Abstract
Objectives
The aim of this study was to investigate the short-term effects of spinal manipulation (SM) on wrist muscle spasticity and manual dexterity in participants with cerebral palsy (CP).
Methods
After baseline examination, 78 participants with spastic CP (7-18 years) without contractures or hyperkinetic syndrome were randomly allocated into 2 groups. The experimental group underwent SM to the cervical, thoracic, and lumbar spine, and the control group received sham SM. A second evaluation was performed 5 minutes postintervention. Wrist muscle spasticity was measured quantitatively with NeuroFlexor (Aggero MedTech AB, Solna, Sweden), a device assessing resistance to passive movements of different velocities. Between-group difference was calculated using the Mann-Whitney U test. Manual dexterity was evaluated by the Box and Block test.
Results
In the experimental group, muscle spasticity was reduced by 2.18 newton from median 5.53 with interquartile range 8.66 to median 3.35 newton with interquartile range 7.19; the difference was statistically significant (P = .002). In the control group, reduction in spasticity was negligible. The between-group difference in change of muscle spasticity was statistically significant (P = .034). Improvement of manual dexterity was not statistically significant (P = .28).
Conclusions
These findings suggest that SM may, in the short term, help to reduce spasticity in participants with CP. Long-term effects of SM on muscle spasticity have yet to be studied.
Key Indexing Terms: Manipulation, Spinal; Musculoskeletal Manipulations; Muscle Spasticity; Cerebral Palsy
Introduction
Muscle spasticity is an important clinical syndrome in people with cerebral palsy (CP) and other neurologic diseases resulting from upper motor neuron lesions.1 It manifests with an increased stretch reflex, which intensifies with movement velocity.2
Spasticity affects motor development and functioning of a child, and its reduction is an important therapeutic target for optimizing motor performance. The range of treatments for excess muscle tone is vast: from simple stretching exercises or pharmacotherapy to surgery.3 However, because of limited effectiveness of conventional treatments, a wide range of complementary and alternative therapies are used for muscle tone management in patients with CP, including spinal manipulation (SM).4, 5
Resent research indicates possible influence of SM on muscle spasticity. The literature points to the effect of SM on spinal cord neural circuits as a factor that possibly modifies stretch reflexes.6, 7 Neural responses to SM have been reported in studies on animal models.8, 9 There is preliminary evidence that SM is followed by a short-term reduction in local spinal muscle electromyographic activity in hypertonic muscles.10 Decrease in motoneuron excitability (H-reflex) after sacroiliac joint manipulation was observed in patients with low back pain.11, 12 There are several clinical studies suggesting the influence of SM on spasticity. Decrease of spasticity after SM was noted in post-stroke patients.13 Reduction in wrist muscle spasticity after SM was also reported in patients with CP.14, 15
In addition, there is growing body of research on the effects of SM on sensory processing, motor output, and functional performance, including hand function.8, 16 Studies suggest possible changes of muscle strength after a single session of manual therapy (MT).17 Improvement of manual dexterity after SM was also noted in patients with CP.18
However, there are no studies directly measuring the relationship between SM and reduction of muscle spasticity. Therefore, the primary aim of this study was to evaluate the effect of SM on muscle spasticity in participants with CP. A secondary aim of this study was to test the hypothesis that SM influences manual dexterity in participants with reduced hand function due to CP.
Methods
Study Design
This was a prospective, randomized controlled trial with 2 groups: experimental (receiving SM) and control (receiving sham of SM).
After the baseline examination, participants were randomized into 2 equal arms (1:1): the SM group (experimental) and the sham group (control). We used stratified block randomization with a block size of 4 by the form of CP (unilateral or bilateral) and level of wrist spasticity (low or high). Stratified block randomization helped to achieve balance between the groups on all studied parameters. Both participants and examiners were blinded to group allocation, only the research coordinator allocating participants to groups and the doctor performing MT were aware of which group the participants belonged to. The second evaluation in both groups was carried out 5 minutes after the intervention.
This study was performed on vulnerable populations: both children and people with disabilities. The necessity of their inclusion was approved by the Medical Ethics Commission of the International Clinic of Rehabilitation, located in Truskavets, Ukraine (Protocol Number N- 2016-09-1), after review of all documents, including study protocol and the informed consent forms. Participants and their legal representatives received comprehensive information about the procedures and study design. Written informed consent was obtained from legal representatives. Where appropriate, based on age and cognitive abilities, participants were asked to give verbal assent. The study was registered at clinicaltrials.gov with identifier NCT03005938.
Statistics
Data analysis was performed with SPSS version 23 software (IBM Corp, Armonk, New York). After descriptive analysis, the normal distribution of variables was verified by means of the Kolmogorov-Smirnov test. Normally distributed variables were described with mean and standard deviation (SD), non-normally distributed with median and interquartile range (IQR). Comparison of baseline values between the groups was performed using the χ2 test for categorical data, independent samples t test for normally distributed continuous data, and the Mann-Whitney U test for non-normally distributed data.
Within-group difference between baseline and postintervention values for non-normally distributed variables was measured with the Wilcoxon signed-ranks test, whereas between-group difference was calculated using the Mann-Whitney U test.
For normally distributed variables, difference between baseline and postintervention within group were measured with the paired samples t test, and difference between experimental and control groups was computed with the independent samples t test, and P < .05 was considered significant in all tests.
Sample size was calculated based on data from the preliminary research on the influence of SM on muscle spasticity,15 with confidence level of 95% and power of 80%. Aimed at detecting the size effect of 1.92 newton in the mild spasticity group, with an SD of 2.92, the estimated sample size was calculated to be at least 37 participants in each group.
Participants
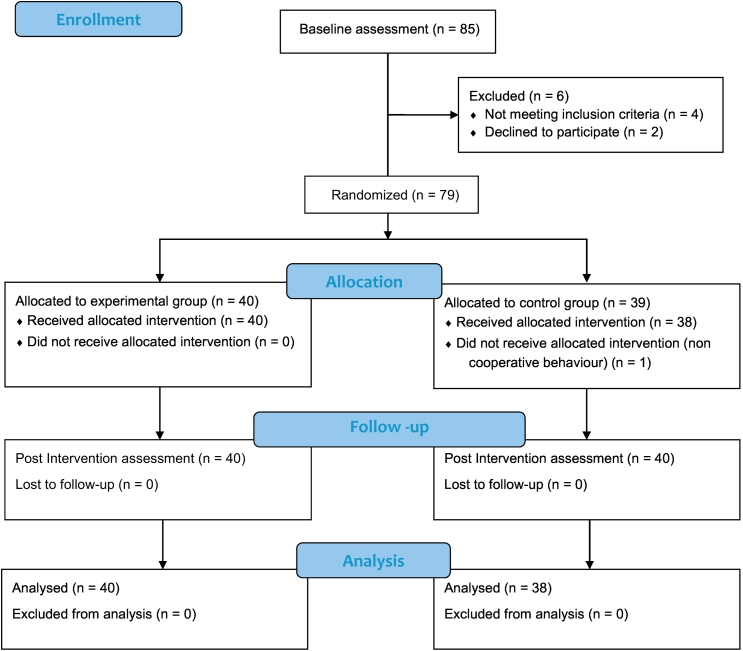
Participants with spastic forms of CP and who were 8 to 18 years of age and admitted to the tertiary care rehabilitation clinic were prescreened during the routine examination and invited to participate in the study. Upon obtaining the informed consent, 85 participants were invited for the baseline assessment. Participants flow is described in the CONSORT 2010 flow Diagram (Fig 1).19
Fig 1.
CONSORT 2010 flow diagram.
The inclusion criteria were spastic forms of CP, age 8 to 18 years, and hand function level I to III according to the Manual Ability Classification System (MACS). The exclusion criteria were dyskinetic or ataxic syndrome, wrist flexion-extension range less than 80° with fingers extended, hyperkinetic movements, startle reflex, Botox injections in hand muscles during the preceding year, antispastic medication during the preceding month, wrist or forearm fracture less than 6 months prior to study, uncooperative behavior, and inability to understand and comply with instructions, in addition to general contraindications to spinal manipulative therapy stated in the World Health Organization guidelines on basic training and safety in chiropractic.20 In addition, the participants must not have received SM within 3 months prior to the study.
At baseline assessment, 6 participants were excluded because they did not meet the inclusion criteria or because of refusal to participate. Forty participants were randomly allocated to the experimental group, where SM was performed, and 39 were allocated to the control group, which received sham of the manipulation. One participant was excluded from the study, because of noncooperative behavior.
Outcome Measures
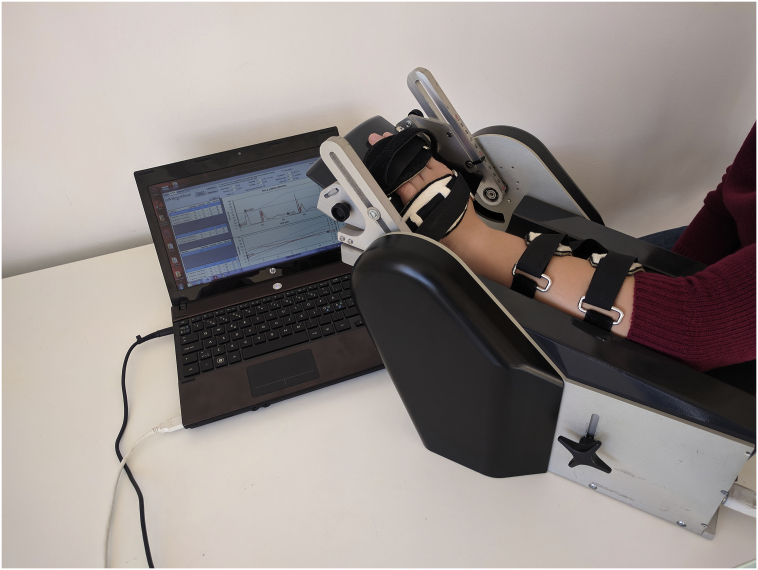
The primary outcome measure was muscle spasticity in the wrist muscles measured at baseline and postintervention. Spasticity was assessed quantitatively with a NeuroFlexor (Aggero MedTech AB, Solna, Sweden) device by measuring the resistance to passive movements of the wrist, performed with different velocity by a computer-controlled step motor (Fig 2). Total resisting force during passive wrist extension consists of the following components: (1) active neural component (NC), the equivalent of spasticity and produced by muscle contractions induced by stretch reflexes and (2) non-neural components, associated with altered properties of muscle and tendons: elastic component (EC) and viscous component (VC).
Fig 2.
NeuroFlexor device for quantitative measurement of muscle tone.
According to the protocol, 1 test session included 5 slow movements and 10 fast movements; dedicated software was used to calculate NC, EC, and VC values. Lower NC values correspond to lower spasticity levels.
Recent studies have indicated that NeuroFlexor is a reliable measurement tool, with high test–retest and inter-rater reliability.21 NeuroFlexor is sensitive enough to measure changes in spasticity during the course of treatment,22 which gives this device an advantage over the more conventional spasticity measurement tool, Modified Ashworth Scale (MAS),23 validity and reliability of which have been questioned by many authors.24
Manual dexterity was the secondary outcome measure, assessed at baseline and after the intervention with the Box and Block test. During the test, participants were given 60 seconds to move as many blocks as possible from 1 compartment of the box to the other, using only the tested hand.25 The score was the number of blocks moved in 1 minute.
The baseline assessment also included neurologic examination with the following standardized measures and classifications:
-
•
Gross motor functions classified according to the Gross Motor Function Classification System26
-
•
Hand function evaluated according to the Manual Ability Classification System (MACS)27
-
•
Wrist joint range of movement assessed using hand-held goniometer
-
•
Wrist muscle spasticity measured by MAS23
-
•
Body weight, body height, and wrist length
Intervention
The study had 2 arms: the experimental group, which underwent SM, described in the present analysis according to the reporting guidelines28 and the control group, which received sham of the SM.
Spinal manipulation was performed by an orthopedic medical doctor certified in MT, with over 10 years of experience, and after 1-hour special training on how to perform sham of SM. Spinal manipulation was performed in a tertiary care rehabilitation clinic, identical for all participants, and carried out according to the description provided in the published manual.29 After manual evaluation, SM was carried out in the thoracic, lumbar, and cervical regions. The intervention encompassed lumbar manipulation because spasticity in CP usually manifests at all levels of the body and not just in the upper extremity. No corrections of spinal lesions (subluxation, mechanical lesion) were performed during the intervention.
Thoracic manipulation was performed in prone position by applying postero-anterior pressure to take up the slack along with a counterclockwise rotation force driving the right hand away from the left. High-velocity, low-amplitude thrust was then applied in the vertical direction while the participant exhaled.
Lumbar spine manipulation was performed in lateral recumbent position with the upper leg flexed at the hip and knee, the lower leg straight, and lumbar spine placed in slight extension. Joint pretension produced by the rotational force was applied to the shoulder and thigh. High-velocity, low-amplitude thrust was delivered, targeting the facet joints in a posterior to anterior direction.
Cervical spine manipulation was conducted in a seated position with the head flexed sideways and slightly rotated and the weight of the head supported by the practitioner’s hand. Traction and side-bending force were employed, and when the slack was taken out and this premanipulation position was determined to be comfortable, a high-velocity, low-amplitude thrust was applied. Lumbar and cervical manipulations were performed symmetrically on both right and left side.
The sham of the SM physically and visually resembles the SM. It encompasses placing the participant in the same positions and performing movements identical to those performed during SM, but without applying substantial force. All of the interventions, both SM and sham of SM, were performed by the same practitioner. Average duration was about 5 minutes.
Results
Participants’ demographic and baseline assessment data are summarized in Table 1. Values of each variable for experimental and control group, as well as between-group difference, are presented. Between-group difference probability (P value) was calculated using the independent samples t test for normally distributed data, χ2 test for binary data, and the Mann-Whitney U test for nonparametric variables.
Table 1.
Baseline Characteristics of 2 Study Groups
| Name/Variable/Characteristic | Experimental Group n = 40 |
Control Group n = 38 |
Between-group Difference |
|---|---|---|---|
| Age (y): mean (SD) | 11.03 (2.49) | 10.72 (2.63) | P = .59a |
| Sex: n (%) | |||
| Male | 17 (42.5) | 23 (59) | P = .14b |
| Female | 23 (57.5) | 16 (41) | |
| Body weight (kg): mean (SD) | 33.7 (11.2) | 35.1 (13.3) | P = .63a |
| Body height (cm): mean (SD) | 140.01 (14.7) | 139.9 (16.25) | P = .96a |
| Wrist length (cm): mean (SD) | 7.68 (0.94) | 7.46 (0.95) | P = .40a |
| Diagnosis: n (%) | |||
| CP spastic bilateral | 34 (85) | 35 (89.7) | P = .53b |
| CP spastic unilateral | 6 (15) | 4 (10.3) | |
| Spasticity level: n (%) | |||
| High | 14 (35) | 14 (35.9) | P = .93b |
| Low | 26 (65) | 25 (64.1) | |
| MACS: n (%) | |||
| Level I | 16 (40.0) | 11 (28.2) | P = .47b |
| Level II | 21 (52.2) | 20 (51.3) | |
| Level III | 3 (7.5) | 7 (17.9) | |
| Level IV | 0 | 1 (2.6) | |
| Level V | 0 | 0 | |
| GMFCS: n (%) | |||
| Level I | 15 (37.5) | 13 (33.3) | P = .77b |
| Level II | 12 (30.0) | 9 (23.1) | |
| Level III | 11 (27.5) | 14 (35.9) | |
| Level IV | 2 (5.0) | 3 (7.7) | |
| Level V | 0 | 0 | |
| Evaluated hand: n (%) | |||
| Left | 19 (47.5) | 23 (59.0) | P = .31b |
| Right | 21 (52.5) | 16 (41.0) | |
| Wrist flexion-extension range (deg.): median (IR) | 148 (14) | 146 (20) | P = .50c |
| Box and block test: mean (SD) | 28.58 (12.28) | 24.03 (12.31) | P = .10a |
| NC (newton): median (IQR) | 5.53 (8.66) | 6.83 (9.10) | P = .61c |
| EC (newton): median (IQR) | 5.59 (4.34) | 4.43 (3.89) | P = .06c |
| VC (newton): median (IQR) | 0.31 (0.79) | 0.3 (0.76) | P = .76c |
EC, elastic component; GMFCS, gross motor function classification system; IQR, interquartile range; MACS, manual ability classification system; NC, neural component; SD, standard deviation; VC, viscous component.
Independent samples t test.
χ2 test
Mann-Whitney U test.
There was no significant difference between experimental and control groups in any of the studied baseline parameters. There was some predominance of the participants with low levels of spasticity, but thanks to the block randomization, they were equally distributed into both groups, thus eliminating possible selection bias.
For gross motor function developmental level (measure according to Gross Motor Function Classification System) and hand function capabilities (measured by MACS), the groups were comparable (P = .47 and P = .77). Children of the experimental group demonstrated slightly better performance on the Box and Block test: mean 28.58 (SD = 12.28) compared to 24.03 (SD = 12.31), but this difference was not statistically significant (P = .10). There was no between-group difference in values of the neural (equivalent of spasticity) and VCs of the muscle tone. Some differences were detected in EC but were still not significant (P = .06). After the intervention, participants were evaluated for the second time. The results are presented in Table 2, Table 3 and Figure 3.
Table 2.
Baseline and Post-intervention Values of the Outcome Measures: Nonparametric Variables
| Outcome/Group | N Cases | Baseline | Post-intervention | Within Group Difference (Probability) | Between Group Difference (Pprobability) |
|---|---|---|---|---|---|
| Neural component: median (IQR) | |||||
| Experimental group | 40 | 5.53 (8.66) | 3.35 (7.19) | P = .002a | P = .034a |
| Control group | 38 | 6.83 (9.10) | 5.73 (11.96) | P = .98 | |
| Elastic component: median (IQR) | |||||
| Experimental group | 40 | 5.53 (4.34) | 5.75 (4.68) | P = .56 | P = .66 |
| Control group | 38 | 4.43 (3.89) | 3.59 (3.32) | P = .23 | |
| Viscous component: median (IQR) | |||||
| Experimental group | 40 | 0.31 (0.79) | 0.20 (0.71) | P = .41 | P = .92 |
| Control group | 38 | 0.3 (0.76) | 0.28 (0.61) | P = .48 | |
IQR, interquartile range.
Statistically significant difference, within-group difference calculated using the Wilcoxon signed-ranks test, between-group difference calculated using the Mann-Whitney U test.
Table 3.
Baseline and Post-intervention Values of the Outcome Measures: Parametric Variables
| Outcome/Group | N Cases | Baseline | Post-intervention | Pre- and Postdifference | 95% Confidence Interval | Within Group Difference (Probability) | Between Group Mean Difference | Between Group Difference (Probability) |
|---|---|---|---|---|---|---|---|---|
| Box and block test: mean (SD) | ||||||||
| Experimental group | 40 | 28.58 (12.29) | 32.68 (12.68) | 4.10 | 5.52-2.68 | P < .001a | 1.05 | P = .28 |
| Control group | 38 | 24.03 (12.31) | 27.11 (13.36) | 3.09 | 4.47-1.69 | P < .001a | ||
SD, standard deviation.
Statistically significant difference.
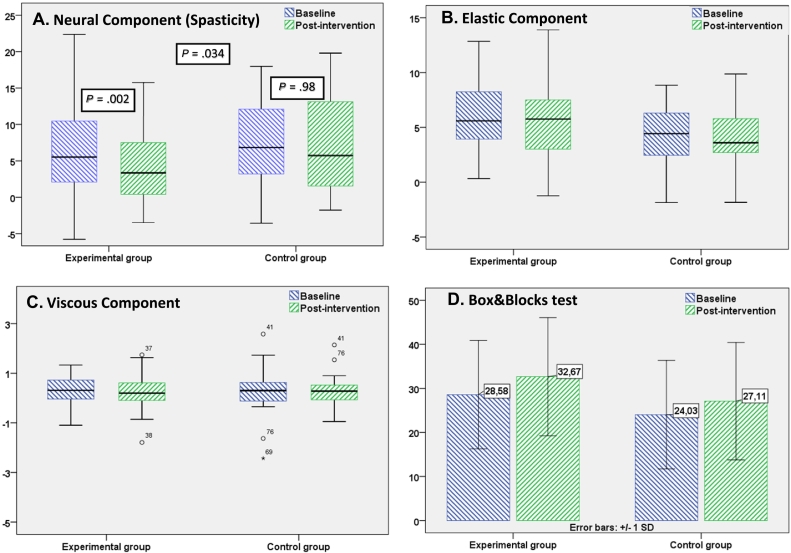
Fig 3.
Baseline and post-intervention outcome measures. In A, B, and C, within group difference calculated using Wilcoxon signed ranks test, between group difference calculated using Mann-Whitney U test, in D within group difference calculated using t test for paired samples. SD, standard deviation.
The primary outcome measure was wrist muscle spasticity, measured quantitatively as a NC of the muscle tone. Statistically significant (P = .002) reduction of NC after SM was noted in the experimental group. Values dropped from the median 5.53 newton (IQR = 8.66) to 3.35 (IQR = 7.19). In the control group, there was only slight reduction of values from the median 6.83 (IQR = 9.10) to 5.7 newton (IQR = 11.96). Comparison between the groups revealed statistically significant difference in spasticity reduction (P = .034).
There was no statistically significant difference in values of the EC of the muscle tone. In the experimental group, the EC changed from 5.53 newton (IQR = 4.34) to 5.75 (IQR = 4.68), and in the control group the EC changed from 4.43 (IQR = 3.89) to 3.59 (IQR = 3.32). Also, no difference was observed while comparing baseline and postintervention values of the VC in both experimental and control groups.
The secondary outcome measure of the study was hand dexterity measured by means of the Box and Block test. Statistically significant difference between baseline and postintervention assessment was measured in both groups (P < .05). In the experimental group, the pre- and postdifference was +4.10 blocks per second (95% confidence interval = 5.52-2.68). In the control group, the pre- and postdifference was +3.01 blocks (95% confidence interval = 4.41-1.69). The experimental group showed a more substantial improvement, with between group difference of 1.05 blocks per minute, but the difference was not statistically significant (P = .28).
Discussion
To our knowledge, this is the first trial to measure the immediate effect of SM on muscle spasticity and hand dexterity in participants with spastic forms of CP. The contribution of this study is that it corroborates the hypothesis that SM may decrease muscle spasticity temporarily in participants with disordered muscle tone regulation, specifically in children with CP.
At present, the mechanisms through which SM alters muscle spasticity are not fully understood. Experimental evidence accumulated to date indicates that SM could have an impact on the afferent neurons from paraspinal tissues and directly influences muscle spindle afferents and Golgi tendon organs, which are directly involved in muscle tone regulation.6, 7, 8, 9, 11, 12 Previous studies conducted on stroke patients and CP children provided only descriptive information about such influence.13, 14, 15
Muscle spasticity was the primary outcome measure of the study. It was measured quantitatively with a NeuroFlexor device, a valid and reliable tool.21, 22 This instrumental method has an advantage over the more traditional tool for spasticity measurement (ie, MAS) because it reduces subjectivity and possible bias.24 Measurement revealed a statistically significant decrease of spasticity after SM in participants in the experimental group. Neural component values dropped from median 5.53 newton (IQR = 8.66) to 3.35 (IQR = 7.19), with P < .05. In the control group, the reduction was subtle and not statistically significant. Comparison between groups revealed statistically significant difference in spasticity reduction (P < .05).
Measurement of elastic and viscous properties of the wrist muscles showed no difference between baseline and postintervention evaluations in neither of the groups. Such results were anticipated because structural changes in the muscle are not supposed to occur in such a short period of time.
The secondary outcome measure was changes of hand dexterity, measured using the Box and Block test, in which a number of wooden blocks are moved from 1 part of the box to the other in 1 minute. While comparing the preintervention and postintervention data, improvement was noted in both groups (P < .05), and this could be attributed to the effects of training. In the experimental group, improvement of hand dexterity was more pronounced, with between-group difference of 1.05 blocks per minute. However, the difference was not statistically significant (P = .28), and we were unable to draw any conclusions about influence of SM on manual dexterity in participants with spasticity syndrome.
Limitations and Future Studies
One limitation of this study was its short-term design. It resulted from difficulties in recruiting people for longer-term observation and ensuring that no other factors would interfere and impact spasticity. The study was conducted during the first half of the day on participants who arrived at the clinic for treatment, and there was only a short period of time in the morning before the beginning of a regular treatment program. However, a longer follow-up period should be implemented in future studies to understand long-term effects of the SM therapy.
Another possible limitation of this study is the fact that the participants in the control group, who had previously undergone SM, might have suspected that they were in the control group, even though they had no direct influence on the results of the second evaluation.
While our trial provides data that supports a relationship between SM and reduction of spasticity, its findings are preliminary. Further studies are needed to deepen the understanding of the neurophysiological underpinnings behind SM and confirm the efficacy of its use to treat spasticity. Our study can potentially expand the range of clinical application of spinal manipulative therapy in the future, but the data to support its implementation in clinical practice are insufficient.
Conclusion
These findings suggest that SM may, in the short term, help to reduce spasticity in participants with CP. Long-term effects of this influence were beyond the scope of the study and will have to be studied in the future. No evidence about the influence of SM on hand dexterity in participants with spasticity was obtained.
Funding Sources and Conflicts of Interest
Research was supported by a grant from the Institute of Medical Rehabilitation, Ukraine (state registration number: 23272758). No conflicts of interest were reported for this study.
Contributorship Information
Concept development (provided idea for the research): O.K.
Design (planned the methods to generate the results): O.K., A.K.
Supervision (provided oversight, responsible for organization and implementation, writing of the manuscript): O.K.
Data collection/processing (responsible for experiments, patient management, organization, or reporting data): A.K., O.M., M.H.
Analysis/interpretation (responsible for statistical analysis, evaluation, and presentation of the results): O.K., A.K.
Literature search (performed the literature search): A.K., O.M., M.H.
Writing (responsible for writing a substantive part of the manuscript): O.K., O.M.
Critical review (revised manuscript for intellectual content, this does not relate to spelling and grammar checking): O.K., A.K., O.M., M.H.
Practical Applications
-
•
This randomized controlled study demonstrated reduction of wrist muscle spasticity after spinal manipulation in participants with cerebral palsy.
-
•
The study provides new data on neurophysiological effects of spinal manipulation.
-
•
Long-term effects of spinal manipulation on spasticity should be studied further.
Alt-text: Unlabelled Box
References
- 1.Bar-On L, Molenaers G, Aertbeliën E. Spasticity and its contribution to hypertonia in cerebral palsy. Biomed Res Int. 2015;2015:317047. doi: 10.1155/2015/317047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lance J. Symposium synopsis. In: Feldman RG, R. Young RR, Koella WP, editors. Spasticity: Disordered Motor Control. Yearbook Medical; Chicago, Illinois: 1980. pp. 485–494. [Google Scholar]
- 3.Tilton AH. Management of spasticity in children with cerebral palsy. Semin Pediatr Neurol. 2009;16(2):82–89. doi: 10.1016/j.spen.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 4.Oppenheim WL. Complementary and alternative methods in cerebral palsy. Dev Med Child Neurol. 2009;51(suppl 4):122–129. doi: 10.1111/j.1469-8749.2009.03424.x. [DOI] [PubMed] [Google Scholar]
- 5.Liptak GS. Complementary and alternative therapies for cerebral palsy. Ment Retard Dev Disabil Res Rev. 2005;11(2):156–163. doi: 10.1002/mrdd.20066. [DOI] [PubMed] [Google Scholar]
- 6.Pickar JG. Neurophysiological effects of spinal manipulation. Spine J. 2002;2(5):357–371. doi: 10.1016/s1529-9430(02)00400-x. [DOI] [PubMed] [Google Scholar]
- 7.Chu J, Allen DD, Pawlowsky S, Smoot B. Peripheral response to cervical or thoracic spinal manual therapy: an evidencebased review with meta-analysis. J Man Manip Ther. 2014;22(4):220–229. doi: 10.1179/2042618613Y.0000000062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reed WR, Long CR, Kawchuk GN, Pickar JG. Neural responses to the mechanical characteristics of high velocity, low amplitude spinal manipulation: effect of specific contact site. Man Ther. 2015;20(6):797–804. doi: 10.1016/j.math.2015.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reed WR, Liebschner MA, Sozio RS, Pickar JG, Gudavalli MR. Neural response during a mechanically assisted spinal manipulation in an animal model: a pilot study. J Nov Physiother Phys Rehabil. 2015;2(2):20–27. doi: 10.17352/2455-5487.000021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Herzog W. The biomechanics of spinal manipulation. J Bodyw Mov Ther. 2010;14(3):280–286. doi: 10.1016/j.jbmt.2010.03.004. [DOI] [PubMed] [Google Scholar]
- 11.Suter E, McMorland G, Herzog W. Short-term effects of spinal manipulation on H-reflex amplitude in healthy and symptomatic subjects. J Manipulative Physiol Ther. 2005;28(9):667–672. doi: 10.1016/j.jmpt.2005.09.017. [DOI] [PubMed] [Google Scholar]
- 12.Dishman JD, Weber KA, Corbin RL. Understanding inhibitory mechanisms of lumbar spinal manipulation using H-reflex and F-wave responses: a methodological approach. J Neurosci Methods. 2012;210(2):169–177. doi: 10.1016/j.jneumeth.2012.07.014. [DOI] [PubMed] [Google Scholar]
- 13.Valente FM, Lupino RA, Ramirez C, Bonvicine C. Evaluation of the effect of spinal manipulation in upper limb spasticity post stroke. Man Ther Posturol Rehabil J. 2014;12 [Google Scholar]
- 14.Kozyavkin VI, Kachmar OO. Rehabilitation outcome assessment methods in Intensive neurophysiological rehabilitation system. Ukrayinskyj Medychnyj Chasopys. 2003;3(35):61–66. [Google Scholar]
- 15.Kachmar O, Voloshyn T, Hordiyevych M. Changes in muscle spasticity in patients with cerebral palsy after spinal manipulation: case series. J Chiropr Med. 2016;15(4):299–304. doi: 10.1016/j.jcm.2016.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haavik H, Murphy B. The role of spinal manipulation in addressing disordered sensorimotor integration and altered motor control. J Electromyogr Kinesiol. 2012;22(5):768–776. doi: 10.1016/j.jelekin.2012.02.012. [DOI] [PubMed] [Google Scholar]
- 17.Petersen S, Domino N, Postma C, Wells C, Cook C. Scapulothoracic muscle strength changes following a single session of manual therapy and an exercise programme in subjects with neck pain. Musculoskelet Care. 2016;14(4):195–205. doi: 10.1002/msc.1132. [DOI] [PubMed] [Google Scholar]
- 18.Kozyavkin V, Lysovych V, Hordiyevych S. Proceedings of the Scientific Practical Conference with International Participation "Cerebral Palsy and Other Movement Disorders in Children.". 2011. Changes of hand function in CP patients during treatment by intensive neurophysiological rehabilitation system; pp. 75–76. Moscow, Russia. [Google Scholar]
- 19.Schulz KF, Altman DG, Moher D. CONSORT 2010 statement: updated guidelines for reporting parallel group randomized trials. Obstet Gynecol. 2010;115(5):1063–1070. doi: 10.1097/AOG.0b013e3181d9d421. [DOI] [PubMed] [Google Scholar]
- 20.World Health Organization . 2005. WHO Guidelines on Basic Training in Chiropractic. Geneva, Switzerland. [Google Scholar]
- 21.Gäverth J, Sandgren M, Lindberg PG, Forssberg H, Eliasson AC. Test-retest and inter-rater reliability of a method to measure wrist and finger spasticity. J Rehabil Med. 2013;45(7):630–636. doi: 10.2340/16501977-1160. [DOI] [PubMed] [Google Scholar]
- 22.Gäverth J, Eliasson AC, Kullander K, Borg J, Lindberg PG, Forssberg H. Sensitivity of the NeuroFlexor method to measure change in spasticity after treatment with botulinum toxin a in wrist and finger muscles. J Rehabil Med. 2014;46(7):629–634. doi: 10.2340/16501977-1824. [DOI] [PubMed] [Google Scholar]
- 23.Bohannon RW, Smith MB. Inter-rater reliability of a modified Ashworth scale of muscle spasticity. Phys Ther. 1987;67(2):206–207. doi: 10.1093/ptj/67.2.206. [DOI] [PubMed] [Google Scholar]
- 24.Fleuren JF, Voerman GE, Erren-Wolters CV. Stop using the Ashworth scale for the assessment of spasticity. J Neurol Neurosurg Psychiatry. 2010;81(1):46–52. doi: 10.1136/jnnp.2009.177071. [DOI] [PubMed] [Google Scholar]
- 25.Mathiowetz V, Volland G, Kashman N, Weber K. Adult norms for the box and block test of manual dexterity. Am J Occup Ther. 1985;39(6):386–391. doi: 10.5014/ajot.39.6.386. [DOI] [PubMed] [Google Scholar]
- 26.Kozyavkin V, Kachmar O, Voloshyn T. Gross motor function classification system for patients with cerebral palsy. Expanded and revised version. Soc Paediatr. 2012;2(3):74–85. [Google Scholar]
- 27.Kachmar O, Kozyavkin V, Voloshyn T, Vityk C, Kalynovych N. Manual ability classification system for children with cerebral palsy: Ukrainian version. Mankovsky J Neurol. 2016;2(4):31–34. [Google Scholar]
- 28.Groeneweg R, Rubinstein SM, Oostendorp RA, Ostelo RW, van Tulder MW. Guideline for Reporting Interventions on Spinal Manipulative Therapy: Consensus on interventions reporting criteria list for spinal manipulative therapy (CIRCLe SMT) J Manipulative Physiol Ther. 2017;40(2):61–70. doi: 10.1016/j.jmpt.2016.10.013. [DOI] [PubMed] [Google Scholar]
- 29.Kozyavkin V, Babadahly M, Lun H. Papuga Publishing House; Lviv, Ukraine: 2012. Intensive Neurophysiological Rehabilitation System — the Kozyavkin Method. A Manual for Rehabilitation Specialists. [Google Scholar]