation devices provide continuous flow (with axial or centrifugal design) [4].
Despite technological improvements (greater durability and easier placement) with the continuous flow design, bleeding and thromboembolism, as well as pump thrombosis, remain frequent complications that may affect the long-term outcome of patients on VAD [[5], [6], [7]]. Indeed, only 30% of patients remain free from bleeding or thromboembolic complications after 1 year from VAD implantation [8].
The event rate per patient-year is ≈0.40 and 0.06 for non-gastrointestinal and gastrointestinal major bleeding [9], 0.04 to 0.09 for pump thrombosis [10], and 0.08 for hemorrhagic or ischemic stroke [9]. The clinical consequences of thrombotic and bleeding complications can be devastating, with ischemic and hemorrhagic stroke remaining among the most common causes of death in these patients (adjusted hazard ratio 6.1; 95% CI, 4.6–7.9) [11]. Even if an increase in pump thrombosis reported in previous studies [[12], [13], [14]] has been mitigated in recently published trials [15, 16], pump thrombosis may be a vexing problem because of older patients and longer duration of life after implantation. Device manufacturers usually suggest the use of a specific antiplatelet or anticoagulant agent, without any strong evidence supporting their indication. Therefore, the antithrombotic therapy in patients with VADs is still evolving to better face the fine balance between bleeding and thrombosis.
The aim of the present review is to summarize the available evidence about the pathophysiological mechanisms contributing to bleeding and thrombosis in VAD patients, the managements that are usually adopted and the future lines of research.
2. Search strategy
Databases including PubMed, OVID, SCOPUS, CINAHL, Cochrane and Web of Science were searched from database inception through to 28 March 2018, using the predefined search terms “assist device”, “thrombosis”, “bleeding”, “antiplatelet therapy”, “anticoagulation”, “antithrombotic management” using Boolean logic operators (AND) and (OR), with exploded headings. Search of the literature was performed without predefined “timeframe” for published articles. No language and study type limits were applied. Reference lists of selected publications were also analyzed for additional linking studies.
3. Pathophysiology of thrombosis and bleeding in LVAD patients
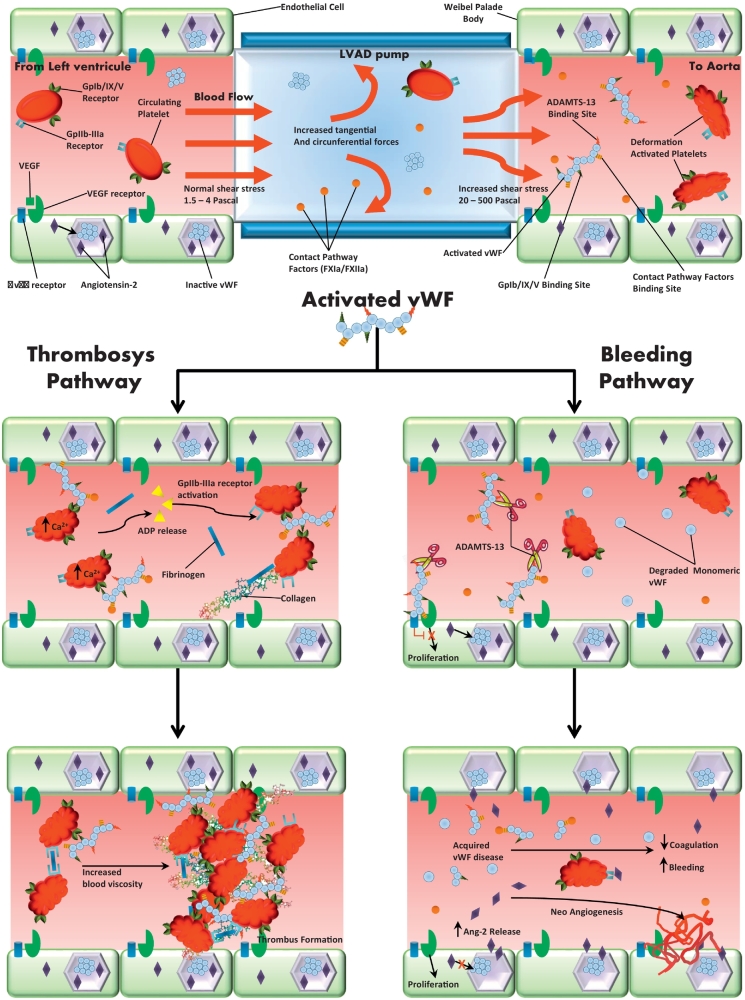
Thrombus formation is a dynamic process that is regulated by components of the hemostatic system, which, under physiological conditions, form blood clots that limit blood loss from damaged vessels [15]. Platelet reactions with the solid surfaces of damaged blood vessels depend on the presence of plasma von Willebrand Factor (vWF) activity [17]. vWF is a multimeric glycoprotein manufactured by the endothelial cells, where it is constitutively released into the circulation and stored into the Weibel-Palade bodies, from where it can be rapidly released upon stimulation [17, 18]. vFW is stored also in the α-granules of megakaryocytes/platelets, from which it is released only upon platelet activation [17, 18]. vWF circulates in the plasma and it is critical for primary hemostasis, being essential for platelet adhesion to the subendothelium of damaged blood vessel, platelet activation and platelet aggregation [19]. Furthermore, it is required to control the formation of vascular network [19].
Rheological variables play a role in thrombus formation, by affecting the ability of platelets to hook up to adhesive proteins that are exposed on the vessel wall (platelet adhesion) or are bound on the surface of other platelets (platelet aggregation) [17]. Shear stress is defined as the force for area unit between the blood laminae. The normal physiologic range of fluid shear stress within the cardiovascular system is 15 to 40 dyne/cm2 (1.5–4 Pa) [20]. Under physiologic shear stress, vWF undergoes a conformational elongation from a globular state to an extended chain conformation with exposure of binding sites for the platelet glycoprotein complex GpIb/IX/V and various subendothelial constituents [21]. The interaction of vWF with platelet GPIb/IX/V causes platelet activation, secretion of ADP, activation of the GpIIb-IIIa receptor and platelet aggregation. However, the same conformation elongation exposes the disulfide bonds of vWF multimers, site of enzymatic degradation cleavage of large, active vWF multimers into small, nonactive vWF fragments by the vWF protease ADAMTS-13 (a disintegrin and metalloproteinase with a thrombospondin type 1 motif, member 13) [[22], [23], [24], [25]]. Therefore, the pathogenesis of bleeding and thrombosis depends on the imbalance between these pro-hemostatic and anti-hemostatic factors.
3.1. Thrombosis
Each VAD has a specific mechanism of blood propulsion, able to produce different hemodynamic profiles associated with changing degrees of pulsatility, turbulence and shear stress. The shear stress that is generated within VADs ranges between 20 and 500 Pa [26, 27].
Shear stress acts as chemical agonists, showing dose and time-response characteristics. The elongated vWF formed under conditions of high shear stress becomes procoagulant through accumulation of contact pathway factors. Accordingly, placement of VADs has been associated with persistent generation of activated contact proteins (FXIa and its co-localized activator FXIIa), that accumulate on the vWF as they form under pathological flow [28] and are absorbed to the surface of VADs via the Vroman effect (according to the original theory, the small molecules are the first to be absorbed on a surface, but they are gradually stifled by proteins with greater affinity) [29]. The threshold evoking a specific response in terms of platelet adhesion and aggregation may change over time depending on the specific interactions between platelets and solid surface and the exposition time [17].
Platelet aggregation induced under supraphysiologic shear stress, as usually occurred in patients with VAD, has peculiar characteristics [[30], [31], [32], [33]]. Thrombelastographic analyses have demonstrated that contact protein pathway activation results in a thrombus that develops strength at a significantly faster rate and that takes significantly longer to lyse than thrombi generated by tissue factor initiated coagulation, by more efficient activation of the thrombin-activatable fibrinolysis inhibitor [34]. It is well known that vWF stress-stimulated aggregation is little affected by inhibition of cycloxygenase by acetylsalicylic acid, whereas it is inhibited by fibrinolytic agents [35,36].Therefore, ideally, agents acting on ADP activity (as P2Y12 inhibitors), blocking GP Ib or GP IIb/IIIa or acting on the fibrinolytic system might be suitable therapeutic target to decrease thrombosis rate in VAD patients.
In experimental studies analyzing platelet-rich plasma under conditions of high shear stress, >50% of activation and aggregation caused by the interaction of vWF with platelet GP Ib could be inhibited by blocking the action of P2Y12 [37].
Similarly, studies in vitro have demonstrated that, under supraphysiologic shear stress, phosphodiesterase (PDE) 3 inhibitors suppress the platelet thrombus formation initiated by the interaction of the platelet receptor GPIb/V/IX with the vWF [38]. The only selective PDE-3 inhibitor available is cilostazol. Several observations conducted in vitro have confirmed the favorable profile of cilostazol in reducing platelet activation under both constant and dynamic device-related conditions [33, 39, 40]. However, as recently reported, FDA has contraindicated the use of cilostazol for patients with any degree of heart failure [33, 41]. Dipyridamole is both a PDE-3 and -5 inhibitor, but studies in vitro have revealed its decreased activity on platelet inhibition under conditions of high shear stress [33].
Finally, a further mechanism leading to thromboembolic complications in VAD patients could be the heat stress. Investigations in this field have revealed hemoconcentration, activation of coagulation with decreased clotting time and increased clot strength and thrombin and d-dimer release [42]. Heat stress may act promoting activation of several inflammatory pathways and inducing the release of chemical agonists as epinephrine. This explains why in this context inhibition of the cyclo-oxygenase enzymes by acetylsalicylic acid, decreasing production of prostaglandins and other inflammatory mediators, is of potential benefit [43].
3.2. Bleeding
On the opposite site of the coin, shear stress may have a role in bleeding diathesis. Acting on the weaker disulfide bonds of vWF multimers, shear stress could tear apart vWF monomers. In parallel, supraphysiologic shear stress is the major mechanism leading to enzymatic degradation cleavage of large, active vWF multimers into small, nonactive vWF fragments by the vWF protease ADAMTS-13 (a disintegrin and metalloproteinase with a thrombospondin type 1 motif, member 13) [30]. As a result, an acquired vWF deficiency develops, thus significantly affecting sprouting angiogenesis. vWF is a ligand for the integrin αvβ3, involved in the regulation of the directionality of cellular migration [22]. Furthermore, vWF drives the formation of Weibel-Palade bodies, which stores regulators of angiogenesis and inflammation including angiopoietin-2. Decreased levels of vWF induce internalization and increased degradation of αvβ3 and increased release of angiopoietin-2 [[22], [23], [24], [25]]. Dysregulated angiopoietin-2 may lead to endothelial cells proliferation and migration throughout the vascular endothelial growth factor (VEGFR)-dependent pathways, with increased neo-angiogenesis and vascularization [18, [22], [23], [24], [25], 44].
Increased angiopoietin-2 release further induces αvβ3 internalization, amplifying cellular proliferation and migration [25]. These multiple cross-talking pathways results in dysplastic vessels and arterio-venous malformations predisposing patients with a VAD to bleeding events [45].
Moreover, diminished pulsatility and increased intraluminal pressure have also been postulated to induce distention of submucosal venous plexus and microvascular perfusion injury of the small bowel [45].
Finally, patients with blood type O have been noted to have lower baseline levels of vWF compared with other blood groups, though the precise mechanism of this regulation remains unknown [46]. However, these slight differences are unlikely to be clinically significant, as reported in studies performed in patients undergoing coronary artery bypass [46]. Moreover, in patients with centrifugal devices, the vWF antigen and vWF activity did not differ among individuals with blood group “O” compared to the other blood groups [47].
These complex relationships (Fig. 1) acknowledge why it is so difficult to find a proper management in patients with VAD support. Summarizing the available evidences from prior published work is challenging because different pumps and center-specific protocols have been adopted, limiting our ability to definitively integrate the available information. Finally, it should always be taken into consideration that risk factors contributing to pump thrombosis are related not only to the pump itself, but even to the patient pre-existing conditions (atrial fibrillation, preexistent thrombus, uncontrolled hypertension, hypercoagulable state as in course of anti-phospholipid antibody syndrome, acute heparin-induced thrombocytopenia or active infection) and patient's compliance with regular medications drugs [48].
Fig. 1.
Thrombosis and bleeding pathways in patients with ventricular assist device. Under physiologic shear stress, vWF undergoes a conformational elongation from a globular state to an extended chain conformation with exposure of binding sites for the platelet glycoprotein complex GpIb/IX/V and various subendothelial constituents. This interaction causes platelet activation, secretion of ADP, activation of the GpIIb-IIIa receptor and platelet aggregation (central box and box on the top left). However, the same conformation elongation exposes the disulfide bonds of vWF multimers and allows the inactivation process by the vWF protease ADAMTS-13 (box on the top right). Placement of VADs may amplify these processes, increasing shear stress and promoting activated contact proteins release. Therefore, the increased activation of vWF may explain the dual pathway activation: increased thrombosis by platelets activation and contact proteins system (pathway on the left) and bleeding diathesis throughout ADAMTS-13 activation and acquired vWF disease (pathway on the right).
4. Antithrombotic strategies
4.1. Secondary prevention of thromboembolic events after VAD implantation: anticoagulant treatment
According to the International Society of Heart and Lung Transplantation guidelines [49] supported by the American Heart Association [50], patients with VAD should receive anti-coagulation with warfarin to maintain an International Normalized Ratio (INR) within a range as specified by each device manufacturer; aspirin (81–325 mg daily) and other antiplatelet drugs may be used in addition to warfarin according to the recommendations of specific device manufacturers, but these indications are mostly based on experts' opinion [49, 50].
Table 1 summarizes the most commonly implanted devices, their specific rheology and the recommended antithrombotic regimen by each device manufacture.
Table 1.
Ventricular assist devices usually implanted in our center and antithrombotic strategies suggested for secondary prevention of thromboembolic events.
| Device | Weight | Type of flow | Range Speed | Material motor can | Material inflow canula | Dose aspirin | Range OAC (INR) |
|---|---|---|---|---|---|---|---|
| HM II | 281 g | Axial | 8000–15,000 | Titanium | Titanium | 100 mg | 2–3 |
| HM III | 180 g | Centrifugal | 4000–9000 | Titanium | Titanium | 100 mg | 2–3 |
| HW | 160 g | Centrifugal | 2400–3200 | Titanium | Titanium | 300 mg | 2,5–3 |
HM: HeartMate, Thoratec Corporation; HW: HeartWare ventricular assist device system, HeartWare.
In the 2012 Rossi et al. published a review including 538 patients with axial-flow VAD (HeartMate II, Jarvik 2000, INCOR, Thoratec assist device). Search strategies involved 758 papers retrieved from 1950 to March 2012. Pooling the results of the 17 papers included in the final analysis, the authors suggested that use of warfarin (INR target 2.5), in association with aspirin at 100 mg/day could be considered the best option [51].
In 2015, Bauman et al. published a systematic review focused on the antithrombotic therapy for left ventricular assist devices (LVADs), both axial and centrifugal, in adults [52]. The authors screened 1276 articles identified throughout database searching and other sources. One-hundred and four articles were assessed for eligibility and 24 studies were included in the qualitative synthesis, reporting outcomes of 2784 LVADs with a range of implantation time between 1 day and 2823 days. Almost all of the studies were deemed of moderate quality, as they were mostly retrospective and without a comparison group. The different antithrombotic strategies adopted and the lack of a standardized definition of bleeding between studies limited the ability to compare antithrombotic regimens. Major bleeding, according to each study definition, was reported in 30% of patients. Stroke and pump thrombosis were reported in 6% and 5% of patients. Death was reported in 20% of patients. The most frequent causes of death were multiorgan failure (31%), infections (15%), ischemic stroke (13%), right heart failure (11%), bleeding (10%), mechanical failure of the device (3%), with fatal bleedings and fatal thromboembolic complications showing similar incidences. The authors concluded that lack of effective INR control may be associated with bleeding and thromboembolic complications and that the optimal antithrombotic regimen in LVAD patients remains undetermined. Moreover, the role of antiplatelet therapy during LVAD support was still unclear.
The most recent evidences of the current literature show that differences in markers of hemolysis and coagulation activation may exist according to the specific VAD implanted [[14], [15], [16], 53]. Actually, the improved hemocompatibility achieved with the new generation devices has led to the conclusion that low-intensity anticoagulation (INR range 1.5–1.9) could be a safe and effective strategy in patients closely monitored with new magnetically levitated centrifugal-flow circulatory pump, associated with a short term (six weeks post-implantation) antiplatelet strategy [14, 15, 54]. However, further studies on larger samples are needed to confirm this hypothesis.
4.2. Secondary prevention of thromboembolic events after VAD implantation: antiplatelet treatment
Even if aspirin is commonly recommended and used as the first-line therapy in patients with LVAD since the first clinical experiences [55], mechanisms of platelet activation in VADs patients (shear-induced platelet aggregation) make the efficacy of aspirin questionable. Furthermore, an experimental study conducted in a small sample of patients with external VAD implantation [56], has documented a non-trivial risk of aspirin hyporesponsiveness, defined by persistent platelet aggregation in 26% of the patients under arachidonic acid exposition. In this study patients on VADs support experienced a persistent inflammatory status as expressed by elevated fibrinogen levels and circulating von Willebrand Factor values. Mechanisms for aspirin hyporesponsiveness are potentially multiple in VAD patients [17, [57], [58], [59], [60]]: a) an increase in platelets turnover may overcome the effect of aspirin on thromboxane A2 synthesis [57]; b) chronic inflammatory status may promote the cyclooxygenase-2 isoform activity, that is less sensible to aspirin acetylation [58]; c) platelet adhesion and activation under high shear stress are poorly sensitive to aspirin [59].
Clopidogrel has been suggested in the case of aspirin intolerance [10] or according to specific indications, mostly in the context of recent thromboembolic events [61]. However, the antiplatelet response to clopidogrel, performed with the platelet function analyzer-100 system and the vasodilator-stimulated phosphoprotein phosphorylation assay, is highly variable, with almost 50% of patients showing insufficient anti-platelet response [53].
The combination of dipyridamole and aspirin is associated with a decreased relative and absolute risk of thromboembolic events in VAD patients of 50% and 9%. However, these data have been reported only in few experiences with axial devices [54] and are inconsistent in the general VAD population.
Prasugrel and ticagrelor, P2Y12 antagonists with more predictable pharmacokinetics than clopidogrel, have not been routinely prescribed in patients with VAD likely for fear of an inacceptable high risk of bleeding events. One small case-series suggested a positive role of ticagrelor in patients with suspected LVADs thrombosis [62]. However, on the basis of our clinical experience, the generalizability of these optimistic results is questionable [63].
4.3. Antithrombotic therapy in patients with thromboembolic events
Thrombolysis has been evaluated in patients with pump thrombosis according to local algorithms based on single-center experiences. However, a systematic review and metaanalysis has not documented a significant increased rate of success in patients with pump thrombosis treated with thrombolytic regimens compared to nonthrombolytic pharmacological management [64]. An extensive review of this issue is out of the present revision.
Glycoprotein IIb/IIIa inhibitors have been evaluated as an alternative to fibrinolysis in patients with suspected device thrombosis. However, patients with confirmed events, failed to have a clinically measurable response to therapy [65, 66].
Direct thrombin inhibitors (DTI) might have a strong rationale in this setting.
Bivalirudin is a specific and reversible DTI used in clinical practice since the early '90s, as an alternative medication to heparin, during percutaneous coronary intervention (PCI) [67]. DTIs are better pharmacological tools, compared to unfractionated heparin (UFH). Their advantages include being independent of antithrombin III levels, better bioavailability, and capacity to inhibit both soluble and clot-bound thrombin, which reduces clot formation and propagation. Furthermore, DTIs inhibit thrombin-induced platelet activation [68].
About 20% of bivalirudin is excreted unchanged in the urine. Drug elimination depends on glomerular filtration rate; therefore, the half-life is 25–30 min in healthy patients but is increased to 1 h in severe renal disease and is estimated to be 3.5 h in end-stage renal disease requiring hemodialysis. Intravenous administration of bivalirudin produces measurable anticoagulation within few minutes with linear increases in prothrombin time, activated partial thromboplastin time (aPTT), activated clotting time (ACT) and thrombin time [68].
Bivalirudin has been approved for use in patients undergoing PCI with or without prior treatment with unfractionated heparin (UFH) and in patients with heparin-induced thrombocytopenia (HIT) and current guidelines support its use in acute coronary syndrome patients [69]. In patients undergoing PCI, it is administered as a bolus of 0.75 mg/kg, followed by a continuous infusion at 1.75 mg/kg/h for the entire procedure. The infusion dose should be reduced in patients with renal insufficiency: 1 mg/kg/h for patients with creatinine clearance <30 ml/min, and 0.25 mg/kg/h for patients in hemodialysis [70]. Mueller et al. reported the successful use of bivalirudin therapy for prevention of hemofilter occlusion during continuous veno-venous hemofiltration (CVVH) in a patient with hepatic and renal dysfunction. The bivalirudin dose was 0.09 mg/kg/h. No bleeding complications were noted [71].
Preliminary experiences in patients with VAD implantation have been favorable. In 2014 Pieri et al. performed a study on 12 consecutive patients who underwent VAD implantation and received primary anticoagulation with Bivalirudin: the starting dose was 0.025 mg/kg/h, the median dose was 0.04 ± 0.026 mg/kg/h and median duration of therapy was 5 days. Patients never received heparin during hospitalization nor had a prior diagnosis of HIT. No patient had thromboembolic complications or major bleeding events and the authors concluded that bivalirudin should be a valuable alternative anticoagulant therapy in VAD patients. Other clinical experiences have confirmed the safety profile in this setting [72].
Evidences to support the use of a DTIs in LVAD are mostly anecdotal. In the small case series reported by Lynne et al. bivalirudin was used as an alternative to heparin in six hemodynamically stable patients admitted to hospital for suspected LVAD thrombosis. The starting dose was between 0.03 and 0.15 mg/kg/h and the maintenance dose ranged from 0.04 to 0.23 mg/kg/h. In these cases, bivalirudin was relatively safe and effective for the treatment of LVAD thrombosis even if a high rate of recurrence was showed [73].
Argatroban is another intravenous DTI used in cases of suspected LVAD thrombosis. It is metabolized by the liver, while it has no renal clearance. In comparison with bivalirudin, argatroban has higher affinity for thrombin with a more effective inhibition of clot-bound thrombin: the dose usually employed is 2 μg/kg/min, aiming at prolonging aPTT to 1.5–3 times the baseline values. Argatroban also prolongs the PT, thus interfering with laboratory monitoring of warfarin, when the two drugs are co-administered [74].
Dabigatran, an oral DTI, have been evaluated in LVAD patients with promising results [75]. However, a recent randomized controlled trial showed an excess of thromboembolic events in dabigatran-treated patients compared to phenprocoumon-treated patients [76].
Even if the dual pathway therapy with direct oral anticoagulant agents combined with P2Y12 inhibitor appears promising in patients with acute coronary syndromes, based on the results of recent randomized trials on dabigatran and rivaroxaban [77, 78], this strategy has not been tested in LVAD patients. Moreover, in view of the unfavorable results in patients with mechanical valves, this option deserves ad hoc well-designed trial.
In our clinical experience all the pharmacological strategies above reported have been attempted in different clinical scenarios. Bivalirudin has been used with a good profile of safety and efficacy in patients at higher risk for both the conditions (suspected pump thrombosis and hemorrhagic events, even cerebral hemorrhagic foci).
5. Conclusions
Antithrombotic management in VADs patients is based on ancient protocols without strong experimental evidence. Large clinical trials with standardized laboratory methods and well-defined protocols are needed in order to evaluate the different antiplatelet and anticoagulant drugs available, suggesting therapeutic strategies to overcome the risk of thrombotic and bleeding events.
Disclosures
None of the authors report any conflict of interest with the specific subject of the study.
Footnotes
These authors take responsibility for all aspects of the reliability and freedom from bias of the data presented and their discussed interpretation.
References
- 1.Ponikowski P., Voors A.A., Anker S.D., Bueno H., Cleland J.G.F., Coats A.J.S., Falk V., González-Juanatey J.R., Harjola V.P., Jankowska E.A., Jessup M., Linde C., Nihoyannopoulos P., Parissis J.T., Pieske B., Riley J.P., Rosano G.M.C., Ruilope L.M., Ruschitzka F., Rutten F.H., van der Meer P., ESC Scientific Document Group 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 2016;37:2129–2200. doi: 10.1093/eurheartj/ehw128. [DOI] [PubMed] [Google Scholar]
- 2.Redfield M.M., Jacobsen S.J., Burnett J.C., Mahoney D.W., Bailey K.R., Rodeheffer R.J. Burden of systolic and diastolic ventricular dysfunction in the community: appreciating the scope of the heart failure epidemic. JAMA. 2003;289:194–202. doi: 10.1001/jama.289.2.194. [DOI] [PubMed] [Google Scholar]
- 3.Maggioni A.P., Dahlström U., Filippatos G., Chioncel O., Crespo Leiro M., Drozdz J., Fruhwald F., Gullestad L., Logeart D., Fabbri G., Urso R., Metra M., Parissis J., Persson H., Ponikowski P., Rauchhaus M., Voors A.A., Nielsen O.W., Zannad F., Tavazzi L., Heart Failure Association of the European Society of Cardiology (HFA) EURObservational Research Programme: regional differences and 1-year follow-up results of the Heart Failure Pilot Survey (ESC-HF Pilot) Eur. J. Heart Fail. 2013;15:808–817. doi: 10.1093/eurjhf/hft050. [DOI] [PubMed] [Google Scholar]
- 4.Patel C.B., Cowger J.A., Zuckermann A. A contemporary review of mechanical circulatory support. J Heart Lung Transplant. 2014;33:667–674. doi: 10.1016/j.healun.2014.02.014. [DOI] [PubMed] [Google Scholar]
- 5.Teuteberg J.J., Slaughter M.S., Rogers J.G., McGee E.C., Pagani F.D., Gordon R., Rame E., Acker M., Kormos R.L., Salerno C., Schleeter T.P., Goldstein D.J., Shin J., Starling R.C., Wozniak T., Malik A.S., Silvestry S., Ewald G.A., Jorde U.P., Naka Y., Birks E., Najarian K.B., Hathaway D.R., Aaronson K.D. The HVAD left ventricular assist device: risk factors for neurological events and risk mitigation strategies. JACC Heart Fail. 2015;3:818–828. doi: 10.1016/j.jchf.2015.05.011. [DOI] [PubMed] [Google Scholar]
- 6.Kirklin J.K., Pagani F.D., Kormos R.L., Stevenson L.W., Blume E.D., Myers S.L., Miller M.A., Baldwin J.T., Young J.B., Naftel D.C. Eighth annual INTERMACS report: special focus on framing the impact of adverse events. J Heart Lung Transplant. 2017;36:1080–1086. doi: 10.1016/j.healun.2017.07.005. [DOI] [PubMed] [Google Scholar]
- 7.Starling R.C., Estep J.D., Horstmanshof D.A., Milano C.A., Stehlik J., Shah K.B., Bruckner B.A., Lee S., Long J.W., Selzman C.H., Kasirajan V., Haas D.C., Boyle A.J., Chuang J., Farrar D.J., Rogers J.G., ROADMAP Study Investigators Risk assessment and comparative effectiveness of left ventricular assist device and medical management in ambulatory heart failure patients: the ROADMAP study 2-year results. JACC Heart Fail. 2017;5:518–527. doi: 10.1016/j.jchf.2017.02.016. [DOI] [PubMed] [Google Scholar]
- 8.Kirklin J.K., Naftel D.C., Pagani F.D., Kormos R.L., Stevenson L.W., Blume E.D., Miller M.A., Baldwin J.T., Young J.B. Sixth INTERMACS annual report: a 10,000-patient database. J Heart Lung Transplant. 2014;33:555–564. doi: 10.1016/j.healun.2014.04.010. [DOI] [PubMed] [Google Scholar]
- 9.Goldstein D.J., Aaronson K.D., Tatooles A.J., Silvestry S.C., Jeevanandam V., Gordon R., Hathaway D.R., Najarian K.B., Slaughter M.S., ADVANCE Investigators Gastrointestinal bleeding in recipients of the HeartWare Ventricular Assist System. JACC Heart Fail. 2015;4:303–313. doi: 10.1016/j.jchf.2014.11.008. [DOI] [PubMed] [Google Scholar]
- 10.Scandroglio A.M., Kaufmann F., Pieri M., Kretzschmar A., Müller M., Pergantis P., Dreysse S., Falk V., Krabatsch T., Potapov E.V. Diagnosis and treatment algorithm for blood flow obstructions in patients with left ventricular assist device. J. Am. Coll. Cardiol. 2016;67:2758–2768. doi: 10.1016/j.jacc.2016.03.573. [DOI] [PubMed] [Google Scholar]
- 11.Parikh N.S., Cool J., Karas M.G., Boehme A.K., Kamel H. Stroke risk and mortality in patients with ventricular assist devices. Stroke. 2016;47:2702–2706. doi: 10.1161/STROKEAHA.116.014049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Muthiah K., Robson D., Macdonald P.S., Keogh A.M., Kotlyar E., Granger E., Dhital K., Spratt P., Jansz P., Hayward C.S. Thrombolysis for suspected intrapump thrombosis in patients with continuous flow centrifugal left ventricular assist device. Artif. Organs. 2013;37:313–318. doi: 10.1111/j.1525-1594.2012.01567.x. [DOI] [PubMed] [Google Scholar]
- 13.Aissaoui N., Börgermann J., Gummert J., Morshuis M. HeartWare continuous-flow ventricular assist device thrombosis: the Bad Oeynhausen experience. J. Thorac. Cardiovasc. Surg. 2012;143:e37–e39. doi: 10.1016/j.jtcvs.2011.12.035. [DOI] [PubMed] [Google Scholar]
- 14.Miller L.W., Rogers J.G. Evolution of left ventricle assist device therapy for advanced heart failure. A review. JAMA Cardiol. 2018 doi: 10.1001/jamacardio.2018.0522. [DOI] [PubMed] [Google Scholar]
- 15.Netuka I., Ivák P., Tučanová Z., Gregor S., Szárszoi O., Sood P., Crandall D., Rimsans J., Connors J.M., Mehra M.R. Evaluation of low-intensity anti-coagulation with a fully magnetically levitated centrifugal-flow circulatory pump-the MAGENTUM 1 study. J Heart Lung Transplant. 2018;37:579–586. doi: 10.1016/j.healun.2018.03.002. [DOI] [PubMed] [Google Scholar]
- 16.Uriel N., Colombo P.C., Cleveland J.C., Long J.W., Salerno C., Goldstein D.J., Patel C.B., Ewald G.A., Tatooles A.J., Silvestry S.C., John R., Caldeira C., Jeevanandam V., Boyle A.J., Sundareswaran K.S., Sood P., Mehra M.R. Hemocompatibility-related outcomes in the MOMENTUM 3 trial at 6 months: a randomized controlled study of a fully magnetically levitated pump in advanced heart failure. Circulation. 2017;135:2003–2012. doi: 10.1161/CIRCULATIONAHA.117.028303. [DOI] [PubMed] [Google Scholar]
- 17.Kroll M.H., Hellums J.D., McIntire L.V., Schafer A.I., Moake J.L. Platelets and shear stress. Blood. 1996;88:1525–1541. [PubMed] [Google Scholar]
- 18.Sadler J.E. Biochemistry and genetics of von Willebrand factor. Annu. Rev. Biochem. 1998;67:395–424. doi: 10.1146/annurev.biochem.67.1.395. [DOI] [PubMed] [Google Scholar]
- 19.Starke R.D., Ferraro F., Paschalaki K.E., Dryden N.H., McKinnon T.A., Sutton R.E., Payne E.M., Haskard D.O., Hughes A.D., Cutler D.F., Laffan M.A., Randi A.M. Endothelial von Willebrand factor regulates angiogenesis. Blood. 2011;117:1071–1080. doi: 10.1182/blood-2010-01-264507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Resnick N., Yahav H., Shay-Salit A., Shushy M., Schubert S., Zilberman L.C., Wofovitz E. Fluid shear stress and the vascular endothelium: for better and for worse. Prog. Biophys. Mol. Biol. 2003;81:177–199. doi: 10.1016/s0079-6107(02)00052-4. [DOI] [PubMed] [Google Scholar]
- 21.Siedlecki C.A., Lestini B.J., Kottke-Marchant K.K., Eppell S.J., Wilson D.L., Marchant R.E. Shear-dependent changes in the three-dimensional structure of human von Willebrand factor. Blood. 1996;88:2939–2950. [PubMed] [Google Scholar]
- 22.White D.P., Caswell P.T., Norman J.C. Alpha v beta3 and alpha5beta1 integrin recycling pathways dictate downstream Rho kinase signaling to regulate persistent cell migration. J. Cell Biol. 2007;177:515–525. doi: 10.1083/jcb.200609004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lobov I.B., Brooks P.C., Lang R.A. Angiopoietin-2 displays VEGF-dependent modulation of capillary structure and endothelial cell survival in vivo. Proc. Natl. Acad. Sci. U. S. A. 2002;99:11205–11210. doi: 10.1073/pnas.172161899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hashizume H., Falcón B.L., Kuroda T., Baluk P., Coxon A., Yu D., Bready J.V., Oliner J.D., McDonald D.M. Complementary actions of inhibitors of angiopoietin-2 and VEGF on tumor angiogenesis and growth. Cancer Res. 2010;70:2213–2223. doi: 10.1158/0008-5472.CAN-09-1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thomas M., Felcht M., Kruse K., Kretschmer S., Deppermann C., Biesdorf A., Rohr K., Benest A.V., Fiedler U., Augustin H.G. Angiopoietin-2 stimulation of endothelial cells induces alphavbeta3 integrin internalization and degradation. J. Biol. Chem. 2010;285:23842–23849. doi: 10.1074/jbc.M109.097543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu G.M., Jin D.H., Zhou J.Y., Zhang Y., Chen H.B., Sun H.S., Hu S.S., Gui X.M. Numerical investigation of the influence of blade radial gap flow on axial blood pump performance. ASAIO J. Jan 5 2018 doi: 10.1097/MAT.0000000000000745. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 27.Chua L.P., Su B., Lim T.M., Zhou T. Numerical simulation of an axial blood pump. Artif. Organs. 2007;31:560–570. doi: 10.1111/j.1525-1594.2007.00422.x. [DOI] [PubMed] [Google Scholar]
- 28.Herbig B.A., Diamond S.L. Pathological von Willebrand factor fibers resist tissue plasminogen activator and ADAMTS13 while promoting the contact pathway and shear-induced platelet activation. J. Thromb. Haemost. 2015;13:1699–1708. doi: 10.1111/jth.13044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vroman L. Effect of adsorbed proteins on the wettability of hydrophilic and hydrophobicsolids. Nature. 1962;196:476–477. doi: 10.1038/196476a0. [DOI] [PubMed] [Google Scholar]
- 30.Bartoli C.R., Restle D.J., Zhang D.M., Acker M.A., Atluri P. Pathologic von Willebrand factor degradation with a left ventricular assist device occurs via two distinct mechanisms: mechanical demolition and enzymatic cleavage. J. Thorac. Cardiovasc. Surg. 2015;149:281–289. doi: 10.1016/j.jtcvs.2014.09.031. [DOI] [PubMed] [Google Scholar]
- 31.Dassanayaka S., Slaughter M.S., Bartoli C.R. Mechanistic pathway(s) of acquired von Willebrand syndrome with a continuous-flow ventricular assist device: in vitro findings. ASAIO J. 2013;59:123–129. doi: 10.1097/MAT.0b013e318283815c. [DOI] [PubMed] [Google Scholar]
- 32.Eckman P.M., John R. Bleeding and thrombosis in patients with continuous-flow ventricular assist devices. Circulation. 2012;125:3038–3047. doi: 10.1161/CIRCULATIONAHA.111.040246. [DOI] [PubMed] [Google Scholar]
- 33.Valerio L., Sheriff J., Tran P.L., Brengle W., Redaelli A., Fiore G.B., Pappalardo F., Bluestein D., Slepian M.J. Routine clinical antiplatelet agents have limited efficacy in modulating hypershear-mediated platelet activation associated with mechanical circulatory support. Thromb. Res. 2018;163:162–171. doi: 10.1016/j.thromres.2017.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nielsen V.G., Kirklin J.K., Holman W.L., Steenwyk B.L., George J.F., Zhou F., Parks D.A., Ellis T.C. Mechanical circulatory device thrombosis: a new paradigm linking hypercoagulation and hypofibrinolysis. ASAIO J. 2008;54:351–358. doi: 10.1097/MAT.0b013e31817f3e03. [DOI] [PubMed] [Google Scholar]
- 35.Federici A.B., Berkowitz S.D., Zimmerman T.S., Mannucci P.M. Proteolysis of von Willebrand factor after thrombolytic therapy in patients with acute myocardial infarction. Blood. 1992;79:3844. [PubMed] [Google Scholar]
- 36.Stricker R.B., Wong D., Shiu D.T., Reyes P.T., Shuman M.A. Activation of plasminogen by tissue plasminogen activator on normal and thrombasthenic platelets: effects on surface proteins and platelet aggregation. Blood. 1986;68:275280. [PubMed] [Google Scholar]
- 37.Goto S., Tamura N., Eto K., Ikeda Y., Handa S. Functional significance of adenosine 5′-diphosphate receptor (P2Y(12)) in platelet activation initiated by binding of von Willebrand factor to platelet GP Ibalpha induced by conditions of high shear rate. Circulation. 2002;105:2531–2536. doi: 10.1161/01.cir.0000016703.93845.af. [DOI] [PubMed] [Google Scholar]
- 38.Yoshida H., Okamura Y., Watanabe N., Ikeda Y., Handa M. Shear-dependent suppression of platelet thrombus formation by phosphodiesterase 3 inhibition requires low levels of concomitant Gs-coupled receptor stimulation. Thromb. Haemost. 2011;105:487–495. doi: 10.1160/TH10-07-0439. [DOI] [PubMed] [Google Scholar]
- 39.Yagi H., Yamaguchi N., Shida Y., Hayakawa M., Matsumoto M., Sugimoto M., Wada H., Tsubaki K., Fujimura Y. Cilostazol down-regulates the height of mural platelet thrombi formed under a high-shear rate flow in the absence of ADAMTS13 activity. Eur. J. Pharmacol. 2012;691:151–155. doi: 10.1016/j.ejphar.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 40.Chi Y.W., Lavie C.J., Milani R.V., White C.J. Safety and efficacy of cilostazol in the management of intermittent claudication. Vasc. Health Risk Manag. 2008;4:1197–1203. doi: 10.2147/vhrm.s3160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.FDA Pletal: full prescribing information. 2015. https://www.accessdata.fda.gov/drugsatfda_docs/label/2015/020863s023lbl.pdf
- 42.Sumann G., Fries D., Griesmacher A., Falkensammer G., Klingler A., Koller A., Streif W., Greie S., Schobersberger B., Schobersberger W. Blood coagulation activation and fibrinolysis during a downhill marathon run. Blood Coagul. Fibrinolysis. 2007;18:435–440. doi: 10.1097/MBC.0b013e328136c19b. [DOI] [PubMed] [Google Scholar]
- 43.Elwood P.C., Gallagher A.M., Duthie G.G., Mur L.A., Morgan G. Aspirin, salicylates, and cancer. Lancet. 2009;373:1301–1309. doi: 10.1016/S0140-6736(09)60243-9. [DOI] [PubMed] [Google Scholar]
- 44.Feng Y., vom Hagen F., Pfister F., Djokic S., Hoffmann S., Back W., Wagner P., Lin J., Deutsch U., Hammes H.P. Impaired pericyte recruitment and abnormal retinal angiogenesis as a result of angiopoietin-2 overexpression. Thromb. Haemost. 2007;97:99–108. [PubMed] [Google Scholar]
- 45.Redondo P., Martínez-Cuesta A., Quetglas E.G., Idoate M. Active angiogenesis in an extensive arteriovenous vascular malformation: a possible therapeutic target? Arch. Dermatol. 2007;143:1043–1045. doi: 10.1001/archderm.143.8.1043. [DOI] [PubMed] [Google Scholar]
- 46.Welsby I.J., Jones R., Pylman J., Mark J.B., Brudney C.S., Phillips-Bute B., Mathew J.P., Campbell M.L., Stafford-Smith M., Cardiothoracic Anesthesiology Research Endeavours (C.A.R.E.), Department of Anesthesiology, Duke University Medical Center ABO blood group and bleeding after coronary artery bypass graft surgery. Blood Coagul. Fibrinolysis. 2007;18:781–785. doi: 10.1097/MBC.0b013e3282f1029c. [DOI] [PubMed] [Google Scholar]
- 47.Esmaeilzadeh F., Wauters A., Wijns W., Argacha J.F., van de Borne P. Effects of HeartWare ventricular assist device on the von Willebrand factor: results of an academic Belgian center. BMC Cardiovasc. Disord. 2016;16:155. doi: 10.1186/s12872-016-0334-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Goldstein D.J., John R., Salerno C. Algorithm for the diagnosis and management of suspected pump thrombus. J Heart Lung Transplant. 2013;32:667–670. doi: 10.1016/j.healun.2013.05.002. [DOI] [PubMed] [Google Scholar]
- 49.Feldman D., Pamboukian S.V., Teuteberg J.J., Birks E., Lietz K., Moore S.A., Morgan J.A., Arabia F., Bauman M.E., Buchholz H.W., Deng M., Dickstein M.L., El-Banayosy A., Elliot T., Goldstein D.J., Grady K.L., Jones K., Hryniewicz K., John R., Kaan A., Kusne S., Loebe M., Massicotte M.P., Moazami N., Mohacsi P., Mooney M., Nelson T., Pagani F., Perry W., Potapov E.V., Eduardo Rame J., Russell S.D., Sorensen E.N., Sun B., Strueber M., Mangi A.A., Petty M.G., Rogers J., International Society for Heart and Lung Transplantation The 2013 International Society for Heart and Lung Transplantation Guidelines for mechanical circulatory support: executive summary. J Heart Lung Transplant. 2013;32:157–187. doi: 10.1016/j.healun.2012.09.013. [DOI] [PubMed] [Google Scholar]
- 50.Peura J.L., Colvin-Adams M., Francis G.S., Grady K.L., Hoffman T.M., Jessup M., John R., Kiernan M.S., Mitchell J.E., O'Connell J.B., Pagani F.D., Petty M., Ravichandran P., Rogers J.G., Semigran M.J., Toole J.M., American Heart Association Heart Failure and Transplantation Committee of the Council on Clinical Cardiology; Council on Cardiopulmonary, Critical Care, Perioperative and Resuscitation; Council on Cardiovascular Disease in the Young; Council on Cardiovascular Nursing; Council on Cardiovascular Radiology and Intervention, and Council on Cardiovascular Surgery and Anesthesia Recommendations for the use of mechanical circulatory support: device strategies and patient selection: a scientific statement from the American Heart Association. Circulation. 2012;126:2648–2667. doi: 10.1161/CIR.0b013e3182769a54. [DOI] [PubMed] [Google Scholar]
- 51.Rossi M., Serraino G.F., Jiritano F., Renzulli A. What is the optimal anticoagulation in patients with a left ventricular assist device? Interact. Cardiovasc. Thorac. Surg. 2012;15:733–740. doi: 10.1093/icvts/ivs297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Baumann Kreuziger L.M., Kim B., Wieselthaler G.M. Antithrombotic therapy for left ventricular assist devices in adults: a systematic review. J. Thromb. Haemost. 2015;13:946–955. doi: 10.1111/jth.12948. [DOI] [PubMed] [Google Scholar]
- 53.Birschmann I., Dittrich M., Eller T., Wiegmann B., Reininger A.J., Budde U., Strüber M. Ambient hemolysis and activation of coagulation is different between HeartMate II and HeartWare left ventricular assist devices. J Heart Lung Transplant. 2014;33:80–87. doi: 10.1016/j.healun.2013.11.010. [DOI] [PubMed] [Google Scholar]
- 54.Mehra M.R., Naka Y., Uriel N., Goldstein D.J., Cleveland J.C., Jr., Colombo P.C., Walsh M.N., Milano C.A., Patel C.B., Jorde U.P., Pagani F.D., Aaronson K.D., Dean D.A., McCants K., Itoh A., Ewald G.A., Horstmanshof D., Long J.W., Salerno C., MOMENTUM 3 Investigators A fully magnetically levitated circulatory pump for advanced heart failure. N. Engl. J. Med. 2017;376:440–450. doi: 10.1056/NEJMoa1610426. [DOI] [PubMed] [Google Scholar]
- 55.Szefner J. Control and treatment of hemostasis in cardiovascular surgery. The experience of La Pitié Hospital with patients on total artificial heart. Int. J. Artif. Organs. 1995;18:633–648. [PubMed] [Google Scholar]
- 56.Houël R., Mazoyer E., Boval B., Kirsch M., Vermès E., Drouet L., Loisance D.Y. Platelet activation and aggregation profile in prolonged external ventricular support. J. Thorac. Cardiovasc. Surg. 2004;128:197–202. doi: 10.1016/j.jtcvs.2003.11.059. [DOI] [PubMed] [Google Scholar]
- 57.Zimmerman N., Kienzle P., Weber A.A., Winter J., Gams E., Schrör K. Aspirin resistance after coronary artery bypass grafting. J. Thorac. Cardiovasc. Surg. 2001;121:982–984. doi: 10.1067/mtc.2001.111416. [DOI] [PubMed] [Google Scholar]
- 58.Weber A.-A., Zimmerman K.C., Meyer-Kichrath J., Schrör K. Cyclooxygenase-2 in human platelets as a possible factor in aspirin resistance. Lancet. 1999;353:900. doi: 10.1016/S0140-6736(99)00498-5. [DOI] [PubMed] [Google Scholar]
- 59.Parker R.I., Gralnick H.R. Effect of aspirin on platelet-von Willebrand factor surface expression on thrombin and ADP-stimulated platelets. Blood. 1989;74:2016–2021. [PubMed] [Google Scholar]
- 60.Ensor C.R., Paciullo C.A., Cahoon W.D., Jr., Nolan P.E., Jr. Pharmacotherapy for mechanical circulatory support: a comprehensive review. Ann. Pharmacother. 2011;45:60–77. doi: 10.1345/aph.1P459. [DOI] [PubMed] [Google Scholar]
- 61.Jennings D.L., Weeks P.A. Thrombosis in continuous-flow left ventricular assist devices: pathophysiology, prevention, and pharmacologic management. Pharmacotherapy. 2015;35:79–98. doi: 10.1002/phar.1501. [DOI] [PubMed] [Google Scholar]
- 62.Oliveira G.H., Al-Kindi S.G., ElAmm C., Qattan M.Y., Deo S., Medalion B., Benatti R.D., Osman M.N., Ginwalla M., Park S.J., Simon D.I. Platelet inhibition with ticagrelor for left ventricular assist device thrombosis. Circ. Heart Fail. 2015;8:649–651. doi: 10.1161/CIRCHEARTFAILURE.115.002096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Morici N., Perna E., Cipriani M., Femia E.A., Oliva F., Frigerio M., Cattaneo M. Ticagrelor for left ventricular assist device thrombosis: a new therapeutic option to be evaluated with caution. Int. J. Cardiol. 2016;15(221):58–59. doi: 10.1016/j.ijcard.2016.06.304. [DOI] [PubMed] [Google Scholar]
- 64.Dang G., Epperla N., Muppidi V., Sahr N., Pan A., Simpson P., Bauman Kreuziger L. Medical management of pump related thrombosis in patients with continuous flow left ventricular assist devices: a systematic review and metaanalysis. ASIAO J. 2017;63:373–385. doi: 10.1097/MAT.0000000000000497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Najjar S.S., Slaughter M.S., Pagani F.D., Starling R.C., McGee E.C., Eckman P., Tatooles A.J., Moazami N., Kormos R.L., Hathaway D.R., Najarian K.B., Bhat G., Aaronson K.D., Boyce SW10, HVAD Bridge to Transplant ADVANCE Trial Investigators An analysis of pump thrombus events in patients in the HeartWare ADVANCE bridge to transplant and continued access protocol trial. J Heart Lung Transplant. 2014;33:23–34. doi: 10.1016/j.healun.2013.12.001. [DOI] [PubMed] [Google Scholar]
- 66.Bellumkonda L., Subrahmanyan L., Jacoby D., Bonde P. Left ventricular assist device pump thrombosis: is there a role for glycoprotein IIb/IIIa inhibitors? ASAIO J. 2014;60:134–136. doi: 10.1097/MAT.0000000000000028. [DOI] [PubMed] [Google Scholar]
- 67.Savonitto S., Morici N. Bivalirudin as an anticoagulant during percutaneous coronary interventions in acute coronary syndromes: strengths and doubts. Rev. Esp. Cardiol. 2011;64:361–364. doi: 10.1016/j.recesp.2011.02.005. [DOI] [PubMed] [Google Scholar]
- 68.Ibanez B., James S., Agewall S., Antunes M.J., Bucciarelli-Ducci C., Bueno H., Caforio A.L.P., Crea F., Goudevenos J.A., Halvorsen S., Hindricks G., Kastrati A., Lenzen M.J., Prescott E., Roffi M., Valgimigli M., Varenhorst C., Vranckx P., Widimský P., ESC Scientific Document Group 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: the Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC) Eur. Heart J. 2018;39:119–177. doi: 10.1093/eurheartj/ehx393. [DOI] [PubMed] [Google Scholar]
- 69.Salter B.S., Weiner M.M., Trinh M.A., Heller J., Evans A.S., Adams D.H., Fischer G.W. Heparin-induced thrombocytopenia: a comprehensive clinical review. J. Am. Coll. Cardiol. 2016;67:2519–2532. doi: 10.1016/j.jacc.2016.02.073. [DOI] [PubMed] [Google Scholar]
- 70.Mueller S.W., MacLaren R., Fish D.N., Kiser T.H. Prefilter bivalirudin for preventing hemofilter occlusion in continuous renal replacement therapy. Ann. Pharmacother. 2009;43:1360–1365. doi: 10.1345/aph.1M179. [DOI] [PubMed] [Google Scholar]
- 71.Pieri M., Agracheva N., Di Prima A.L., Nisi T., De Bonis M., Isella F., Zangrillo A., Pappalardo F. Primary anticoagulation with bivalirudin for patients with implantable ventricular assist devices. Artif. Organs. 2014;38:342–346. doi: 10.1111/aor.12168. [DOI] [PubMed] [Google Scholar]
- 72.Kantorovich A., Fink J.M., Militello M.A., Bauer S.R., Soltesz E.G., Moazami N. Comparison of anticoagulation strategies after left ventricular assist device implantation. ASAIO J. 2016;62:123–127. doi: 10.1097/MAT.0000000000000317. [DOI] [PubMed] [Google Scholar]
- 73.Sylvia L.M., Ordway L., Pham D.T., DeNofrio D., Kiernan M. Bivalirudin for treatment of LVAD thrombosis: a case series. ASAIO J. 2014;60:744–747. doi: 10.1097/MAT.0000000000000122. [DOI] [PubMed] [Google Scholar]
- 74.Badiye A., Hernandez G.A., Chaparro S. Argatroban as novel therapy for suspected thrombosis in patients with continuous-flow left ventricle assist device and hemolysis. ASAIO J. 2014;60:361–365. doi: 10.1097/MAT.0000000000000067. [DOI] [PubMed] [Google Scholar]
- 75.Terrovitis J.V., Ntalianis A., Kapelios C.J., Vakrou S., Diakos N., Katsaros L., Tsamatsoulis M., Kaldara E., Charitos C., Nanas J.N. Dabigatran etexilate as second-line therapy in patients with a left ventricular assist device. Hell. J. Cardiol. 2015;56:20–25. [PubMed] [Google Scholar]
- 76.Andreas M., Moayedifar R., Wieselthaler G., Wolzt M., Riebandt J., Haberl T., Angleitner P., Schlöglhofer T., Wiedemann D., Schima H., Laufer G., Zimpfer D. Increased thromboembolic events with dabigatran compared with vitamin K antagonism in left ventricular assist device patients: a randomized controlled pilot trial. Circ. Heart Fail. 2017;10(5) doi: 10.1161/CIRCHEARTFAILURE.116.003709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gibson C.M., Mehran R., Bode C., Halperin J., Verheugt F.W., Wildgoose P., Birmingham M., Ianus J., Burton P., van Eickels M., Korjian S., Daaboul Y., Lip G.Y., Cohen M., Husted S., Peterson E.D., Fox K.A. Prevention of bleeding in patients with atrial fibrillation undergoing PCI. N. Engl. J. Med. 2016;375:2423–2434. doi: 10.1056/NEJMoa1611594. [DOI] [PubMed] [Google Scholar]
- 78.Cannon C.P., Bhatt D.L., Oldgren J., Lip G.Y.H., Ellis S.G., Kimura T., Maeng M., Merkely B., Zeymer U., Gropper S., Nordaby M., Kleine E., Harper R., Manassie J., Januzzi J.L., Ten Berg J.M., Steg P.G., Hohnloser S.H., Investigators R-DPSCa Dual antithrombotic therapy with dabigatran after PCI in atrial fibrillation. N. Engl. J. Med. 2017;377:1513–1524. doi: 10.1056/NEJMoa1708454. [DOI] [PubMed] [Google Scholar]