Abstract
Background:
Breath acetone is reported to be a noninvasive biomarker for heart failure. However, the measurement of this metabolite requires expertize and is not standardized yet. Acetone is also released from the skin; thus, measuring acetone as a skin gas may be easier than breath analysis.
Methods:
We analyzed skin acetone collected from 41 hospitalized patients with cardiovascular diseases. Passive samplers were used to measure skin acetone emission. Passive sampler was softly fixed on the surface of forearm skin for 10 hour at night.
Results:
Skin acetone emission ranged from 0.00 to 2.70 ng/cm2/h, and was significantly correlated with blood ketone bodies (r = 0.377, p = 0.017).
Conclusions:
This is the first study to analyze skin gas in patients with cardiovascular diseases. Skin acetone emission was found to reflect blood ketone bodies. It is feasible to measure skin acetone emission for reflecting blood ketone bodies in patients with cardiovascular diseases.
Keywords: Skin acetone, cardiovascular diseases, ketone body, biogas analysis
Introduction
The ketone body is an important fuel source for the heart in heart failure1). Blood ketone bodies are correlated with heart failure2). Acetone is a volatile component of the ketone bodies and detected in the human breath. Breath acetone concentration has been proposed as a useful noninvasive biomarker for cardiovascular diseases3-7). However, the measurement of this metabolite requires expertise and is not standardized yet. Acetone in the body is not only released via the mouth, but also from the skin. Therefore, the measurement of acetone as a skin gas may be easier than breath analysis because skin gas collection is simple.
Skin gas emission has been reported to be easily measured by the use of a passive sampler8). However, the feasibility of skin acetone measurement in patients with cardiovascular diseases has not yet been investigated. Therefore, we investigated the feasibility of skin gas emission in patients with cardiovascular diseases.
Methods
This study prospectively included 41 patients who were hospitalized between September 2016 and March 2017 in Fukushima Medical University Hospital. Baseline data of sex, age, body mass index, diagnosis, past medical history, and current medication were collected at the time of enrollment in this study. The study protocol was approved by the institutional ethics committee of Fukushima Medical University. Written informed consent was provided by all patients.
Skin gas analysis and blood sampling
All patients were receiving a hospital diet with an average total energy count of 1,400 to 1,950 kcal/day. A passive sampler (GASTEC Co., Kanagawa, Japan, Figure 1A) consisting of a trapping filter, a polyethylenetereohthalate dish, and a stopper (Figure 1B) was used to sample skin acetone emission. The sampler was softly fixed on the surface of the right or left forearm skin using a band, and was left there for 10 hours overnight (Figure 1C). All patients were in a fasting state during sampling. After sampling, the samplers were collected and kept at 4 degrees until use. The passive samplers were analyzed according to the manufacture’s instruction in a blinded manner. The skin acetone analysis by a passive sampler was established by a previous study8). Blood sampling of total ketone bodies were analyzed on the same day as the skin acetone analysis in a fasting state.
Fig. 1.
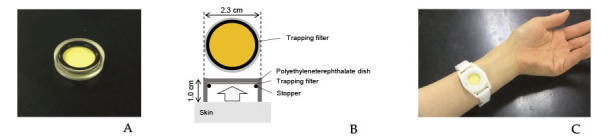
A. A passive sampler (GASTEC Co., Kanagawa, Japan).
B. The passive sampler consisted of a trapping filter, a polyethyleneterephthalate dish, and a stopper.
C. The passive sampler is softly fixed on the surface of the patient’s forearm skin using a band.
Statistical analysis
Data were analyzed using the Statistical Package for Social Sciences version 24 (SPSS Inc., Chicago, IL, USA). Quantitative data are expressed as mean ± SD, and median and interquartile range. The statistical significance of differences was analyzed using Students’ t-test for parametric continuous variables, and the Mann-Whitney U-test for nonparametric continuous variables. Correlation between skin acetone emission and blood total ketone bodies was analyzed using Spearman’s correlation analysis. Values of P < 0.05 were considered statistically significant.
Results
The study population was as follows: 12 patients with myocardial infarction; six with pulmonary hypertension; five with cardiomyopathy; five with arrhythmia; five with valvular heart disease; four with angina pectoris; two with acute decompensated heart failure, one with congenital heart disease, and one with acute myocarditis.
In the study population (n = 41), the mean age was 65 ± 13 years. 25 patients was male. The mean body mass index was 19 ± 4 kg/m2. 21 patients had hypertension, 13 patients had diabetes mellitus, and 30 patients had dyslipidemia. The mean estimated glomerular filtration rate was 59 ± 26 mL/min/1.73 m2. The median (interquartile range) level of brain natriuretic peptide was 66 (28 - 192) pg/mL, and the median (interquartile range) level of total ketone bodies was 90 (38 - 203) μmL/L. The mean left ventricular ejection fraction was 54 ± 14%. 17 patients had angiotensin-converting enzyme inhibitor or angiotensin-receptor blocker. 18 patients had β-blocker.
We were able to measure skin acetone emission in all patients, the median of which was 0.05 ng/cm2/h, ranging from 0.00 to 2.70 ng/cm2/h (Figure 2A). Skin acetone emission was significantly correlated with blood total ketone bodies (r = 0.377, p = 0.017, Figure 2B).
Fig. 2.
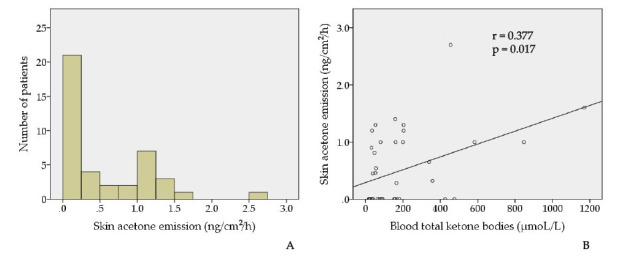
A. Skin acetone emission ranged from 0.00 to 2.70 ng/cm2/h.
B. Skin acetone emission was significantly correlated with blood total ketone bodies (r = 0.377, p = 0.017).
Discussion
The present study is the first to measure skin acetone emission in patients with cardiovascular diseases. We found that skin acetone emission was significantly correlated with blood total ketone bodies in patients with cardiovascular diseases.
Breath analysis is a noninvasive tool and has been tested in patients with cardiovascular diseases3-5). However, there is currently no standardized method for breath sampling9). There are different methods including type of sampling (total, or alveolar), sampling duration, breath hold, and type of collection bag10). Thus, the repeatability and reproducibility of breath analysis are sometimes questionable. An alternative biogas analysis is skin gas examination. Skin gas sampling by a passive sampler is a simpler method than breath sampling, and is easy to perform because the method only requires fixing a passive sampler to the skin of the patient for several hours.
We demonstrated in the present study that it is feasible to measure skin gas emission for reflecting blood ketone bodies in patients with cardiovascular diseases. Skin acetone seems to be generated from a capillary vessel through skin because acetone is a volatile component of ketone bodies in the blood. Ketone body is known to be the fuel of the failing heart1). Since elevation of blood ketone bodies is associated with decompensation or severity of heart failure, skin acetone emission might be a non-invasive marker of heart failure2).
Blood ketone bodies requires routine blood collection, which can accompanied by pain and may cause several complications such as nerve injury. Development of less invasive examination is needed. Skin acetone analysis is superior to blood ketone body collection because of its non-invasive method.
The correlation between skin acetone emission and blood total ketone bodies was significant, however, the correlation coefficient was 0.377. So, the skin acetone emission could not directly reflect blood total ketone bodies. The number of sweat gland and skin moisture might affect skin acetone emission. Mechanical reasons such as a fail in long-term contact of the device to skin and reproducibility of the measurements could also be other reasons for the low correlation coefficient.
A limitation of the current study is its small sample size. Because of the small sample size, we were unable to clarify any significant analysis between specific cardiovascular diseases including heart failure, and brain natriuretic peptide, and skin acetone emission. A larger study is required to establish the significance of skin acetone emission in patients with cardiovascular diseases.
In conclusion, in the current study we found that, in patients with cardiovascular diseases, skin acetone emission measurement is feasible to reflect blood total ketone bodies.
Conflict of interest
GASTEC Co. provided passive samplers for this study.
Funding
This work was supported, in part, by a grant from the Japanese Society for the Promotion of Science (No. 15K19401 to T.Y.).
References
- 1.Aubert G, Martin OJ, Horton JL, et al. The Failing Heart Relies on Ketone Bodies as a Fuel. Circulation, 133: 698-705, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lommi J, Kupari M, Koskinen P, et al. Blood ketone bodies in congestive heart failure. J Am Coll Cardiol, 28: 665-672, 1996. [DOI] [PubMed] [Google Scholar]
- 3.Cikach FS, Jr., Tonelli AR, Barnes J, et al. Breath analysis in pulmonary arterial hypertension. Chest, 145: 551-558, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marcondes-Braga FG, Batista GL, Bacal F, et al. Exhaled Breath Analysis in Heart Failure. Curr Heart Fail Rep, 13: 166-171, 2016. [DOI] [PubMed] [Google Scholar]
- 5.Yokokawa T, Sugano Y, Shimouchi A, et al. Exhaled Acetone Concentration Is Related to Hemodynamic Severity in Patients With Non-Ischemic Chronic Heart Failure. Circ J, 80: 1178-1186, 2016. [DOI] [PubMed] [Google Scholar]
- 6.Marcondes-Braga FG, Batista GL, Gutz IG, et al. Impact of Exhaled Breath Acetone in the Prognosis of Patients with Heart Failure with Reduced Ejection Fraction (HFrEF). One Year of Clinical Follow-up. PLoS One, 11: e0168790, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marcondes-Braga FG, Gutz IG, Batista GL, et al. Exhaled acetone as a new biomaker of heart failure severity. Chest, 142: 457-466, 2012. [DOI] [PubMed] [Google Scholar]
- 8.Sekine Y, Toyooka S, Watts SF. Determination of acetaldehyde and acetone emanating from human skin using a passive flux sampler—HPLC system. J Chromatogr B Analyt Technol Biomed Life Sci, 859: 201-207, 2007. [DOI] [PubMed] [Google Scholar]
- 9.O’Hara ME, O’Hehir S, Green S, et al. Development of a protocol to measure volatile organic compounds in human breath: a comparison of rebreathing and on-line single exhalations using proton transfer reaction mass spectrometry. Physiol Meas, 29: 309-330, 2008. [DOI] [PubMed] [Google Scholar]
- 10.Horvath I, Barnes PJ, Loukides S, et al. A European Respiratory Society technical standard: exhaled biomarkers in lung disease. Eur Respir J, 49, 2017. [DOI] [PubMed] [Google Scholar]


