Abstract
The ascomycetous dark septate endophytic (DSE) fungi characterized by their melanized hyphae can confer abiotic stress tolerance in their associated plants in addition to improving plant growth and health. In this study inoculation of the DSE fungus Nectria haematococca Berk. & Broome significantly improved all the plant growth parameters like the plant height, stem girth, leaf characteristics and plant biomass of drought-stressed tomato. Root characters like the total root length, primary root diameter, 2nd order root number and diameter, root hair number and length were also significantly influenced by the fungal inoculation. Nevertheless, N. haematococca inoculation did not affect root colonization by native arbuscular mycorrhizal (AM) fungi and no significant correlation existed between the AM and DSE fungal variables examined. The proline accumulation in shoots of N. haematococca inoculated plants was significantly higher than uninoculated plants. The present study clearly indicates for the first time the ability of the DSE fungus, N. haematococca in inducing the drought stress tolerance and promoting the growth of the host plant under water stress.
Keywords: Water stress, DSE fungus, AM fungi, Plant growth, Proline, Root length, Root hairs
Introduction
A diverse range of fungi colonizes plant roots growing in natural soils. Of these, the dark septate endophytic (DSE) fungi which are often the conidial or sterile ascomycetous fungi, colonize healthy root tissues of plants, both inter and intracellularly without causing any obvious negative effects, such as tissue distortion, or forming any typical mycorrhizal structures [1]. Melanization of the septate hyphae and microsclerotia gives DSE their characteristic dark colour. Studies have also shown that the colonizing septate hyphae of the DSE fungi at times may also be hyaline [1]. However, the hyaline hyphae were found to be continuous with stained or melanized structures [1]. Several studies have demonstrated the plant growth promoting ability of DSE fungi in different plant species (see [1] and references therein); however, the role of these fungi in imparting stress tolerance in plants against different types of biotic or abiotic stresses is not adequately explored. Further, Newsham [1] in his meta-analysis on the response of plants to DSE fungal association emphasized the need to understand how the parameters like plant height and root length which determine the fitness in plants growing under natural conditions respond to DSE inoculation, as there are few studies that have assessed these variables. The arbuscular mycorrhizal (AM) fungi belonging to Glomeromycota are the most common colonizers of plant roots than DSE fungi and both these fungi often coexist within the same root system [2]. Arbuscular mycorrhizal fungi aids plants in the acquisition of water and nutrients especially the phosphorus (P), nitrogen (N) and other micronutrients from the soil in exchange for host carbon. These fungi also protect the host plant against various types of environmental stresses [3]. Therefore it is essential to understand the interaction between these fungi as they share the same niche within plant roots.
Nectria haematococca Berk. & Broome, the perfect state of the ascomycetous fungus Fusarium solani (Mart.) Sacc. belongs to the “F. solani species complex” that includes more than 50 species [4]. Many members of this complex cause disease in more than 100 genera of plants and are also opportunistic pathogens of humans [5]. Nevertheless, N. haematococca occurs as endophytes in roots of several plant species [5, 6]. Nectria haematococca is the dominant (18.4%) endophyte colonizing the roots of Panax ginseng Meyer [6] and metabolites of this fungus possess antibacterial activity against bacterial pathogens [7]. We had earlier isolated N. haematococca from healthy root tissues of Chrysanthemum indicum L., growing in Western Ghats region of southern India. The test plant tomato (Solanum lycopersicum L.) used in the present study is not only a widely used vegetable in the world; it is also a popular research material and a model plant in many biological studies. Previous studies do indicate that roots of tomato can be simultaneously colonized by both AM and DSE fungi [8]. As root colonizing DSE fungi are known to improve plant growth and stress tolerance in plants we investigated the effect of the fungal endophyte N. haematococca on the growth of tomato plants subjected to drought stress together with the status of natural association with AM fungi.
Materials and Methods
Seed Material, Fungus and Plant Culture
Seeds of tomato (var. CO-2) were obtained from Tamil Nadu Agricultural University, Coimbatore, India. Six seeds were sown in each plastic containers (9 × 6 cm) containing 273.5 g of normal field soil. Alfisol soil for the experiment was collected from the Botanical Garden of the Botany Department, Bharathiar University, Coimbatore, India. The Alfisol soil had a pH of 7.30 (1:3, soil: water), an electrical conductivity of 0.40 dSm−1, 119 mg/kg of total N, 9.4 mg/kg of available P and 320 mg/kg of exchangeable potassium (K) as determined by standard methods [9]. After germination, the seedlings were thinned to four seedlings per container.
The seeded containers were irrigated with tap water to maintain the soil moisture at field capacity. N. haematococca strain RSL02 (GenBank: KU355862.1) isolated from the roots of C. indicum and maintained on Potato Dextrose Agar (PDA) medium at 26 ± 2 °C was used in the study.
Estimation of AM and DSE Fungal Propagules in the Experimental Soil
The number of AM and DSE fungal propagules per gram of the experimental soil was quantified using the most probable number (MPN) technique [10] and were expressed as the number of AM/DSE infective propagules per gram of soil. The experimental soil had 180 propagules of AM fungi and 107 propagules of DSE fungi per gram of air-dried dry soil.
Preparation of DSE Fungal Inoculum
A carrier-based inoculum of N. haematococca was prepared and used for the study. The fungus was cultured in potato dextrose broth for 2 weeks. The fungal mat was washed, dried and mixed with the carrier talcum powder in the ratio of 1:10. To this mixture, 5 g of carboxy methyl cellulose per 50 g of the carrier was added, mixed and stored under refrigeration at 4 °C.
Experimental Design
The drought stress tolerance of tomato plants was assessed with and without inoculation of the fungal endophyte in unsterilized soil. Three grams of the fungus inoculum containing 6 ×106 colony forming units per gram was layered 1 cm below the seeding hole. Experimental treatments included: negative control—only unsterilized soil without carrier or fungal inoculum, positive control—unsterilized soil with carrier and N. haematococca inoculated —unsterilized soil inoculated with carrier-based N. haematococca inoculum. Ten replicates were maintained for each treatment with four plants in each container (replication) during the experimental period. The treatments were arranged in a completely randomized block design and the plants were maintained under normal irrigation (field capacity) until 6 weeks of growth in a poly house under natural conditions. After 6 weeks, the plants were subjected to five continuous drought cycles before harvest. The drought cycle was induced by withholding water for 2 days allowing the soil to completely dry before watering.
Harvest and Measurements
After 2 days of the fifth drought cycle, the plants were harvested and five replicates from each treatment were chosen randomly for the measurement of shoot and root growth parameters. Leaf area was measured according to Pandey and Singh [11]. Proline content of the plant samples (shoot and root) was estimated according to Sadasivam and Manickam [12] and expressed for fresh weight using the formula: Proline (µmoles/g tissue) = (µg proline/ml × ml toluene/115.5) × (5/g sample); where 115.5 is the molecular weight of proline.
A weighed portion of each root system was taken and fixed in formalin-acetic acid-alcohol (FAA) solution for the assessment of the endophytic fungal colonization. The root characters like numbers and diameter of 1st and 2nd order root were measured with three root bits in each (of the five) replicate and the root hairs number and diameter were also measured with root bits of 1 cm size. These root characters were measured by keeping the fresh or fixed root bits in a clean glass slide with cover glass over it in an Olympus BX 51 bright field microscope (×10) fitted with a calibrated ocular scale. The total root length was determined according to Newman [13].
Preparation of Roots for Endophytic Fungal Assessment
Fixed roots were washed, cut into 1-cm fragments, cleared in 2.5% KOH at 90 °C, acidified with 5 N HCl and stained with trypan blue (0.05% in lactoglycerol) overnight. The percentage of root length colonized or containing different DSE and AM fungal structures was quantified according to McGonigle et al. [14].
Statistical Analysis
All the data were subjected to one-way or two-way analysis of variance (ANOVA) after testing the data for homogeneity (Levene’s test) using SPSS (version 16.0). Means were compared with Duncan’s multiple range test (P < 0.05). The data on AM and DSE fungal colonization were arcsine square root transformed prior to statistical analysis. Pearson’s correlation coefficient was calculated to assess the relationship between plant growth parameters, AM and DSE fungal variables.
Results and Discussion
Plant Growth Promotion
The results of this study show the potential and feasibility of using the DSE fungus, N. haematococca to improve plant growth under drought stress as inoculation of this fungus significantly and positively improved all the growth parameters of tomato under water limiting condition (Table 1). This is in accordance with studies where inoculation of DSE fungi has been shown to improve the growth of tomato and other plant species under normal or stressed conditions [1, 15]. Fungal inoculation increased plant height (Table 1) similar to the observations reported in rice (Oryza sativa L.) [16] and sorghum [Sorghum bicolor (L.) Moench] [17] inoculated with different DSE fungi under drought. Contrary to the observations of Vergara et al. [15] where the stem thickness of tomato failed to vary with inoculation of three different unidentified DSE fungi, the stems of N. haematococca inoculated tomato plants in the present study was thicker over the different controls (Table 1).
Table 1.
Influence of Nectria haematococca on growth characters of drought stressed tomato plants in an unsterilized Alfisol soil
| Treatment | Plant height (cm) | Stem girth (cm) | Leaf number (per plant) | Leaflet number (per leaf) | Leaf area (sq.cm/leaf) |
|---|---|---|---|---|---|
| NC | 6.50 ± 0.50b | 0.56 ± 0.07b | 5.05 ± 0.18b | 10.00 ± 0.45b | 4.05 ± 0.45b |
| PC | 6.93 ± 0.16b | 0.65 ± 0.03b | 3.55 ± 0.367c | 9.50 ± 0.79b | 3.30 ± 0.40b |
| NH | 9.48 ± 0.28a | 0.84 ± 0.04a | 6.15 ± 0.40a | 17.40 ± 0.79a | 7.65 ± 0.33a |
| (F2,14) | 22.287*** | 8.708** | 45.032*** | 45.032*** | 34.424*** |
Means in a column followed by a same letter(s) are not significantly (P > 0.05) different according to Duncan’s Multiple Range Test
NC Negative control, PC Positive control, NH-N. haematococca inoculated
**, ***Significant at 0.1% level and 0.01% level
Leaf Characteristics
The leaf characteristics of the negative control plants were higher compared to the positive control plants (Table 1). The influence of carrier talc on plant growth appears to be an indirect effect as its application to soil or culture medium has been shown to affect soil fungal and bacterial populations variedly [18]. Tomato plants inoculated with N. haematococca had more leaves, leaflets per leaf and leaf area (Table 1). This positive influence of N. haematococca on tomato leaf characteristics is similar to those observed by Vergara et al. [15] for DSE fungi colonized tomato. Many DSE fungi including Fusarium are known to produce phytohormones that affect leaf development [19]. Moreover, Vergara et al. [15] speculated that the increased cell division and cell enlargement due to higher K and N contents were responsible for the increased total leaf area of DSE fungi colonized tomato plants.
Root Characteristics
Under drought stress, overall root characteristics of tomato plants differed significantly (Table 2). Of these, total root length, the diameter of primary roots, numbers and diameter of 2nd order lateral roots, root hair numbers and length varied significantly with different treatments. While the number and diameter of 1st order lateral roots and root hair diameter were not influenced by N. haematococca inoculation (Table 2), an increase in root length is also reported in Epimedium wushanense T. S. Ying inoculated with DSE fungi [20]. These observations are in direct contrast to a study where inoculation of the DSE fungus DSE48 reduced the root length and thickness of the tomato plant [21]. Likewise, the number and length of root hairs of N. haematococca inoculated plants was significantly higher when compared to both the controls (Table 2). Though the effect of DSE fungi on root hair characteristics is unknown, Vergara et al. [16] speculated that the increased root biomass and nutrient uptake in DSE fungi inoculated rice was due to more lateral and secondary roots and root hairs. Changes in root architecture by N. haematococca clearly suggest that DSE fungi can modulate root architecture like the mycorrhizal fungi thereby increasing the efficiency of the plant root systems.
Table 2.
Influence of Nectria haematococca on root characteristics of tomato plants under drought stress conditions in unsterilized soil
| Treatment | Total root length (cm) | Primary root diameter (µm) | Lateral root | Root hairs | |||||
|---|---|---|---|---|---|---|---|---|---|
| Number (per plant) | Diameter (µm) | Number (per cm root) | Length (µm) | Diameter (µm) | |||||
| 1st order | 2nd order | 1st order | 2nd order | ||||||
| NC | 21.92 ± 1.31a | 294.00 ± 17.04b | 26.55 ± 2.14a | 17.50 ± 1.73b | 162.67 ± 9.51a | 104.00 ± 6.78b | 122.67 ± 24.62b | 129.20 ± 3.09a | 11.33 ± 0.82a |
| PC | 24.40 ± 1.52ab | 216.00 ± 6.09a | 29.20 ± 1.83a | 15.40 ± 1.96b | 146.00 ± 20.37a | 88.80 ± 2.98a | 103.33 ± 39.48b | 130.40 ± 1.33a | 10.67 ± 0.66a |
| NH | 27.71 ± 0.93a | 309.33 ± 20.02b | 32.80 ± 1.65a | 47.25 ± 2.05a | 176.17 ± 20.56a | 104.17 ± 9.71b | 384.33 ± 88.57a | 168.80 ± 2.96b | 15.50 ± 2.26a |
| (F2,14) | 5.143* | 10.314** | 2.783 ns | 85.974*** | 0.738ns | 10.970** | 7.383* | 75.952*** | 3.308ns |
Means in a column followed by a same letter(s) are not significantly (P > 0.05) different according to Duncan’s Multiple Range Test
NC Negative control, PC Positive control, NH-N. haematococca inoculated
***Significant at 0.1% level, *Significant at 5% level, ns non significant
Biomass
Root and shoot dry weights were found to be significantly higher for N. haematococca inoculated plants than the control plants (Table 3) as shown in recent studies by Vergara et al. [15, 16] and in the meta-analysis on plant growth response to DSE fungal inoculation [1, 22]. In contrast, some studies have reported a neutral or negative response for shoot biomass of DSE fungal inoculated plants [1]. Although different mechanisms like the facilitation of nutrient uptake by the host plant, increased synthesis of phytohormones (abscisic acid), protection against soil-borne pathogens and modification of the antioxidant activities [1, 15, 16] have been speculated for the DSE fungal mediated plant growth, the actual mechanisms behind this are rather unclear.
Table 3.
Influence of Nectria haematococca on biomass and root/shoot ratio of drought stressed tomato plants in an unsterilized Alfisol soil
| Treatment | Shoot dry weight (mg) | Root dry weight (mg) | Root/shoot ratio |
|---|---|---|---|
| NC | 17.14 ± 2.27b | 7.17 ± 1.18b | 0.42 ± 0.04a |
| PC | 21.26 ± 2.06b | 10.94 ± 1.79b | 0.51 ± 0.08a |
| NH | 50.43 ± 3.38a | 25.47 ± 2.17a | 0.59 ± 0.11a |
| (F2,14) | 47.44*** | 30.04*** | 1.05ns |
Means in a column followed by a same letter(s) are not significantly (P > 0.05) different according to Duncan’s Multiple Range Test
NC Negative control, PC Positive control, NH-N. haematococca inoculated
***Significant at 0.01% level, ns non significant
R/S Ratios
One of the strategies plants often adapt to water or nutrient limiting conditions is to increase in their R/S ratios to capture more resources [23]. Drought stress increased the R/S ratios of N. haematococca inoculated tomato plants compared to control plants (Table 3). This clearly shows that N. haematococca inoculated tomato plants portioned more resources to the roots than uninoculated plants. The allocation of more resources to increase root growth during drought stress in different plant species is dependent upon various plant and environmental factors [23].
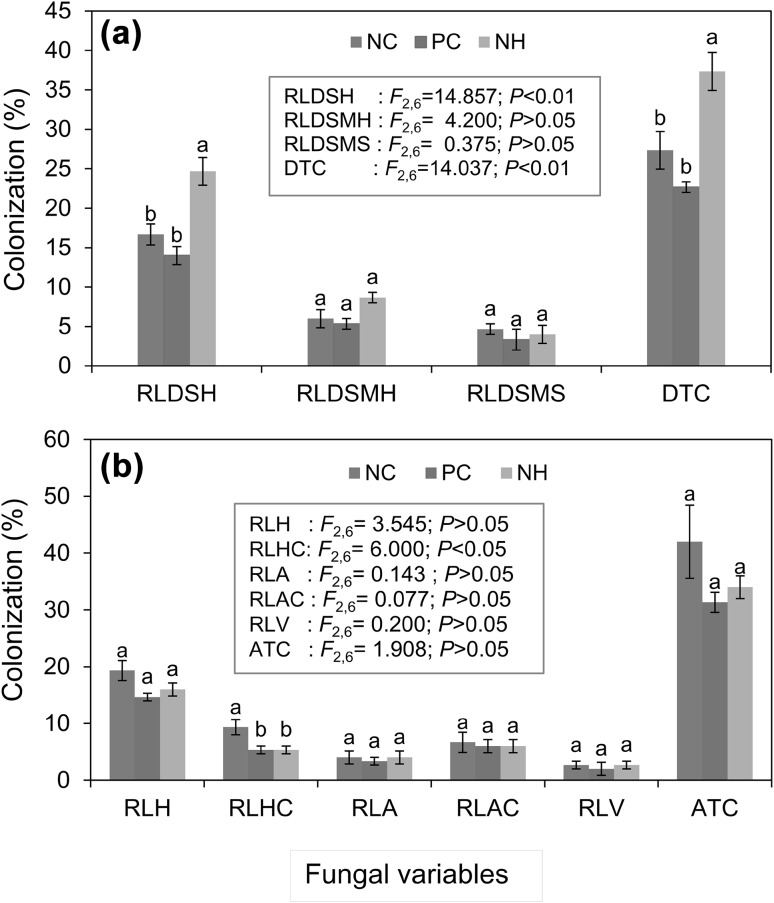
DSE Fungal Colonization
Though N. haematococca inoculation failed to significantly affect the percentage of root length with moniliform hyphae and microsclerotia it positively influenced root length with dark septate hyphae and total colonization under water stress (Fig. 1a). Contrarily drought negatively affected DSE fungal colonization in citrus (Citrus tangerina Tanaka) [24] and sorghum [17]. In the present study, the negative carrier effect on DSE colonization observed is in line with Walter et al. [25] who showed that the properties of the inoculum including the carrier can affect the soil colonization by the fungus Trametes versicolor (L.) Lloyd.
Fig. 1.
Root colonization with natural arbuscular mycorrhizae (a) and dark septate endophyte (b) fungal colonization of tomato plants inoculated with Nectria haematococca. Error bars indicate ± standard errors. Bars bearing same letter for a fungal structure are not significantly (P > 0.05) different according to Duncan’s Multiple Range test. RLH, RLHC, RLA, RLAC, RLV and ATC, represent root length (RL) with hyphae, hyphal coils, arbuscules, arbusculate coils, vesicle and total colonization, respectively. RLDSH, RLDSMH, RLDSMS, DTC, represent RL with dark septate hyphae, moniliform hyphae, microsclerotia and total colonization respectively. NC, PC and NH, represent negative control, positive control and N. haematococca inoculated, respectively
Native AM Fungal Colonization
Contrary to the observations where exudates of Fusarium sp. significantly inhibited mycorrhizal development [26], the decline in total root colonization or the root length containing different AM fungal structures of the indigenous AM fungi in N. haematococca inoculated tomato roots under drought stress was not significant (Fig. 1b). Moreover, the lack of DSE fungal influence on native AM fungi is also evidenced by the lack of correlation between these fungal variables (Table 4). This is similar to studies where a lack of response in AM colonization to DSE fungal inoculation has been reported [27]. In contrast to other AM fungal structures, the percentage root length with hyphal coils was significantly higher for N. haematococca inoculated plants (Fig. 1b). Like arbuscules, hyphal coils are modified fungal hyphae that provide a large interfacial area for resource exchange between the symbionts [3].
Table 4.
Pearson’s correlation coefficients for the percentage root length colonization of dark septate endophytic and arbuscular mycorrhizal fungal variables in tomato under drought stress (n = 9)
| Fungal parameters | Dark septate endophytic fungia | |||
|---|---|---|---|---|
| RLDSH | RLDMH | RLDMS | DTC | |
| Arbuscular mycorrhizal fungib | ||||
| RLH | 0.011 | 0.354 | 0.204 | 0.157 |
| RLHC | − 0.103 | 0.000 | 0.354 | 0.010 |
| RLA | 0.378 | 0.533 | 0.185 | 0.472 |
| RLAC | − 0.145 | 0.316 | 0.274 | 0.048 |
| RLV | 0.466 | 0.438 | 0.000 | 0.466 |
| ATC | 0.089 | 0.400 | 0.300 | 0.250 |
aRLDSH, RLDMH, RLDMS and DTC, root length containing dark septate fungal hyphae, moniliform hyphae, microsclerotia and total colonization respectively
bRLH, RLHC, RLA, RLAC, RLV and ATC, root length containing arbuscular mycorrhizal fungal hyphae, hyphal coils, arbuscules, arbusculate coils, vesicles and total colonization respectively
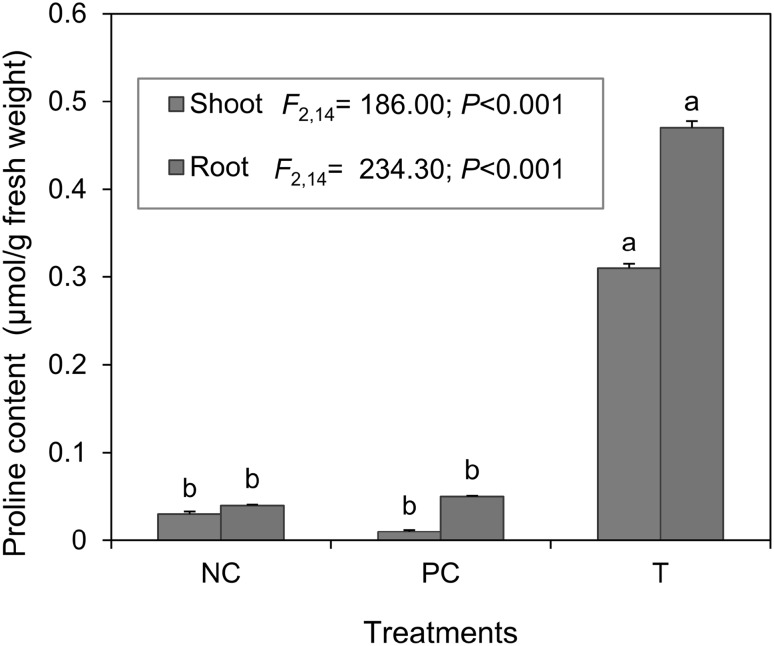
Proline Content
Proline content in roots and shoots of tomato was significantly influenced by N. haematococca inoculation (Fig. 2). This is in accordance with Bayat et al. [28] who showed that Festuca arundinacea plants infected by Neotyphodium coenophialum accumulated more proline in their shoots than uninfected plants under drought. Generally, plants proline content increases under stress conditions to maintain the osmotic potential and is a reliable indicator of environmental stress in tomato [29]. Therefore these observations clearly establish that proline accumulation play an important role in DSE fungi mediated alleviation of stress tolerance in plants [30].
Fig. 2.
Proline content of root and shoot tissues of tomato plants inoculated with Nectria haematococca. Error bars indicate ± standard errors. Bars bearing same letter for a fungal structure are not significantly (P > 0.05) different according to Duncan’s Multiple Range test. NC, PC and NH, represent negative control, positive control and N. haematococca inoculated, respectively
Conclusion
From the observations of the present study, it could be concluded that inoculation of the DSE fungus N. haematococca may induce tolerance in the host plants. Synthesis of secondary metabolites is one of the important defense mechanisms in plants and some evidence also show that secondary metabolites associated with DSE fungal association could modulate plant growth under drought stress conditions. The present study clearly demonstrated for the first time that N. haematococca symbiosis can enhance tolerance of the tomato plants against drought stress. The study also highlighted that in spite of improving plant growth and proline accumulation; N. haematococca inoculation did not adversely affect mycorrhizal association in tomato plants under stress conditions. As a result, N. haematococca inoculation could serve as an effective tool for improving crop production under drought stress. Nevertheless, this has to be tested using different crop species and diverse soil types.
Authors’ Contributions
TM conceived the research and designed the experiment, PPSV carried out the experiment, and TM and PPSV wrote the manuscript. All authors read and approved the final manuscript.
Compliance with Ethical Standards
Conflict of interest
The Authors declared that they have no conflict of interests.
References
- 1.Newsham KK. A meta-analysis of plant responses to dark septate root endophytes. New Phytol. 2011;190:783–793. doi: 10.1111/j.1469-8137.2010.03611.x. [DOI] [PubMed] [Google Scholar]
- 2.Muthukumar T, Sathiyadash K, Valarmathi V. Arbuscular mycorrhizal and dark septate endophyte fungal associations in plants of different vegetation types in Velliangiri hills of Western Ghats, Southern India. Acta Bot Hung. 2018;60:185–222. doi: 10.1556/034.60.2018.1-2.9. [DOI] [Google Scholar]
- 3.Smith SE, Read D. Mycorrhizal symbiosis. London: Academic Press; 2008. [Google Scholar]
- 4.Coleman JJ, Rounsley SD, Rodriguez-Carres M, Kuo A, Wasmann CC, GrimwoodJ Schmutz J, Taga M, White GJ, Zhou S, Schwartz DC, Freitag M, Ma LJ, VanEtten HD. The genome of Nectria haematococca: contribution of supernumerary chromosomes to gene expansion. PLoS Genet. 2009;5(8):e1000618. doi: 10.1371/journal.pgen.1000618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Summerell BA, Laurence MH, Liew ECY, Leslie JF. Biogeography and phylogeography of Fusarium: a review. Fungal Divers. 2010;44:3–13. doi: 10.1007/s13225-010-0060-2. [DOI] [Google Scholar]
- 6.Wu H, Yang HY, You XL, Li YH. Diversity of endophytic fungi from roots of Panax ginseng and their saponin yield capacities. SpringerPlus. 2013;2:107. doi: 10.1186/2193-1801-2-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Banhos EF, Souza AQL, Andrade JC, Souza ADL, Koolen HHF, Albuquerque PM. Endophytic fungi from Myrcia guianensis at the Brazilian Amazon: distribution and bioactivity. Braz J Microbiol. 2014;245:153–161. doi: 10.1590/S1517-83822014005000027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Muthukumar T, Sathya R. Endorhizal fungal association and colonization patterns in Solanaceae. Polish Bot J. 2017;62:287–299. doi: 10.1515/pbj-2017-0016. [DOI] [Google Scholar]
- 9.Jackson ML. Soil chemical analysis. New Delhi: Prince Hall of India (P) Limited; 1973. [Google Scholar]
- 10.Feldmann F, Idezak E. Inoculum production of vesicular-arbuscular mycorrhizal fungi for use in tropical nurseries. In: Norris JR, Read DJ, Varma AK, editors. Techniques for Mycorrhiza. I. Research methods in microbiology. London: Academic Press; 1992. pp. 799–817. [Google Scholar]
- 11.Pandey S. K., Singh Hema. A Simple, Cost-Effective Method for Leaf Area Estimation. Journal of Botany. 2011;2011:1–6. doi: 10.1155/2011/658240. [DOI] [Google Scholar]
- 12.Sadasivam S, Manickam A. Biochemical methods. 2. New Delhi: New Age International (P) Limited Publishers; 1996. pp. 42–43. [Google Scholar]
- 13.Newman EI. A method for estimating the total length of root in a sample. J Appl Ecol. 1966;3:139–145. doi: 10.2307/2401670. [DOI] [Google Scholar]
- 14.McGonigle TP, Miller MH, Evans DG, Fairchild GL, Swan JA. A new method which gives an objective measure of colonization of roots by vesicular-arbuscular mycorrhizal fungi. New Phytol. 1990;115:495–501. doi: 10.1111/j.1469-8137.1990.tb00476.x. [DOI] [PubMed] [Google Scholar]
- 15.Vergara C, Araujo KEC, Urquiaga S, Schultz N, Balieiro FC, Medeiros PS, Santos LA, Xavier GR, Zilli Jerri E. Dark septate endophytic fungi help tomato to acquire nutrients from ground plant material. Front Microbiol. 2017 doi: 10.3389/fmicb.2017.02437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vergara C, Araujo KEC, Alves LS, Souza SR, Santos LA, Santa-Catarina C, Silva KD, Pereira GMD, Xavier GR, Zilli JÉ. Contribution of dark septate fungi to the nutrient uptake and growth of rice plants. Braz J Microbiol. 2018;49:67–78. doi: 10.1016/j.bjm.2017.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang Q, Gong M, Yuan J, Hou Y, Zhang H, Wang Y, Hou X. Dark septate endophyte improves drought tolerance in sorghum. Int J Agric Biol. 2017;19:53–60. doi: 10.17957/IJAB/15.0241. [DOI] [Google Scholar]
- 18.Umamaheswari T, Srimeena N, VasanthiN, Cibichakravarthy B, Anthoniraj S, Karthikeyan S (2016) Silica as biologically transmutated source for bacterial growth similar to carbon. Matts Arc 10.19185/matters.201511000005
- 19.Di X, Takken FLW, Tintor N. How phytohormones shape interactions between plants and the soil-borne fungus Fusarium oxysporum. Front Plant Sci. 2016 doi: 10.3389/fpls.2016.00170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhu ZB, Fan JY, Guo QS, Liu ZY, Zhu GS. The growth and medicinal quality of Epimedium wushanense are improved by an isolate of dark septate fungus. Pharm Biol. 2015;53:1344–1351. doi: 10.3109/13880209.2014.982296. [DOI] [PubMed] [Google Scholar]
- 21.Andrade-Linares DR, Grosch R, Restrepo S, Krumbein A, Franken P. Effects of dark septate endophytes on tomato plant performance. Mycorrhiza. 2011;21:413–422. doi: 10.1007/s00572-010-0351-1. [DOI] [PubMed] [Google Scholar]
- 22.Alberton O, Kuyper TW, Summerbell RC. Dark septate root endophytic fungi increase growth of Scots pine seedlings under elevated CO2 through enhanced nitrogen use efficiency. Plant Soil. 2010;328:459–470. doi: 10.1007/s11104-009-0125-8. [DOI] [Google Scholar]
- 23.Xu W, Cui K, Xu A, Nie L, Huang J, Peng S. Drought stress condition increases root to shoot ratio via alteration of carbohydrate partitioning and enzymatic activity in rice seedlings. Acta Physiol Plant. 2015;37:9. doi: 10.1007/s11738-014-1760-0. [DOI] [Google Scholar]
- 24.Wu QS, Zou YN. Beneficial roles of arbuscular mycorrhizas in citrus seedlings at temperature stress. Sci Hortic. 2010;125:289–293. doi: 10.1016/j.scienta.2010.04.001. [DOI] [Google Scholar]
- 25.Walter M, Boyd-Wilson K, Boul L, Ford C, McFadden D, Chong B, Pinfold J. Field-scale bioremediation of pentachlorophenol by Trametes versicolor. Int Biodeterior Biodegrad. 2005;56:51–57. doi: 10.1016/j.ibiod.2005.05.003. [DOI] [Google Scholar]
- 26.Elsharkawy MM, Shimizu M, Takahashi H, Hyakumachi M. The plant growth-promoting fungus Fusarium equiseti and the arbuscular mycorrhizal fungus Glomus mosseae induce systemic resistance against Cucumber mosaic virus in cucumber plants. Plant Soil. 2012;361:397–409. doi: 10.1007/s11104-012-1255-y. [DOI] [Google Scholar]
- 27.Saravesi K, Ruotsalainen AL, Cahill JF. Contrasting impacts of defoliation on root colonization by arbuscular mycorrhizal and dark septate endophytic fungi of Medicago sativa. Mycorrhiza. 2014;24:239–245. doi: 10.1007/s00572-013-0536-5. [DOI] [PubMed] [Google Scholar]
- 28.Bayat F, Mirlohi A, Khodambashi M. Effects of endophytic fungi on some drought tolerance mechanisms of tall fescue in a hydroponics culture. Russ J Plant Physiol. 2009;56:563–570. doi: 10.1134/S1021443709040104. [DOI] [Google Scholar]
- 29.Claussen W. Proline as a measure of stress in tomato plants. Plant Sci. 2005;168:241–248. doi: 10.1016/j.plantsci.2004.07.039. [DOI] [Google Scholar]
- 30.Verbruggen N, Hermans C. Proline accumulation in plants: a review. Amino Acids. 2008;35:753–759. doi: 10.1007/s00726-008-0061-6. [DOI] [PubMed] [Google Scholar]