Abstract
Italian ryegrass is one of main feed for livestock animals/birds. It has potential antioxidant metabolites that can improve their health and protect them against various infectious diseases. In this work, we studied synthesis of silver nanoparticles assisted by forage crop Lolium multiflorum as a green synthesis way. Potential antibacterial efficacy of these synthesized nanosized silver nanoparticles against poultry pathogenic bacteria was then studied. Aqueous extract of IRG was used as reducing agent for bio-reduction of silver salt to convert Ag+ to Ag0 metallic nano-silver. Size, shape, metallic composition, functional group, and crystalline nature of these synthesized silver nanoparticles were then characterized using UV–Vis spectrophotometer, FESEM, EDX, FT-IT, and XRD, respectively. In addition, antibacterial effects of these synthesized AgNPs against poultry pathogenic bacteria were evaluated by agar well diffusion method. UV–Vis spectra showed strong absorption peak of 440–450 nm with differ reaction time ranging from 30 min to 24 h. FESEM measurements revealed particles sizes of around 20–100 nm, majority of which were spherical in shape while a few were irregular. These biosynthesized silver nanoparticles using IRG extract exhibited strong antibacterial activities against poultry pathogenic microorganisms, including Pseudomonas aeruginosa, Salmonella typhi, Escherichia coli, and Bacillus subtilis. Overall results confirmed that IRG plant extract possessed potential bioactive compounds for converting silver ions into nanosized silver at room temperature without needing any external chemical for redox reaction. In addition, such synthesized AgNPs showed strong antibacterial activities against pathogenic bacteria responsible for infectious diseases in poultry.
Keywords: Forage crop, Italian ryegrass, Silver nanoparticles, Biological way, XRD, Poultry pathogenic bacteria
Introduction
In the last two decades, nanomaterial syntheses have been gradually increasing because of their potential applications in the field of biomedicine, bio-nanotechnology, biosensor, optoelectronics, energy, and so on. Nanoparticles also have extensive applicability in medicine and commercial industries to improve human life and environment [1]. Generally, silver nanoparticles exhibit novel properties than their macroscopic counterparts. These nano-sized substances have larger surface area, thus having more contact with microbial cell walls. Many studies have shown that silver-based compounds are highly toxic to foreign bodies without disturbing the host system. A wide range of biological resources such as plants, algae, bacteria, and fungi are involved in bio-reduction of metal ions into metal nanoparticles [2]. These resources contain a different cluster of bioactive substances that are precursor molecules involved in the conversion of bulk metal into nano-sized particles. Recently, it has been shown that organic sources play a potential role in the synthesis of metal nanoparticles with large surface area, specific size, and shapes. In particular, plant mediated synthesis of nanoparticles is a one-step procedure without requiring external stabilizing or reducing agents. The reduction rate of metal ions is fast without involving any specific vacuum techniques, unlike physical or chemical synthesis procedure [3]. Besides, biogenic approaches are environmentally friendly, reproducible, and highly stable.
Different types of antibiotics are used to control bacterial and fungal infections for farm animals, human, and aquaculture Zoonos. However, frequent and continuous usages of multiple drugs have led to development of antibiotic resistant in pathogens. These multidrug resistant (MDR) pathogenic bacteria have emerged as one of the biggest health threats. To overcome this situation, scientists including nanobiotechnologists are searching for novel antibiotics to deal with MDR Gram-negative bacteria effectively. Nano-sized particles have potent properties in binding to cell walls of Gram-negative bacteria, thus controlling the replication process of pathogenic organisms [4, 5].
Lolium multiflorum (IRG) is an important annual grass used in livestock animal feed production. It is a highly palatable forage that contains more than 60% digestible dry matter and 20% crude protein [6]. L. multiflorum is a monocot flowering grass from family Poaceae. It is cultivated for silage. It is also a highly nutritious stock feed for livestock animals in winter season. L. multiflorum is widely used as feed for ruminants. It has been reported that the high nutritional value of IRG could increase meat quality of livestock animals. Furthermore, phytochemical screening of IRG extracts has shown a cluster of phytoconstituents such as phenolics, flavanoids, fattyacid, alkaloids, terpinoids, and minerals present in purified fractions. We have previously reported that chloroform extract of IRG exhibits stimulated adipogenesis function in in vitro in 3T3-L1 cells [7]. IRG extract also possesses potential antioxidant and anti-inflammatory effects closely correlated with the content of polyphenolic compounds from plant extract [8]. Hwang et al. [9] have reported that IRG silage shows significantly higher reactive oxygen species (ROS) scavenging activity compared to other forage crops such as sorghum, barley, and alfalfa. In addition, IRG chloroform extract can suppress the production of tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6) in lipopolysaccharide (LPS)-stimulated macrophages in an in vivo model. This study aims to synthesize silver nanoparticles using forage grass plant IRG aqueous-ethanol extract as potent reducing and stabilizing agent. Structural characterization of these synthesized silver nanoparticles was then performed using various techniques such as UV–Vis spectrophotometer, FESEM, EDX, FT-IR, and XRD. In addition, antibacterial activities of these biosynthesized AgNPs capped with IRG extract against poultry pathogenic bacteria were determined.
Materials and Methods
Chemicals Used for Experiments
Silver nitrate and sodium salts (monobasic and dibasic) were obtained from Sigma-Aldrich chemical Co., Korea. Microbiological media, agar, and KCTC culture were purchased from Himedia, India or NIAS, Seonghwan, Korea. All glassware were cleaned well and sterilized at 121 °C for 15 min before use for experiments.
Preparation of L. multiflorum Aqueous Medium
Biosynthesis process was conducted as previously described by Shankar et al. [10] with slight modification for biochemical parameters. Briefly, 5 g of finely sieved IRG plant powder was added to 100 ml of double distilled water in a 250 ml beaker and kept on a water bath at 70 °C for 20 min. After that, the broth was cooled down at room temperature and centrifuged at 8000 rpm for 20 min at 4 °C. The filtrate was stored at 4 °C for further study. It was used as a capping and stabilizing agent for the synthesis of AgNPs.
Then 10 ml of IRG silage extract was added into 90 ml of distilled water of 1 mM silver nitrate and kept in an incubator/shaker at 37 °C and 80 rpm. The reduction of AgNO3 to Ag+ ions was monitored by the formation of color from pale green to colloidal brownish black. The development of AgNPs in the colloidal mixture was further confirmed by spectrophotometric analysis. After complete reduction, the solution was centrifuged at 8000 rpm for 15 min at 4 °C. The pellet sample was frozen in an external deep freezer at − 80 °C for 6 h and stored properly for future use.
Characterization of Synthesized Ag Nanoparticles Using Bio-physical Techniques
The biosynthesis of silver nanoparticle by IRG silage was monitored visually. Absorption spectrum of the reaction mixture and plant extract was characterized by UV–visible spectrophotometer (T80 UV–Vis spectrophotometer PGI, Beijing, China) with scanning range from 250 to 750 nm at different time intervals. Field emission scanning electron microscopy (FESEM) was used to analyze morphology and size of synthesized silver nanoparticles using FESEM JEOL 6386 (Japan) at 10 kV. Elemental composition analysis for these synthesized silver nanoparticles was performed using energy dispersive X-ray analysis (EDX) along with FESEM. Fourier transform-infrared red spectrophotometer (8400S, Shimadzu, Japan) was used to identify the presence of functional group in these silver nanoparticles. Fine powdered AgNPs were added with potassium bromide (KBr) at 1% concentration (w/w). They were pelletized. Spectrum was recorded in wave number ranging from 500 to 3000−1. IRG bioactive compounds embedded with silver nanoparticles crystalline nature were analyzed by X-ray diffraction (X’Pert Pro, PANalytical, USA). XRD spectra were obtained at a voltage of 40 kV and a current of 30 mA with Cu Kα radiation (λ = 1.54 Å). Diffracted intensities were recorded from 0° to 80° 2θ angles [11].
Bio-activities of Synthesized AgNPs Against Poultry Pathogenic Bacteria
To determine their antibacterial activities, the following pathogenic organisms causing infection in livestock poultry farms were selected: Escherichia coli (KTCC), Salmonella typhi, Staphylococcus aureus, and Pseudomonas aeruginosa. Antibacterial activities of IRG synthesized silver nanoparticles were tested individually for the mentioned above microorganisms. Preliminary evaluation of antibacterial activity of these IRG mediated silver nanoparticles was performed using agar well diffusion method [12]. Briefly, a loopful of bacterial strain was plated using sterile L rod into MHA agar plate. After that, 6 mm wells were made on the MHA agar plate and aliquot of synthesize silver nanoparticles, plant extracts, and AgNO3 was poured into the well. IRG ethanol-aqueous extract and streptomycin (30 µg/ml) were used as controls. Petri dishes were incubated at 37 ± 1 °C for 24 h. The antibacterial activity was determined by measuring the zone of inhibition in diameters (mm). MIC concentration was defined as the lowest concentration of the drug that completely inhibited microbial growth in a test system. MIC concentration was determined by micro-broth dilution method [13]. Briefly, 100 µl of each bacterial suspension was prepared (106 CFU/ml) and inoculated into tubes containing nutrient broth with different concentrations (12.5–250 µg/ml) of silver nanoparticles. After incubation at 37 °C for 24 h, the MIC of each sample was determined by measuring OD value at 600 nm using a microplate reader.
Statistical Analysis
The experiments were conducted in triplicate and the statistical significance was assessed by the SPSS software (version. 6, N.Y., USA) using one way analysis of variance (ANOVA) followed by student t test (P < 0.05). The data were expressed as mean values ± standard deviation.
Results and Discussion
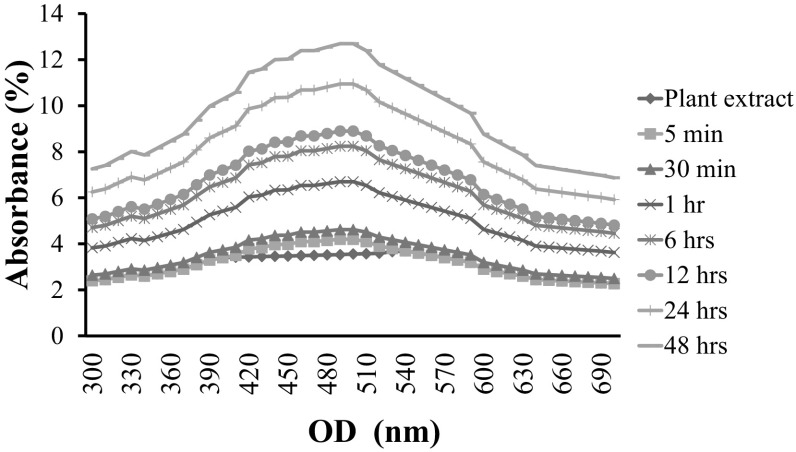
In the present investigation, synthesis of AgNPs assisted by IRG bioactive compounds was reported. One-pot synthesis of AgNPs was carried out at room temperature after adding silver nitrate solution with plant extract. The appearance of gray color indicated the occurrence of redox-reaction and the formation of AgNPs in the synthesized medium. The color intensity was gradually increased with increasing time. This was due to the excitation of surface plasmon resonance in the nano-solution. UV–visible spectra showed a strong absorption peak at 445–455 nm and widening of the peak, indicating that nano-sized materials were poly-dispersed (Fig. 1). Similarly, Bankalgi et al. [14] have reported that UV–visible absorption spectra of phenolics coated silver nanoparticles using Cassia javanica predominantly show peaks at 400–500 nm due to SPR phenomenon.
Fig. 1.
UV–Vis spectra of IRG synthesized AgNPs with varying time dependent nanoparticles production
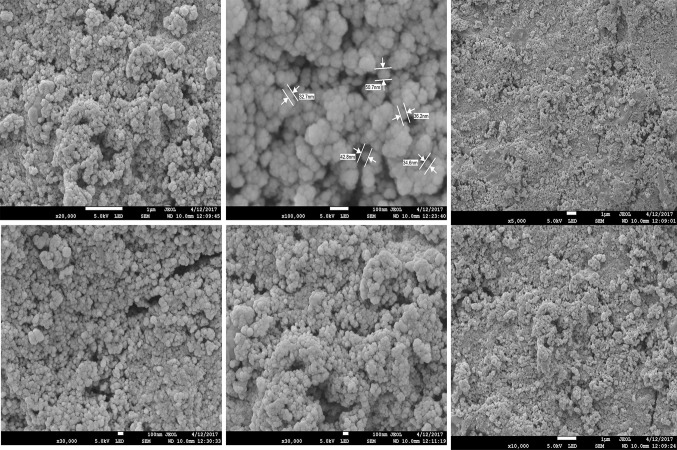
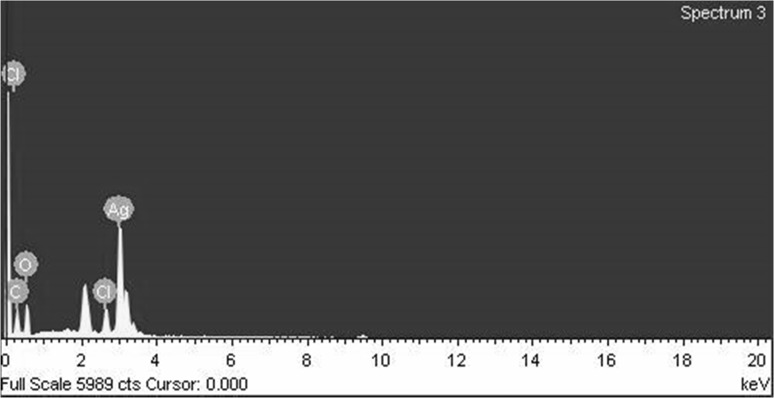
FESEM analysis revealed that these synthesized AgNPs had spherical shape with polydispersity. Almost all nanoparticles were irregularly spherical in shapes. Their sizes ranged from 2 to 100 nm (Fig. 2). The size and shape of nano-sized materials are known to have perceptive impact in conjugation with specific drug and target site action [15]. EDX graph showed strong absorption peak at approximately 3 keV. Along with silver, other metal compositions such as Fe, C, O, and N were also found. This might be due to their presence in the IRG extract. EDX elemental composition results of AgNPs are shown in Fig. 3.
Fig. 2.
Morphology of IRG synthesized AgNPs by field emission scanning electron microscope
Fig. 3.
EDX spectra of biosynthesized AgNPs using IRG extract
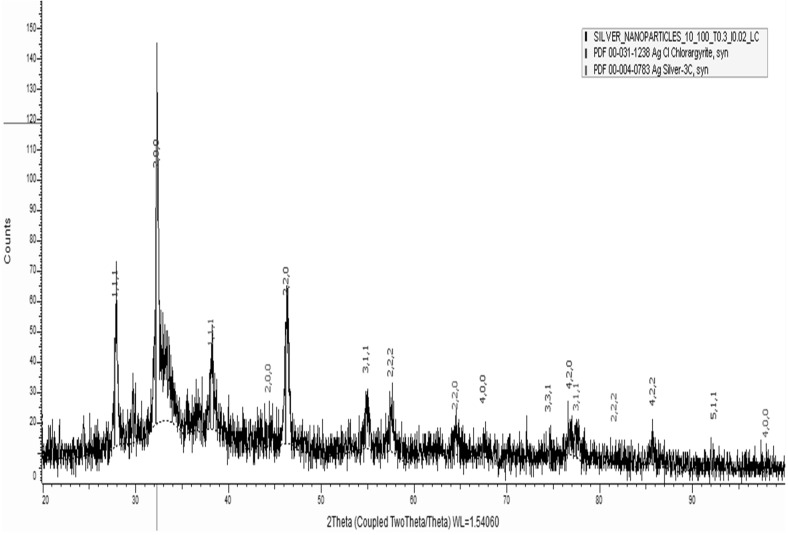
XRD spectra were indexed. Characteristic peaks of AgNPs had a face centered cubic structure. All peaks of XRD pattern matched with similar standard Ag (JC-PDS No: 03-0921). XRD spectra showed diffraction peaks of (111), (200), (220), (222), (311) and (400) planes, suggesting that IRG synthesized silver nanoparticles were crystalline in nature with fcc structure. Assigned peaks denoted at 2θ = 27.76, 33.45, 46.51 correspond to the formation of bio-organic phases of polycrystalline AgNPs (Fig. 4). Similarly, XRD spectra of plant synthesized silver nanoparticles peaks at 2θ degree of 38.1°, 44.3°, 64.4° and 77.7° corresponding to (111), (200), (220) and (311) crystalline planes of the face centered cubic crystalline structure have been reported by Benakashani et al. [16].
Fig. 4.
FT-IR absorption spectra of IRG synthesized AgNPs
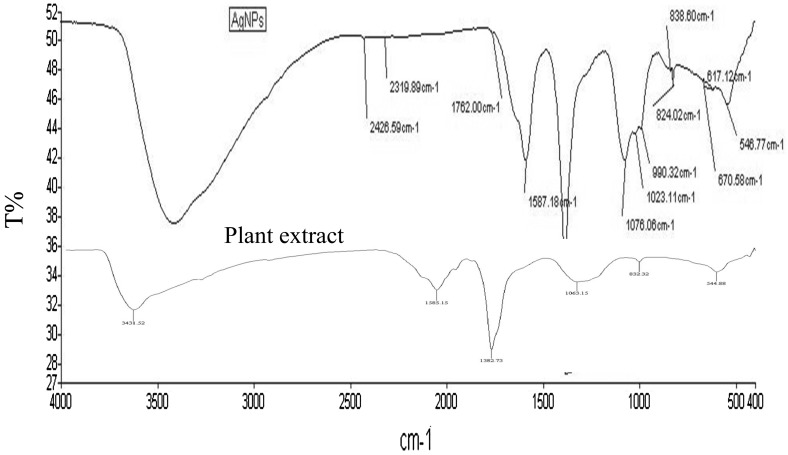
FT-IR spectra can show chemical compositions of AgNPs surface. As can be seen in Fig. 5, peaks appeared at 1762 cm−1 (C=H-stretching vibrations of aldehyde), 1587 cm−1 (C=C multiple band stretching strong vibrations of aromatic compounds), 1076 cm−1 (C=O bending vibrations of carbonyl group), 1029 cm−1 (C–O stretching vibrations of ethers), 824 and 670 cm−1 (bending strong vibrations of alkenes). Their corresponding vibration and intensity values confirmed the presence of phenolics, flavonoids, alkaloids, sugar, and protein in these silver nanoparticles and IRG crude extract. Fatimah, [17] has also carried out FT-IT studies for biosynthesized silver nanoparticles sample and showed that the peak at 3292 cm−1 represents the OH stretching vibration from phenolic compounds in the extract while three peaks at 2849, 1375 and 1043 cm−1 are most likely attributable to NH stretching vibration of amide, C–O stretch, and C–N stretching of amines, respectively. Therefore, phenolics, flavonoids, and aldehydes groups might reduce ions into metallic nanoparticles.
Fig. 5.
XRD spectra of synthesized AgNPs using IRG extract
IRG and grass herbage are important diet for domestic birds and animals [18]. In South Korea, IRG has been cultivated approximately 120,000 ha/year. It is a forage crop with major productivity and high feed value. IRG is also one natural food for poultry and livestock. This green feed provides high nutritional value such as protein, vitamins, phenolics, flavonoids, alkaloids, and fatty acids to poultry husbandry. On the other hand, IRG doped silver nanoparticles might be eco-friendly with less adverse effects. It can be uses for wide applications in food, agriculture, and feed industries. According to the literature, biosynthesized silver nanoparticles are generally considered as safe and their use in food and cosmetics products is recommended [17]. One major cause of poultry infection is through contaminated feed. For instance, several pathogenic bacteria such as Salmonella, Clostridia, and Bacillus sp. are involved in the contamination of feed and poultry products. Different physical and chemical methods can be used to control feed contamination from bacterial and fungal infections. Interestingly, several chemical substances may be able to disinfect contaminated feed. These commercial products are synthesized from organic and inorganic acids such as formaline, acetic acid, and essential oils. They might have adverse effects on livestock animals and humans. Due to this issue, new bio-derived antibiotic is needed to control MDR pathogens. Metallic silver has potent antibacterial effect as proved earlier. When bulk metallic silver is changed to nano-scale, its antimicrobial activity is increased due to the larger surface area and ultrafine particles. Biologically derived nano-silver is also a good candidate as a harmless feed disinfectant.
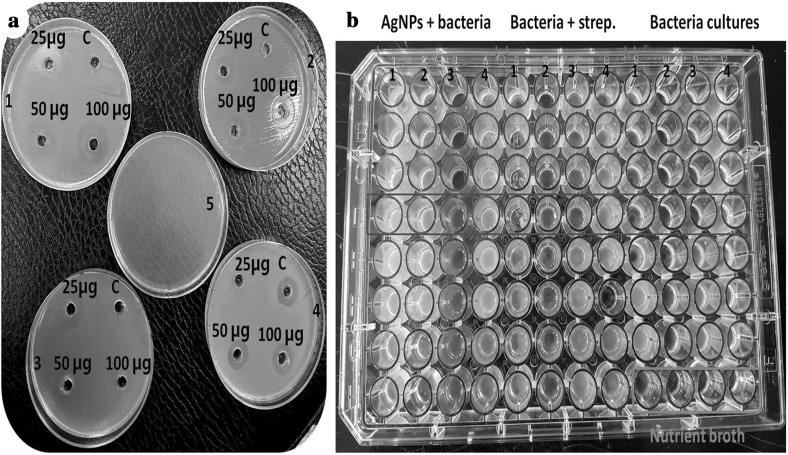
Briefly, antibacterial activities of IRG mediated AgNPs, IRG crude extract, and streptomycin as a standard drug were compared by agar well diffusion assay. Antibacterial results of IRG synthesized AgNPs are shown in Table 1. Generally, IRG mediated silver nanoparticles had significantly higher activities against Gram-negative bacteria compared to the standard drug or the positive control. These biosynthesized AgNPs demonstrated higher antibacterial activity against all tested microbes compared to the control. AgNPs exhibited higher activity against S. typhi (15 mm), P. aeruginosa (13 mm), E. coli (14 mm), and Bacillus subtilis (15 mm) based on zone inhibition (Fig. 6a). Higher ratio of AgNPs exhibited higher antibacterial activity. Their activities varied depending on the concentration AgNPs and bacterial strains. This phenomenon clearly indicates that AgNPs synthesized with IRG extract have potent antibacterial activities against poultry pathogenic bacteria. They might be considered as natural source antibiotics for livestock industries with medicinal uses. Results of MIC for antibacterial activity of these biosynthesized AgNPs are shown in Table 1 (Fig. 6b). MIC values of AgNPs and other tested samples against pathogenic bacteria were in the range of 12.5–250 µg/ml. AgNPs showed an MIC value of 125 µg/ml for P. aeruginosa and S. typhi. Mohanta et al. [13] have also determined antibacterial activities of biosynthesized silver nanoparticles against Gram-positive bacteria B. subtilis and Gram-negative E. coli and P. aeruginosa by agar cup method. Synthesized metallic silver nanoparticles showed growth inhibition above 99% against both Gram-positive and Gram-negative strains. Their MIC was 74.26 ± 0.14 μg/ml against P. aeruginosa and 94.43 ± 0.42 μg/ml against B. subtilis by serial dilution method. Our present finding showed IRG as a potential source for cost effective synthesis of AgNPs by green chemistry way. These biological synthesized silver nanoparticles were highly active against poultry pathogenic bacteria compared to commercial antibiotics or individual samples such as plant extract and AgNO3. Therefore, these AgNPs could be use as alternative disinfectants in livestock industries or for other pharmaceutical purposes.
Table 1.
IRG extract synthesized silver nanoparticle tested against poultry pathogenic bacteria by agar well diffusion method
| Bacterial strains | Zone of inhibition (mm) | MIC (µg/ml) | |||
|---|---|---|---|---|---|
| 100 µg/ml | 50 µg/ml | 25 µg/ml | Streptomycin | ||
| Escherichia coli | 14 | 12 | 7 | 9 | 50 |
| Bacillus subtilis | 15 | 11 | 9 | 14 | 25 |
| Pseudomonas aeruginosa | 13 | 12 | 10 | 11 | 25 |
| Salmonella typhi | 15 | 13 | 8 | 10 | 50 |
Fig. 6.
a Agar well diffusion method of IRG synthesized AgNPs against 24 h grown pathogenic bacteria cultures (C-streptomycin; AgNPs: 25, 50 and 100 µg/ml concentrations; 1, 2, 3, 4 and 5—E. coli, B. subtilis, P. aurogenosa, S. typhi and control respectively). b The MIC assay of IRG mediated AgNPs tested against poultry pathogenic bacteria
Conclusion
This is the first report on forage crop IRG (L. multiflorum) extract used for the synthesis of silver nanoparticles by biological approaches. From this study, we observed that forage crop IRG extract contained a cluster of bioactive substances that could act as potential organic capping and reducing agents to reduce bulk silver into nano silver by green chemistry way. These silver nanoparticles successfully mediated by IRG showed significant activity against poultry disease causing pathogenic bacteria such as E. coli, Staphylococcus aureus, P. aeruginosa, and Salmonella typhi compared to standard antibiotic drug. Therefore, IRG aqueous-ethanol extract can be use as reducing and capping agent for eco-friendly and cost effective synthesis of silver nanoparticles. In addition, these synthesized metallic silver nanoparticles could be used as cost effective organic feed disinfectants for livestock industries in future.
Acknowledgements
The authors are grateful to the Rural Development Administration, Republic of Korea (Cooperative Research Program for Agriculture Science and Technology Development) for funding this research (Title: Technical development to increase the utilization of Italian ryegrass in livestock; Project No. PJ010903042017).
Compliance with Ethical Standards
Conflict of interest
The authors have no conflict of interest relevant to this study to disclose.
References
- 1.Ashraf JM, Ansari MA, Khan HM, Alzohairy MA, Choi I. Green synthesis of silver nanoparticles and characterization of their inhibitory effects on AGEs formation using biophysical techniques. Sci Rep. 2016;6:20414. doi: 10.1038/srep20414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Donnarumma G, Fusco A, Morganti P, Palombo M, Anniboletti T, Ciotto PD, Baroni A, Chianese A. Advanced medications made by green nanocomposites. Int J Res Pharm Nano Sci. 2016;5:261–270. [Google Scholar]
- 3.Naik RR, Stringer SJ, Agarwal G, Jones SE, Stone MO. Biomimetic synthesis and patterning of silver nanoparticles. Nat Mater. 2002;1:169–172. doi: 10.1038/nmat758. [DOI] [PubMed] [Google Scholar]
- 4.Farhadi S, Ajerloo B, Mohammadi A. Green biosynthesis of spherical silver nanoparticles by using date palm (Phoenix dactylifera) fruit extract and study of their antibacterial and catalytic activities. Acta Chim Slov. 2017;64:129–143. doi: 10.17344/acsi.2016.2956. [DOI] [PubMed] [Google Scholar]
- 5.Huo Y, Singh P, Kim YJ, Soshnikova V, Kang J, Markus J, Ahn S, Castro-Aceituno V, Mathiyalagan R, Chokkalingam M, Bae KS, Yang DC. Biological synthesis of gold and silver chloride nanoparticles by Glycyrrhiza uralensis and in vitro applications. Artif Cells Nanomed Biotechnol. 2017;4:1–13. doi: 10.1080/21691401.2017.1307213. [DOI] [PubMed] [Google Scholar]
- 6.Özelçam H, Kırkpınar F, Tan K. Chemical composition, in vivo digestibility and metabolizable energy values of caramba (Lolium multiflorum cv. caramba) fresh, silage and hay. Asian Australas J Anim Sci. 2015;28:1427–1432. doi: 10.5713/ajas.15.0074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Valan Arasu M, Ilavenil S, Kim DH, Gun Roh S, Lee JC, Choi KC. In vitro and in vivo enhancement of adipogenesis by Italian ryegrass (Lolium multiflorum) in 3T3-L1 cells and mice. PLoS ONE. 2014;9:e85297. doi: 10.1371/journal.pone.0085297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Choi KC, Son YO, Hwang JM, Kim BT, Chae M, Lee JC. Antioxidant, anti-inflammatory and anti-septic potential of phenolic acids and flavonoid fractions isolated from Lolium multiflorum. Pharm Biol. 2017;55:611–619. doi: 10.1080/13880209.2016.1266673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hwang JM, Choi KC, Bang SJ, Son YO, Kim BT, Kim DH, Choi GJ, Kim DH, Shi X, Lee JC. Anti-oxidant and anti-inflammatory properties of methanol extracts from various crops. Food Sci Biotechnol. 2013;22:265–272. doi: 10.1007/s10068-013-0076-y. [DOI] [Google Scholar]
- 10.Shankar SS, Ahmad A, Sastry M. Geranium leaf assisted biosynthesis of silver nanoparticles. Biotechnol Prog. 2003;19:1627–1631. doi: 10.1021/bp034070w. [DOI] [PubMed] [Google Scholar]
- 11.Badkas A, Frank E, Zhou Z, Jafari M, Chandra H, Sriram V, Lee JY, Yadav JS. Modulation of in vitro phagocytic uptake and immunogenicity potential of modified Herceptin®-conjugated PLGA-PEG nanoparticles for drug delivery. Colloids Surf B Biointerfaces. 2018;162:271–278. doi: 10.1016/j.colsurfb.2017.12.001. [DOI] [PubMed] [Google Scholar]
- 12.Dawn A, Chandra H, Ade-Browne C, Yadav J, Kumari H. Multifaceted supramolecular interactions from C-Methylresorcin[4]arene lead to an enhancement in in vitro antibacterial activity of gatifloxacin. Chem Eur J. 2017;23:18171–18179. doi: 10.1002/chem.201704291. [DOI] [PubMed] [Google Scholar]
- 13.Dakal TC, Kumar A, Majumdar RS, Yadav V. Mechanistic basis of antimicrobial actions of silver nanoparticles. Front Microbiol. 2016;16:1831. doi: 10.3389/fmicb.2016.01831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bankalgi SC, Londonkar RL, Madire U, Tukappa NKA. Biosynthesis, characterization and antibacterial effect of phenolics-coated silver nanoparticles using Cassia javanica L. J Clust Sci. 2016;27:1485–1497. doi: 10.1007/s10876-016-1016-9. [DOI] [Google Scholar]
- 15.Mohanta YK, Panda SK, Bastia AK, Mohanta TK. Biosynthesis of silver nanoparticles from Protium serratum and investigation of their potential impacts on food safety and control. Front Microbiol. 2017;8:626. doi: 10.3389/fmicb.2017.00626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Benakashani F, Allafchian AR, Jalali SAH. Biosynthesis of silver nanoparticles using Capparis spinosa L. leaf extract and their antibacterial activity. Karbala Int J Mod Sci. 2016;2:251–258. doi: 10.1016/j.kijoms.2016.08.004. [DOI] [Google Scholar]
- 17.Fatimah I. Green synthesis of silver nanoparticles using extract of Parkia speciosa Hassk pods assisted by microwave irradiation. J Adv Res. 2016;7:961–969. doi: 10.1016/j.jare.2016.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sipe GR, Polk HD. Japanese tendergreen mustard, Italian rye grass and oats as a source of green feed for laying hens. Poult Sci. 1941;20:406–412. doi: 10.3382/ps.0200406. [DOI] [Google Scholar]