Abstract
Uveitis (UVT), an inflammatory disease of the eye significantly contributes to vision impairment and blindness. Uveitis is associated with systemic infectious and autoimmune diseases, but in most cases, the aetiology remains unidentified. Dysbiosis in the gut microbiome has been implicated in autoimmune diseases, inflammatory diseases, cancers and mental disorders. In a mice model of autoimmune UVT, it was observed that manipulating the gut microbiome reduces the inflammation and disease severity. Further, alterations in the bacterial gut microbiome and their metabolites were reported in UVT patients from a Chinese cohort. Hence, it is worth comparing the bacterial gut microbiome of UVT patients with that of healthy controls (HC) to ascertain whether dysbiosis of the gut microbiome has implications in UVT. Our analyses showed reduced diversity of several anti-inflammatory organisms including Faecalibacterium, Bacteroides, Lachnospira, Ruminococcus and members of Lachnospiraceae and Ruminococcaceae families, and enrichment of Prevotella (proinflammatory) and Streptococcus (pathogenic) OTUs in UVT microbiomes compared to HC. In addition, decrease in probiotic and antibacterial organisms was observed in UVT compared to HC microbiomes. Heatmap and PCoA plots also indicated significant variations in the microbiomes of UVT versus HC. This is the first study demonstrating dysbiosis in the gut bacterial communities of UVT patients in an Indian cohort and suggests a role of the gut microbiome in the pathophysiology of UVT.
Electronic supplementary material
The online version of this article (10.1007/s12088-018-0746-9) contains supplementary material, which is available to authorized users.
Keywords: Uveitis, Gut microbiome, Dysbiosis, V3–V4 region of 16S rRNA gene, Illumina Miseq
Introduction
Uveitis (UVT) is a vision threatening inflammatory disease of the uveal tract (iris, ciliary body and choroid) and adjacent structures (retina, vitreous humor and optic nerve) of the eye. UVT is responsible for about 25% of blindness in the developing world [1] and affects people in the working age group (20–60 years) thus contributing to the socio-economic burden [2]. Generally, UVT is associated with either a systemic infection or an immunological disease. But, in 50% of the cases, the eye is the only affected organ [3] and the aetiology remains unknown and such cases of UVT are referred to as “idiopathic Uveitis”. Corticosteroids and cycloplegics are given to reduce inflammation and pain but recurrence of the disease is common.
Microbiome refers to the multi-species community of microorganisms (bacteria, fungi and viruses) residing in or on the body surface that varies in abundance and composition from niche to niche [4]. Alterations in the gut microbiome (dysbiosis) have been implicated in several diseases like auto-immune diseases (diabetes, rheumatoid arthritis, muscular dystrophy, multiple sclerosis, and fibromyalgia) [5], inflammatory diseases (obesity, enterocolitis, inflammatory bowel disease, and vaginosis) [6, 7], cancers and mental disorders [8, 9], probably mediated by the interaction of the gut microbiome with host immune system [10]. Several bacterial species within the gut microbiome that are either beneficial or deleterious to the host have also been identified. For example, Akkermansia muciniphila reduces diabetes in humans, Faecalibacterium prausnitzii protects mice against intestinal inflammation [11] whereas Klebsiella pneumoniae and Proteus mirabilis have been implicated in inducing spontaneous and maternally transmitted colitis in mice [12], and segmented filamentous bacteria were shown to drive autoimmune arthritis [13]. However, studies on the involvement of the gut microbiome in ocular diseases are rare. Studies on mice have implicated gut microbiome with autoimmune Uveitis [14] and had demonstrated that high-fat and high-glycemic index diet [15] induced gut dysbiosis drives inflammation and angiogenesis associated with age-related macular degeneration (AMD). Similar studies implicating dysbiosis in the human gut microbiome in dry eye disease in Sjögren syndrome patients [16], AMD patients [17] and more recently in Uveitis patients from a Chinese cohort [18] were reported. In the present study, the gut bacterial microbiomes of healthy controls (HC) and UVT patients categorized as idiopathic or autoimmune UVT were characterized from a south Indian population to ascertain whether dysbiosis in the gut microbiome is associated with UVT. Organisms that were either enriched or depleted in UVT patients compared to HC were also identified so as to help in disease management.
Materials and Methods
Recruitment of Subjects
Patients with UVT (n = 13) were recruited from individuals attending an eye hospital, the L. V. Prasad Eye Institute, Hyderabad, India. Only individuals with idiopathic (n = 10) and auto-immune (n = 3) UVT were recruited and those with infectious Uveitis or those having a systemic infection were eliminated to avoid confounding factors like infection that may also influence gut microbiome/UVT. Out of the 13 UVT patients, 10 were idiopathic UVT and 3 patients with Vogt–Koyanagi–Harada disease constituted the autoimmune group. Such an approach of using only idiopathic and autoimmune UVT patients would help to rule out that the changes that are observed in the microbiome due to inflammation are due to UVT and not due to the infection that caused inflammatory changes. UVT individuals with a systemic disease involvement like diabetes as judged by examination of blood glucose level, tuberculosis as judged by Mantoux, syphilis checked by Venereal Disease Research Laboratory test, HIV by HIV positive test, serum ACE (angiotensin converting enzyme level) for sarcoidosis and complete blood profile for any other systemic infection were not recruited in the study. Exclusion criteria for UVT patients included any significant ocular disease such as diabetic retinopathy (proliferative or non-proliferative), wet age-related macular degeneration, myopic degeneration with active sub-foveal choroidal neovascularization and presence of any form of ocular malignancy in either eye including choroidal melanoma. The UVT patients included 11 women and 2 men who were between 28 and 62 years of age, with mean ± SD age of 44.54 ± 12.64 years. HC (n = 13) who were age (22–62 years with mean ± SD age of 43.08 ± 12.99 years; P = 0.774), sex (male 2; P = 1.000; female 11; P = 0.441), ethnicity (P = 1.000) and diet (P = 1.000) matched with the UVT group were only included. The HC subjects were without any ocular pathology. The HC and UVT patients were from Telangana state in India. Exclusion criteria for all HC and UVT subjects was as follows: participants who had taken probiotics, prebiotics or antibiotics 3 months prior to sample collection; or who had undergone gastrointestinal tract surgery; or having diseases like diabetes, hypertension, obesity, inflammatory bowel disease, any form of malignancy; or having prolonged constipation or diarrhea. Informed consent was taken from all the study subjects prior to sample collection. The study protocols were approved by the Institutional Review Board of L. V. Prasad Eye Institute, Hyderabad (Ethics Ref. No. LEC 06-14-060) and all methods were performed in accordance with relevant guidelines and regulations.
Fecal Sample Collection and DNA Extraction
The stool samples were collected by the subjects in a sterile container (HiMedia, India) and frozen at − 80 °C until processing. Genomic DNA was extracted from 300 mg of homogenized fecal samples using QIAamp DNA stool minikit (Qiagen, Hilden, North Rhine-Westphalia, Germany) according to the instructions provided by the manufacturer. DNA extractions were performed in triplicate; equal volume of DNA from each replicate was pooled together and used for PCR amplification and sequencing. Genomic DNA was checked for quality on a 0.8% agarose gel and quantified using Qubit® 2.0 fluorometer (Life Technologies, India).
PCR Amplification, Illumina Library Preparation and Amplicon Sequencing
V3–V4 region of bacterial 16S rRNA gene was amplified using the primers 5′-CCTACGGGNGGCWGCAG-3′ and 5′-GACTACHVGGGTATCTAATCC-3′. The standard Illumina protocol was used to generate the bacterial microbiome libraries. The libraries were sequenced using 2 × 250 bp chemistry on an Illumina MiSeq at the Xcelris Genomics Pvt. Ltd., Ahmedabad, India.
Taxonomic Classification of Sequenced Reads
Paired-end reads of each sample were first demultiplexed into separate files (Fastq) and, combined into contigs using FLASH software [19]. Sequence reads were quality filtered using Prinseq-lite (reads with mean Phred score < 25 were removed). Chimeric sequences were removed using Usearch61. The leftover high quality reads (HQ) were used for OTU picking using QIIME. UCLUST run with default parameters was used for clustering sequences with 97% similarity. GreenGenes 13.8 OTUs clustered at 97% identity was used as the reference OTU database for bacteria. Taxonomic assignments for denovo clustered OTUs (denovo-OTUs) were obtained using MOTHUR (version v.1.29.2) and the Wang Classifier [20] with a bootstrap of 80%. Samples in which ≥ 60% of sequences were assigned to an OTU were only considered for further analysis. Sparse OTUs (representing < 0.001% of the total HQ reads) were not considered for further analysis.
Diversity Analyses of Bacterial Microbiome Samples
R-Vegan 2.4-2package (http://vegan.r-forge.r-project.org/) was used for rarefaction curves and for calculating the alpha diversity indices (Shannon diversity, Simpson index, observed number of OTUs and Chao1) for all the microbiomes. OTUs which were present in over 80% of samples with a minimum abundance of 0.01% in the sample were assigned as core OTUs.
Identification of Differentially Abundant Taxonomic Groups
Wilcoxon signed rank test (with Benjamini Hochberg (BH) corrected P < 0.05 as significant) was implemented to identify significantly different taxonomic groups in the bacterial microbiomes at various levels of hierarchy including phylum, family, genus and OTU. Further to assess the variation between the healthy controls (HC), idiopathic Uveitis (UVT_ID) and autoimmune Uveitis (UVT_AD), a Kruskal–Wallis test was first performed to identify significantly different taxonomic groups (BH corrected P < 0.05) in the gut microbiomes of the three groups. Subsequently to assess the variation in the above three groups, post hoc Wilcoxon tests (BH corrected P < 0.05) were performed between the microbiomes of HC vs. UVT_ID, HC vs. UVT_AD and UVT_ID vs. UVT_AD.
Differences in the HC and UVT bacterial microbiomes, at the OTU level, were also visualized through a PCoA plot using ade4 package in R (v3.2.5). JSD was used as a distance metric (http://enterotyping.embl.de/enterotypes.html). K-means clustering (k = 2) was also performed with the data to generate the PCoA plot to identify clusters on the PCoA plot.
Correlation Network Between Bacterial Genera
Two interactive networks specific to the HC and UVT microbiomes were generated to infer bacteria-bacteria interaction (both positive and negative interactions) at the genus level based on pair-wise correlations between abundances using Spearman correlation coefficient (r). Cytoscape [21] and CoNet [22] were used to visualize and analyse the network.
Results
Gut Bacterial Communities in Healthy Subjects and UVT Individuals
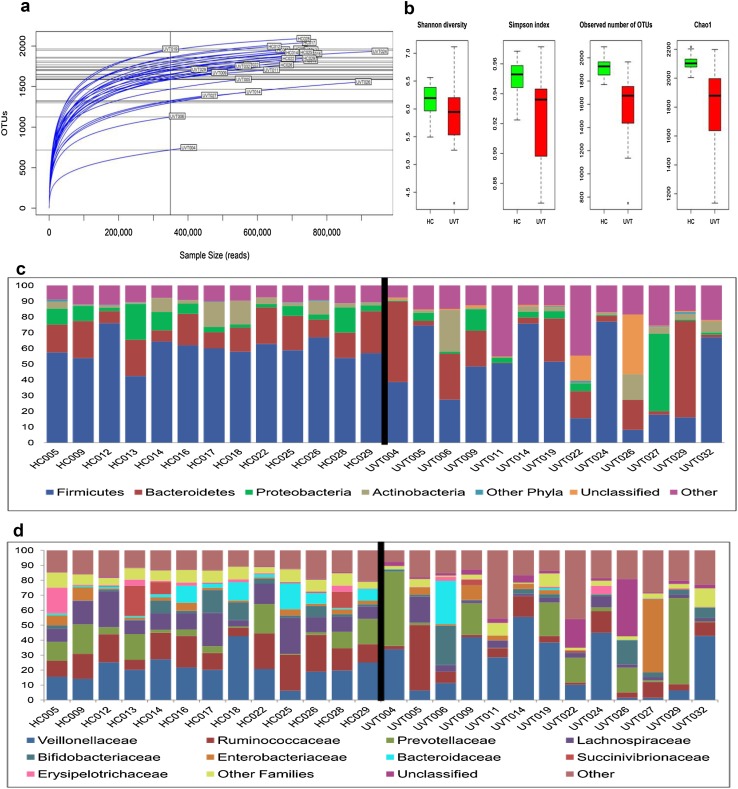
Twenty-six gut bacterial microbiomes (13 HC and 13 UVT) were generated and the average reads per microbiome were 765,389 (Online Resource 1). From the 26 microbiomes, 2992 OTUs were identified and consisted of 1380 reference and 1612 denovo-OTUs respectively (Online Resource 2). Rarefaction curves of all microbiomes were tending towards saturation indicating that the sequencing depth and coverage is adequate to capture the diversity in the microbiomes (Fig. 1a). Alpha diversity measured using Shannon diversity index, Simpson index (evenness), number of observed OTUs and Chao1 index (richness) indicated that both the number of species and diversity was higher in HC subjects compared to UVT patients (Fig. 1b).
Fig. 1.
a Rarefaction curves of the 26 gut bacterial microbiomes from healthy controls (HC) and Uveitis (UVT) patients. b Box-plots illustrating alpha diversity indices (Shannon diversity, Simpson index, Observed number of OTUs and Chao1) in gut microbiomes of healthy controls (HC) and Uveitis (UVT) patients. Median values (horizontal line) and interquartile ranges have been indicated in the plots. c Abundance of different bacterial phyla in the gut microbiomes of healthy controls (HC) and Uveitis (UVT) patients. “Other phyla” includes phyla with < 1% mean abundance. “Other” includes singletons, unassigned reads and sparse OTUs (< 0.001% of total abundance). d Abundance of different bacterial families in gut microbiomes of healthy controls (HC) and Uveitis (UVT) patients. “Other families” includes families with < 1% mean abundance. “Other” includes singletons, unassigned reads and sparse OTUs (< 0.001% of total abundance)
In the studied cohort, the bacterial gut microbiomes were dominated by the phyla Firmicutes, Bacteroidetes, Proteobacteria and Actinobacteria (Fig. 1c, Online Resource 3). The abundance of these four dominant phyla in HC and UVT microbiomes was not statistically significant. However, several low abundant phyla (mean abundance < 1%) showed significant variation (P < 0.05). A total of 63 bacterial families were identified in the microbiomes out of which 38 and 5 respectively were enriched in HC and UVT patients (BH corrected P < 0.05) (Fig. 1d, Online Resource 4). The predominant families were Veillonellaceae, Ruminococcaceae, Prevotellaceae and Lachnospiraceae among which Ruminococcaceae and Lachnospiraceae were enriched only in HC compared to UVT.
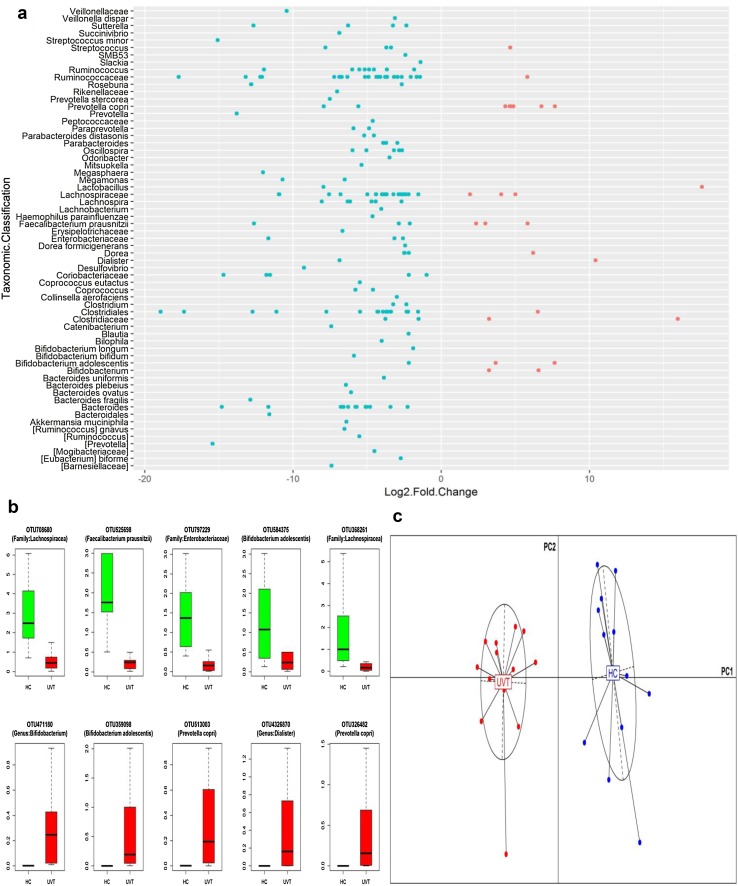
We also identified 171 and 34 core OTUs in HC and UVT samples respectively (Online Resource 5). At the core OTU level also, Ruminococaceae and Lachnospiraceae were increased in HC (24 and 22 OTUs respectively). Further, at the OTU level also we observed that more OTUs were enriched in HC (180 OTUs) compared to UVT where 24 OTUs were enriched (Fig. 2a). OTUs enriched in HC were affiliated to Lachnospiraceae (27 OTUs), Ruminococcaceae (25 OTUs), Clostridiales (16 OTUs) and Bacteroides (12 OTUs) where as in UVT microbiomes, Prevotella copri with 6 OTUs was predominantly enriched (Fig. 2a, b). PCoA plot (Fig. 2c) based on the relative abundances of differentiating OTUs in the HC and UVT gut bacterial microbiomes showed two separate clusters formed by HC and UVT microbiomes thus illustrating the distinct patterns of bacterial composition occurring in the HC and UVT gut microbiomes.
Fig. 2.
a Bacterial OTUs exhibiting significant (BH corrected P < 0.05) differential abundance in the gut microbiomes of Uveitis patients (UVT) compared to healthy controls (HC). OTUs having a median abundance of > 0.01% in at least one group of samples (HC or UVT) have been depicted. Out of 204 OTUs, 180 were enriched in HC and 24 were enriched in UVT patients. Data is represented as log2 fold change. Each circle denotes an OTU; OTUs to the right of zero line and shown in pink are enriched in UVT compared to HC and OTUs to the left of zero line and shown in blue are depleted in UVT compared to HC. OTUs are organized according to the lowest taxonomic classification along the y-axis. b Bacterial OTUs exhibiting significant (BH corrected P < 0.05) differential abundance in the gut microbiomes of healthy controls (HC, green) and Uveitis patients (UVT, red). Only the top 5 abundant OTUs enriched in HC or UVT samples have been depicted. The median abundances and the interquartile ranges have been indicated in the plots. c Principal Coordinate Analysis (PCoA) based on JSD (Jensen–Shannon divergence) distances between bacterial OTU abundance in the microbiomes of healthy controls (HC, blue) and Uveitis patients (UVT, red). Samples plotted along first two principal coordinates PC1 and PC2 showed distinct clustering of healthy controls (HC) and Uveitis (UVT) microbiomes
Differentially Abundant Bacterial Communities in the Gut Microbiomes of Healthy Controls and Uveitis Patients
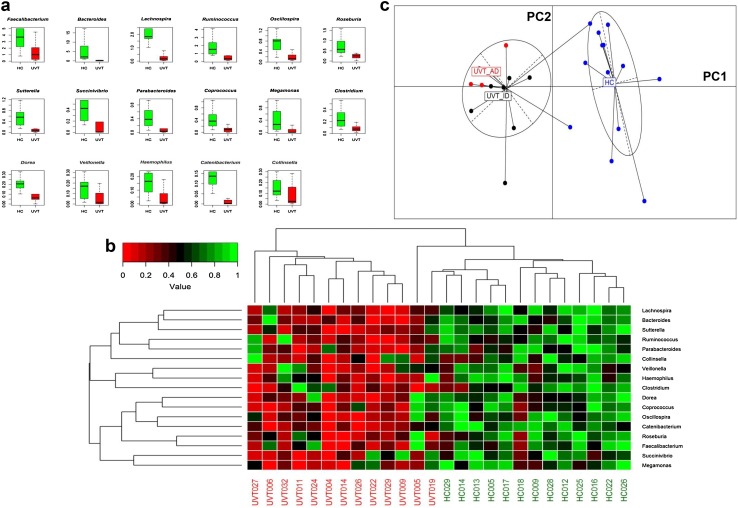
At the genera level, Faecalibacterium, Bacteroides, Lachnospira and Ruminococcus (median abundance > 1%) were abundantly enriched in HC compared to UVT patients gut microbiomes (Fig. 3a). No genera were enriched in UVT patients. Heatmap constructed with the 17 most abundant genera also showed a clear separation of HC and UVT microbiomes into two clusters (Fig. 3b). Two UVT microbiomes clustered away from the remaining UVT microbiomes and appeared to be more related to the HC microbiomes.
Fig. 3.
a Bacterial genera exhibiting significant (BH corrected P < 0.05) differential abundance in gut microbiomes of healthy controls (HC) compared to Uveitis (UVT) patients. Genera having a median abundance of > 0.1% in at least one group of samples (HC or UVT) have been depicted. Median abundances (horizontal line) and interquartile ranges have been indicated in the plots. b Two dimensional heatmap depicting rank normalized abundances (scaled between 0 and 1) of the 17 bacterial genera (median abundance > 0.1% in at least one group [HC or UVT] which were differentially abundant in the gut microbiomes of healthy controls (HC) compared to the Uveitis (UVT) patients. The discriminating genera as well as the samples (HC and UVT) were arranged along the two dimensions (axes) based on hierarchical clustering. c Principal Coordinate Analysis (PCoA) based on OTU abundances in the bacterial microbiomes of healthy controls (HC, blue), idiopathic Uveitis patients (UVT_ID, black) and autoimmune Uveitis patients (UVT_AD, red). JSD was used as a distance metric. Except 2 samples, all healthy controls (HC) samples clustered together, and all Uveitis patients (UVT_ID and UVT_AD) irrespective of their aetiology clustered together when plotted along the first two principal coordinates PC1 and PC2
Out of the 13 microbiomes in the UVT group, 10 were from patients with idiopathic UVT (UVT_ID) and the remaining 3 were from autoimmune UVT (UVT_AD) patients. Therefore, it was necessary to identify the differences in the microbiomes between UVT_ID, UVT_AD and HC. Kruskal–Wallis test was performed to identify the differentially abundant OTUs (BH corrected P < 0.05) among the three groups and subsequently a PCoA plot was constructed with the identified differentiating OTUs. Two clusters were seen in the PCoA plot, one formed by HC and the other formed by UVT_ID and UVT_AD together (Fig. 3c). This indicates that UVT_ID and UVT_AD groups have similar bacterial gut microbiome composition irrespective of their aetiology that was distinct from the HC microbiome. We also observed that two HC samples clustered with the UVT samples.
Further, post hoc pairwise Wilcoxon tests were performed between HC vs. UVT_ID, HC vs. UVT_AD and UVT_ID vs. UVT_AD to know whether idiopathic and autoimmune aetiology of the disease have any effect on the observed dysbiosis in UVT patients. Several significantly different (BH corrected P < 0.05) OTUs were identified between HC vs. UVT_ID as well as HC vs. UVT_AD samples, but no OTUs were identified that were significantly different between UVT_ID and UVT_AD samples (Online Resource 6) indicating that the microbiomes of idiopathic and autoimmune UVT patients were similar and influenced the observed dysbiosis in the gut bacterial microbiome of UVT patients compared to HC.
Interactions of Bacterial Genera in the Gut Microbiomes of Healthy Subjects and Uveitis Patients
Complex positive and negative interactions were observed within the bacterial population in the HC (Fig. 4a) and UVT microbiomes (Fig. 4b). Two major hub genera (interactions ≥ 10) could be identified in HC network viz. Prevotella and Oscillospira and they showed negative interactions with 14 and 10 other bacterial genera respectively. In contrast, in the UVT network, 15 hub genera (interactions ≥ 10) were identified among which Megasphaera, Prevotella and Dialister were predominant (interactions ≥ 15) and showed negative interactions with other bacterial genera.
Fig. 4.
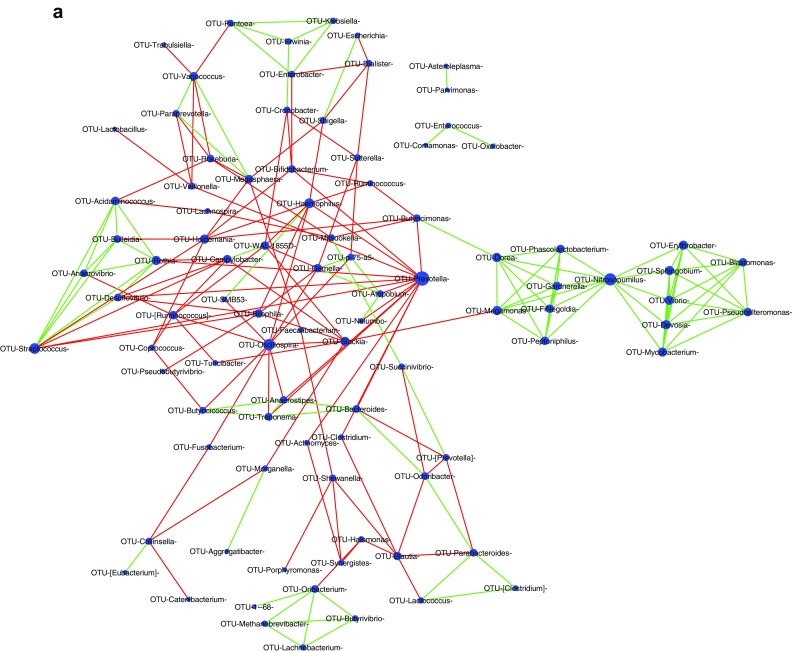
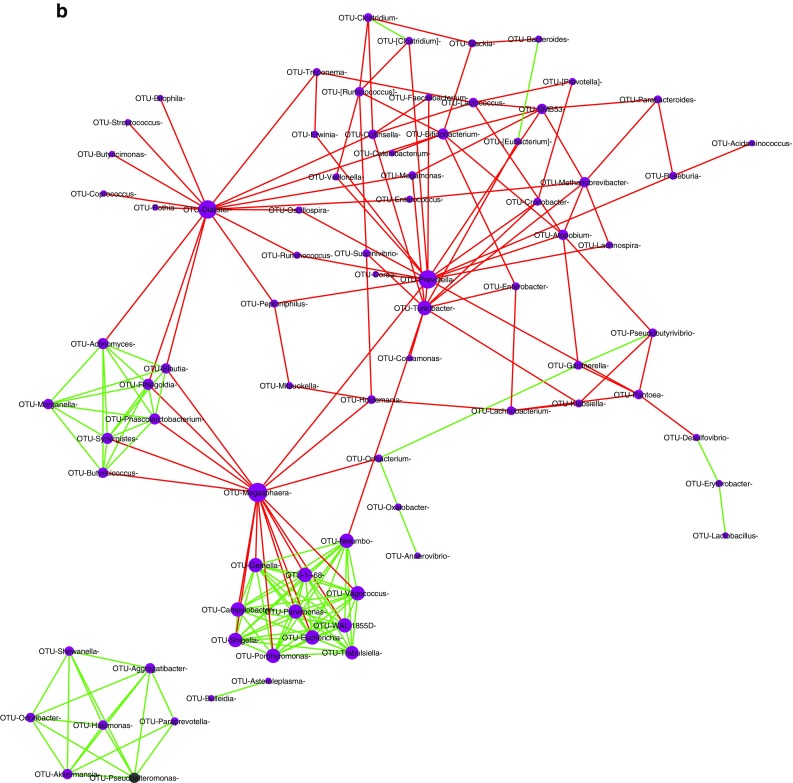
a Bacterial interaction network in the gut microbiome of healthy controls (HC) showing significant co-occurrence and co-exclusion relationships at genus level. Each node represents a genus and the node sizes in the network correspond to their degree of interaction. The positive and negative correlations/associations have been indicated with green and red edges respectively. b Bacterial interaction network in the gut microbiome of Uveitis (UVT) patients showing significant co-occurrence and co-exclusion relationships at genus level. Each node represents a genus and the node sizes in the network correspond to their degree of interaction. The positive and negative correlations/associations have been indicated with green and red edges respectively
Discussion
UVT causes 10–15% of severe visual impairment in the Western world and nearly 25% of blindness in the developing world [1, 3]. The most challenging task of management of UVT is related to the fact that it has multiple aetiologies like it could be due to infection of the eye, systemic infection including viral infection, autoimmune manifestation as in Vogt–Koyanagi–Harada disease and it could be idiopathic UVT. Uveitis has also been categorized as anterior, intermediate and posterior depending on whether the site of inflammation is in the anterior chamber, vitreous, retina or choroid respectively and is known as panuveitis when it simultaneously involves all the above sites. However, in all the above cases the underlying factor is that it manifests as an inflammation of the uveal tract and adjacent structures of the eye. Therefore, in the present study we have considered UVT mainly as an inflammatory disease irrespective of the site of manifestation and chose to analyze the gut microbiomes of only individuals with idiopathic and autoimmune UVT to avoid confounding factors that could be caused due to infections of the eye and systemic infections which may also cause inflammation of the eye. Recruiting individuals with idiopathic UVT (UVT_ID) and autoimmune UVT (UVT_AD) was yet another challenge and especially when the two groups in the cohort (HC vs. UVT) were to be matched for age, sex, diet, ethnicity and region of origin. The entire process of recruitment took close to two years and eventually we could analyse the gut microbiomes of only 13 HC and 13 UVT individuals.
Studies that showed association of human gut microbiome with ocular diseases are rare and have been reported only recently in AMD patients [17], in dry eye in Sjögren syndrome patients [16] and more recently in Uveitis patients [18] from a Chinese population. Two earlier studies also showed association of gut microbiome with autoimmune Uveitis [14] in mice. In the present study, we analysed the gut microbiomes of HC and UVT patients in a south Indian population to identify whether dysbiosis was associated with UVT patients. The sequencing depth (an average of 765,389 reads/microbiome), the rarefaction analysis and stringent quality filtering methods applied were sufficient to make genuine comparisons between the HC and UVT gut microbiomes (Online Resource 1, Fig. 1a). We observed an overall decrease in the diversity and abundance of the bacterial communities in the gut microbiomes of UVT patients compared to HC (Fig. 1b) thus confirming the recent findings of Huang et al. [18] in Chinese patients with Uveitis. Decrease in the diversity and abundance in the bacterial microbiomes was also reported in patients with AMD [17], dry eye disease in Sjögren syndrome [16] and IBD [23]. It was suggested that reduced diversity may favour the emergence of pathogenic bacteria that mediate inflammation [16]. Further analyses of the gut microbiomes of HC and UVT patients clearly indicated dysbiosis in the gut microbiomes of UVT patients compared to the control as judged by the decrease in diversity and abundance at the phyla, OTU, family and genera level. Dysbiosis was further confirmed by the heatmap and PCoA analysis that demonstrated that UVT and HC microbiomes are distinctly different.
In the bacterial gut microbiomes of both HC and UVT, bacteria affiliated to the phylum Firmicutes (Fig. 1c) and the families Ruminococcaceae and Lachnospiraceae were the most abundant as observed earlier in Chinese and Indian population [24–26]. We also found that several bacteria like Lachnospira, Ruminococcus, Bacteroides, Dialister, Dorea, Blautia, Clostridium, Coprococcus, Bifidobacterium adolescentis, Oscillospira, Odoribacter, Veillonella dispar, Faecalibacterium prausnitzii, Akkermansia municiphala, Mitsuokella, Magasphaera and Roseburia which are known butyrate producers and contribute to anti-inflammatory response in the host [27, 28] were increased several folds in HC microbiomes compared to UVT. These dominant genera in HC microbiomes are likely to be beneficial to the host. Decrease in bacteria that produce short chain fatty acids (SCFAs) which confer several health benefits to the host in the microbiomes has also been reported in the gut microbiome of dry eye patients [16], Crohn’s disease patients [29] and ulcerative colitis [30]. Thus, it may be concluded that in UVT subjects, the decrease in gut bacteria with anti-inflammatory properties may contribute or exacerbate the inflammatory reaction. In addition, Bifidobacterium adolescentis and Bifidobacterium longum, which are probiotic and anti-inflammatory and thus are beneficial to human host [27, 31] are also enriched in HC. This is in accordance with a recent study by Kim et al. [32] who demonstrated that in mice with autoimmune uveitis a consortium of probiotic bacteria (Lactobacillus caseii, Lactobacillus acidophilus, Bifidobacterium bifidum and Streptococcus thermophilus) reduced the concentration of pro-inflammatory cytokines and predicted that this may attenuate autoimmune uveitis. Several other OTUs such as Enterobacteriaceae, Coriobacteriaceae, Bacteroides plebeius, Bacteriodes ovatus, Parabacteroides, Paraprevotella, Haemophilus, Catenibacterium, Slackia, Succinivibrio, Sutturella, Bilophila, Desulfovibrio, Bacteroides uniformis and Collinsella aerofaciens were also significantly enriched in HC and substantially reduced in UVT patients (Fig. 2a). We do not know the physiological relevance of these enrichments in HC vs. UVT patients. It was also observed by Anand et al. [27], a few SCFA producing bacteria viz. Faecalibacterium, Roseburia were present in the guts of diseased individuals, but their abundances were less than half when compared to healthy controls implying that abundance is important.
In UVT patients, Prevotella copri which is pro-inflammatory and associated with rheumatoid arthritis, an autoimmune disease [3] was enriched. That P. copri has an autoimmune aetiology is relevant to the present study since it may imply that idiopathic UVT in the present cohort with dominance of P. copri is of autoimmune aetiology. This assumption of gut bacterial involvement in autoimmune Uveitis is further supported by studies in a mouse model of autoimmune Uveitis which showed that gut bacteria activate retina specific T-cells that trigger autoimmune Uveitis [33]. Earlier studies have also indicated that bacteria affiliated to the genus Prevotella exhibit significant inter-individual variation in the gut microbiota and increased proportions of Prevotella are associated with a vegetarian diet rich in plant-derived products such as the diet in Indians [34]. The abundance of Prevotella in the present cohort was not significantly different implying that the diets of the individuals in the two groups were similar. In addition, enrichment of Streptococcus OTU, a pathogenic genus was observed in UVT patients (Fig. 2a). The other OTUs enriched in UVT patients were affiliated to Lachnospiraceae, Ruminococcaceae, Clostridiaceae, Bifidobacterium, Bifidobacterium adolescentis, Faecalibacterium prausnitzii, Lactobacillus, Dorea and Dialister. Thus, overall it would appear that increase in bacteria with pro-inflammatory and pathogenic characteristics in UVT patients may be responsible for the inflammatory response seen in UVT patients and increase in bacteria with ant-inflammatory, probiotic and antibacterial properties would prevent the inflammatory response in HC individuals (Table 1).
Table 1.
Functional characteristics of bacterial genera in the gut microbiomes of healthy controls (HC) and Uveitis (UVT) patients involved in inflammation and pathogenesis based on the abundance of discriminating OTUs as in Fig. 2a
| Sl. no. | Function | Bacteria | Abundance in HC | Abundance in UVT | References |
|---|---|---|---|---|---|
| 1 | Anti-inflammatory | dAkkermansia muciniphila, a,b,d,eBacteroides, Bifidobacterium adolescentis, a,d,eBlautia, a,b,dClostridium, aCoprococcus, aCoprococcus eutactus, d,eDialister, dDorea, aFaecalibacterium prausnitzii, aLachnospira, Lactobacillus, eMegamonas, aMegasphaera, Mitsuokella, aOdoribacter, d,eParabacteroides, eParaprevotella, aRoseburia, dRuminococcus, dStreptococcus, a,d,eVeillonella dispar, Lachnospiraceae, Ruminococcaceae, Veillonellaceae, Bacteroidales, Clostridiales | Increased | Decreased | [27] |
| 2 | Pro-inflammatory | Bacteroides fragilis, Bacteroides ovatus, cBilophila | Increased | Decreased | [3, 36] |
| Prevotella copri | Decreased | Increased | [3] | ||
| 3 | Probiotic | Bacteriodes, Bifidobacterium adolescentis, Bifidobacterium bifidum, Bifidobacterium longum, Blautia, Collinsella aerofaciens, Dialister, Lactobacillus, Megamonas, Oscillospira, Ruminococcus, Veillonella, Streptococcus | Increased | Decreased | [37, 38] |
| 4 | Antibacterial | Bacteroides, Bacteroides fragilis, Lactobacillus, Bifidobacterium adolescentis, Bifidobacterium bifidum, Bifidobacterium longum, Blautia | Increased | Decreased | [37, 39, 40] |
aBased on the capability to produce butyrate
bBased on single strain
cBased on studies on single species
dBased on the capability to produce acetate
eBased on the capability to produce propionate
Complex positive and negative interactions were observed within the bacterial population in the HC (Fig. 4a) and UVT microbiomes (Fig. 4b). It was anticipated that in HC microbiomes, bacterial interactions would lead to increase in anti-inflammatory organisms, decrease in pro-inflammatory organisms, increase in SCFA producers and decrease in pathogens. Accordingly it was observed that in HC microbiomes Prevotella and Oscillospira showed together negative interactions with several pathogenic bacteria like Fusobacterium, Rothia, Slackia, Treponema, Actinomyces, Atopobium, Haemophilus and Streptococcus. Such negative interactions on pathogens would benefit the gut of HC. But it was also surprising that Prevotella showed competition with SCFA producers Butyricimonas, Anaerostipes, Veillonella and Clostridium and with probiotic Bifidobacterium and, Oscillospira also negatively influenced Butyricicoccus which is a probiotic organism and Anaerostipes which is a butyrate producer. Such interactions where in the same organism have both a beneficial effect and a detrimental effect is difficult to interpret though the outcome could depend on the severity of interaction.
In contrast, in the gut microbiome of UVT patients, interactions were as anticipated and supported increase in pro-inflammatory organisms, decrease in anti-inflammatory organisms, decrease in SCFA producers and increase in pathogens. It was observed that Dialister, which produces SCFAs negatively interacts with anti-inflammatory Oscillospira, and with SCFA producers like Blautia, Ruminococcus, Butyricimonas and Coprococcus; another genus Megasphaera also negatively interacted with beneficial organisms like Butyricicoccus (probiotic), Blautia and Oribacterium (SCFA producers) and Prevotella negatively interacted with several beneficial bacteria including Ruminococcus, Megasphaera, Roseburia, Dorea, Veillonella, Lachnospira, Faecalibacterium (SCFA producers), with anti-inflammatory Oscillospira and with pathogenic organisms like Erwinia, Cronobacter, Peptoniphilus and Atopobium.
Conclusion
This is the first study that demonstrates dysbiosis in the gut bacterial communities of UVT patients compared to healthy human subjects from a south Indian population. Several beneficial bacteria that are anti-inflammatory, probiotic and antibacterial were enriched in healthy subjects, whereas, the pro-inflammatory and pathogenic bacteria were enriched in the guts of UVT patients. These changes may probably contribute to or exacerbate the inflammatory reaction in the ocular tissues of UVT patients probably by modulating the host immune system. The data also suggests the possibility of developing alternate strategies for addressing inflammatory disease of the eye by manipulating the gut microbiome by the use of probiotics and nutritional supplements, a strategy also proposed for pulmonary infectious disease like Tuberculosis [35].
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The study was supported by grants received from the Hyderabad Eye Research Foundation. We would like to thank Dr. Annie Mathai for helping in recruitment of Uveitis patients.
Conflict of interest
The authors declare that they have no conflict of interest.
Informed Consent
Informed consent was taken from all the study subjects prior to sample collection. The study protocols were approved by the Institutional Review Board of L. V. Prasad Eye Institute, Hyderabad (Ethics Ref. No. LEC 06-14-060) and all methods were performed in accordance with relevant guidelines and regulations.
References
- 1.Abdulaal MR, Abiad BH, Hamam RN. Uveitis in the aging eye: incidence, patterns, and differential diagnosis. J Ophthalmol. 2015 doi: 10.1155/2015/509456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sharma SM, Fu DJ, Xue K. A review of the landscape of targeted immunomodulatory therapies for non-infectious uveitis. Ophthalmol Ther. 2017 doi: 10.1007/s40123-017-0115-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Horai R, Sen HN, Caspi RR. Commensal microbiota as a potential trigger of autoimmune uveitis. Expert Rev Clin Immunol. 2017;13:291–293. doi: 10.1080/1744666x.2017.1288098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shivaji S. We are not alone: a case for the human microbiome in extra intestinal diseases. Gut Pathog. 2017;9:13. doi: 10.1186/s13099-017-0163-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tlaskalova-Hogenova H, Stepankova R, Kozakova H, Hudcovic T, Vannucci L, Tuckova L, Rossmann P, Klimesova K, Pribylova J, Bartova J, Sanchez D, Fundova P, Borovska D, Srutkova D, Zidek Z, Schwarzer M, Drastich P, Funda DP. The role of gut microbiota (commensal bacteria) and the mucosal barrier in the pathogenesis of inflammatory and autoimmune diseases and cancer: contribution of germ-free and gnotobiotic animal models of human diseases. Cell Mol Immunol. 2011;8:110–120. doi: 10.1038/cmi.2010.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aas J, Gessert CE, Bakken JS. Recurrent Clostridium difficile colitis: case series involving 18 patients treated with donor stool administered via a nasogastric tube. Clin Infect Dis. 2003;36:580–585. doi: 10.1086/367657. [DOI] [PubMed] [Google Scholar]
- 7.Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444:1027–1131. doi: 10.1038/nature05414. [DOI] [PubMed] [Google Scholar]
- 8.Zackular JP, Baxter NT, Iverson KD, Sadler WD, Petrosino JF, Chen GY, Schloss PD. The gut microbiome modulates colon tumorigenesis. mBio. 2013;4:e00692–00613. doi: 10.1128/mBio.00692-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Malan-Muller S, Valles-Colomer M, Raes J, Lowry CA, Seedat S, Hemmings SMJ. The gut microbiome and mental health: implications for anxiety- and trauma-related disorders. OMICS 22. 2017 doi: 10.1089/omi.2017.0077. [DOI] [PubMed] [Google Scholar]
- 10.Kau AL, Ahern PP, Griffin NW, Goodman AL, Gordon JI. Human nutrition, the gut microbiome, and immune system: envisioning the future. Nature. 2011;474:327–336. doi: 10.1038/nature10213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Everard A, Belzer C, Geurts L, Ouwerkerk JP, Druart C, Bindels LB, Guiot Y, Derrien M, Muccioli GG, Delzenne NM, de Vos WM, Cani PD. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc Natl Acad Sci USA. 2013;110:9066–9071. doi: 10.1073/pnas.1219451110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garrett WS, Gallini CA, Yatsunenko T, Michaud M, DuBois A, Delaney ML, Punit S, Karlsson M, Bry L, Glickman JN, Gordon JI, Onderdonk AB, Glimcher LH. Enterobacteriaceae act in concert with the gut microbiota to induce spontaneous and maternally transmitted colitis. Cell Host Microbe. 2010;8:292–300. doi: 10.1016/j.chom.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu HJ, Ivanov II, Darce J, Hattori K, Shima T, Umesaki Y, Littman DR, Benoist C, Mathis D. Gut-residing segmented filamentous bacteria drive autoimmune arthritis via T helper 17 cells. Immunity. 2010;32:815–827. doi: 10.1016/j.immuni.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nakamura YK, Metea C, Karstens L, Asquith M, Gruner H, Moscibrocki C, Lee I, Brislawn CJ, Jansson JK, Rosenbaum JT, Lin P. Gut microbial alterations associated with protection from autoimmune uveitis. Invest Ophthalmol Vis Sci. 2016;57:3747–3758. doi: 10.1167/iovs.16-19733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rowan S, Jiang S, Korem T, Szymanski J, Chang ML, Szelog J, Cassalman C, Dasuri K, McGuire C, Nagai R, Du XL, Brownlee M, Rabbani N, Thornalley PJ, Baleja JD, Deik AA, Pierce KA, Scott JM, Clish CB, Smith DE, Weinberger A, Avnit-Sagi T, Lotan-Pompan M, Segal E, Taylor A. Involvement of a gut-retina axis in protection against dietary glycemia-induced age-related macular degeneration. Proc Natl Acad Sci USA. 2017;114:E4472–E4481. doi: 10.1073/pnas.1702302114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de Paiva CS, Jones DB, Stern ME, Bian F, Moore QL, Corbiere S, Streckfus CF, Hutchinson DS, Ajami NJ, Petrosino JF, Pflugfelder SC. Altered mucosal microbiome diversity and disease severity in sjögren syndrome. Sci Rep. 2016;6:23561. doi: 10.1038/srep23561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zinkernagel MS, Zysset-Burri DC, Keller I, Berger LE, Leichtle AB, Largiadèr CR, Fiedler GM, Wolf S. Association of the intestinal microbiome with the development of neovascular age-related macular degeneration. Sci Rep. 2017;7:40826. doi: 10.1038/srep40826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang X, Ye Z, Cao Q, Su G, Wang Q, Deng J, Zhou C, Kijlstra A, Yang P. Gut microbiota composition and fecal metabolic phenotype in patients with acute anterior uveitis. Invest Ophthalmol Vis Sci. 2018;59:1523–1531. doi: 10.1167/iovs.17-22677. [DOI] [PubMed] [Google Scholar]
- 19.Magoc T, Salzberg SL. FLASH: fast length adjustment of short reads to improve genome assemblies. Bioinformatics. 2011;27:2957–2963. doi: 10.1093/bioinformatics/btr507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schloss PD, Westcott SL, Ryabin T, Hall JR, Hartmann M, Hollister EB, Lesniewski RA, Oakley BB, Parks DH, Robinson CJ, Sahl JW, Stres B, Thallinger GG, Van Horn DJ, Weber CF. Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl Environ Microbiol. 2009;75:7537–7541. doi: 10.1128/aem.01541-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shannon P, Markiel A, Ozier O, Baliga NS, Wang JT, Ramage D, Amin N, Schwikowski B, Ideker T. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 2003;13:2498–2504. doi: 10.1101/gr.1239303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Faust K, Raes J. CoNet app: inference of biological association networks using Cytoscape. F1000Res. 2016;5:1519. doi: 10.12688/f1000research.9050.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Frank DN, St. Amand AL, Feldman RA, Boedeker EC, Harpaz N, Pace NR. Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc Natl Acad Sci USA. 2007;104:13780–13785. doi: 10.1073/pnas.0706625104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dehingia M, Thangjam Devi K, Talukdar NC, Talukdar R, Reddy N, Mande SS, Deka M, Khan MR. Gut bacterial diversity of the tribes of India and comparison with the worldwide data. Sci Rep. 2015;5:18563. doi: 10.1038/srep18563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bhute S, Pande P, Shetty SA, Shelar R, Mane S, Kumbhare SV, Gawali A, Makhani H, Navandar M, Dhotre D, Lubree H, Agarwal D, Patil R, Ozarkar S, Ghaskadbi S, Yajnik C, Juvekar S, Makharia GK, Shouche YS. Molecular characterization and meta-analysis of gut microbial communities illustrate enrichment of Prevotella and Megasphaera in Indian subjects. Front Microbiol. 2016;7:660. doi: 10.3389/fmicb.2016.00660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fang X, Wang X, Yang S, Meng F, Wei H, Chen T. Evaluation of the microbial diversity in amyotrophic lateral sclerosis using high-throughput sequencing. Front Microbiol. 2016;7:1479. doi: 10.3389/fmicb.2016.01479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Anand S, Kaur H, Mande SS. Comparative in silico analysis of butyrate production pathways in gut commensals and pathogens. Front Microbiol. 2016;7:1945. doi: 10.3389/fmicb.2016.01945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Richards JL, Yap YA, McLeod KH, Mackay CR, Marino E. Dietary metabolites and the gut microbiota: an alternative approach to control inflammatory and autoimmune diseases. Clin Transl Immunol. 2016;5:e82. doi: 10.1038/cti.2016.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kang S, Denman SE, Morrison M, Yu Z, Dore J, Leclerc M, McSweeney CS. Dysbiosis of fecal microbiota in Crohn’s disease patients as revealed by a custom phylogenetic microarray. Inflamm Bowel Dis. 2010;16:2034–2042. doi: 10.1002/ibd.21319. [DOI] [PubMed] [Google Scholar]
- 30.Machiels K, Joossens M, Sabino J, De Preter V, Arijs I, Eeckhaut V, Ballet V, Claes K, Van Immerseel F, Verbeke K, Ferrante M, Verhaegen J, Rutgeerts P, Vermeire S. A decrease of the butyrate-producing species Roseburia hominis and Faecalibacterium prausnitzii defines dysbiosis in patients with ulcerative colitis. Gut. 2014;63:1275–1283. doi: 10.1136/gutjnl-2013-304833. [DOI] [PubMed] [Google Scholar]
- 31.Leser T, Myling-Petersen D, Wellejus A, Brockmann E, Olsen J, Rehdin S (2016) Probiotic strains of Bifidobacterium adolescentis. In: Conference proceedings of IPC 2016, p 108
- 32.Kim J, Choi SH, Kim YJ, Jeong HJ, Ryu JS, Lee HJ, Kim TW, Im SH, Oh JY, Kim MK. Clinical effect of IRT-5 probiotics on immune modulation of autoimmunity or alloimmunity in the eye. Nutrients. 2017;9:1166. doi: 10.3390/nu9111166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Horai R, Zarate-Blades CR, Dillenburg-Pilla P, Chen J, Kielczewski JL, Silver PB, Jittayasothorn Y, Chan CC, Yamane H, Honda K, Caspi RR. Microbiota-dependent activation of an autoreactive T cell receptor provokes autoimmunity in an immunologically privileged site. Immunity. 2015;43:343–353. doi: 10.1016/j.immuni.2015.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.De Filippo C, Cavalieri D, Di Paola M, Ramazzotti M, Poullet JB, Massart S, Collini S, Pieraccini G, Lionetti P. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc Natl Acad Sci USA. 2010;107:14691–14696. doi: 10.1073/pnas.1005963107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sood U, Bajaj A, Kumar R, Khurana S, Kalia VC. Infection and microbiome: impact of tuberculosis on human gut microbiome of Indian cohort. Indian J Microbiol. 2018;58:123–125. doi: 10.1007/s12088-018-0706-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lukiw WJ. Bacteroides fragilis lipopolysaccharide and inflammatory signaling in Alzheimer’s disease. Front Microbiol. 2016;7:1544. doi: 10.3389/fmicb.2016.01544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yun JH, Yim DS, Kang JY, Kang BY, Shin EA, Chung MJ, Kim SD, Baek DH, Kim K, Ha NJ. Identification of Lactobacillus ruminus SPM0211 isolated from healthy Koreans and its antimicrobial activity against some pathogens. Arch Pharm Res. 2005;28:660–666. doi: 10.1007/BF02969355. [DOI] [PubMed] [Google Scholar]
- 38.Ferrario C, Taverniti V, Milani C, Fiore W, Laureati M, De Noni I, Stuknyte M, Chouaia B, Riso P, Guglielmetti S. Modulation of fecal clostridiales bacteria and butyrate by probiotic intervention with Lactobacillus paracasei DG varies among healthy adults. J Nutr. 2014;144:1787–1796. doi: 10.3945/jn.114.197723. [DOI] [PubMed] [Google Scholar]
- 39.Avelar KE, Pinto LJ, Antunes LC, Lobo LA, Bastos MC, Domingues RM, Ferreira MC. Production of bacteriocin by Bacteriodes fragilis and partial characterization. Lett Appl Microbiol. 1999;29:264–268. doi: 10.1046/j.1365-2672.1999.00603.x. [DOI] [PubMed] [Google Scholar]
- 40.Chatzidaki-Livanis M, Coyne MJ, Comstock LE. An antimicrobial protein of the gut symbiont Bacteroides fragilis with a MACPF domain of host immune proteins. Mol Microbiol. 2014;94:1361–1374. doi: 10.1111/mmi.12839. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.