Abstract
Aims:
We aimed to investigate the relationship of colorectal cancer prognosis and inflammatory parameters, including neutrophil-to-lymphocyte ratio (NLR) and lymphocyte-to-monocyte ratio (LMR), with reference to circulating myeloid-derived suppressor cells (MDSCs) in the current study.
Patients and Methods:
Thirty-five patients who underwent curative-intent surgery were enrolled. A receiver-operating characteristic curve (ROC) was used to assess the usefulness of candidates for prognostic factors. Recurrence-free survival (RFS) was calculated using the Kaplan-Meier method, and the candidates for prognostic factors were assessed by a Cox proportional hazard model.
Results:
ROC curve analyses determined cutoff values for NLR and LMR as 2.9 and 2.4, respectively. The percentage of MDSCs in patients with LMR ≤ 2.4 was statistically higher than in those with LMR > 2.4 (p = 0.012). The patients with LMR ≤ 2.4 exhibited a statistically lower RFS than those with LMR > 2.4 (p = 0.008). These results were also observed in patients with stage II + III disease. LMR was an independent prognostic factor of RFS in colorectal cancer patients (hazard ratio: 7.757, 95% confidence interval: 1.462-41.152, p = 0.016).
Conclusion:
Lower LMR was associated with poor prognosis in colorectal cancer patients; whereas, higher circulating MDSCs were observed in patients with lower LMR.
Keywords: lymphocyte-to-monocyte ratio, neutrophil-to-lymphocyte-ratio, myeloid-derived suppressor cells, colorectal cancer
Introduction
Colorectal cancer is the third most commonly diagnosed cancer worldwide in males and the second most in females, with an estimated 1.4 million cases and 693,900 deaths occurring in 20121). One of the hallmarks of cancer is angiogenesis in conjunction with systemic and local inflammation2). The relationship between inflammation and carcinogenesis is well known, especially in high risk of colorectal carcinogenesis in patients with ulcerative colitis. Systemic chronic inflammation has been reported to play roles in the suppression of anti-tumor immunity, carcinogenesis, and tumor progression3,4). In search of a prognostic factor independent of tumor staging, there has been accumulating evidence reporting that inflammation- or nutritional factor-based prognostic scores, such as the Glasgow Prognostic score (GPS) based on C-reactive protein (CRP) and albumin, are useful in predicting patients’ prognosis5). With regard to colorectal cancer, neutrophil-to-lymphocyte ratio (NLR)6-10) and lymphocyte-to-monocyte ratio (LMR)11-15) are two of the most investigated surrogate markers of prognosis of colorectal cancer patients. A high NLR and a low LMR have been reported to be associated with poor overall survival (OS)6,9,11-15) and recurrence-free survival (RFS)8,11,13-15).
On the other hand, myeloid-derived suppressor cells (MDSCs) have been identified as key mediators in the negative regulation of immune responses. MDSCs are a heterogeneous population of myeloid origin, which exhibit a potent immunosuppressive activity against T-cell response16). A partial block in the differentiation of immature myeloid cells has been reported to occur in certain pathological conditions, such as cancer, infectious diseases, sepsis, trauma, hematopoietic stem cell transplantation and some autoimmune diseases, resulting in the expansion of MDSCs17). Several studies have shown that elevated circulating MDSCs correlated with worse prognosis in various cancers including colorectal cancer18-20). However, the relationship between these markers and other parameters, such as inflammatory or nutritional markers, remains to be elucidated. Thus, we aimed to investigate the relationship of colorectal cancer prognosis and inflammatory parameters, including NLR and LMR, with reference to circulating MDSCs in the current study.
Materials and Methods
Patients
Blood samples were collected from 48 patients before starting treatment between February 2011 and August 2013. Among these, 35 patients who underwent curative-intent surgery were enrolled into the present study, while the remaining 13 patients were excluded due to metastatic disease. Following surgery, the final stage of the patients was determined pathologically according to the TNM classification system of malignant tumors published by the International Union Against Cancer, 8th edition21). Table 1 shows patient characteristics. Preoperative chemoradiation therapy (CRT) (S-1 of 100 mg/body surface area (m2) with irradiation of 50.4 Gy) was performed on eight patients with inferior rectal cancer including two stage I, four stage II and two stage III patients. Adjuvant chemotherapy was performed on 17 patients including 11 stage III patients, three stage II patients with CRT, two stage II with intensive lymphatic invasion and one stage I with CRT. The study protocol was approved by the ethics committee of Fukushima Medical University, and written informed consent was obtained from all enrolled patients.
Table 1.
Patient characteristics
| Category | N | % | |
| Gender | |||
| Male | 20 | 57.1 | |
| Female | 15 | 42.9 | |
| T | |||
| 1 | 2 | 5.7 | |
| 2 | 8 | 22.9 | |
| 3 | 16 | 45.7 | |
| 4a | 9 | 25.7 | |
| 4b | 0 | 0.0 | |
| N | |||
| 0 | 21 | 60.0 | |
| 1a | 3 | 8.6 | |
| 1b | 5 | 14.3 | |
| 1c | 0 | 0.0 | |
| 2a | 4 | 11.4 | |
| 2b | 2 | 5.7 | |
| Stage | |||
| I | 9 | 25.7 | |
| IIA | 6 | 17.1 | |
| IIB | 6 | 17.1 | |
| IIC | 0 | 0.0 | |
| IIIA | 0 | 0.0 | |
| IIIB | 12 | 34.3 | |
| IIIC | 2 | 5.7 | |
| Location | |||
| Cecum | 0 | 0.0 | |
| Ascending | 5 | 14.3 | |
| Transverse | 4 | 11.4 | |
| Descending | 1 | 2.9 | |
| Sigmoid | 5 | 14.3 | |
| Superior rectal | 5 | 14.3 | |
| Middle rectal | 6 | 17.1 | |
| Inferior rectal | 10 | 28.6 |
Measurements of Parameters
Blood samples were collected before starting treatment. Patient nutritional status was determined by measuring serum concentrations of total protein (TP), albumin, retinol binding protein (RBP), transthyretin (TTR), and transferrin. These parameters were measured at the Central Clinical Laboratory of Fukushima Medical University Hospital. As for the inflammatory parameters, C-reactive protein (CRP), white blood cell count (WBC), neutrophil, lymphocyte, and monocyte counts, as well as neutrophil-to-lymphocyte ratio (NLR) and lymphocyte-to-monocyte ratio (LMR), were used.
MDSCs are most commonly defined as CD14−CD11b+ cells or cells that express the common myeloid marker CD3322). Thus, MDSCs were defined as CD14−CD11b+CD33+ cells in the present study as described in our previous studies. Peripheral blood mononuclear cells (PBMC) were separated on Ficoll-Hypaque gradient (Pharmacia-Biotech, Uppsala, Sweden). The isolated PBMC were washed twice with RPMI-1640 (Wako Pure Chemical Industries Ltd., Osaka, Japan), then labeled with fluorescent isothiocyanate-conjugated anti-CD14 (Abeam, Cambridge, UK), phycoerythrin-conjugated anti-CD11b (Beckman Coulter Inc., Marseille, France) and phycoerythrin cyanin 5.1-conjugated anti-CD33 (Beckman Coulter Inc., Marseille, France), each diluted in phosphate-buffered saline (PBS) to a concentration of 10 and 50 μg/ml. Cells were incubated with the antibodies for 20 min at 4°C and were then washed with PBS. Data acquisition and analysis were performed with the FACSAria II flow cytometer (BD Biosciences, Mountain View, CA, USA) using Flow Jo software (TreeStar Inc., Ashland, OR, USA).
Statistical Analysis
Data are presented as frequencies or percentages for categorical variables and mean ± SD for continuous variables, unless otherwise indicated. For categorical clinical variables, differences between the groups were evaluated by Fisher’s exact test. The differences in mean values between the groups were analyzed using the Mann-Whitney U test. Associations between the two variables were quantified using Spearman’s rank correlation coefficient. The receiver operating characteristic (ROC) curve was used to evaluate the usefulness of the examined parameters as a prognostic factor for RFS. In a ROC curve, Y-axis shows true positive rate (sensitivity) and X-axis represents false positive rate (1-specificity) at each measured parameter. Thus, the left upper corner is ideal because it represents both sensitivity and specificity equal to 1.0. Therefore, the nearest coordinate point to the left upper corner is usually selected as a coordinate point of cutoff value. The mean observation period was 50.4 months (median: 59.8, range: 1.3-84.5). The final assessment of disease status was made on July 31st, 2017. OS and RFS were calculated using the Kaplan-Meier method and differences between the groups were assessed by the log-rank test. Factors found to be significant in the univariate analysis were subjected to a multivariate analysis using a Cox proportional hazard model to identify the independent predictors of prognosis. A two-sided P value of < 0.05 was considered to indicate statistically significant differences. All statistical calculations were performed using SPSS® version 24 (IBM Japan, Tokyo, Japan).
Results
Analysis using a ROC curve
Fig. 1 shows ROC curves of NLR and LMR for predicting the recurrence of colorectal cancer. The area under the curve for NLR was 0.726, and a level of 2.9 was determined as the cutoff value (sensitivity = 0.857, and specificity = 0.720). The area under the curve for LMR was 0.719. A cutoff value of LMR determined at a level of 2.4 from the nearest coordinate point to the left upper corner (sensitivity = 0.714, and 1-specificity = 0.179).
Fig. 1.
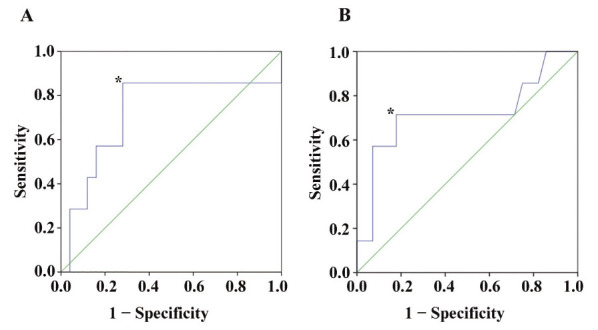
Receiver-operating characteristic (ROC) curves. (A): The area under the ROC curve for NLR was 0.726, and a level of 2.9 was determined as the cutoff value* (sensitivity = 0.857, and specificity = 0.720). (B): The area under the ROC curve for LMR was 0.719, and a level of 2.4 was determined as the cutoff value from the nearest coordinate point* (sensitivity = 0.714, and 1- specificity = 0.179) to the left upper corner.
Relationships between LMR and other parameters
As shown in Fig. 2A and 2B, LMR exhibited a statistically significant inverse correlation with NLR (r = −0.685, p < 0.001), and with MDSCs (r = −0.531, p = 0.003). The percentage of MDSCs in patients with LMR ≤ 2.4 (median: 3.2%, range: 0.8-17.5%) was statistically higher than in those with LMR > 2.4 (median: 0.9%, range: 0.4-9.4%) (Fig. 3A, p = 0.012).
Fig. 2.
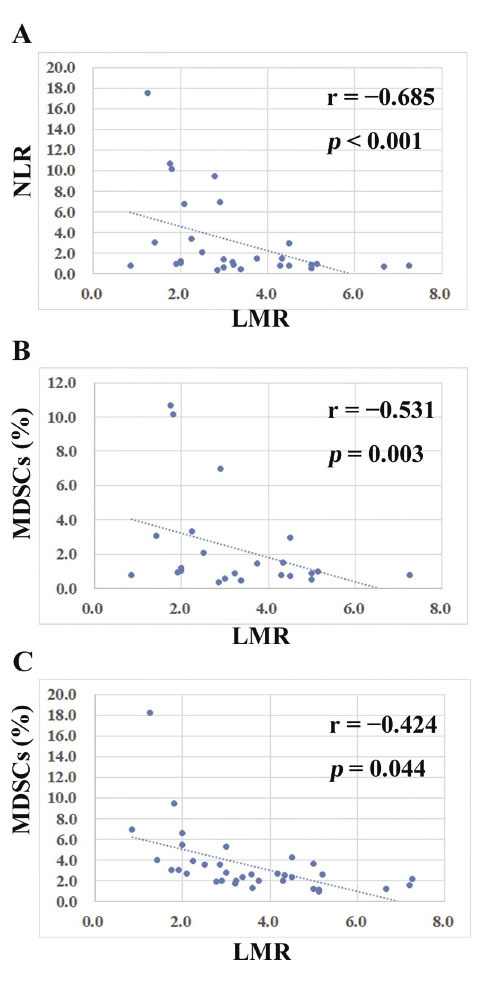
Relationships between LMR and other parameters. (A): LMR exhibited a statistically significant inverse correlation with NLR (r = −0.685, p < 0.001). (B): LMR showed a statistically significant inverse correlation with MDSCs (r = −0.531, p = 0.003). (C): LMR exhibited a statistically significant inverse correlation with MDSCs in patients with stage II + III disease (r = −0.424, p = 0.044). LMR: lymphocyte-to-monocyte ratio, NLR: neutrophil-to-lymphocyte-ratio, MDSCs: myeloid-derived suppressor cells.
Fig. 3.
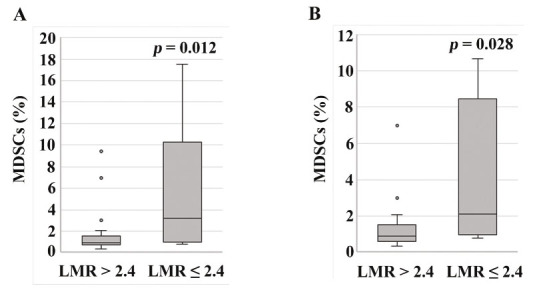
MDSC percentage according to LMR levels. (A): The percentage of MDSCs in the patients with LMR ≤ 2.4 (median: 3.2%, range: 0.8-17.5%) was statistically higher than in those with LMR > 2.4 (median: 0.9%, range: 0.4-9.4%) (p = 0.012). (B): In the analysis in the patients with stage II + III disease, the percentage of MDSCs in patients with LMR ≤ 2.4 (median: 2.1%, range: 0.8-10.7%) was statistically higher than in those with LMR > 2.4 (median: 0.9%, range: 0.4-7.0%) (p = 0.028).
Table 2 summarizes patient characteristics according to NLR or LMR. The average age of patients with NLR ≥ 2.9 (58.6 ± 11.6 years) was significantly lower than that of patients with NLR < 2.9 (67.9 ± 13.5) (p = 0.043). The incidence of T3 + T4 in the patients with NLR ≥ 2.9 was statistically higher in those with NLR < 2.9 (p = 0.028). The incidence of > stage IIA in the patients with NLR ≥ 2.9 was statistically higher in those with NLR < 2.9 (p = 0.007). The incidence of adjuvant chemotherapy in the patients with NLR ≥ 2.9 was also statistically higher than in those with NLR < 2.9 (p = 0.006). On the other hand, the incidence of CRT in the patients with LMR ≤ 2.4 was also statistically higher than in those with LMR > 2.4 (p = 0.027).
Table 2.
Patient characteristics according to NLR or LMR
| NLR < 2.9
(n = 21) |
NLR ≥ 2.9
(n = 14) |
p | LMR > 2.4
(n = 25) |
LMR ≤ 2.4
(n = 10) |
p | |||
| Age | 67.9 ± 13.5 | 58.6 ± 11.6 | 0.043 | 66.3 ± 13.0 | 59.0 ± 13.6 | 0.146 | ||
| Gender | 0.728 | 1.000 | ||||||
| Male | 11 | 9 | 14 | 6 | ||||
| Female | 10 | 5 | 11 | 4 | ||||
| T | 0.028 | 0.686 | ||||||
| T1 + T2 | 9 | 1 | 8 | 2 | ||||
| T3 + T4 | 12 | 13 | 17 | 8 | ||||
| N | 0.483 | 1.000 | ||||||
| negative | 14 | 7 | 15 | 6 | ||||
| positive | 7 | 7 | 10 | 4 | ||||
| Stage | 0.007 | 0.134 | ||||||
| ≤ IIA | 13 | 2 | 13 | 2 | ||||
| > IIA | 8 | 12 | 12 | 8 | ||||
| Ly | 0.461 | 1.000 | ||||||
| 0 | 8 | 3 | 8 | 3 | ||||
| 1 | 13 | 11 | 17 | 7 | ||||
| V | 1.000 | 0.393 | ||||||
| 0 | 5 | 4 | 5 | 4 | ||||
| 1 | 16 | 10 | 20 | 6 | ||||
| Histology | 0.288 | 0.123 | ||||||
| Well | 6 | 7 | 7 | 6 | ||||
| Moderate | 15 | 7 | 18 | 4 | ||||
| CRT | 0.221 | 0.027 | ||||||
| − | 18 | 9 | 22 | 5 | ||||
| + | 3 | 5 | 3 | 5 | ||||
| Adj | 0.006 | 0.146 | ||||||
| − | 15 | 3 | 15 | 3 | ||||
| + | 6 | 11 | 10 | 7 |
NLR: neutrophil-to-lymphocyte ratio, LMR: lymphocyte-to-monocyte ratio, Ly: lymphatic vessel invasion, V: microscopic vascular invasion, Well: well-differentiated adenocarcinoma, Moderate: moderately-differentiated adenocarcinoma, CRT: preoperative chemoradiation therapy, Adj: adjuvant chemotherapy.
OS and RFS
Patients with NLR ≥ 2.9 showed a statistically lower RFS than those with NLR < 2.9 (Fig. 4A, p = 0.007), whereas there were no statistically significant differences in OS (p = 0.423). The patients with LMR ≤ 2.4 exhibited a statistically lower RFS than those with LMR > 2.4 (Fig. 4B, p = 0.008), whereas there were no statistically significant differences in OS (p = 0.430). Since the median percentage of MDSCs in the patients with LMR ≤ 2.4 was 3.2 %, RFS was evaluated between the patients with MDSCs < 3.2% and those with MDSCs ≥ 3.2%; however, there were no statistically significant differences (p = 0.156).
Fig. 4.
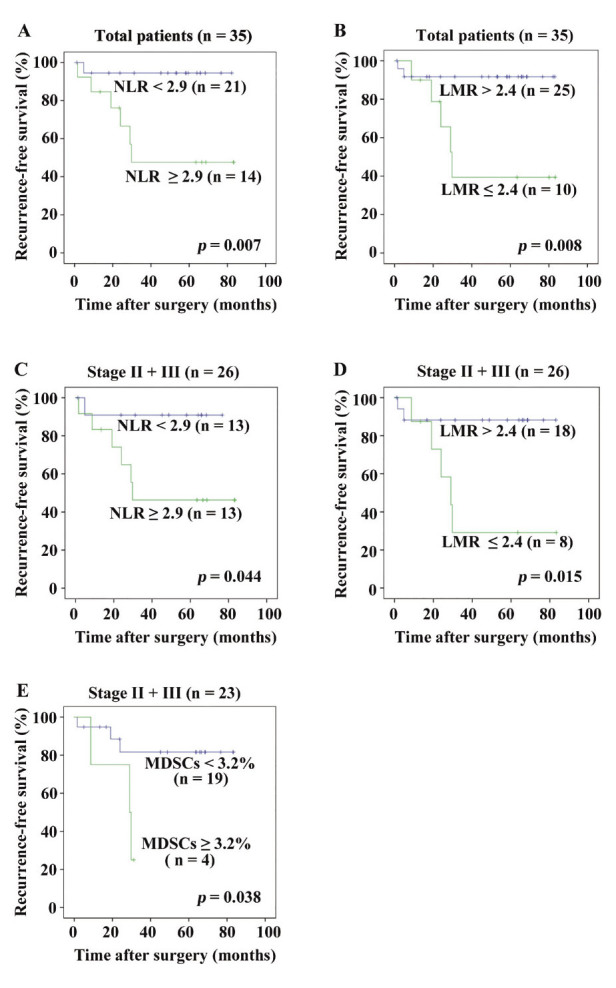
RFS using the Kaplan-Meier method statistically assessed by the log-rank test. (A): Patients with NLR ≥ 2.9 showed a statistically lower RFS than those with NLR < 2.9 (p = 0.007). (B): Patients with LMR ≤ 2.4 exhibited a statistically lower RFS than those with LMR > 2.4 (p = 0.008). (C): When analyzed in stage II + III disease patients, those with NLR ≥ 2.9, or MDSCs ≥ 3.2% showed a statistically lower RFS than those with NLR < 2.9 (p = 0.044). (D): When analyzed in stage II + III disease patients, those with LMR ≤ 2.4 showed a statistically lower RFS than those with LMR > 2.4 (p = 0.015). (E): When analyzed in stage II + III disease patients, those with MDSCs ≥ 3.2% showed a statistically lower RFS than those with MDSCs < 3.2% (p = 0.038). MDSCs could not be examined in three out of 26 patients with stage II + III disease.
Cox proportional hazard model
Table 3 shows the results using a Cox proportional hazard model. Dichotomization was performed as follows: gender (male vs. female), T factor (T1 + T2 vs. T3 + T4), N factor (negative vs. positive), stage (≤ IIA vs. > IIA), adjuvant chemotherapy (negative vs. positive), CRT (negative vs. positive), lymphatic vessel invasion (negative vs. positive), microscopic vascular invasion (negative vs. positive), histology (well differentiated vs. moderately differentiated), MDSCs (< 3.2% vs. ≥ 3.2%), NLR (< 2.9 vs. ≥ 2.9), and LMR (> 2.4 vs. ≤ 2.4). In the univariate analysis, NLR (HR: 10.134, 95% confidence interval: 0.079-2.106, p = 0.032) and LMR (HR: 7.888, 95% confidence interval: 1.501-41.459, p = 0.015) exhibited statistically significant differences. LMR and NLR were confounding factors of each other. Thus, NLR and LMR were separately introduced into the multivariate analysis with N factor which p factor showed < 0.1 in the univariate analysis. Then, only LMR was an independent prognostic factor of RFS in colorectal cancer patients (hazard ratio: 7.757, 95% confidence interval: 1.462-41.152, p = 0.016).
Table 3.
Cox proportional hazard model
| Univariate analysis | Multivariate analysis | ||||||
| HR | 95% CI | p | HR | 95% CI | p | ||
| Gender | 0.408 | 0.079-2.106 | 0.285 | ||||
| T | 33.276 | 0.029-37863.615 | 0.352 | ||||
| N | 4.559 | 0.883-23.537 | 0.070 | 3.997 | 0.768-20.793 | 0.100 | |
| Stage | 54.016 | 0.118-24800.875 | 0.202 | ||||
| CRT | 2.207 | 0.493-9.871 | 0.300 | ||||
| Adj | 2.384 | 0.462-12.295 | 0.299 | ||||
| Ly | 1.203 | 0.566-2.557 | 0.632 | ||||
| V | 2.396 | 0.272-21.112 | 0.431 | ||||
| Histology | 0.900 | 0.549-1.474 | 0.675 | ||||
| MDSCs | 3.013 | 0.607-14.945 | 0.177 | ||||
| NLR | 10.134 | 1.214-84.565 | 0.032 | ||||
| LMR | 7.888 | 1.501-41.459 | 0.015 | 7.757 | 1.462-41.152 | 0.016 | |
HR: hazard ratio, 95% CI: 95% confidence interval, CRT: preoperative chemoradiation therapy, Adj: adjuvant chemotherapy, Ly: lymphatic vessel invasion, V: microscopic vascular invasion, MDSCs: myeloid-derived suppressor cells, NLR: neutrophil-to-lymphocyte ratio, LMR: lymphocyte-to-monocyte ratio. NLR and LMR were confounding factors of each other. Thus, NLR and LMR were separately introduced into the multivariate analysis with N factor which p factor showed < 0.1 in the univariate analysis. Only LMR was an independent prognostic factor of RFS in colorectal cancer patients.
Subgroup analysis
A subgroup analysis was performed in patients with stage II + III disease. There were no statistically significant differences in clinicopathological characteristics between patients with LMR ≤ 2.4 and those with LMR > 2.4 (data not shown). LMR exhibited a statistically significant inverse correlation with MDSCs (Fig. 2C, r = −0.424, p = 0.044). The percentage of MDSCs in patients with LMR ≤ 2.4 (median: 2.1%, range: 0.8-10.7%) was statistically higher than in those with LMR > 2.4 (median: 0.9%, range: 0.4-7.0%) (Fig. 3B, p = 0.028). When analyzed in stage II + III patients (Figs. 4C, 4D and 4E), those with NLR ≥ 2.9, LMR ≤ 2.4 or MDSCs ≥ 3.2% showed a statistically lower RFS than those with NLR < 2.9, LMR > 2.4 or MDSCs < 3.2% (p = 0.044, p = 0.015or p = 0.038, respectively).
Discussion
In the current study, a lower LMR was associated with poorer RFS, and LMR ≤ 2.4 was an independent prognostic factor for RFS in colorectal cancer patients. A higher NLR exhibited poorer RFS; however, it could not be a prognostic factor in the multivariate analysis using a Cox proportional hazard model. With regard to LMR, a lower LMR was associated with higher MDSCs. In the subgroup analysis in the patients with stage II + III disease, a higher NLR, lower LMR and higher MDSCs showed a poorer RFS, and a lower LMR was also associated with higher MDSCs. Previous studies have reported that a lower LMR was associated with poor OS as well as RFS; however, we could not prove this in the present study.
The peripheral lymphocyte count has been reported to reflect the responsiveness of the entire immune system of a patient23,24). A high number of tumor-infiltrating lymphocytes (TIL) at the site of the tumor have been reported to be associated with a good prognosis25). On the other hand, neutrophilia has been reported to be associated with disease severity, and neutrophils may promote a tumor-favorable environment by the suppression of lymphocyte-mediated cytolysis26). Tumor-associated neutrophils (TAN) have dual aspects: N1, anti-tumor phenotype and N2, protumor phenotype27). TGF-β can enhance tumor growth by inducing N2 neutrophils, and after blockade of TGF-β, neutrophils become N1. Thus, TAN may have quite different effects on tumor cells according to tumor types28). Since NLR is derived from peripheral neutrophil and lymphocyte count, it is easily understandable that higher NLR is associated with poor prognosis in various cancers.
Monocytes play an important role in tumor progression. The peripheral blood monocyte count has been reported to be associated with poor clinical outcomes in colorectal cancer28). Tumor-associated macrophages (TAM), which originate from peripheral blood monocytes29), are also a double-edged sword: M1, anti-tumor phenotype and M2, protumor phenotype27), which is the main population of TAM. Since LMR is derived from the peripheral lymphocyte and monocyte count, it is acceptable to suggest that lower LMR is associated with poor clinical outcomes. In the present study, the patients with lower LMR exhibited a poorer prognosis than those with higher LMR. Interestingly, this result could be confirmed in the patients with stage II + III disease. Current clinical guidelines recommend adjuvant chemotherapy for patients with stage III and those with high-risk stage II; however, the necessity to administer adjuvant chemotherapy should be considered for patients with lower LMR.
MDSCs consist of two large groups of cells: granulocytic or polymorphonuclear MDSCs (defined as CD11b+CD14−CD15+ or CD11b+CD14−CD66b+), and monocytic MDSCs (defined as CD11b+CD14+HLA-DR−/low). Thus, MDSCs in the current study (defined as CD14−CD11b+CD33+) contains more immature progenitors, and we could not define which subtype of MDSCs accounted for the increased number of MDSCs in the current study. Several studies have shown elevated circulating MDSCs in colorectal cancer patients19,20,22). In the present study, LMR exhibited a statistically significant inverse correlation with MDSCs. Furthermore, MDSCs ≥ 3.2% showed a statistically lower RFS than those with MDSCs < 3.2% in patients with stage II + III disease. Increased MDSCs may contribute to increase of peripheral monocyte count, resulting in low LMR. The relationship between increased MDSCs and low LMR remains to be elucidated.
Several limitations exist in the current study. Firstly, there was a relatively small number of enrolled patients. Secondary, we did not examine immunohistochemical studies to evaluate TIL, TAN and TAM. Thirdly, the subtype of MDSCs could not be defined. There have been several reports on the relationship between LMR and prognosis in colorectal cancer patients; however, this is the first study to demonstrate the relationship between LMR and MDSCs in colorectal cancer patients.
References
- 1.Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin, 65: 87-108, 2015. [DOI] [PubMed] [Google Scholar]
- 2.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell, 144: 646-674, 2011. [DOI] [PubMed] [Google Scholar]
- 3.Blackwill F, Mantovani A. Cancer and inflammation: implications for pharmacology and therapeutics. Clin Pharmacol Ther, 87: 401-406, 2010. [DOI] [PubMed] [Google Scholar]
- 4.Coussens LM, Werb Z. Inflammation and cancer. Nature, 420: 860-867, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McMillan DC. The systemic inflammation-based Glasgow Prognostic Score: A decade of experience in patients with cancer. Cancer Treat Rev, 39: 534-540, 2013. [DOI] [PubMed] [Google Scholar]
- 6.Absenger G, Szkandera J, Pichler M, et al. A derived neutrophil to lymphocyte ratio predicts clinical outcome in stage II and III colon cancer patients. Br J Cancer, 109: 395-400, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Templeton AJ, McNamara MG, Šeruga B, et al. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: a systematic review and meta-analysis. J Natl Cancer Inst, 106: dju124, 2014. [DOI] [PubMed] [Google Scholar]
- 8.Galizia G, Lieto E, Zamboli A, et al. Neutrophil to lymphocyte ratio is a strong predictor of tumor recurrence in early colon cancers: A propensity score-matched analysis. Surgery, 158: 112-120, 2015. [DOI] [PubMed] [Google Scholar]
- 9.Turner N, Wong HL, Templeton A, et al. Analysis of local chronic inflammatory cell infiltrate combined with systemic inflammation improves prognostication in stage II colon cancer independent of standard clinicopathologic criteria. Int J Cancer, 138: 671-678, 2016. [DOI] [PubMed] [Google Scholar]
- 10.Rashtak S, Ruan X, Druliner BR, et al. Peripheral Neutrophil to Lymphocyte Ratio Improves Prognostication in Colon Cancer. Clin Colorectal Cancer, 16: 115-123, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stotz M, Pichler M, Absenger G, et al. The preoperative lymphocyte to monocyte ratio predicts clinical outcome in patients with stage III colon cancer. Br J Cancer, 110: 435-440, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chan JC, Chan DL, Diakos CI, et al. The Lymphocyte-to-Monocyte Ratio is a Superior Predictor of Overall Survival in Comparison to Established Biomarkers of Resectable Colorectal Cancer. Ann Surg, 265: 539-546, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu Q, Hu T, Zheng E, Deng X, Wang Z. Prognostic role of the lymphocyte-to-monocyte ratio in colorectal cancer: An up-to-date meta-analysis. Medicine (Baltimore), 96: e7051, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guo YH, Sun HF, Zhang YB, et al. The clinical use of the platelet/lymphocyte ratio and lymphocyte/monocyte ratio as prognostic predictors in colorectal cancer: a meta-analysis. Oncotarget, 8: 20011-20024, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shibutani M, Maeda K, Nagahara H, Iseki Y, Ikeya T, Hirakawa K. Prognostic significance of the preoperative lymphocyte-to-monocyte ratio in patients with colorectal cancer. Oncol Lett, 13: 1000-1006, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ostrand-Rosenberg S. Myeloid-derived suppressor cells: more mechanisms for inhibiting antitumor immunity. Cancer Immunol Immunother, 59: 1593-600, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol, 9: 162-174, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Diaz-Montero CM, Finke J, Montero AJ. Myeloid-derived suppressor cells in cancer: therapeutic, predictive, and prognostic implications. Semin Oncol, 41: 174-184, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Toor SM, Syed Khaja AS, El Salhat H, et al. Increased Levels of Circulating and Tumor-Infiltrating Granulocytic Myeloid Cells in Colorectal Cancer Patients. Front Immunol, 7: 560, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang B, Wang Z, Wu L, et al. Circulating and tumor-infiltrating myeloid-derived suppressor cells in patients with colorectal carcinoma. PLoS One, 8: e57114, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brierley JD. Colon and rectum. In: TNM classification of malignant tumours. 8th ed. Wiley Blackwell, Oxford, 73-76, 2017. [Google Scholar]
- 22.Ohki S, Shibata M, Gonda K, et al. Circulating myeloid-derived suppressor cells are increased and correlate to immune suppression, inflammation and hypoproteinemia in patients with cancer. Oncol Rep, 28: 453-458, 2012. [DOI] [PubMed] [Google Scholar]
- 23.Cézé N, Thibault G, Goujon G, et al. Pre-treatment lymphopenia as a prognostic biomarker in colorectal cancer patients receiving chemotherapy. Cancer Chemother Pharmacol, 68: 1305-1313, 2011. [DOI] [PubMed] [Google Scholar]
- 24.Kitayama J, Yasuda K, Kawai K, Sunami E, Nagawa H. Circulating lymphocyte is an important determinant of the effectiveness of preoperative radiotherapy in advanced rectal cancer. BMC Cancer, 11: 64, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mei Z, Liu Y, Liu C, et al. Tumour-infiltrating inflammation and prognosis in colorectal cancer: systematic review and meta-analysis. Br J Cancer, 110: 1595-1605, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell, 140: 883-899, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fridlender ZG, Sun J, Kim S, et al. Polarization of tumor-associated neutrophil phenotype by TGF-beta: “N1” versus “N2” TAN. Cancer Cell, 16: 183-194, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sasaki A, Kai S, Endo Y, et al. Prognostic value of preoperative peripheral blood monocyte count in patients with colorectal liver metastasis after liver resection. J Gastrointest Surg, 11: 596-602, 2007. [DOI] [PubMed] [Google Scholar]
- 29.Mantovani A, Bottazzi B, Colotta F, Sozzani S, Ruco L. The origin and function of tumor-associated macrophages. Immunol Today, 13: 265-270, 1992. [DOI] [PubMed] [Google Scholar]


