Abstract
In recent years, investigations of microbial flora associated with fish gut have deepened our knowledge of the complex interactions occurring between microbes and host fish. The gut microbiome not only reinforces the digestive and immune systems in fish but is itself shaped by several host-associated factors. Unfortunately, in the past, majority of studies have focused upon the structure of fish gut microbiome providing little knowledge of effects of these factors distinctively and the immense functional potential of the gut microbiome. In this review, we have highlighted the recently gained insights into the diversity and functions of the fish gut microbiome. We have also delved on the current approaches that are being employed to study the fish gut microbiome with an aim to collate all the knowledge gained and make accurate conclusions for their application based perspectives. The literature reviewed indicated that the future research should shift towards functional microbiomics to improve the maximum sustainable yield in aquaculture.
Keywords: Fish, Microbiome, Gut, Metagenomics, High-throughput sequencing
Introduction
Due to their vast ecological adaptations, microorganisms present enormous diversity, thereby fascinating the microbiologists to explore their residence in and on the animal bodies. After first insights into diversity of microbial communities were given by the “Human Microbiome Project”, it followed that most of the bacterial symbionts and commensals populate the gastro-intestinal (GI) tracts and their interactions among themselves and with the host finds great significance to human biology [1]. Since then, intensive research has contributed to an enhanced understanding of gut microbiome for its potential complexity and functional contributions only to develop as an attractive area of research among other vertebrates. Representing more than half of the vertebrates with vast ecological diversities and distinctive structural features within their intestinal tracts [2, 3], fish has justifiably emerged to be a significant class for the examination of the confederation of microorganisms with their hosts. In the past, the traditional culture-dependent methods [4–7] and the use of Denaturing Gradient Gel Electrophoresis (DGGE) and Temporal Temperature Gradient Gel Electrophoresis (TTGE) techniques [8, 9] revealed very low fraction of these significant microorganisms. While these approaches only aimed to reveal the “variety” constituted in the microbiome, some early studies also correlated the taxonomic abundance of fish gut flora with specific host functions [10, 11]. Over the years, the culture based assessments have led to identification of several probiotic and pathogenic bacterial strains and still continue being employed in studies of fish gut microbiome [12–14]. In recent years, however, the culture-independent methods have replaced these traditional techniques as the direct mining of community DNA hold promise to unveil the low abundance and rare taxa [15–19]. While the next generation sequencing (NGS) platforms have vested ease in deciphering the whole community structure, they also compel researchers to devise ways to mine the complete functional potential of fish gut microbiome. Further, to test the efficiency and sustainability of the beneficial flora is an emerging need. In 2016, the Food and Agriculture Organisation (FAO) of the United Nations reported that the aquaculture yield worldwide has been increasing. However, at the same time, about 31.4% of the commercial wild fish stocks were reported to be fished at biologically unsustainable levels in 2013. As the demand of fish for human consumption continues to increase, the aquaculture research must focus on the application of latest technologies to improve the maximum sustainable yield (MSY). The gut microbiome is known to play crucial roles in development of fish immune system and aid in optimal nutrient absorption [20]. Despite this, the use of NGS platforms in studies aimed at exploiting the beneficial flora to improve overall fish health lags far behind. Too often, these studies only focus on their species specificity and effect on fish health while neglecting the change in the overall gut microbiome structure. The microbiome is influenced by a myriad of factors and it is difficult to ascertain the individual effects of each of these factors. Tarnecki et al, recently summarized the different factors affecting fish gut microbiome while also highlighting the potential sources of bias in the results from sample processing [21]. Apparently, the choice of the study design also greatly impacts the results. It is therefore important to contemplate at the study design prior to noting the important inferences. In this review, we aim to summarize the different study designs currently being employed in order to delineate the influence of the different selective pressures affecting fish gut microbiome. The important findings from recent metagenomics studies are noted. The review also discusses the future perspectives of fish gut microbiome research and sheds light on the need to focus on applied microbiomics.
Composition of Fish Gut Microbiome
The colonization of fish gut starts early in the larval stage and is continuously driven towards achievement of a complex assemblage of gut associated microbes [22]. Approximately 108 bacterial cells belonging to over 500 different species are reported to populate the fish GI tract, which are dominated by aerobes or facultative anaerobes although strict aaerobes have also been detected [7, 23]. While considering a metagenomic sample, the diversity is defined in terms of number of OTUs. OTUs are number of clustered similar sequences (> 97%) that define a taxonomic unit on the basis of divergence. OTU analyses are done to reveal the alpha diversity (within-sample diversity) and beta diversity (diversity among different samples) while deciphering the composition of gut microbiome. Most studies corroborate to the domination of bacterial sequences in NGS sequencing data from fish gut sources with negligible representatives of archaeal and eukaryal origins [17, 19, 24].
To date, the analyses of sequencing data have revealed a peculiarly low phylogenetic diversity with Proteobacteria, Firmicutes and Bacteroidetes representing up to 90% of the fish intestinal microbiota across different species and Fusobacteria, Actinobacteria, and Verrucomicrobia among the represented phyla [20, 24–31]. This is not very surprising with our knowledge of the challenges imposed by the gut environment onto the microorganisms to whittle down the diversity in the niche. The diversity generally increases as the diet of the fish changes from carnivorous to omnivorous to herbivorous [19]. The composition also differs due to different environmental conditions. Acinetobacter, Aeromonas, Flavobacterium, Lactococcus, and Pseudomonas, obligate anaerobes Bacteroides, Clostridium, and Fusobacterium, and members of family Enterobacteriaceae dominate the gut of freshwater species [32]. The guts of marine fish are dominated by Aeromonas, Alcaligenes, Alteromonas, Carnobacterium, Flavobacterium, Micrococcus, Moraxella, Pseudomonas and Vibrio [32]. A summary of the major bacterial phyla composing the gut microbiome as revealed by studies in different species can be referred in review by Llewellyn et al, [33] and much recent studies in Table 1. Abundance of similar bacterial phyla irrespective of the taxonomic position or geographical location of the fish indicates a role of microbiome in important host functions such as nutrient absorption, digestion and generation of immune response. Further, the structural similarity of fish gut microbiome with that of the mammals indicates towards the vertebrate core gut microbiotas.
Table 1.
List of some recent studies concerning fish gut microbiome. In all cases, the targeted region used for amplicon sequencing is the hypervariable region(s) of bacterial 16S rRNA gene sequence which is denoted by its standard code(s). AU and AL, here, stand for autochthonous and allochthonous microbiomes respectively
| Host | NGS platform | Amplicon/shotgun Sequencing | Microbiome | Study design | Problem addressed | Dominant phyla | Important inferences | References |
|---|---|---|---|---|---|---|---|---|
| Silurus meridionalis | Illumina Hiseq | Amplicon: V4–V5 | AU and AL | Gut microbiome of lab-reared fish over developmental stages | Effect of host age and—associated factors on gut microbiome | Tenericutes, Fusobacteria, Proteobacteria, and Bacteroidetes | Microbial diversity increases with host age; is significantly Abundance of Tenericutes decrease while Fusobacteria, Proteobacteria and increase with host age |
[107] |
| Silurus meridionalis | Illumina HiSeq | Amplicon: V4–V5 | AL | Gut microbiome of stomach and intestine at different time intervals after feeding | Spatial and temporal microbial dynamics within gut | Fusobacteria, Firmicutes, Proteobacteria, and Bacteroidetes | Distinguishable communities between stomach and intestine; higher diversity in stomach. Firmicutes increase and Fusobacteria decrease after feeding |
[108] |
| Danio rerio | Illumina Miseq | Amplicon: V4 | Not defined | Microbiome of formulated diet fed group compared with that of control | Effect of gluten formulated diet (GFD) on gut microbiome | Proteobacteria, Actinobacteria, Firmicutes and Bacteroidetes dominated control samples. Planctomycetes, Fusobacteria, and Verrucomicrobia dominated GFD samples | Legionellales, Rhizobiaceae, and Rhodobacter abundant in GFD fed group. Cobalamin synthesizing Bacteroides and Lactobacillus present in control group and absent in experimental group | [109] |
| Siganus fuscescens | Illumina Miseq | Amplicon: V4 | AU and AL | Microbiome of different regions in GI tract | Differences at the level of individuals, gut locations and sample types | Proteobacteria, Cyanobacteria and Firmicutes | Midgut communities highly diverse for both adherent and non-adherent microbiome; Greater diversity of adherent microbiome accounting for its active selection by host | [110] |
| Gambusia affinis | Illumina HiSeq | Amplicon: V4 | AU and AL | Effect of broad range antibiotic rifampicin on gut microbiome | Microbiome composition and its response towards antibiotic stress | Proteobacteria, Planctomycetaceae; Myroides genus of Flavobacteria dominated during treatment | Antibiotic treatment lowers the diversity and unstably alters the composition to become antibiotic resistant during treatment while the microbiome takes long to recover | [111] |
| Oncorhynchus mykiss | Illumina Miseq | Amplicon: V6–V8 | AU and AL | Juvenile fish fed with plant- and animal-derived dietary proteins | Role of dietary nutrients source in microbiome composition | Proteobacteria, Firmicutes, Bacteroidetes, Fusobacteria and Actinobacteria | Gut microbial diversity decreases as the source of nutrient derivation in diet is restricted | [72] |
| Carassius auratus | Illumina Miseq | Amplicon: V4–V5 | AU and AL | ‘Red-operculum’ disease affected individuals compared with healthy individuals | Differences in healthy and diseased gut microbiome | Fusobacteria, Proteobacteria and Bacteriodetes | Distinct differences in microbiome composition in two groups; potential of some species as disease-specific bacterial signatures | [112] |
| Ctenopharyngodon idellus, Megalobrama amblycephala, Carassius auratus, Hypophthalmichthys molitrix and H. nobilis, | Pyrosequencing | Amplicon: V4 | AL | Species with three different feeding habits raised under identical husbandry conditions | Relationship among host evolutionary distance, gut microbiota and metabolic profiles | Fusobacteria, Proteobacteria, Bacteroidetes and Firmicutes | Gut microbiome composition as well as metabolite profiles are significantly altered by host species and feeding behaviour | [71] |
| Danio rerio | Illumina Hiseq | Amplicon: V4 | Not defined | Adaptive immune compromised individuals compared with wild-type individuals | Ecological filtering of microbiome composition by adaptive immunity of the host | Proteobacteria, Fusobacteria and Actinobacteria | Functional adaptive immunity affects the neutral assembly processes; Adaptive immunity specifies the microbiome composition in each host which otherwise seems to be much similar; Co-housing individuals of different genotypes increases the microbiome diversity | [113] |
| Salmo salar L. | Illumina Miseq | Amplicon: V3 and V4 | AL | Lab reared and commercial freshwater fish farm | Effect of habitat on microbiome composition | Firmicutes, Proteobacteria, Tenericutes | Presence of core gut microbial flora regardless of the habitat type indicating operational host selective forces | [61] |
| Salmo salar | Ion Torrent | Amplicon: V1 and V2 | AU and AL | Reference and experimental groups fed with alternative protein sources | Effect of alternative diets on microbiome structure and function | Firmicutes, Proteobacteria, Fusobacteria, Bacteroidetes, Actinobacteria | Significantly different adherent and non-adherent communities; Non-adherent microbiome much diverse and diet-dependent than adherent microbiome | [114] |
| Oreochromis niloticus | Illumina MiSeq | Amplicon: V4–V5 | AL | Cadmium(Cd)-exposed, Cd-exposed and probiotic fed, only probiotic fed and control groups | Toxic effects of pollutants on microbiome and application of probiotics for treatment | Fusobacteria, Proteobacteria, Bacteroidetes and Firmicutes | Probiotic supplementation improved the gut health status in Cd-exposed fish to prevent death | [115] |
| Carassius auratus gibelio | Illumina Miseq | Amplicon: V4 | AL | Development of microbiota in a bacteria-free fish gut ecosystem over a year | Factors governing colonization of germ- free gut | Proteobacteria, Fusobacteria and Firmicutes | Gut microbial diversity increases as the fish develop and is less affected by surrounding environment than by host diet and development | [62] |
| Oncorhynchus mykiss | Illumina MiSeq | Amplicon: V4 | AL | Fish from different sources i.e. reared in farm and in aquarium | Complexity of microbiome composition in differently sourced individuals of a species | Tenericutes, Firmicutes, Proteobacteria, Spirochaetae Bacteroidetes | Similar gut microbiota regardless of source; shaped by host factors; Differences in composition highlight the habitat specific taxa | [116] |
| Antarctic Fish spp.: Trematomus bernacchii, Chionodraco hamatus, Gymnodraco acuticeps and Pagothenia borchgrevinki |
Illumina Miseq | Amplicon: V4–V5 | AL | Analysis of gut microbiota | Complexity of microbiome composition in different species | Proteobacteria, Actinobacteria, Firmicutes,Thermi,Bacteriodetes and Tenericutes | Gut microbial communities in different species are not exactly same but also not different altogether | [117] |
| Ictalurus punctatus | Illumina MiSeq | Amplicon: V4 | AU and AL | Microbiome at different life stages with different diet formulations to study gut microbiota across developmental ontogeny | Influence of age as well as the dynamic dietary and environmental factors on gut microbiome | Bacteroidetes, Firmicutes, Fusobacteria, and Proteobacteria | Age has a significant influence on the intestinal microbiota; Water microbiota strongly influence gut microbiota at early life stages | [118] |
| Oreochromis niloticus | Illumina HiSeq | Amplicon: V4 | AU and AL | Gut microbiome of cultured fish in axenic, probiotic-supplemented and later active life phases | Effect of short-term probiotic administration on gut microbiome | Firmicutes, Actinobacteria and Proteobacteria (control groups) | Probiotic administration for a short period significantly affects the gut microbiota composition at later stages of life | [119] |
| Dicentrarchus labrax | Pyrosequencing | Amplicon: V3–V4 | AU | Gut microbiome of two different nutritionally stressed groups and effect on host’s growth or resistance to hypoxia | Correlation between gut microbiota composition, dietary stress and host’s health | Proteobacteria, Bacteroidetes, Actinobacteria and Firmicutes | Gut microbiome is dependent upon the host life history or genetic background; Different nutritional stresses affect host microbiome and health differently | [120] |
|
Ctenopharyngodon idellus,
Siniperca chuatsi, Silurus meridionalis, Carnis megalobramae, Carassius auratus Cyprinus carpio and Canna micropeltes |
Illumina Miseq | Amplicon: V4 | AL | Assembly of gut microbiota in larval and adult fish | Effect of ecological processes on gut microbiota assemby | Proteobacteria, Firmicutes and Bacteroidetes | Gut environment and other host development processes shape the microbiome | [44] |
| Danio rerio | Illumnina Hiseq | Amplicon: V4 | AU and AL | Survey of intestinal bacteria at key developmental time points | Microbiome composition across developmental stages | Proteobacteria, Firmicutes, Fusobacteria, Actinobacteria and Bacteroidetes | Environmental factors more strongly influence microbiome at early life stages | [121] |
| Salmo salar | Illumina HiSeq | Amplicon: V6 | AU and AL | Influence of alternative diet on microbiome, recirculating waters and biofilters | Effect of alternative feeds on microbiome in fish cultured in recirculating water systems | Proteobacteria and others | Gut microbiome differs by diet treatment but communities in biofilters remain stable independent of diet; Gut communities less diverse than those of water and biofilters | [122] |
|
Megalobrama amblycephala, Ctenopharyngodon idellus, Siniperca chuatsi, Culter alburnus, Cyprinus carpio, Carassius auratus, Hypophthalmichthys molitrix, H. nobilis |
Illumina MiSeq | Amplicon: V4 | AL | Influence of trophic level on the gut microbiome | Differences in gut microbiota in herbivorous, carnivorous, omnivorous and filter feeding species | Proteobacteria, Firmicutes, Fusobacteria, Acidobacteria, Bacteroidetes, Actinobacteria, Verrucomicrobia and Cyanobacteria | Trophic level strongly influence the microbiome composition of fish from same habitats; Evidence of a large core gut microbiota in multiple species | [19] |
| Salmo salar L. | Pyrosequencing | Amplicon: V3 to V6 | AL | Effect of diet on the gut microbiome | Diet as a factor controlling microbiome composition | Bacteroidetes, Firmicutes and Proteobacteria | Diet, time of sampling and host specific factors influence the microbes | [123] |
| Oreochromis niloticus | Pyrosequencing | Amplicon: V1–V2 | AU and AL | Gut microbiome development in larvae | Impact of rearing environment on microbiota assembly in early life stages | Proteobacteria and Actinobacteria | Water microbial communities strongly shape those in the gut; Correlation between water and gut microbial community dynamics | [63] |
| Salmo salar | Illumina MiSeq | Amplicon: V4 | AL | Gut microbiomes of freshwater and marine specimens | Effect of geographical distance on microbiome | Proteobacteria, Firmicutes, Bacteriodetes and Actinobacteria | Geographical distance has less impact on gut microbiome; Diversity and identity of microbial communities is more strongly determined by life-cycle stage | [124] |
| Pimephales promelas | Illumina MiSeq | Amplicon: V3–V4 | AU and AL | Gut microbiome of triclosan exposed fish | Effect of antimicrobial compounds on gut microbiome | Proteobacteria, Bacteroidetes and Fusobacteria | Microbiome is significantly altered even at low level of environmental changes but has strong resilience power | [125] |
Functional Potential of Fish Gut Microbiome
Till date, majority of studies on fish gut microbiome are restricted to diversity analysis and most of our knowledge comes from its correlation with the necessary host functions (Table 1). In this regard, computational tools have been developed that may provide important predictions of the functional capabilities of the community, once the taxonomic composition is deciphered using marker gene based approach e.g. PICRUSt [34] and Tax4Fun [35]. The most widely studied functional attributes of microbiota include digestion and immunity. In grass carp, the ability to digest plant matter is long been associated with the higher abundance of cellulolytic bacteria in the gut of herbivorous species [20, 36, 37]. Li et al, showed that gut bacterial community of grass carp is dominated by cellulolytic Aeromonas, followed by Enterobacter, Enterococcus, Citrobacter, Bacillus, Raoultella, Klebsiella, Hydrotalea, Pseudomonas, Brevibacillus and that an increase in intake of plant-fibre increases the diversity of cellulolytic bacteria [36]. Cellulose degrading bacteria Clostridium, Aeromonas, Cellulomonas and Bacteroides along with other nitrogen fixing species are reported to provide assimilable carbon in the wood eating fish Panaque nigrolineatus [38, 39]. Clostridia also dominate the gut microbial flora in different marine herbivorous fish species [40]. In contrast to the cellulolytic function of microbiome in herbivorous species, lipase and protease producing bacteria and trypsin activity are observed to be much higher in carnivorous species [19], which further confirms the role of microbiota in host digestion. In Atlantic salmon fed a plant-based diet, Lactic acid producing bacteria (LAB) are shown to be present in higher abundance as compared to those fed with fishmeal-based diets suggesting their potential role in digestion [41]. Besides digestion, changes in microbiome composition resulting from environmental stress results in challenged immunity in the host. The gut microbiota produce important short-chain fatty acids (SCFAs) while breakdown of complex sugars which are absorbed in the intestine by simple diffusion or specific receptors and confer resistance against pathogenic invaders [42, 43]. Therefore, the functional repertoire of gut microbiota appears to be synergic with the host needs.
Fate of Colonizing Members and the Factors Affecting Fish Gut Microbiome
The gut colonization may either be driven by (1) stochastic or neutral assembly that derives from random dispersal of microorganisms or chance events that land the microbes into the intestine that are solely responsible for the final shape of intestinal community; or (2) deterministic or non- neutral model assumes that the assembly is determined by the host selective pressures, active dispersal by the host and host-microbe and microbe–microbe interactions. Studies on zebrafish, Danio rerio, herbivorous Ctenopharyngodon idellus, carnivorous Chinese perch, Siniperca chuatsi, and catfish Silurus meridionalis over developmental time suggest that the gut colonization among larvae is governed by seeding from surrounding environments which then transits to be progressively determined by the non-neutral processes as the host matures to become adult [44, 45]. Therefore, suggesting stochastic towards deterministic colonization of GI tract.
Though the host GI tract provides for shelter of microbes, certain adaptations are demonstrated to be exigent for this possession. Ley et al. [46], intricately reviewed the demands imposed on microbial flora by the GI tract viz. in order to firmly attach to the mucosal epithelium of gut wall, microorganisms must possess cell surface molecules for adhesion; the efficient utilization of all the nutrients calls for the production of an enzyme arsenal; the microbes must also be armed with genetic tools for adapting to the ecological plasticity offered by the GI tract and for immunity against bacteriophages. The fittest microorganisms that are able to meet the ecological demands grow and survive in the GI tract and are able to appropriate most niche space to become permanent dwellers, also referred to as “autochthonous”. While others, known as “allochthonous” are visitors of the gut that derive from the surrounding environment [22]. Thus, microbes associate with the host in diverse ways which may be simply fortuitous at first and may become obligatory later. The adherence of the bacteria onto the epithelial cells is considered an essential factor in determining their effector functions within host. A link between the biofilm forming ability of bacteria and successful colonization and functions of gut flora is suggested [47, 48]. The ability to form biofilms helps bacteria to survive in hostile environment offered by the GI tract and is seen in cases of both pathogenic and probiotic bacteria [49, 50]. To unravel the allochthonous communities, fecal samples are generally examined while mucosal epithelium scraped after rinsing are used for analyzing the adherent residents. So far, studies on characterization and comparison of the two community types suggest presumably different composition and that both serve different purposes within the GI tract [51]. High species richness in the digesta (allochthonous) is observed as compared to the mucosa (autochthonous) [52]. This can be understood from the knowledge of the exasperating conditions within the GI tract outlined above. Further, there are evidences that the microbial composition also differs in different regions along the GI tract, the foregut communities being significantly different from hindgut communities [53]. However, the degree by which these two population types differ is not completely understood and needs further clarification.
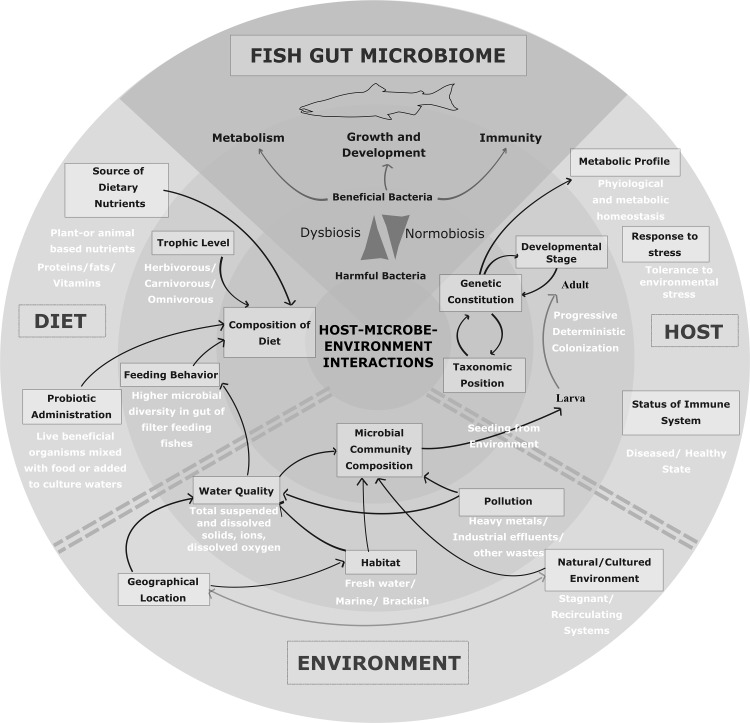
Interplay of a variety of factors determines the constitution of the fish gut microbiome. Quality of surrounding water and microbial communities directly influence the gut microbiome of fish [24, 54]. Strong evidences of host genetics, developmental stage, immune status and other host specific pressures on the gut microbiome also persist [33, 45, 53, 55, 56]. In addition, the diet also shapes the gut microbiome which displays differential composition with difference in dietary intake [38, 57–60]. Hence, the factors influencing gut microbiome may be broadly grouped into three classes, which are (1) ecology and environmental conditions, (2) host specific and (3) trophic level and/or feeding behavior as illustrated in Fig. 1. Each of these factors are discussed in detail in the following section.
Fig. 1.
Factors influencing the diversity and function of the gut microbiome of fish. The factors may be broadly categorized into environmental, diet-associated or host—associated. All these intrinsic and extrinsic factors are responsible for either healthy state (normobiosis) or altered microbiota (dysbiosis) both of which affect the growth and development of the fish host
Study Designs Currently Employed to Study the Factors Affecting Fish Gut Microbiome
While some recent attempts highlight both the environmental and host associated factors to be significantly contributing to the microbial composition in gut [61], others suggest a stronger influence of the host selective pressures [62]. The exact degree with which each of these factors influence the gut microbiome is not known. Clearly, it is difficult to distinguish the host specific and environmental effects on fish gut microbiota. The difference in feeding behavior of different species adds to the problem of investigating the role of each of these factors. Several different approaches are currently employed to study the degree of their influence on fish gut microbiome. Most approaches rely on the fact that if strongly determined by the environment, the gut microbiome must vary among members of a species, both spatially and temporally, in correlation with the surrounding waters and vice versa if strongly shaped by host selective pressures. The different approaches that are currently employed to study the different determining factors are outlined below.
Microbial Composition and Quality of Surrounding Waters
Due to being constantly exposed to alterations in water quality resulting from various anthropogenic and natural causes, the microbial communities in GI tract of fish should change repeatedly. Thus, a comparison of the water quality and host gut microbiome is expected to improve the understanding of environmental influence. Shared Operational Taxonomic Units (OTUs) between water and gut microflora have suggested the possible role of surrounding water in steering of gut microbiota [63]. Salinity of water also largely determines the microbial composition in fish gut as revealed from analysis of a large number of species with different ecological needs for survival [24]. Studies on wild and lab-reared invasive carp species also establish environment as a key factor in shaping the gut microbiome [64]. On the contrary, studies also suggest otherwise that the abundant microbial taxa in surrounding waters are not found in the gut of habitant fish and vice versa suggesting a much stronger influence of host associated factors than environment [65].
Species that are known to survive in different stressed habitats are considered apt to assess if a change in quality of surrounding waters other than microbial composition also affect the host gut microbiota. For instance, the endogenous microbiota in Amazonian tambaqui, Colossoma macropomum that is tolerant to significant variation in pH levels has been shown to be significantly altered at experimental low pH levels; however, it also displays a strong resilience once pH levels are restored [66].
Variations in Different Cohabiting Species
The second approach employs the use of different species inhabiting same water systems. Many studies have revealed a larger influence of host selective pressures and trophic level in constraining the gut microbiota than the environment as different cohabiting species are observed to comprise different microflora in gut. For instance, cohabiting species of silver carp, grass carp, bighead carp, and blunt snout bream revealed distinct gut microbiome composition [67] providing for the influence of host specific factors on the gut micro-organismal communities. Similar results were obtained in paddle fish, Polyodon spathala and bighead carp, Aristichthys nobilis with similar feeding behavior when fed same food and reared in same pond [68]. Thus host associated factors outplay the influence of environment.
Variations Within a Species at Different Geographical Locations
Third approach employs studying the microbiome of a single species found in different geographical locations to study if it is defined by a core set of microbial communities that stably reside in the GI tract due to host-specific selective pressures or if the environment shapes the communities. In this regard, zebrafish, Danio rario, has been extensively studied to establish the strong influence of host associated factors that shape the core gut microbiome of the species [25]. Although differences occur, however, the peculiarly low diversity presented by the gut microbiome of different fish species of ecologically and geographically different origins with respect to the bacterial phyla further warrants the argument of host selective pressures on the gut microbiome [19, 23, 26, 27, 69, 70].
Variations at Different Trophic Levels and Feeding Behavior
The microbial diversity in GI tract increases as the diet changes from carnivorous to omnivorous to herbivorous [53]. Cellulose-decomposing bacteria such as Anoxybacillus, Leuconostoc, Clostridium, Actinomyces, and Citrobacter populate the gut of herbivorous species such as grass carp, Ctenopharyngodon idellus [20]. On the other hand, carnivorous species are found to harbor lipase and protease-producing bacteria such as Halomonas [19]. Feeding behavior is also shown to affect the gut microbiome composition in a habitat in closely related but different species. Filter feeding fishes display higher diversity as they filter large volumes of water and cover large areas by swimming rapidly [71].
Administration of Different Dietary Components
The effect of dietary intake on gut flora is not only restricted to the nutritional composition but also the source of nutrients. In general, plant-derived dietary proteins have been linked to significantly reduced diversity of microbial flora [28] with an increase in relative abundance of Lactobacillales, Bacillales and Pseudomonadales [72]. While animal-derived proteins nurture Bacteroidales, Clostridiales, Vibrionales, Fusobacteriales and Alteromonadales in the gut [72]. A study by Mansfield et al., revealed that fish fed with synthetic casein based diet have larger diversity as compared to those fed with fish meal or soyabean meal based diet [73]. Effects of a large number of other nutritional components have been reviewed in detail [74].
Patterns of Gut Colonization in Gnotobiotic Models
The use of gnotobiotic fish models incomparably fit to serve the purpose of delineating the processes that fabricate the gut microbiome structure. Analysis of patterns of colonization in germ-free zebrafish GI tract revealed that the host responses may be attributed to specific bacterial members of the community [23]. Transplantation experiments of gut microbial flora between gnotobiotic zebrafish and mouse and between gnotobiotic mouse and zebrafish hint towards reconstruction of the microbiome after transplantation to resemble that of conventionally raised fish thereby concealing the microbial communities which were transplanted in their gut [55].
Thus, it can be inferred that the microbial composition of water influences the fish gut microbiome to some extent. In addition, diet and genetic variations among individual hosts also shape the gut microbiome. To elucidate the exact degree of influence by each factor would be an interesting area of study. Even though the microbiome composition of two individuals might differ at the species level, there occur significant level of similarity among the microbial genes that are shared which suggest for a molecular link between the microbial genes and host functions [75]. Thus, there is an emerging need for multifaceted analyses to clearly demarcate the host-, environment- and feeding behavior derived manifestations of the gut microbiome. It may be viewed as environment, host specific factors and diet, act in concert to constrain the acquisition of gut microbiota.
Profiling the Fish Gut Microbiome
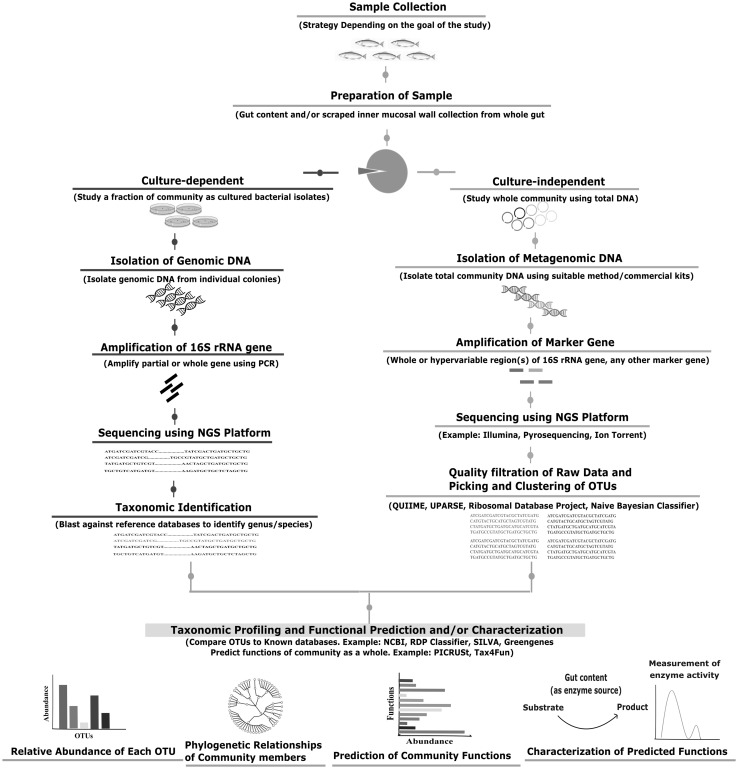
An illustration of the steps followed to analyze the gut microbiome of a fish species by direct analysis of the community DNA is shown in Fig. 2. The choice of amplicon or shotgun sequencing depends on the goal of the study. To date, the studies of fish gut microbiome have remained limited to deciphering the composition. However, shotgun assessments of the gut microbial genetic repertoire are needed to provide crucial insights into their functional potential and can be further aided by the genomic sequencing and analysis of the cultured isolates as has been investigated in other niches [76–85].
Fig. 2.
Schematic representation of the workflow for analyzing the fish gut microbiome. The currently employed procedures for fish gut microbiome studies include the traditional culture-dependent analysis as well as culture-independent analysis of the total DNA obtained directly from gut contents and mucosal wall. The culture-dependent techniques widely use sequencing of 16S rRNA gene to identify bacteria. For defining the uncultured microbiota, amplification and sequencing of whole or partial [hypervariable region(s)] of 16S rRNA gene is widely employed. Highly similar sequences are then grouped into Operational Taxonomic Units (OTUs) and compared against databases. The widely used databases and tools include NCBI [126], QIIME [127], UPARSE [128], Silva [129], Green Genes [130], RDP Classifier [131] and Naïve Bayesian Classifier [132]. The community is thus profiled based on the relative abundance of each OTU and their phylogenetic relationships. Using advanced ‘omic’ tools, functions of the community can also be predicted such as PICRUSt [34] or Tax4Fun [35]. Important microbial functions may then be characterized through wet lab experiments
The gut produces and receives an arsenal of enzyme secretions while performing functions such as grinding the food mechanically and chemically and extracting and absorbing nutrients, which creates a major challenge in extraction of quality community DNA from the microbial populations. For instance, bile salts and complex polysaccharides present in gut inhibit the downstream processes of PCR amplification [86]. Therefore, a DNA extraction method should be employed after envisaging (1) the correct representation of genomes from entire community with no over or under-representation of individual microbial populations, (2) its efficiency on the sample source (foregut, midgut or hindgut) and type (luminal contents only or gut wall with contents), (3) the design of study which involves the type of sequencing i.e., whole genome or targeted sequencing and (4) the quality data required for downstream analysis. The choice of DNA extraction method may strongly influence the analysis of the community composition. There are convincing evidences that a method for extracting DNA does not conform to changes in host species or sample type due to variability in the ingested food and microbial load in the host GI tract [87–90]. As the ingested food may vary among individual hosts and may comprise of semi-digested particulate matter and bones, the availability of the starting mass of fecal or gut content samples for DNA extraction needs to be taken into consideration while comparing the extraction protocols to avoid false interpretations. Perceivable differences are reported to occur in the purity and concentrations of the extracted DNA employing different methods [88]. Further, the purity of extracted DNA does not guarantee a successful amplification and sequencing [88]. Sample preparations pose as a source of variability in testing the extraction efficiency of a method. Processing the entire GI tract along with the luminal content may result in isolation of a large amount of eukaryotic DNA from fish, therefore, diluting the bacterial DNA for sequencing and analysis. The structural composition of microbiota also affects precise extraction of DNA as the gram-positive bacteria are more difficult to lyse than gram-negative bacteria due to presence of a thick peptidoglycan layer in their cell walls. This spurs a necessity to ensure complete lysis of all bacterial cells in the community by the chosen extraction method.
It is critical to use an appropriate method of extraction of microbial community DNA which lays the foundation for accurate characterization of the gut microbiota both structurally and functionally. Essentially, the sample source and type need to be emphasized upon before relying upon the results from DNA extraction methods comparison studies as not all the results are comparable owing to the sample variability.
Host-Microbe Interactions in Fish Gut
The gut-associated microbes might have potential beneficial or harmful effects on the host. When the beneficial micro-organisms constitute majority of the microbiome, a state of “normobiosis” prevails. Any perturbations in its normal composition result in “dysbiosis” in which state the harmful microbes predominate giving rise to a diseased condition. While on one hand host intrinsic factors and environment shape the gut microbiome, the microbiota has equal influence on the host biology. Thus, a three way interaction involving host- microbe-environment operates to maintain homeostasis in the fish gut.
Colonization of the gut with specific microorganisms to gain insights into the effects of microbiota on host cellular responses in mice revealed that they modulate the expression of several cellular genes that endows host with important functions including metabolism, nutrient absorption, immune response generation, and intestinal maturation [1]. Similar findings have been obtained using zebrafish models [23, 91, 92] and in other fish species where microorganisms demonstrated to regulate metabolism [29]. Studies on transgenic fast-growing common carp, Cyprinus carpio L. suggest an important role of gut flora on growth of fish and that the Firmicutes confer fast growth of fish over Bacteroidetes [29]. The microbial members are known to aid in digestion of cellulose in herbivorous fishes [20, 22, 60]. They are also known to influence innate immune responses in fish [32] and educate the host-immune system for better protection against pathogenic invaders. These findings provide strong perspectives of the interactions between resident microorganisms and their host fish.
Conclusion and Future Perspectives: Towards Applied Research
The gut microbiota is influenced by a myriad of factors but the appeal of each of these factors on the behavior and physiology of fish remain poorly understood. For drawing meaningful conclusions, one of the crucial challenges is to establish a correlation of microbiome structure and function with health status, age, genetic background, geographical location and other individual differences of the host. Furthermore, creating a comparative picture based on studies employing different methods of DNA extraction from different sample types within a species needs careful analyses. As the marker gene based approach limits the analysis to predictions of microbiome structure, there has emerged a need for complete shotgun sequencing efforts to fully explore the metabolic potential of the gut microflora and uncover functional variation with diet or host associated factors.
Fish are affected by several pathogenic bacteria including Aeromonas, Edwardsiella, Pseudomonas, Flavobacterium, Vibrio and Streptococcus and Yersinia causing diseases in different tropical freshwater fishes [93, 94]. These are often treated with antibiotics. However, their overuse has encouraged antibiotic resistance which makes the fight against pathogens even more difficult [93]. Pathogenicity of these bacteria such as Aeromonas hydrophila, A. salmonicida, Vibrio anguillarum, V. harveyi, Yersinia ruckeri and Tenacibaculum maritimum is mediated by quorum sensing systems that are important for biofilm formation [95]. The use of quorum quenching enzymes has also been successful in reducing pathogenicity of these pathogens [96–98]. Bacteria derived from fish microbiome have also been shown to produce natural products that inhibit formation of biofilms and therefore promote detachment of gram-negative pathogens [50]. However, more efforts focusing on their application in fish GI tracts are needed. From the recent studies on isolation of Bdellovibrios that naturally feed on gram-negative bacteria from experimental diseased fish models, we envisage the use of these microbial predators as natural cure of diseases [99–101]. Bdellovibrios are also found to be prevalent in intestines of human and other animals and have been reported from diverse habitats [76, 102–104]. The emerging concept of “forward microbiomics” that involves manipulating the gut flora to promote fish health finds important applications in aquaculture. Several Lactobacillus species have been identified as probiotics in fish and other animals including humans [105, 106]. However, their potential needs to be fully explored. Collating inferences from different studies would help in identifying microbial biomarkers and would augment the application of probiotics. It is becoming increasingly clear that the microbiome affects its host in more than one ways and its study is thought to bring a plenitude of understanding of its functional potential in the host and expand current notions of the fish gut microbiome.
Acknowledgements
The authors acknowledge funds from ICAR-National Bureau of Agriculturally Important Microorganisms NBAIM. CT and SN gratefully acknowledge Council of Scientific & Industrial Research (CSIR) and Department of Biotechnology (DBT), Govt. of India respectively, for providing doctoral fellowships.
Author Contributions
RL and RKN conceived the idea. CT wrote the manuscript and SN helped shape the manuscript. RL and RKN critically reviewed the manuscript.
Compliance with Ethical Standards
Conflict of interest
The authors declare no conflict of interest.
Contributor Information
Rup Lal, Phone: (91) 11-27666254, Email: ruplal@gmail.com.
Ram Krishan Negi, Phone: (91) 11-27666254, Email: negi_gkv@rediffmail.com.
References
- 1.Hooper LV, Gordon JI. Commensal host-bacterial relationships in the gut. Science. 2001;292:1115–1118. doi: 10.1126/science.1058709. [DOI] [PubMed] [Google Scholar]
- 2.Nelson JS. Fishes of the world. 4. Hoboken: Wiley; 2006. [Google Scholar]
- 3.Suyehiro Y. A study on the digestive system and feeding habits of fish. Jap J Zool. 1942;10:1–303. [Google Scholar]
- 4.Al-Harbi AH, Uddin MN. Seasonal variation in the intestinal bacterial flora of hybrid tilapia (Oreochromis niloticus x Oreochromis aureus) cultured in earthern ponds in Saudi Arabia. Aquaculture. 2004;229:37–44. doi: 10.1016/S0044-8486(03)00388-0. [DOI] [Google Scholar]
- 5.Ringo E, Olsen RE, Mayhew TM, Myklebust R. Electron microscopy of the intestinal microflora of fish. Aquaculture. 2003;227:395–415. doi: 10.1016/j.aquaculture.2003.05.001. [DOI] [Google Scholar]
- 6.Amann RI, Ludwig W, Schleifer KH. Phylogenetic identification and in situ detection of individual microbial cells without cultivation. Microbiol Rev. 1995;59:143–169. doi: 10.1128/mr.59.1.143-169.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Romero J, Navarrete P. 16S rDNA-based analysis of dominant bacterial populations associated with early life stages of coho salmon (Oncorhynchus kisutch) Microb Ecol. 2006;51:422–430. doi: 10.1007/s00248-006-9037-9. [DOI] [PubMed] [Google Scholar]
- 8.Reveco FE, Øverland M, Romarheim OH, Mydland LT. Intestinal bacterial community structure differs between healthy and inflamed intestines in Atlantic salmon (Salmo salar L.) Aquaculture. 2014;420–421:262–269. doi: 10.1016/j.aquaculture.2013.11.007. [DOI] [Google Scholar]
- 9.Navarrete P, Espejo RT, Romero J. Molecular analysis of microbiota along the digestive tract of juvenile Atlantic salmon (Salmo salar L.) Microb Ecol. 2009;57:550–561. doi: 10.1007/s00248-008-9448-x. [DOI] [PubMed] [Google Scholar]
- 10.Stickney RR, Shumway SE. Occurrence of cellulase activity in the stomachs of fishes. J Fish Biol. 1974;6:779–790. doi: 10.1111/j.1095-8649.1974.tb05120.x. [DOI] [Google Scholar]
- 11.Sugita H, Miyajima C, Deguchi Y. The vitamin B12-producing ability of the intestinal microflora of freshwater fish. Aquaculture. 1991;92:267–276. doi: 10.1016/0044-8486(91)90028-6. [DOI] [Google Scholar]
- 12.Nayak SK. Probiotics and immunity: a fish perspective. Fish Shellfish Immunol. 2010;29:2–14. doi: 10.1016/j.fsi.2010.02.017. [DOI] [PubMed] [Google Scholar]
- 13.Ayo-Olalusi CI, Oresegun A, Bernard E. Screening of Lactic acid bacteria from the gut of Chrysichthys nigrodigitatus for use as probiotics in aquaculture production. J Fish Aquat Sci. 2014;9:478–482. doi: 10.3923/jfas.2014.478.482. [DOI] [Google Scholar]
- 14.Asaduzzaman M, Iehata S, Akter S, Kader MA, Ghosh SK, Khan MNA, Abol-Munafi AB. Effects of host gut-derived probiotic bacteria on gut morphology, microbiota composition and volatile short chain fatty acids production of Malaysian Mahseer Tor tambroides. Aquac Rep. 2018;9:53–61. doi: 10.1016/j.aqrep.2017.12.003. [DOI] [Google Scholar]
- 15.Star B, Haverkamp THA, Jentoft S, Jakobsen KS. Next generation sequencing shows high variation of the intestinal microbial species composition in Atlantic cod caught at a single location. BMC Microbiol. 2013;13:248. doi: 10.1186/1471-2180-13-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xing MX, Hou ZH, Yuan JB, Liu Y, Qu Y, Liu B. Taxonomic and functional metagenomic profiling of gastrointestinal tract microbiome of the farmed adult turbot (Scophthalmus maximus) FEMS Microbiol Ecol. 2013;86:432–443. doi: 10.1111/1574-6941.12174. [DOI] [PubMed] [Google Scholar]
- 17.Xia JH, Lin G, Fu GH, Wan ZY, Lee M, Wang L, Liu XJ, Yue GH. The intestinal microbiome of fish under starvation. BMC Genom. 2014;15:266. doi: 10.1186/1471-2164-15-266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zarkasi KZ, Abell GC, Taylor RS, Neuman C, Hatje E, Tamplin ML, Katouli M, Bowman JP. Pyrosequencing-based characterization of gastrointestinal bacteria of Atlantic salmon (Salmo salar L.) within a commercial mariculture system. J Appl Microbiol. 2014;117:18–27. doi: 10.1111/jam.12514. [DOI] [PubMed] [Google Scholar]
- 19.Liu H, Guo X, Gooneratne R, Lai R, Zeng C, Zhan F, Wang W. The gut microbiome and degradation enzyme activity of wild freshwater fishes influenced by their trophic levels. Sci Rep. 2016;6:24340. doi: 10.1038/srep24340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu S, Wang G, Angert ER, Wang W, Li W, Zou H. Composition, diversity, and origin of the bacterial community in grass carp intestine. PLoS One. 2012;7:e30440. doi: 10.1371/journal.pone.0030440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tarnecki AM, Burgos FA, Ray CL, Arias CR. Fish intestinal microbiome: diversity and symbiosis unravelled by metagenomics. J Appl Microbiol. 2017;123:2–17. doi: 10.1111/jam.13415. [DOI] [PubMed] [Google Scholar]
- 22.Nayak SK. Role of gastrointestinal microbiota in fish. Aquac Res. 2010;41:1553–1573. doi: 10.1111/j.1365-2109.2010.02546.x. [DOI] [Google Scholar]
- 23.Rawls JF, Samuel BS, Gordon JI. Gnotobiotic zebrafish reveal evolutionarily conserved responses to the gut microbiota. Proc Natl Acad Sci USA. 2004;101:4596–4601. doi: 10.1073/pnas.0400706101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sullam KE, Essinger SD, Lozupone CA, O’Connor MP, Rosen GL, Knight R, Kilham SS, Russell JA. Environmental and ecological factors that shape the gut bacterial communities of fish: a meta-analysis. Mol Ecol. 2012;21:3363–3378. doi: 10.1111/j.1365-294X.2012.05552.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roeselers G, Mittge EK, Stephens WZ, Parichy DM, Cavanaugh CM, Guillemin K, Rawls JF. Evidence for a core gut microbiota in the zebrafish. ISME J. 2011;5:1595–1608. doi: 10.1038/ismej.2011.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ringo E, Sperstad S, Myklebust R, Refstie S, Krogdahl A. Characterisation of the microbiota associated with intestine of Atlantic cod (Gadus morhua L.)—the effect of fish meal, standard soybean meal and a bioprocessed soybean meal. Aquaculture. 2006;261:829–841. doi: 10.1016/j.aquaculture.2006.06.030. [DOI] [Google Scholar]
- 27.Givens CE, Ransom B, Bano N, Hollibaugh JT. A fish tale: comparison of the gut microbiomes of 12 finfish and 3 shark species. Mar Ecol Prog Ser. 2014;518:209–223. doi: 10.3354/meps11034. [DOI] [Google Scholar]
- 28.Desai AR, Links MG, Collins SA, Mansfield GS, Drew MD, Van Kessel AG, Hill JE. Effects of plant-based diets on the distal gut microbiome of rainbow trout (Oncorhynchus mykiss) Aquaculture. 2012;350–353:134–142. doi: 10.1016/j.aquaculture.2012.04.005. [DOI] [Google Scholar]
- 29.Li X, Yan Q, Xie S, Hu W, Yu Y, Hu Z. Gut microbiota contributes to the growth of fast-growing transgenic common carp (Cyprinus carpio L.) PLoS One. 2013;8:e64577. doi: 10.1371/journal.pone.0064577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Carda-Diéguez M, Mira A, Fouz B. Pyrosequencing survey of intestinal microbiota diversity in cultured sea bass (Dicentrarchus labrax) fed functional diets. FEMS Microbiol Ecol. 2013;87:451–459. doi: 10.1111/1574-6941.12236. [DOI] [PubMed] [Google Scholar]
- 31.Ingerslev HC, von Gersdorff Jørgensen L, Lenz Strube M, Larsen N, Dalsgaard I, Boye M, Madsen L. The development of the gut microbiota in rainbow trout (Oncorhynchus mykiss) is affected by first feeding and diet type. Aquaculture. 2014;424–425:24–34. doi: 10.1016/j.aquaculture.2013.12.032. [DOI] [Google Scholar]
- 32.Gómez GD, Balcázar JL. A review on the interactions between gut microbiota and innate immunity of fish. FEMS Immunol Med Microbiol. 2008;52:145–154. doi: 10.1111/j.1574-695X.2007.00343.x. [DOI] [PubMed] [Google Scholar]
- 33.Llewellyn MS, Boutin S, Hoseinifar SH, Derome N. Teleost microbiomes: the state of the art in their characterization, manipulation and importance in aquaculture and fisheries. Front Microbiol. 2014;5:207. doi: 10.3389/fmicb.2014.00207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Langille MGI, Zaneveld J, Caporaso JG, McDonald D, Knights D, Reyes J, Clemente JC, Burkepile DE, Vega Thurber RL, Knight R, Beiko RG, Huttenhower C. Predictive functional profiling of microbial communities using 16S rRNA marker gene sequences. Nat Biotechnol. 2013;31:814–821. doi: 10.1038/nbt.2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Aßhauer KP, Wemheuer B, Daniel R, Meinicke P. Tax4Fun: predicting functional profiles from metagenomic 16S rRNA data. Bioinformatics. 2015;31:2882–2884. doi: 10.1093/bioinformatics/btv287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li H, Zheng Z, Cong-xin X, Bo H, Chao-yuan W, Gang H. Isolation of cellulose-producing microbes from the intestine of grass carp (Ctenopharyngodon idellus) Environ Biol Fishes. 2009;86:131–135. doi: 10.1007/978-90-481-3458-8_19. [DOI] [Google Scholar]
- 37.Li H, Wu S, Wirth S, Hao Y, Wang W, Zou H, Li W, Wang G. Diversity and activity of cellulolytic bacteria, isolated from the gut contents of grass carp (Ctenopharyngodon idellus) (Valenciennes) fed on Sudan grass (Sorghum sudanense) or artificial feedstuffs. Aquac Res. 2016;47:153–164. doi: 10.1111/are.12478. [DOI] [Google Scholar]
- 38.McDonald R, Schreier HJ, Watts JE. Phylogenetic analysis of microbial communities in different regions of the gastrointestinal tract in Panaque nigrolineatus, a wood-eating fish. PLoS One. 2012;7:e48018. doi: 10.1371/journal.pone.0048018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Watts JEM, McDonald R, Daniel R, Schreier HJ. Examination of a culturable microbial population from the gastrointestinal tract of the wood-eating loricariid catfish Panaque nigrolineatus. Diversity. 2013;5:641–656. doi: 10.3390/d5030641. [DOI] [Google Scholar]
- 40.Clements KD, Pasch IBY, Moran D, Turner SJ. Clostridia dominate 16S rRNA gene libraries prepared from the hindgut of temperate marine herbivorous fishes. Mar Biol. 2007;150:1431–1440. doi: 10.1007/s00227-006-0443-9. [DOI] [Google Scholar]
- 41.Catalan N, Villasante A, Wacyk J, Ramírez C, Romero J. Fermented soybean meal increases lactic acid bacteria in gut microbiota of Atlantic Salmon (Salmo salar) Probiot Antimicrob Proteins. 2017 doi: 10.1007/s12602-017-9366-7. [DOI] [PubMed] [Google Scholar]
- 42.Montalban-Arques A, De Schryver P, Bossier P, Gorkiewicz G, Mulero V, Gatlin DM, Galindo-Villegas J. Selective manipulation of the gut microbiota improves immune status in vertebrates. Front Immunol. 2015;6:512. doi: 10.3389/fimmu.2015.00512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Maji A, Misra R, Dhakan DB, Gupta V, Mahato NK, Saxena R, Mittal P, Thukral N, Sharma E, Singh A, Virmani R, Gaur M, Singh H, Hasija Y, Arora G, Agrawal A, Chaudhry A, Khurana JP, Sharma VK, Lal R, Singh Y. Gut microbiome contributes to impairment of immunity in pulmonary tuberculosis patients by alteration of butyrate and propionate producers. Environ Microbiol. 2018;20:402–419. doi: 10.1111/1462-2920.14015. [DOI] [PubMed] [Google Scholar]
- 44.Yan Q, Li J, Yu Y, Wang J, He Z, Van Nostrand JD, Kempher ML, Wu L, Wang Y, Liao L, Li X, Wu S, Ni J, Wang C, Zhou J. Environmental filtering decreases with fish development for the assembly of gut microbiota. Environ Microbiol. 2016;18:4739–4754. doi: 10.1111/1462-2920.13365. [DOI] [PubMed] [Google Scholar]
- 45.Burns AR, Stephens WZ, Stagaman K, Wong S, Rawls JF, Guillemin K, Bohannan BJ. Contribution of neutral processes to the assembly of gut microbial communities in the zebrafish over host development. ISME J. 2016;10:655–664. doi: 10.1038/ismej.2015.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ley RE, Peterson DA, Gordon JI. Ecological and evolutionary forces shaping microbial diversity in the human intestine. Cell. 2006;124:837–848. doi: 10.1016/j.cell.2006.02.017. [DOI] [PubMed] [Google Scholar]
- 47.Rieu A, Aoudia N, Jego G, Chluba J, Yousfi N, Briandet R, Deschamps J, Gasquet B, Monedero V, Garrido C, Guzzo J. The biofilm mode of life boosts the anti-inflammatory properties of Lactobacillus. Cell Microbiol. 2014;16:1836–1853. doi: 10.1111/cmi.12331. [DOI] [PubMed] [Google Scholar]
- 48.de Vos WM. Microbial biofilms and the human intestinal microbiome. NPJ Biofilms Microbiomes. 2015;1:15005. doi: 10.1038/npjbiofilms.2015.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kalia VC, Prakash J, Koul S, Ray S. Simple and rapid method for detecting biofilm forming bacteria. Indian J Microbiol. 2017;57:109–111. doi: 10.1007/s12088-016-0616-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sanchez LM, Cheng AT, Warner CJ, Townsley L, Peach KC, Navarro G, Shikuma NJ, Bray WM, Riener RM, Yildiz FH, Linington RG. Biofilm formation and detachment in gram-negative pathogens is modulated by select bile acids. PLoS One. 2016;11:e0149603. doi: 10.1371/journal.pone.0149603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Larsen AM, Mohammed HH, Arias CR. Comparison of DNA extraction protocols for the analysis of gut microbiota in fishes. FEMS Microbiol Lett. 2014;362:fnu031. doi: 10.1093/femsle/fnu031. [DOI] [PubMed] [Google Scholar]
- 52.Gajardo K, Rodiles A, Kortner TM, Krogdahl Å, Bakke AM, Merrifield DL, Sørum H. A high-resolution map of the gut microbiota in Atlantic salmon (Salmo salar): a basis for comparative gut microbial research. Sci Rep. 2016;6:30893. doi: 10.1038/srep30893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ye L, Amberg J, Chapman D, Gaikowski M, Liu WT. Fish gut microbiota analysis differentiates physiology and behavior of invasive Asian carp and indigenous American fish. ISME J. 2014;8:541–551. doi: 10.1038/ismej.2013.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wong S, Rawls JF. Intestinal microbiota composition in fishes is influenced by host ecology and environment. Mol Ecol. 2012;21:3100–3102. doi: 10.1111/j.1365-294X.2012.05646.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rawls JF, Mahowald MA, Ley RE, Gordon JI. Reciprocal gut microbiota transplants from zebrafish and mice to germ-free recipients reveal host habitat selection. Cell. 2006;127:423–433. doi: 10.1016/j.cell.2006.08.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hennersdorf P, Kleinertz S, Theisen S, Abdul-Aziz MA, Mrotzek G, Palm HW, Saluz HP. Microbial diversity and parasitic load in tropical fish of different environmental conditions. PLoS One. 2016;11:e0151594. doi: 10.1371/journal.pone.0151594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Miyake S, Ngugi DK, Stingl U. Diet strongly influences the gut microbiota of surgeonfishes. Mol Ecol. 2015;24:656–672. doi: 10.1111/mec.13050. [DOI] [PubMed] [Google Scholar]
- 58.Scott KP, Gratz SW, Sheridan PO, Flint HJ, Duncan SH. The influence of diet on the gut microbiota. Pharmacol Res. 2013;69:52–60. doi: 10.1016/j.phrs.2012.10.020. [DOI] [PubMed] [Google Scholar]
- 59.Bolnick DI, Snowberg LK, Hirsch PE, Lauber CL, Knight R, Caporaso JG, Svanback R. Individuals’ diet diversity influences gut microbial diversity in two freshwater fish (threespine stickleback and Eurasian perch) Ecol Lett. 2014;17:979–987. doi: 10.1111/ele.12301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Saha S, Roy RN, Sen SK, Ray AK. Characterization of cellulase-producing bacteria from the digestive tract of tilapia, Oreochromis mossambica (Peters) and grass carp, Ctenopharyngodon idella (Valenciennes) Aquac Res. 2006;37:380–388. doi: 10.1111/j.1365-2109.2006.01442.x. [DOI] [Google Scholar]
- 61.Dehler CE, Secombes CJ, Martin SA. Environmental and physiological factors shape the gut microbiota of Atlantic salmon parr (Salmo salar L.) Aquaculture. 2017;467:149–157. doi: 10.1016/j.aquaculture.2016.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Li X, Zhou L, Yu Y, Ni J, Xu W, Yan Q. Composition of Gut microbiota in the gibel carp (Carassius auratus gibelio) varies with host development. Microb Ecol. 2017;74:239–249. doi: 10.1007/s00248-016-0924-4. [DOI] [PubMed] [Google Scholar]
- 63.Giatsis C, Sipkema D, Smidt H, Heilig H, Benvenuti G, Verreth J, Verdegem M. The impact of rearing environment on the development of gut microbiota in tilapia larvae. Sci Rep. 2015;5:18206. doi: 10.1038/srep18206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Eichmiller JJ, Hamilton MJ, Staley C, Sadowsky MJ, Sorensen PW. Environment shapes the fecal microbiome of invasive carp species. Microbiome. 2016;4:44. doi: 10.1186/s40168-016-0190-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Schmidt VT, Smith KF, Melvin DW, Amaral-Zettler LA. Community assembly of a euryhaline fish microbiome during salinity acclimation. Mol Ecol. 2015;24:2537–2550. doi: 10.1111/mec.13177. [DOI] [PubMed] [Google Scholar]
- 66.Sylvain FÉ, Cheaib B, Llewellyn M, Correia TG, Fagundes DB, Val AL, Derome N. pH drop impacts differentially skin and gut microbiota of the Amazonian fish tambaqui (Colossoma macropomum) Sci Rep. 2016;6:32032. doi: 10.1038/srep32032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Li X, Yu Y, Feng W, Yan Q, Gong Y. Host species as a strong determinant of the intestinal microbiota of fish larvae. J Microbiol. 2012;50:29–37. doi: 10.1007/s12275-012-1340-1. [DOI] [PubMed] [Google Scholar]
- 68.Li XM, Zhu YJ, Yan QY, Ringø E, Yang DG. Do the intestinal microbiotas differ between paddlefish (Polyodon spathala) and bighead carp (Aristichthys nobilis) reared in the same pond? J Appl Microbiol. 2014;117:1245–1252. doi: 10.1111/jam.12626. [DOI] [PubMed] [Google Scholar]
- 69.Wong S, Waldrop T, Summerfelt S, Davidson J, Barrows F, Kenney PB, Welch T, Wiens GD, Snekvik K, Rawls JF, Good C. Aquacultured rainbow trout (Oncorhynchus mykiss) possess a large core intestinal microbiota that is resistant to variation in diet and rearing density. Appl Environ Microbiol. 2013;79:4974–4984. doi: 10.1128/AEM.00924-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sullam KE, Rubin BE, Dalton CM, Kilham SS, Flecker AS, Russell JA. Divergence across diet, time and populations rules out parallel evolution in the gut microbiomes of Trinidadian guppies. ISME J. 2015;9:1508–1522. doi: 10.1038/ismej.2014.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Li T, Long M, Li H, Gatesoupe FJ, Zhang X, Zhang Q, Feng D, Li A. Multi-omics analysis reveals a correlation between the host phylogeny, gut microbiota and metabolite profiles in cyprinid fishes. Front Microbiol. 2017;8:454. doi: 10.3389/fmicb.2017.00454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Michl SC, Ratten JM, Beyer M, Hasler M, LaRoche J, Schulz C. The malleable gut microbiome of juvenile rainbow trout (Oncorhynchus mykiss): diet-dependent shifts of bacterial community structures. PLoS One. 2017;12:e0177735. doi: 10.1371/journal.pone.0177735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mansfield GS, Desai AR, Nilson SA, Van Kessel AG, Drew MD, Hill JE. Characterization of rainbow trout (Oncorhynchus mykiss) intestinal microbiota and inflammatory marker gene expression in a recirculating aquaculture system. Aquaculture. 2010;307:95–104. doi: 10.1016/j.aquaculture.2010.07.014. [DOI] [Google Scholar]
- 74.Ringø E, Zhou Z, Vecino JLG, Wadsworth S, Romero J, Krogdahl Å, Olsen RE, Dimitroglou A, Foey A, Davies S, Owen M, Lauzon HL, Martinsen LL, De Schryver P, Bossier P, Sperstad S, Merrifield DL. Effect of dietary components on the gut microbiota of aquatic animals. A never-ending story? Aquac Nutr. 2016;22:219–282. doi: 10.1111/anu.12346. [DOI] [Google Scholar]
- 75.Rajendhran J, Gunasekaran P. Human microbiomics. Indian J Microbiol. 2010;50:109–112. doi: 10.1007/s12088-010-0034-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sangwan N, Lambert C, Sharma A, Gupta V, Khurana P, Khurana JP, Sockett RE, Gilbert JA, Lal R. Arsenic rich Himalayan hot spring metagenomics reveal genetically novel predator-prey genotypes. Environ Microbiol Rep. 2015;7:812–823. doi: 10.1111/1758-2229.12297. [DOI] [PubMed] [Google Scholar]
- 77.Sangwan N, Lata P, Dwivedi V, Singh A, Niharika N, Kaur J. Comparative metagenomic analysis of soil microbial communities across three hexachlorocyclohexane contamination levels. PLoS One. 2012;7:e46219. doi: 10.1371/journal.pone.0046219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Dwivedi V, Sangwan N, Nigam A, Garg N, Niharika N, Khurana P, Khurana JP, Lal R. Draft genome sequence of Thermus sp. RL isolated from hot water spring located atop the Himalayan ranges at Manikaran, India. J Bacteriol. 2012;194:3534–3535. doi: 10.1128/JB.00604-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Singh AK, Sangwan N, Sharma A, Gupta V, Khurana JP, Lal R. Draft genome sequence of Sphingobium quisquiliarum P25T, a novel Hexachlorocylohexane (HCH) degrading bacterium isolated from the HCH dumpsite. Genome Announc. 2013;1:e00717-12. doi: 10.1128/genomeA.00717-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mukherjee U, Kumar R, Mahato NK, Khurana JP, Lal R. Draft genome sequence of Sphingobium sp. HDIPO4, an avid degrader of Hexachlorocyclohexane. Genome Announc. 2013;1:e00749-13. doi: 10.1128/genomeA.00749-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kaur J, Verma H, Tripathi C, Khurana JP, Lal R. Draft genome sequence of a Hexachlorocyclohexane-degrading bacterium, Sphingobium baderi strain LL03T. Genome Announc. 2013;1:e00751-13. doi: 10.1128/genomeA.00751-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Dua A, Malhotra J, Saxena A, Khan F, Lal R. Devosia lucknowensis sp. nov., a bacterium isolated from Hexachlorocyclohexane (HCH) contaminated pond soil. J Microbiol. 2013;51:689–694. doi: 10.1007/s12275-013-2705-9. [DOI] [PubMed] [Google Scholar]
- 83.Dua A, Sangwan N, Kaur J, Saxena A, Kohli P, Gupta AK, Lal R. Draft genome sequence of Agrobacterium sp. Strain UHFBA-218, isolated from rhizosphere soil of crown gall-infected cherry rootstock colt. Genome Announc. 2013;1:e00302–e00313. doi: 10.1128/genomeA.00302-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Negi V, Lata P, Sangwan N, Gupta S, Das S, Rao DLN, Lal R. Draft genome sequence of Hexachlorocyclohexane (HCH)-degrading Sphingobium lucknowense strain F2, isolated from the HCH dumpsite. Genome Announc. 2014;2:e00788-14. doi: 10.1128/genomeA.00788-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sharma A, Hira P, Shakarad M, Lal R. Draft genome sequence of Cellulosimicrobium sp. MM, isolated from arsenic rich microbial mats of a Himalayan hot spring. Genome Announc. 2014;5:e01020-14. doi: 10.1128/genomeA.01020-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Schrader C, Schielke A, Ellerbroek L, Johne R. PCR inhibitors—occurrence, properties and removal. J Appl Microbiol. 2012;113:1014–1026. doi: 10.1111/j.1365-2672.2012.05384.x. [DOI] [PubMed] [Google Scholar]
- 87.Kashinskaya EN, Andree KB, Simonov EP, Solovyev MM. DNA extraction protocols may influence biodiversity detected in the intestinal microbiome: a case study from wild Prussian carp. Carassius gibelio. FEMS Microbiol Ecol. 2017;93:fiw240. doi: 10.1093/femsec/fiw240. [DOI] [PubMed] [Google Scholar]
- 88.Hart ML, Meyer A, Johnson PJ, Ericsson AC. Comparative evaluation of DNA extraction methods from feces of multiple host species for downstream next-generation sequencing. PLoS One. 2015;10:e0143334. doi: 10.1371/journal.pone.0143334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Henderson G, Cox F, Kittelmann S, Miri VH, Zethof M, Noel SJ, Waghorn GC, Janssen PH. Effect of DNA extraction methods and sampling techniques on the apparent structure of cow and sheep rumen microbial communities. PLoS One. 2013;8:e74787. doi: 10.1371/journal.pone.0074787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mirsepasi H, Persson S, Struve C, Andersen LOB, Petersen AM, Krogfelt KA. Microbial diversity in fecal samples depends on DNA extraction method: easyMag DNA extraction compared to QIAamp DNA stool mini kit extraction. BMC Res Notes. 2014;7:50. doi: 10.1186/1756-0500-7-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kanther M, Rawls JF. Host-microbe interactions in the developing zebrafish. Curr Opin Immunol. 2010;22:10–19. doi: 10.1016/j.coi.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Cheesman SE, Guillemin K. We know you are in there: conversing with the indigenous gut microbiota. Res Microbiol. 2007;158:2–9. doi: 10.1016/j.resmic.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 93.Kalia VC. Microbes, antimicrobials and resistance: the battle goes on. Indian J Microbiol. 2014;54:1–2. doi: 10.1007/s12088-013-0443-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Austin B. Taxonomy of bacterial fish pathogens. Vet Res. 2011;42:20. doi: 10.1186/1297-9716-42-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kalia VC. Quorum sensing inhibitors: an overview. Biotechnol Adv. 2013;31:224–245. doi: 10.1016/j.biotechadv.2012.10.004. [DOI] [PubMed] [Google Scholar]
- 96.Koul S, Kalia VC. Multiplicity of quorum quenching enzymes: a potential mechanism to limit quorum sensing bacterial population. Indian J Microbiol. 2017;57:100–108. doi: 10.1007/s12088-016-0633-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kalia VC, Purohit HJ. Quenching the quorum sensing system: potential antibacterial drug targets. Crit Rev Microbiol. 2011;37:121–140. doi: 10.3109/1040841X.2010.532479. [DOI] [PubMed] [Google Scholar]
- 98.Chu W, Lu F, Zhu W, Kang C. Isolation and characterization of new potential probiotic bacteria based on quorum-sensing system. J Appl Microbiol. 2011;110:202–208. doi: 10.1111/j.1365-2672.2010.04872.x. [DOI] [PubMed] [Google Scholar]
- 99.Willis AR, Moore C, Mazon-Moya M, Krokowski S, Lambert C, Till R, Mostowy S, Sockett RE. Injections of predatory bacteria work alongside host immune cells to treat Shigella infection in Zebrafish larvae. Curr Biol. 2016;26:3343–3351. doi: 10.1016/j.cub.2016.09.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Cao H, He S, Wang H, Hou S, Lu L, Yang X. Bdellovibrios, potential biocontrol bacteria against pathogenic Aeromonas hydrophila. Vet Microbiol. 2012;154:413–418. doi: 10.1016/j.vetmic.2011.07.032. [DOI] [PubMed] [Google Scholar]
- 101.Chu WH, Zhu W. Isolation of Bdellovibrio as biological therapeutic agents used for the treatment of Aeromonas hydrophila infection in fish. Zoonoses Public Health. 2010;57:258–264. doi: 10.1111/j.1863-2378.2008.01224.x. [DOI] [PubMed] [Google Scholar]
- 102.Feng S, Tan CH, Cohen Y, Rice SA. Isolation of Bdellovibrio bacteriovorus from a tropical wastewater treatment plant and predation of mixed species biofilms assembled by the native community members. Environ Microbiol. 2016;18:3923–3931. doi: 10.1111/1462-2920.13384. [DOI] [PubMed] [Google Scholar]
- 103.Oyedara OO, De Luna-Santillana EJ, Olguin-Rodriguez O, Guo X, Mendoza-Villa MA, et al. Isolation of Bdellovibrio sp. from soil samples in Mexico and their potential applications in control of pathogens. Microbiologyopen. 2016;5:992–1002. doi: 10.1002/mbo3.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Iebba V, Santangelo F, Totino V, Nicoletti M, Gagliardi A, et al. Higher prevalence and abundance of Bdellovibrio bacteriovorus in the human gut of healthy subjects. PLoS One. 2013;8:e61608. doi: 10.1371/journal.pone.0061608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.He S, Ran C, Qin C, Li S, Zhang H, de Vos WM, Ringø E, Zhou Z. Anti-infective effect of adhesive probiotic Lactobacillus in fish is correlated with their spatial distribution in the intestinal tissue. Sci Rep. 2017;7:13195. doi: 10.1038/s41598-017-13466-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Koul S, Kalia VC. Comparative genomics reveals biomarkers to identify Lactobacillus species. Indian J Microbiol. 2016;56:265–276. doi: 10.1007/s12088-016-0605-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zhang Z, Li D, Refaey MM, Xu W, Tang R, Li L. Host age affects the development of southern catfish gut bacterial community divergent from that in the food and rearing water. Front Microbiol. 2018;9:495. doi: 10.3389/fmicb.2018.00495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zhang Z, Li D, Refaey MM, Xu W. High spatial and temporal variations of microbial community along the southern catfish gastrointestinal tract: insights into dynamic food digestion. Front Microbiol. 2017;8:1531. doi: 10.3389/fmicb.2017.01531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Koo H, Hakim JA, Powell ML, Kumar R, Eipers PG, Morrow CD, Crowley M, Lefkowitz EJ, Watts SA, Bej AK. Metagenomics approach to the study of the gut microbiome structure and function in zebrafish Danio rerio fed with gluten formulated diet. J Microbiol Methods. 2017;135:69–76. doi: 10.1016/j.mimet.2017.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Nielsen S, Walburn JW, Vergés A, Thomas T, Egan S. Microbiome patterns across the gastrointestinal tract of the rabbitfish Siganus fuscescens. PeerJ. 2017;5:e3317. doi: 10.7717/peerj.3317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Carlson JM, Leonard AB, Hyde ER, Petrosino JF, Primm TP. Microbiome disruption and recovery in the fish Gambusia affinis following exposure to broad-spectrum antibiotic. Infect Drug Resist. 2017;10:143–154. doi: 10.2147/IDR.S129055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Li T, Li H, Gatesoupe FJ, She R, Lin Q, Yan X, Li J, Li X. Bacterial signatures of “Red-Operculum” disease in the gut of crucian carp (Carassius auratus) Microb Ecol. 2017;74:510–521. doi: 10.1007/s00248-017-0967-1. [DOI] [PubMed] [Google Scholar]
- 113.Stagaman K, Burns AR, Guillemin K, Bohannan BJ. The role of adaptive immunity as an ecological filter on the gut microbiota in zebrafish. ISME J. 2017;11:1630–1639. doi: 10.1038/ismej.2017.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Gajardo K, Jaramillo-Torres A, Kortner TM, Merrifield DL, Tinsley J, Bakke AM, Krogdahl Å. Alternative protein sources in the diet modulate microbiota and functionality in the distal intestine of Atlantic salmon (Salmo salar) Appl Environ Microbiol. 2017;83:e02615–e02616. doi: 10.1128/AEM.02615-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Zhai Q, Yu L, Li T, Zhu J, Zhang C, Zhao J, Zhang H, Chen W. Effect of dietary probiotic supplementation on intestinal microbiota and physiological conditions of Nile tilapia (Oreochromis niloticus) under waterborne cadmium exposure. Antonie Van Leeuwenhoek. 2017;110:501–513. doi: 10.1007/s10482-016-0819-x. [DOI] [PubMed] [Google Scholar]
- 116.Lyons PP, Turnbull JF, Dawson KA, Crumlish M. Phylogenetic and functional characterization of the distal intestinal microbiome of rainbow trout Oncorhynchus mykiss from both farm and aquarium settings. J Appl Microbiol. 2017;122:347–363. doi: 10.1111/jam.13347. [DOI] [PubMed] [Google Scholar]
- 117.Song W, Li L, Huang H, Jiang K, Zhang F, Chen X, Zhao M, Ma L. The gut microbial community of Antarctic fish detected by 16S rRNA gene sequence analysis. Biomed Res Int. 2016;2016:3241529. doi: 10.1155/2016/3241529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Bledsoe JW, Peterson BC, Swanson KS, Small BC. Ontogenetic characterization of the intestinal microbiota of channel catfish through 16S rRNA gene sequencing reveals insights on temporal shifts and the influence of environmental microbes. PLoS One. 2016;11:e0166379. doi: 10.1371/journal.pone.0166379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Giatsis C, Sipkema D, Ramiro-Garcia J, Bacanu GM, Abernathy J, Verreth J, Smidt H, Verdegem M. Probiotic legacy effects on gut microbial assembly in tilapia larvae. Sci Rep. 2016;6:33965. doi: 10.1038/srep33965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Gatesoupe FJ, Huelvan C, Le Bayon N, Le Delliou H, Madec L, Mouchel O, Quazuguel P, Mazurais D, Zambonino-Infante JL. The highly variable microbiota associated to intestinal mucosa correlates with growth and hypoxia resistance of sea bass, Dicentrarchus labrax, submitted to different nutritional histories. BMC Microbiol. 2016;16:266. doi: 10.1186/s12866-016-0885-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Stephens WZ, Burns AR, Stagaman K, Wong S, Rawls JF, Guillemin K, Bohannan BJM. The composition of the zebrafish intestinal microbial community varies across development. ISME J. 2016;10:644–654. doi: 10.1038/ismej.2015.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Schmidt V, Amaral-Zettler L, Davidson J, Summerfelt S, Good C. Influence of fishmeal-free diets on microbial communities in Atlantic Salmon (Salmo salar) recirculation aquaculture systems. Appl Environ Microbiol. 2016;82:4470–4481. doi: 10.1128/AEM.00902-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Zarkasi KZ, Taylor RS, Abell GC, Tamplin ML, Glencross BD, Bowman JP. Atlantic Salmon (Salmo salar L.) gastrointestinal microbial community dynamics in relation to digesta properties and diet. Microb Ecol. 2016;71:589–603. doi: 10.1007/s00248-015-0728-y. [DOI] [PubMed] [Google Scholar]
- 124.Llewellyn MS, McGinnity P, Dionne M, Letourneau J, Thonier F, Carvalho GR, Creer S, Derome N. The biogeography of the atlantic salmon (Salmo salar) gut microbiome. ISME J. 2016;10:1280–1284. doi: 10.1038/ismej.2015.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Narrowe AB, Albuthi-Lantz M, Smith EP, Bower KJ, Roane TM, Vajda AM, Miller CS. Perturbation and restoration of the fathead minnow gut microbiome after low-level triclosan exposure. Microbiome. 2015;3:6. doi: 10.1186/s40168-015-0069-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.NCBI Resource Coordinators Database resources of the National Center for Biotechnology Information. Nucleic Acids Res. 2016;44:D7–D19. doi: 10.1093/nar/gkv1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, et al. QIIME allows analysis of high-throughput community sequencing data. Nat Methods. 2010;7:335–336. doi: 10.1038/nmeth.f.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Edgar RC. UPARSE: highly accurate OTU sequences from microbial amplicon reads. Nat Methods. 2013;10:996–998. doi: 10.1038/nmeth.2604. [DOI] [PubMed] [Google Scholar]
- 129.Pruesse E, Quast C, Knittel K, Fuchs BM, Ludwig W, Peplies J, Glöckner FO. SILVA: a comprehensive online resource for quality checked and aligned ribosomal RNA sequence data compatible with ARB. Nucleic Acids Res. 2007;35:7188–7196. doi: 10.1093/nar/gkm864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.DeSantis TZ, Hugenholtz P, Larsen N, Rojas M, Brodie EL, Keller K, Huber T, Dalevi D, Hu P, Andersen GL. Greengenes, a chimera-checked 16S rRNA gene database and workbench compatible with ARB. Appl Environ Microbiol. 2006;72:5069–5072. doi: 10.1128/AEM.03006-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Cole JR, Wang Q, Cardenas E, Fish J, Chai B, Farris RJ, Kulam-Syed-Mohideen AS, McGarrell DM, Marsh T, Garrity GM, Tiedje JM. The Ribosomal Database Project: improved alignments and new tools for rRNA analysis. Nucleic Acids Res. 2009;37:D141–D145. doi: 10.1093/nar/gkn879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Rosen GL, Reichenberger ER, Rosenfeld AM. NBC: the Naive Bayes classification tool webserver for taxonomic classification of metagenomic reads. Bioinformatics. 2011;27:127–129. doi: 10.1093/bioinformatics/btq619. [DOI] [PMC free article] [PubMed] [Google Scholar]