Abstract
Currently considerable research both in life and in environmental sciences is dedicated to chemosensors able to detect metals of biological interest such as zinc and iron or other toxic and carcinogenic, as cadmium, mercury, chromium, lead. Recently, a new chemosensor strategy of “single chemosensor for multiple metals” has emerged. For this scope, many fluorescent sensors for Cd(II) and Zn(II) have been designed and synthetized, as ligand systems or in polymeric matrices [1], [2], [3]. The data presented in this article include experimental data on the of a pyridyl/phenolic/benzothiazole functionalized colorimetric receptor (BPAP) and its selectively recognise Fe(III) and Fe(II) ions with visible, naked eye colour changes and fluorometric selectivity towards Zn2+ and Cd2+ ions in aqueous medium.
This article is submitted as a companion paper to Caruso et al. (2018) [4].
Specifications Table
| Subject area | Chemistry, Materials Science |
| More specific subject area | Electro-optic field sensors |
| Type of data | Crystal data and structure refinement, NMR spectrum, tables and figure |
| How data was acquired | NMR recorded in DMSO using Bruker Spectrometers operating at 400 MHz. |
| UV-Visible and fluorescence spectra recorded with JASCO spectrometers. | |
| Single crystals X-ray structural analysis performed on a BrukerNoniusKappaCCD diffractometer equipped with Oxford Cryostream apparatus. | |
| Data format | Raw data and their elaborations |
| Experimental factors | The data concerns structural information, UV/Vis calculation and some spectroscopic raw data |
| Experimental features | Elaboration of X-ray diffraction data and UV/Vis curves |
| Data source location | Naples, Italy |
| Data accessibility | Data is within this article |
Value of the data
-
•
The data show some molecular structure of BPAP along a and c axes.
-
•
The data report relevant structural data of BPAP and its zinc complex (lengths and angles).
-
•
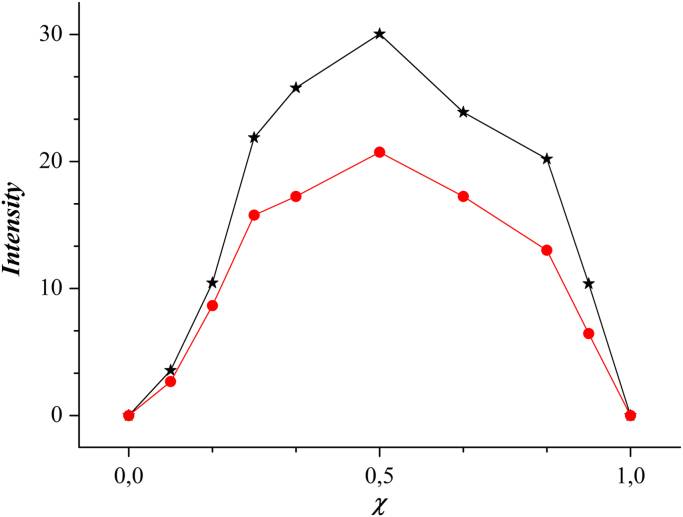
The data report Job׳s plot analysis for the binding Zn(II) and Cd(II) with ligand system.
-
•
1H NMR and 13C NMR of BPAP and BPAP metal complexes are reported.
1. Data
The data presented in this article are related to the research article entitled “A real-time tripodal colorimetric/fluorescence sensor for multiple target metal ions” [4]. Recently an impressive progress has been done toward the design and synthesis of novel sensitive ligands and fluorescent materials [5], [6], [7], [8]. The data presented here include experimental data on the of a pyridyl/phenolic/benzothiazole functionalized colorimetric receptor (BPAP) and its selectively recognise Fe(III) and Fe(II) ions with visible, naked eye colour changes and fluorometric selectivity towards Zn2+ and Cd2+ ions in aqueous medium.
The following data are a necessary support for the identification of materials and properties of the ligand system and its complexes.
2. Experimental design, materials and methods
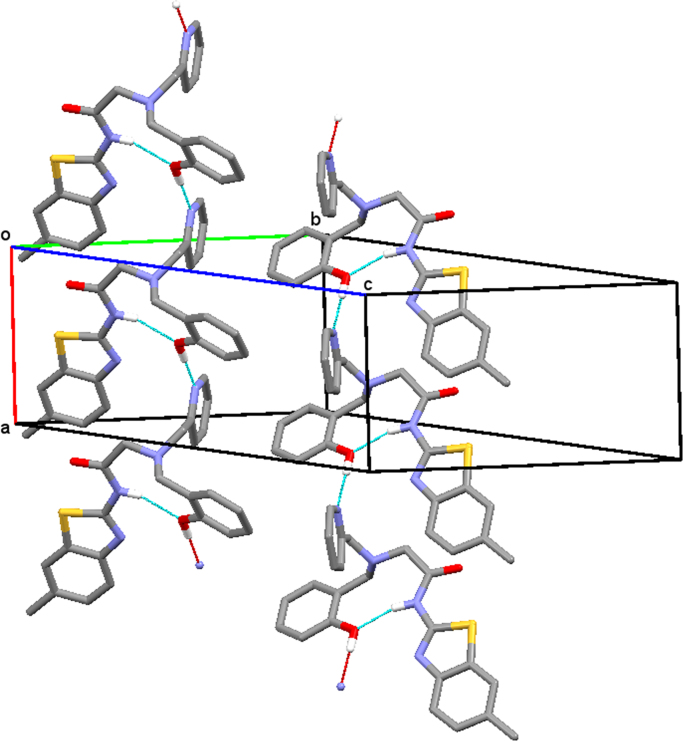
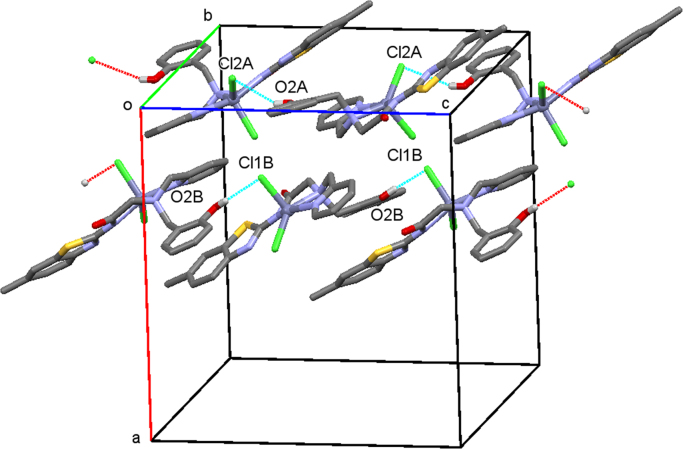
Structural analysis of single crystals of ligand and its Zinc complex has been performed on a BrukerNoniusKappaCCD diffractometer equipped with Oxford Cryostream apparatus (graphite monochromated MoKα radiation, λ = 0.71073 Å, CCD rotation images, thick slices, φ and ω scans to fill asymmetric unit). Semiempirical absorption corrections (SADABS [9]) were applied. Both the two structures were solved by direct methods (SIR97 program [10]) and anisotropically refined by the full matrix least-squares method on F2 against all independent measured reflections using SHELXL-2016 [11] and WinGX software [12]. Crystal data and structure refinement details are reported in Table 1. Relevant bond lengths and angle are reported in Table 2. The figures were generated using ORTEP-3 [13] and Mercury CSD 3.9 [14] programs. Molecular structure of BPAP along a axis is shown in Fig. 1. Molecular structure of the complex Zn-BPAP along c axis is shown in Fig. 2.
Table 1.
Structural data and refinement details for BPAP and Zn-BPAP.
| BPAP | Zn-BPAP | |
|---|---|---|
| CCDC number | 1582069 | 1582070 |
| Empirical formula | C23H22N4O2S | C23H22Cl2N4 O2SZn |
| Formula weight | 418.50 | 554.77 |
| Temperature (K) | 298(2) | 173(2) |
| Wavelength (Å) | 0.71073 | 0.71073 |
| Crystal system (Å) | Triclinic | Monoclinic |
| Space group | P -1 | P21/c |
| a (Å) | 7.2950(15) | 18.542(5) |
| b (Å) | 16.3670(18) | 14.650(3) |
| c (Å) | 18.592(2) | 17.217(2) |
| ⎕ (°) | 77.506(8) | 90. |
| ⎕ (°) | 87.794(11) | 92.373(13) |
| ⎕ (°) | 85.826(11) | 90. |
| Volume (Å3) | 2160.9(6) | 4672.8(17) |
| Z | 4 | 8 |
| Dcalc (Mg/m3) | 1.286 | 1.577 |
| ⎕ (mm−1) | 0.177 | 1.399 |
| F(000) | 880 | 2272 |
| Crystal size (mm) | 0.480 × 0.150 × 0.020 | 0.200 × 0.060 × 0.040 |
| θ range (°) | 2.333 to 25.996 | 2.602 to 27.022 |
| Limiting indices | −8≤h≤8, −20≤k≤20, −22≤l≤22 | −23≤h≤23, −18≤k≤18, −21≤l≤21 |
| Reflections collected / unique | 19,458 / 8234 [R(int) = 0.0671] | 39,344 / 10,013 [R(int) = 0.1524] |
| Refinement method | Full-matrix least-squares on F 2 | Full-matrix least-squares on F 2 |
| Data / restraints / parameters | 8234 / 0 / 556 | 10,013 / 0 / 609 |
| Goodness-of-fit on F2 | 1.034 | 1.079 |
| Final R indices [I>2sigma(I)] | R1 = 0.0628, wR2 = 0.1340 | R1 = 0.0787, wR2 = 0.1561 |
| R indices (all data) | R1 = 0.1416, wR2 = 0.1695 | R1 = 0.1891, wR2 = 0.2013 |
| Largest diff. peak and hole (eA−3) | 0.448 and −0.283 | 0.800 and −0.878 |
Table 2.
Selected bond lengths (Å) and angles (°).
|
BPAP |
Zn-BPAP |
|||
|---|---|---|---|---|
| Molecule A | Molecule B | Molecule A | Molecule B | |
| C9-O1 | 1.219(4) | 1.212(4) | 1.221(9) | 1.220(9) |
| C9-N2 | 1.3578(5) | 1.357(5) | 1.372(9) | 1.345(10) |
| S1-C8 | 1.746(3) | 1.742(4) | 1.724(8) | 1.734(7) |
| S1-C6 | 1.734(4) | 1.730(4) | 1.754(7) | 1.747(7) |
| Zn1-N2 | 2.207(6) | 2.213(6) | ||
| Zn1-N3 | 2.182(6) | 2.165(6) | ||
| Zn1-N4 | 2.157(6) | 2.140(6) | ||
| Zn1-Cl1 | 2.250(3) | 2.289(2) | ||
| Zn1-Cl2 | 2.288(3) | 2.282(2) | ||
| C8-N2-C9 | 126.2(3) | 126.0(3) | 116.4(6) | 117.0(6) |
| N2-C9-C10 | 115.8(3) | 115.3(3) | 113.6(6) | 114.1(7) |
| O1-C9-N2 | 122.4(4) | 123.2(4) | 125.7(7) | 126.6(7) |
Fig. 1.
Chains of H bonded molecules of BPAP along a axis. Only the hydroxy H atom is drawn for clarity.
Fig. 2.
Chains of Zn-BPAP molecules running along c axis direction. Only the hydroxy H atom is drawn for clarity.
All crystal data were deposited at Cambridge Crystallographic Data Centre with assigned number CCDC 1582069 (BPAP) and 1582070 (Zn-BPAP). These data can be obtained free of charge from www.ccdc.cam.ac.uk/data_request/cif.
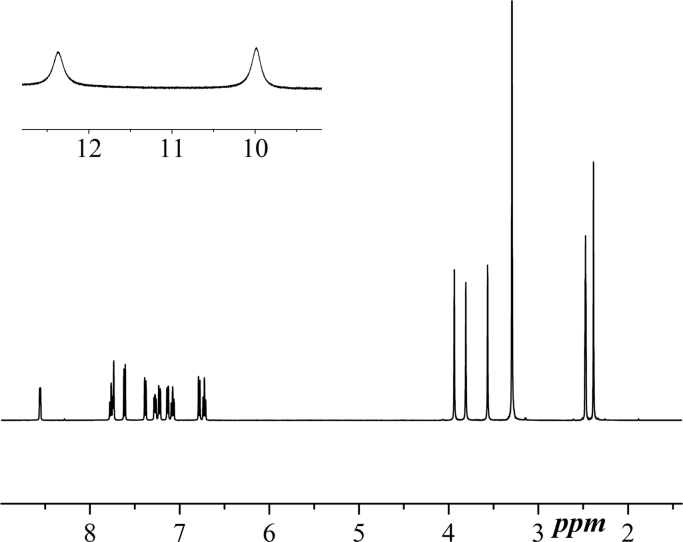
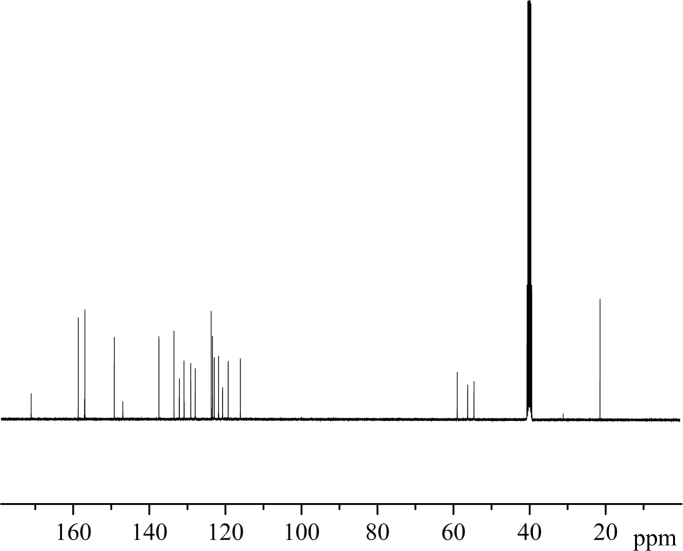
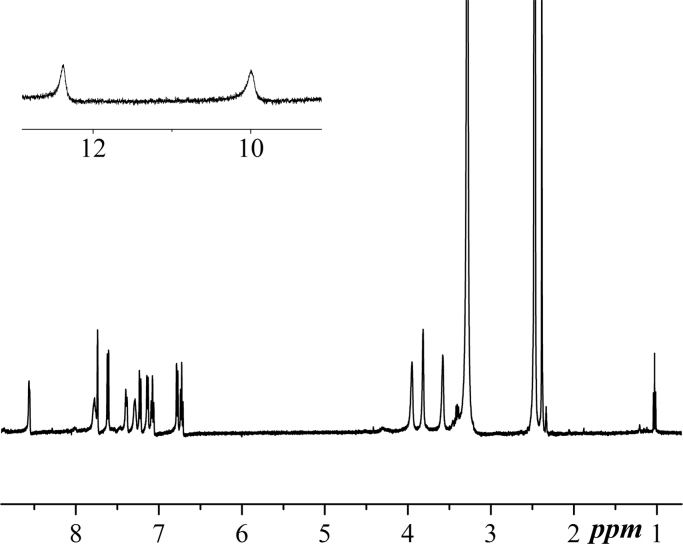
NMR spectra were recorded in DMSO using a Bruker Spectrometer operating at 400 MHz. For BPAP, both 1H and 13C NMR are reported in Fig. 3, Fig. 4. In Fig. 5, 1H NMR spectrum of Zn-BPAP is shown.
Fig. 3.
1H NMR spectrum of BPAP.
Fig. 4.
13C NMR spectrum of BPAP.
Fig. 5.
1H NMR spectrum of Zn-BPAP.
Job׳s plot measurement of Zn2+ and Cd2+ (Fig. 6) has been performed on 500μM solutions of Zn(II) chloride (or Cd(II) chloride) in bidistilled water (pH 6.25) and 500μM of BPAP in ethanol. Volumes of 3.00, 2.75, 2.50, 2.00, 1.50, 1.00, 0.50, 0.25 and 0 mL of the solution of ligand were taken and transferred to vials and volumes of 0, 0.25, 0.50, 1.00, 1.50, 2.00, 2.50, 2.75, 3.00 mL of metal ion added, each vial having a total volume of 3.0 mL. Fluorescence spectra were recorded at room temperature after shaking each vial for a few seconds.
Fig. 6.
Job׳s plot analysis for the binding Zn(II) (black curve) and Cd(II) (red curve) with BPAP.
Acknowledgements
Financial support from the Italian Ministry of Education, University and Research (MIUR) [grant number 300395FRB16] is gratefully acknowledged.
Footnotes
Transparency data associated with this article can be found in the online version at https://doi.org/10.1016/j.dib.2018.06.096.
Transparency document. Supplementary material
Supplementary material
.
References
- 1.Caruso U., Panunzi B., Roviello A., Tingoli M., Tuzi A. Two aminobenzothiazole derivatives for Pd (II) and Zn (II) coordination: synthesis, characterization and solid state fluorescence. Inorg. Chem. Commun. 2011;14(1):46–48. [Google Scholar]
- 2.Borbone F., Caruso U., Causà M., Fusco S., Panunzi B., Roviello A. Series of O, N, O‐tridentate ligands zinc (II) complexes with high solid‐state photoluminescence quantum yield. Eur. J. Inorg. Chem. 2014;2014(16):2695–2703. [Google Scholar]
- 3.Caruso U., Panunzi B., Roviello A., Tuzi A. Fluorescent metallopolymers with Zn (II) in a Schiff base/phenoxide coordination environment. Inorg. Chem. Commun. 2013;29:138–140. [Google Scholar]
- 4.Diana R., Caruso U., Concilio S., Piotto S., Tuzi A., Panunzi B. A real-time tripodal colorimetric/fluorescence sensor for multiple target metal ions. Dyes Pigment. 2018;155:249–257. doi: 10.1016/j.dib.2018.06.096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Borbone F., Caruso U., Palma S.D., Fusco S., Nabha S., Panunzi B. High solid state photoluminescence quantum yields and effective color tuning in polyvinylpyridine based zinc (II) metallopolymers. Macromol. Chem. Phys. 2015;216(14):1516–1522. [Google Scholar]
- 6.Borbone F., Caruso U., Concilio S., Nabha S., Panunzi B., Piotto S. Mono-, di-, and polymeric pyridinoylhydrazone ZnII complexes: structure and photoluminescent properties. Eur. J. Inorg. Chem. 2016;2016(6):818–825. [Google Scholar]
- 7.Borbone F., Caruso U., Concilio S., Nabha S., Piotto S., Shikler R. From cadmium (II)-aroylhydrazone complexes to metallopolymers with enhanced photoluminescence. A structural and DFT study. Inorg. Chim. Acta. 2017;458:129–137. [Google Scholar]
- 8.Acierno D., Amendola E., Bellone S., Ferrara L., Iannelli P., Neitzert H.-C. Synthesis and luminescent properties of a new class of nematic oxadiazole containing poly-ethers for PLED. J. Noncryst. Solids. 2004;338:278–282. [Google Scholar]
- 9.SADABS B. 1; Bruker AXS Inc., Madison, Wisconsin, USA. 2001.
- 10.Altomare A., Burla M.C., Camalli M., Cascarano G.L., Giacovazzo C., Guagliardi A. SIR97: a new tool for crystal structure determination and refinement. J. Appl. Crystallogr. 1999;32(1):115–119. [Google Scholar]
- 11.Sheldrick G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C: Struct. Chem. 2015;71(1):3–8. doi: 10.1107/S2053229614024218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Farrugia L.J. WinGX and ORTEP for Windows: an update. J. Appl. Crystallogr. 2012;45(4):849–854. [Google Scholar]
- 13.Farrugia L.J. ORTEP-3 for Windows-a version of ORTEP-III with a Graphical User Interface (GUI) J. Appl. Crystallogr. 1997;30(5) (565-) [Google Scholar]
- 14.Macrae C.F., Bruno I.J., Chisholm J.A., Edgington P.R., McCabe P., Pidcock E. Mercury CSD 2.0–new features for the visualization and investigation of crystal structures. J. Appl. Crystallogr. 2008;41(2):466–470. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material