Abstract
Cannabis use is typically initiated during adolescence and is a significant risk factor for the development of cocaine use in adulthood. However, no preclinical studies have examined the effects of adolescent cannabinoid exposure on cocaine dependence in adulthood using the escalation model of cocaine self-administration and the assessment of negative emotional states. In the present study, we found that exposure to the cannabinoid receptor agonist WIN55,212-2 (WIN) in adolescence produced irritability-like behavior and psychomotor cross-sensitization to cocaine in adolescence. In adulthood, rats were allowed to self-administer cocaine. The acquisition of cocaine self-administration was lower in rats with adolescent WIN exposure compared with controls. However, both WIN-exposed and control rats escalated their cocaine intake at the same rate, had similar responding under a progressive-ratio schedule of reinforcement, and had similar psychomotor responses to cocaine. Interestingly, the increase in irritability-like behavior that was previously observed in adolescence after WIN exposure persisted into adulthood. Whether the persisting increase in irritability-like behavior after WIN exposure has translational relevance remains to be studied. In summary, these results suggest that psychoactive cannabinoid exposure during adolescence is unlikely to have a major effect on the escalation of cocaine intake or the development of compulsive-like responding per se in adulthood in a rat model of cocaine self-administration. However, whether the persisting irritability-like behavior may predispose an individual to mood-related impairments in adulthood or predict such impairments warrants further investigation.
Introduction
Cannabis is one of the most frequently used psychoactive substances in the world1 and is the subject of major debates between proponents of the gateway hypothesis2 and advocates of legalization. Proponents of the gateway hypothesis have argued that epidemiological studies indicate that the early use of cannabis is an important risk factor for initiating cocaine use, that cannabis dependence predicts cocaine dependence3–6, that cannabis use may be associated with poor cognitive and psychiatric outcomes in adulthood7–9, and that major changes in legalization of the possession, sale, and cultivation of cannabis in the United States may exacerbate these poor outcomes by increasing the level of cannabis use in adolescents and young adults10. Cannabis use has been increasingly prevalent among adolescents and young adults. Currently, its use exceeds that of tobacco smoking among adolescents in the United States, in which 37.1% of high school seniors in 2017 reported using cannabis within the past year11. Advocates of legalization and medicinal use argue that it is unclear whether the relationship between prior cannabis use and later cocaine use or cocaine use disorder is caused by cannabis use per se or other drug-associated factors, such as concomitant psychiatric disorders and socioeconomic status5,12. However, epidemiological studies cannot establish causal relationships between the pharmacological effects of exposure to cannabis and the development of cocaine use3,13.
Preclinical studies provide a controlled way to study causal relationships between early-life cannabinoid exposure and cocaine use, including compulsive-like use, later in life. Previous studies reported that exposure to the cannabinoid receptor agonist WIN55,212-2 (WIN) during adolescence decreased the reactivity of dopaminergic neurons to WIN (i.e., tolerance), produced cross-tolerance to cocaine in adolescence14, and produced cross-sensitization to the psychomotor effects of cocaine in adolescence but not in adulthood15. This effect appears to be mediated by the modulation of eukaryotic initiation factors in the brain15. Such modifications of key neural substrates may reprogram the adolescent brain and make it more susceptible to the later use of other illicit drugs, such as cocaine2,16. However, other groups found that prior treatment with either the main psychoactive constituent of cannabis (Δ9- tetrahydrocannabinol [THC]) or WIN had no effect on behavioral responses to amphetamine in either adolescence or adulthood17. However, in the study by Ellgren et al.17, cannabinoid exposure lasted only 5 days, the doses of cannabinoid were low, and the animals were injected only once per day. A major limitation of these preclinical studies is the use of an animal model of cocaine exposure (psychomotor sensitization) that reflects neither the direct acquisition of cocaine use (measured by self-administration) nor the compulsive nature of cocaine use disorder (measured by the escalation of self-administration).
To address this issue, we tested the effect of adolescent exposure to the cannabinoid receptor agonist WIN on key addiction-related behaviors using a more complex animal model of drug addiction. The model included measures of irritability-like behavior, which has recently been used as a measure of the negative emotional state in animal models of addiction18–20. We also assessed cocaine-induced locomotion (i.e., cross-sensitization) in adolescence and adulthood and the acquisition of cocaine self-administration under conditions of short access (1 h) and long access (6 h; i.e., escalation of cocaine self-administration) in adulthood. The long-access model represents a comprehensive model of human addiction21 because it produces the escalation of cocaine intake that is associated with the emergence of negative emotional states22 and compulsive-like responding despite adverse consequence23–26.
Materials and Methods
Animals
Male Sprague-Dawley rats (Charles River, Wilmington, MA, USA; n = 18) were housed 2–3 per cage on a reverse 12 h/12 h light/dark cycle (lights off at 8:00 AM) in a temperature (20–22 °C)- and humidity (45–55%)-controlled animal facility with ad libitum access to water and food. All of the experiments were designed to minimize animal suffering and the number of animals used. All of the procedures were conducted in adherence to the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee of The Scripps Research Institute. The rats were considered adolescent until postnatal day (PND) 6027.
Drugs
WIN55,212-2 mesylate (Tocris, Minneapolis, MN, USA) was dissolved in vehicle that contained 2% Tween 80, 2% ethanol, and saline and injected intraperitoneally (i.p.) in a volume of 1 ml/kg of body weight. Cocaine HCl (National Institute on Drug Abuse, Bethesda, MD, USA) was dissolved in 0.9% saline (Hospira, Lake Forest, IL, USA) at a dose of 0.5 mg/kg/0.1 ml infusion and self-administered intravenously.
Drug treatment
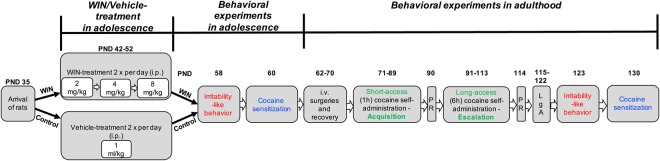
The experimental design of WIN exposure and behavioral testing during adolescence and later in adulthood is illustrated in Fig. 1. Prior to treatment, the rats were divided into two groups (n = 9/group) that were matched for body weight and food and water intake. These parameters were also monitored throughout WIN treatment. The rats began treatment with WIN or vehicle during adolescence (PND42). Control rats received a corresponding volume of vehicle. Increasing doses of WIN (2 mg/kg, PND42-44; 4 mg/kg, PND45-48; 8 mg/kg, PND49-52) or vehicle were administered twice daily for 11 consecutive days. The WIN dose regimen was chosen according to the literature for subchronic adolescent cannabinoid treatment28–30 and because it has been shown to induce psychomotor cross-sensitization to the effects of cocaine in adolescence15.
Figure 1.
Experimental design of WIN exposure and behavioral testing during adolescence and later in adulthood. LgA, long-access; PND, postnatal day; PR, progressive-ratio schedule of reinforcement; WIN, cannabinoid receptor agonist WIN55,212-2.
Irritability-like behavior
All behavioral testing was conducted during the dark phase. To test irritability-like behavior after WIN exposure, we used the bottle-brush test, based on the experimental method that was designed previously for mice31,32 and slightly modified to better monitor rat behavior19. Currently, this model is increasingly used by both our laboratory and others as a measure of negative emotional states in animal models of addiction18–20. This method has advantages over other behavioral paradigms that measure aggressive/defensive behaviors, such as the social dominance/subordination paradigms and resident/intruder confrontation paradigm, because the experimenter has greater control over the mechanical stimulus and thus better precision in ensuring uniform provocation. Furthermore, the “social” factor in eliciting agonistic behavior and the risk of physical injury during an agonistic encounter are both circumvented in the bottle-brush test. The mechanical stimulus of the moving bottle-brush has also been found to be more effective in provoking these behaviors compared with either deceased or stuffed animals32.
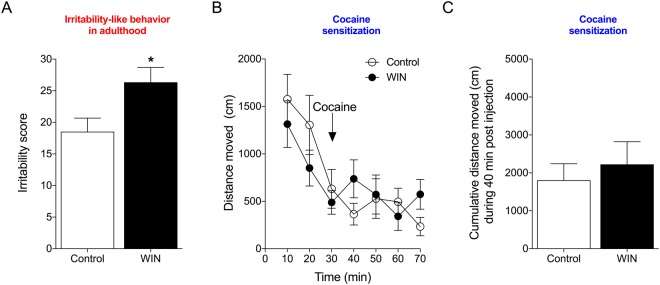
In the present study, the animals were randomized, and three trained observers scored the rats’ behaviors in real-time as described below. The observers were blinded to treatment of the animals. Testing consisted of ten 10-s trials with 10-s intertrial intervals in plastic cages (27 cm × 48 cm × 20 cm) with clean bedding. A bottle-brush was rotated rapidly toward the rat’s whiskers. Both aggressive responses (smelling, biting, boxing, following, and exploring the bottle-brush) and defensive responses (escaping, digging, jumping, climbing, defecation, vocalization, and grooming) were recorded. The behavioral responses were chosen based on Riittinen et al. (1986)32 and Lagerspetz and Portin (1968)33. Total aggressive and defensive scores were calculated for each animal based on the average score of the observers. Both aggressive and defensive behaviors were summed to calculate the total irritability score. Irritability-like behavior reflects a composite measure of aggressive vs. defensive responses. Irritability-like behavior was assessed 6 days after the last injection of WIN/vehicle in adolescence (PND58) and again in adulthood 18 h into withdrawal after the escalation of cocaine self-administration (PND123).
Cocaine sensitization
Locomotor stimulation by acute cocaine administration was assessed in rats with prior exposure to either vehicle or WIN. This test was performed in adolescence (PND60) to model the previous finding of WIN-induced cocaine cross-sensitization in adolescence15 and again in adulthood (PND130). After 30 min habituation to the acrylic experimental chamber (43.2 cm × 43.2 cm × 30.5 cm; Med Associates, St. Albans, VT, USA), the rats were injected with cocaine (10 mg/kg, i.p.), and locomotor activity was recorded for 40 min under red light. Locomotor activity was recorded as the distance traveled (in centimeters) using a video camera that was connected to the ANY-maze Video Tracking System 5.11 (Wood Dale, IL, USA).
Intravenous catheterization
The rats were anesthetized by isoflurane inhalation, and intravenous catheters were aseptically inserted in the right jugular vein using a modified version of a procedure that was described previously33–35. The right jugular vein was punctured with a 22-gauge needle, and the tubing was inserted and secured inside the vein by tying the vein with suture thread. The catheter assembly consisted of an 18 cm length of MicroRenathane tubing (0.023 inch inner diameter, 0.037 inch outer diameter; Braintree Scientific, Braintree, MA, USA) that was attached to a guide cannula (Plastics One, Roanoke, VA, USA). The guide cannula was bent at a near right angle, embedded in dental acrylic, and anchored with a mesh (1 mm thick, 2 cm square). The catheter exited through a small incision on the back, and the base was sealed with a small plastic cap and metal cover cap. The catheters were flushed daily with heparinized saline (10 U/ml of heparin sodium; American Pharmaceutical Partners, Schaumburg, IL, USA) in 0.9% bacteriostatic sodium chloride (Hospira, Lake Forest, IL, USA) that contained 20 mg/0.2 ml of the antibiotic Cefazolin (Hospira, Lake Forest, IL, USA).
Operant training and escalation of cocaine self-administration in adulthood
Self-administration in adulthood was performed in operant conditioning chambers (Med Associates, St. Albans, VT, USA) that were enclosed in lit, sound-attenuating, ventilated environmental cubicles. The front door and back wall of the chambers were constructed of transparent plastic, and the other walls were opaque metal. Each chamber was equipped with two retractable levers that were located on the front panel. Cocaine was delivered through plastic catheter tubing that was connected to an infusion pump, which was activated by responses on the right (active) lever. Responses on the left (inactive) lever were recorded but did not have any scheduled consequences. Activation of the pump resulted in the delivery of 0.1 ml of cocaine (0.5 mg/kg/0.1 ml). A computer controlled fluid delivery and behavioral data recording. The rats were first trained to self-administer cocaine under a fixed-ratio 1 (FR1) schedule of reinforcement in daily 1-h sessions. Each active lever press resulted in the delivery of one cocaine dose. A 20-s timeout (TO) period followed each cocaine infusion. During the TO period, responses on the active lever did not have scheduled consequences. This TO period occurred concurrently with illumination of a cue light that was located above the active lever to signal delivery of the positive reinforcement. The rats were trained to self-administer cocaine in 14 sessions (5 days/week) until a stable baseline of reinforcement was achieved (≤10% variation over the last three sessions). The criterion for the acquisition of cocaine self-administration was defined as the intake of at least 2.5 mg/kg cocaine in the 1-h self-administration session, requiring at least five lever presses. This criterion was adapted from previous publications36,37. After the 14-session acquisition period, the rats were subjected to fourteen 6-h cocaine self-administration sessions to allow them to escalate their cocaine intake22. To study the motivation to seek cocaine, a progressive-ratio (PR) schedule of reinforcement was used, in which the response requirement began at one lever press/infusion and increased exponentially according to the following equation38: lever presses/infusion = [5 × e(infusion number × 0.2)] − 5. The session duration was limited to 6 h or ended when a rat failed to achieve the response requirement within 1 h. The PR sessions were conducted after the training/acquisition phase and again after a stable level of escalation was achieved. In the course of the experiment, which lasted for more than 3 months, some rats were excluded at different stages of the experiment. Two rats (one in the control group and one in the WIN group) were excluded during the acquisition phase because of the failure of catheter patency. In the course of the escalation phase, three rats (one in the control group and two in the WIN group) were excluded from the study because of the failure of catheter patency at the end of the study, and one rat in the vehicle group died unexpectedly during the day off from cocaine self-administration before the study was completed, thus leaving n = 6 rats/group for the final analysis. The exclusion of those data did not affect the results of the statistical analysis.
Statistical analysis
The effects of WIN on body weight and cocaine self-administration were analyzed using two-way repeated-measures analysis of variance (ANOVA). Food and water intake during WIN exposure, irritability-like behavior, 40-min cumulative scores for the cocaine challenge, and the PR data were analyzed using unpaired two-tailed Student’s t-test. The acquisition of cocaine self-administration was analyzed using Mantel-Cox survival analysis. Significant effects in the ANOVA were followed by Bonferroni’s multiple-comparison post hoc test. Values of p < 0.05 were considered statistically significant. The sample size (n = 6–9) was chosen based on a pre hoc power analysis (Power = 0.8, p < 0.05) to detect effect sizes (Cohen’s d = 1.3–1.6) that were similar to previously published effect sizes and demonstrated the effect of exposure to cannabinoid receptor agonists on cocaine sensitization (Cohen’s d = 1.6)15 and cocaine self-administration (Cohen’s d = 1.7)39. The sample size in the present study was also similar to previous studies that employed the escalation model of drug self-administration40–44. The level of intake in the present study was also comparable to previous studies that employed the escalation model of cocaine self-administration with larger sample sizes34,35,45.
Results
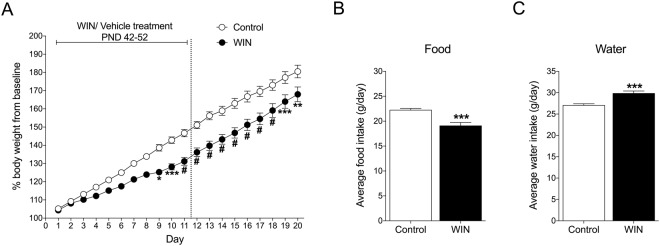
WIN decreases body weight and food intake but increases water intake in adolescence
During WIN treatment, body weight and food and water intake were monitored daily. A significant difference in the percentage of body weight gain was observed between WIN- and vehicle-treated rats (Fig. 2A). Weight gain in WIN-treated rats was lower than in vehicle-treated rats, confirmed by a significant interaction (F19,304 = 8.244, p < 0.0001) in the two-way repeated-measures ANOVA. The Bonferroni multiple-comparison post hoc analysis revealed that the weight gain difference between groups was significant on days 9–21 (p < 0.05 for day 9, p < 0.01 for day 20, p < 0.001 for days 10 and 19, p < 0.0001 for days 11–18). WIN-treated rats also exhibited a reduction of average food intake during WIN treatment compared with controls, confirmed by Student’s two-tailed unpaired t-test (t20 = 4.647, p = 0.0002; Fig. 2B). Compared with controls, WIN-treated rats had significantly higher water intake during treatment (t20 = 4.794, p = 0.0001; Fig. 2C).
Figure 2.
(A) Chronic WIN treatment in adolescence decreased body weight. *p < 0.05, **p < 0.01, ***p < 0.001; #p < 0.0001 (two-way repeated-measures ANOVA followed by Bonferroni’s multiple-comparison post hoc test). (B) Chronic WIN treatment in adolescence decreased food intake. (C) Chronic WIN treatment in adolescence increased water intake. The data are expressed as mean ± SEM. ***p < 0.001 (Student’s unpaired two-tailed t-test; n = 9).
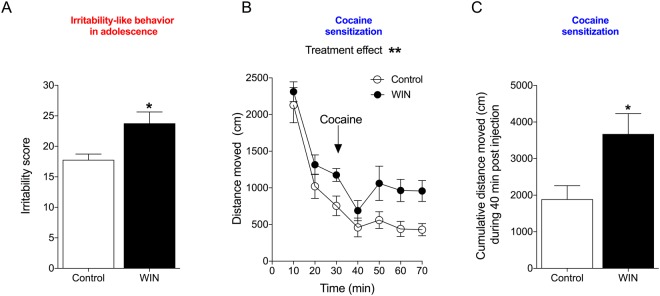
WIN induces irritability-like behavior and cross-sensitization to cocaine in adolescence
In adolescence, 6 days after the last WIN/vehicle treatment (PND58), rats that were treated with WIN exhibited an increase in irritability-like behavior compared with controls, measured by the total irritability score. This was analyzed by Student’s two-tailed unpaired t-test (t16 = 2.873, p = 0.0111; Fig. 3A). The effect-size and power analyses also confirmed that adolescent WIN treatment increased irritability-like behavior in adolescence (Cohen’s d = 1.35, Power = 0.93 at a level of confidence of p < 0.05). The individual scores during adolescence for each recorded behavior are listed in Table 1.
Figure 3.
Irritability-like behavior and cocaine-induced cross-sensitization in adolescent rats. (A) Irritability-like behavior increases after 6 days of abstinence from WIN. *p < 0.05 (Student’s two-tailed unpaired t-test). (B) WIN induces behavioral cross-sensitization to the psychostimulant effect of cocaine (10 mg/kg, i.p.). **p < 0.01, significant effect of treatment (two-way repeated-measures ANOVA). (C) The 40-min cumulative locomotor activity score during the cocaine challenge demonstrated that WIN-treated rats were more stimulated by cocaine. *p < 0.05 (Student’s two-tailed unpaired t-test; n = 9).
Table 1.
Individual irritability-like behaviors (mean ± SEM) during adolescence and adulthood after WIN exposure.
| ADOLESCENCE | ADULTHOOD | |||||||
|---|---|---|---|---|---|---|---|---|
| Control | WIN | df = 16 | p | Control | WIN | df = 10 | p | |
| Escape | 8.4 ± 0.9 | 8.6 ± 0.6 | t = 0.2 | p = 0.8s4 | 7.4 ± 1.2 | 8.7 ± 0.4 | t = 1.0 | p = 0.34 |
| Digging | 0 ± 0 | 0.7 ± 0.4 | t = 1.5 | p = 0.16 | 0.1 ± 0.1 | 0.2 ± 0.2 | t = 0.3 | p = 0.79 |
| Jumping | 0.4 ± 0.3 | 0.5 ± 0.2 | t = 0.3 | p = 0.76 | 0.2 ± 0.2 | 0.0 ± 0.0 | t = 1.0 | p = 0.34 |
| Climbing | 3.4 ± 0.7 | 4.4 ± 0.5 | t = 1.3 | p = 0.21 | 4.5 ± 1.0 | 6.3 ± 0.4 | t = 1.8 | p = 0.10 |
| Defecation | 0.2 ± 0.1 | 1.3 ± 0.4 | t = 2.8 | *p = 0.01 | 0.2 ± 0.2 | 1.2 ± 0.5 | t = 1.9 | p = 0.09 |
| Vocalization | 0.1 ± 0.1 | 0.0 ± 0.0 | t = 1.5 | p = 0.15 | 0.1 ± 0.1 | 0.1 ± 0.1 | t = 0.5 | p = 0.66 |
| Grooming | 0.3 ± 0.1 | 0.7 ± 0.4 | t = 0.8 | p = 0.42 | 2 ± 0.9 | 0.7 ± 0.3 | t = 1.4 | p = 0.19 |
| Smelling | 1.1 ± 0.2 | 2.1 ± 0.5 | t = 1.8 | p = 0.09 | 1.4 ± 0.4 | 2.6 ± 0.7 | t = 1.6 | p = 0.14 |
| Biting | 0.2 ± 0.1 | 0.4 ± 0.2 | t = 0.7 | p = 0.52 | 0.0 ± 0.0 | 0.6 ± 0.5 | t = 1.34 | p = 0.24 |
| Boxing | 1.8 ± 0.4 | 1.5 ± 0.4 | t = 0.5 | p = 0.60 | 1.1 ± 0.7 | 1.7 ± 0.4 | t = 0.8 | p = 0.47 |
| Following | 1.2 ± 0.4 | 2.2 ± 0.6 | t = 1.5 | p = 0.18 | 2.4 ± 0.5 | 3.6 ± 0.7 | t = 1.3 | p = 0.21 |
| Exploration | 0.7 ± 0.2 | 1.4 ± 0.4 | t = 1.7 | p = 0.10 | 1.1 ± 0.4 | 1.7 ± 0.5 | t = 1.0 | p = 0.34 |
| Total | 17.8 ± 1.0 | 23.8 ± 1.9 | t = 2.9 | *p = 0.011 | 20.5 ± 1.9 | 27.3 ± 2.3 | t = 2.3 | *p = 0.048 |
On PND60, the rats were placed in the open field for 30 min of habituation, after which they were challenged with cocaine (10 mg/kg, i.p.), and locomotor activity was monitored for 40 min (Fig. 3B). The two-way repeated-measures ANOVA revealed significant effects of treatment (F1,16 = 10.57, p = 0.0050) and time (F6,96 = 35.71, p < 0.0001) but no treatment × time interaction (F6,96 = 0.5967, p = 0.7623). No significant differences were found between groups in cumulative locomotor activity during 30-min habituation in adolescence (t16 = 1.558, p = 0.1387). Rats with prior exposure to WIN exhibited an increase in activity during the cocaine challenge compared with controls, reflected by the 40-min cumulative locomotor activity score during the cocaine challenge (Fig. 3C). This was confirmed by Student’s two-tailed t-test (t16 = 2.655, p = 0.0173). The effect-size and power analyses also confirmed that adolescent WIN treatment increased locomotor activity during the cocaine challenge in adolescence (Cohen’s d = 1.23, Power = 0.90 at a level of confidence of p < 0.05).
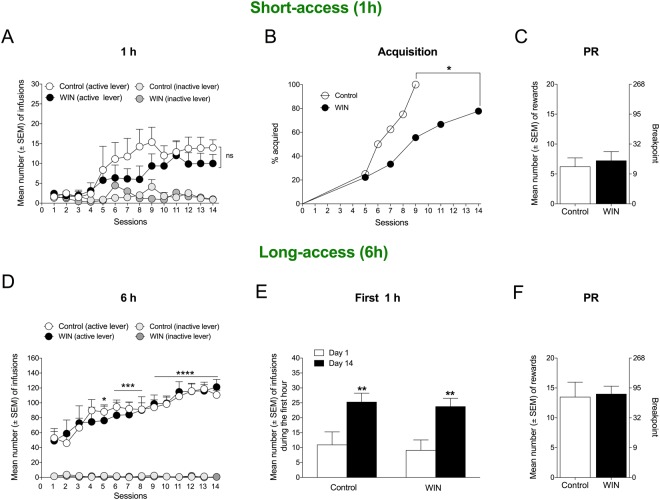
WIN exposure in adolescence decreases the acquisition of cocaine self-administration but has no effect on escalation
Nineteen days after the last WIN treatment, the rats had reached adulthood and were trained to self-administer cocaine. Although there was a trend toward lower levels of cocaine self-administration during acquisition (1-h session) in WIN-exposed rats compared with controls, no significant difference was found between controls and WIN-treated rats, confirmed by the lack of an interaction in the two-way repeated-measures ANOVA (F13,182 = 0.7133, p = 0.7488, n = 8; Fig. 4A). A significant effect of time was observed (F13,182 = 0.6285, p < 0.0001), demonstrating an increase in self-administration, with no effect of treatment (F1,14 = 1.208, p = 0.2902). However, WIN-treated animals exhibited slower acquisition of cocaine self-administration compared with controls. Only 50% of the WIN-treated rats acquired cocaine self-administration by day 9, whereas 100% of the control rats acquired cocaine self-administration by day 9. The acquisition criterion was a minimum of five lever presses (2.5 mg/kg cocaine) during the short-access session. The significant difference between groups was confirmed by Mantel-Cox survival analysis (log rank χ2 = 4.211, p = 0.0402; Fig. 4B). The motivation to self-administer cocaine at the end of the acquisition period (PND114), measured by PR responding, was unaltered by prior WIN treatment compared with controls (t14 = 0.482, p = 0.6372; Fig. 4C). During 14 long-access (6-h) sessions of cocaine self-administration, both control rats and WIN-treated rats gradually escalated their cocaine intake. The two-way repeated-measures ANOVA confirmed a significant effect of time (F13,130 = 14.28, p < 0.0001) but no effect of treatment (F1,10 = 0.003261, p = 0.9556) and no treatment × time interaction (F13,130 = 0.5473, p = 0.7913). The one-way repeated-measures ANOVA, followed by Bonferroni’s multiple-comparison post hoc test, revealed the significant escalation of cocaine self-administration that started in the fifth 6-h session (p < 0.05 for session 5, p < 0.001 for sessions 6–8, p < 0.0001 for sessions 9–14). The two-tailed paired Student’s t-test indicated the same level of escalation in the first hour of the 6-h session (i.e., the loading phase) in control rats (t5 = 6.85, p = 0.0010) and WIN-treated rats (t5 = 5.474, p = 0.0028; Fig. 4E). Finally, adolescent WIN treatment had no effect on PR responding after the escalation of cocaine self-administration compared with controls (t10 = 0.018, p = 0.8608; Fig. 4F). The effect-size analysis showed that adolescent WIN treatment had no effect on cocaine escalation (Cohen’s d = 0.12), the loading-dose of cocaine self-administration (Cohen’s d = 0.22), or compulsive-like responding for cocaine before escalation (Cohen’s d = 0.24) or after escalation (Cohen’s d = 0.10), measured by PR responding.
Figure 4.
Cocaine self-administration in adulthood. (A) Cocaine self-administration on a fixed-ratio 1 (FR1) schedule during daily 1-h sessions (n = 8). (B) WIN treatment in adolescence decreased the acquisition of cocaine self-administration in adulthood. Acquisition was defined as the first session during which the rat consumed at least 2.5 mg/kg cocaine (five lever presses) in 1 h. *p < 0.05 (Mantel-Cox survival analysis). (C) Progressive-ratio (PR) responding for cocaine after short access was unaffected by prior WIN treatment. The data are expressed as the mean ± SEM number of rewards (left y-axis) or breakpoint (right y-axis; n = 8). (D) Prior WIN treatment had no effect on the escalation of cocaine intake during daily 6-h self-administration sessions. *p < 0.05, ***p < 0.001, ****p < 0.0001, significant difference from baseline (one-way repeated-measures ANOVA followed by Bonferroni’s multiple-comparison post hoc test). (E) The first hour of the 6-h self-administration session (i.e., the loading phase) demonstrated the significant escalation of cocaine intake during 14 sessions compared with day 1 in both vehicle- and WIN-exposed rats. **p < 0.01 (Student’s two-tailed unpaired t-test). (F) Progressive-ratio (PR) responding for cocaine after long access (6 h) was unaffected by prior WIN treatment. The data are expressed as the mean ± SEM number of rewards (left y-axis) or breakpoint (right y-axis; n = 6).
WIN-induced irritability-like behavior but not cocaine cross-sensitization persists into adulthood
Irritability-like behavior was measured again in adulthood after the escalation of cocaine self-administration, 18 h into abstinence from cocaine. The calculated interobserver agreement demonstrated that the observers of behavior were in high agreement (r = 0.9350). In the control group, the baseline irritability score before cocaine self-administration was 17.8 ± 0.96 (mean ± SEM). After the escalation of cocaine self-administration, the irritability score was 20.5 ± 1.9, confirming that cocaine withdrawal per se did not affect irritability-like behavior. However, in rats with adolescent exposure to WIN, the increase in irritability-like behavior persisted into adulthood (27.3 ± 2.3). The increase in the total irritability score was confirmed by Student’s two-tailed unpaired t-test (t10 = 2.253, p = 0.048; Fig. 5A). The effect-size and power analyses also confirmed that adolescent WIN treatment increased irritability-like behavior in adulthood (Cohen’s d = 1.42, Power = 0.89 at a level of confidence of p < 0.05). The individual scores for each recorded behavior during adulthood are listed in Table 1.
Figure 5.
Irritability-like behavior and cocaine-induced locomotor activity in adult rats. (A) Prior treatment with WIN increased irritability-like behavior 18 h after the last cocaine self-administration session. *p < 0.05 (Student’s two-tailed unpaired t-test). (B) Prior WIN treatment did not alter cocaine-induced (10 mg/kg, i.p.) locomotor activity in adulthood. (C) The 40-min cumulative locomotor activity score during the cocaine challenge was unaffected by prior WIN treatment. n = 6.
Six days after the last cocaine self-administration session, the rats were again placed in the open field for 30 min of habituation, after which they were challenged with cocaine (10 mg/kg, i.p.), and locomotor activity was monitored for 40 min (Fig. 5B). The two-way repeated-measures ANOVA revealed a significant effect of time (F6,60 = 11.93, p < 0.0001) but no effect of treatment (F1,10 = 0.04206, p = 0.8416) and no treatment × time interaction (F6,60 = 1.82, p = 0.1103). No significant differences were found between groups in cumulative locomotor activity during 30-min habituation in adulthood (t10 = 0.9881, p = 0.3464). Rats with prior exposure to WIN exhibited no difference in 40-min cumulative locomotor activity scores during the cocaine challenge compared with control rats (t10 = 0.5659, p = 0.5839; Fig. 5C). The effect-size analysis also confirmed that adolescent WIN treatment had no effect on locomotor activity during the cocaine challenge in adulthood (Cohen’s d = 0.33).
At the end of the escalation phase, the mean ± SEM body weight was 432.5 ± 22.9 g in the control group and 400.83 ± 29.4 g in the WIN group, demonstrating that the WIN-induced reduction of body weight during adolescence recovered in adulthood.
Discussion
The present study found that adolescent WIN exposure (i) increased irritability-like behavior in adolescence, which persisted into adulthood, (ii) induced cross-sensitization to the locomotor-stimulating effect of cocaine in adolescence, which did not persist into adulthood, (iii) decreased the speed of acquisition but not the rate of cocaine self-administration in adulthood, and (iv) had no effect on the escalation of cocaine self-administration in adulthood. Overall, these results demonstrate that although cannabinoid exposure in adolescence induces irritability-like behavior and cross-sensitization to the psychostimulant effect of cocaine during adolescence, it does not promote cocaine self-administration once the animals reach adulthood. However, the effect of adolescent WIN exposure on cocaine self-administration in adolescence was not investigated in the present study because the animals reached adulthood by the time they had recovered from the surgeries that were required for self-administration.
Reductions of both body weight and food intake were observed during WIN treatment. Although the activation of cannabinoid receptors typically produces an increase in food intake in adulthood46, accumulating evidence suggests that adolescent exposure to THC or WIN in rats decreases food intake and body weight15,28,29,47. The increase in water intake during WIN exposure in the present study confirms the role of cannabinoid receptors in homeostatic responses that regulate not only energy homeostasis but also fluid balance48.
Irritability, anxiety, and dysphoria are key negative emotional states that characterize the withdrawal syndrome in humans, which arises when access to the drug is prevented and contributes to drug relapse49. Irritability has also been reported to be greater in adolescents at higher risk for substance use50. Irritability-like behavior has also been shown to increase during withdrawal from alcohol and nicotine in rodents18–20. However, to our knowledge, whether early exposure to cannabinoids affects irritability-like behavior has not been studied in animal models. In the present study, we found that WIN exposure induced irritability-like behavior in adolescence and adulthood, suggesting that cannabinoid exposure in adolescence induces long-lasting neurobehavioral adaptations that can persist months after WIN exposure. However, further studies are needed to investigate whether this finding has translational relevance. An alternative explanation is that, despite blind randomization of the subjects to the two groups, the increase in irritability-like behavior that was observed in WIN-treated rats may be attributable to preexisting differences in irritability-like behavior. Further studies are needed to investigate whether this finding has translational relevance. Numerous human studies demonstrate that early cannabis use is associated with greater vulnerability to the later development of drug addiction and psychiatric illness51. A recent study reported a pivotal role for cannabinoid receptors as molecular mediators of adolescent behavior and suggested that cannabinoid receptors may be important in adolescent-onset mental health disorders52. Chronic adolescent exposure to WIN has also been shown to induce anxiety-like behavior in rats53. However, contradictory findings have also been published, with either no change54 or even a decrease55 in anxiety-like behavior after cannabinoid exposure in adolescence. Rats that were exposed to cannabis smoke were also reported to exhibit a decrease in anxiety-like behavior56. Interestingly, a previous study also demonstrated that long-term cognitive and behavioral dysfunction that was induced by adolescent THC exposure could be prevented by concurrent cannabidiol treatment57. Importantly, WIN acts as a full cannabinoid receptor agonist, in contrast to THC, which only acts as a partial agonist. Moreover, cannabis is known to consist of dozens of additional phytocannabinoids apart from THC. Furthermore, different strains of cannabis differ in their THC content, and THC levels in cannabis have increased year after year because of consumer demand, thus making direct comparisons of human data across time and across studies difficult. Nevertheless, we chose this model of early cannabinoid exposure and followed it precisely because it has been shown to induce cocaine cross-sensitization, thus supporting the gateway hypothesis15. Further studies are needed to investigate whether the long-term irritability-like behavior that was observed in the present study can be prevented by concurrent cannabidiol treatment or whether adolescent exposure to cannabis smoke induces long-lasting irritability-like behavior in rats.
Epidemiological data consistently document that cannabis exposure precedes the use of other illicit drugs6,12. However, epidemiological data cannot provide causal evidence of this sequence. Animal models are particularly useful for studying effects that are related to cross-sensitization because they allow sequential administrations of the studied drugs while controlling for confounding variables. Several studies have reported behavioral cross-sensitization between cannabinoids and stimulants in rodents58–61. WIN treatment during adolescence in rats induces long-lasting cross-tolerance to morphine, cocaine, and amphetamine14, potentiates amphetamine-induced psychomotor sensitization62, and induces cocaine-induced psychomotor sensitization in adolescence15. WIN exposure also leads to increases in methylenedioxymethamphetamine-induced63 and cocaine-induced64 conditioned place preference. In the present study, WIN exposure in adolescence induced cross-sensitization to the stimulatory effect of cocaine in adolescence. However, this effect was no longer present in adulthood when the rats had self-administered cocaine for several weeks, suggesting that cannabinoid exposure in adolescence may increase the psychomotor effects of cocaine during the first exposure to cocaine, but this effect is not necessarily long-lasting.
Cannabinoid exposure increased irritability-like behavior and the psychomotor effects of cocaine, but it did not promote the acquisition or escalation of cocaine self-administration. Indeed, we observed the slower acquisition of cocaine self-administration with 1-h short-access to cocaine in male rats with prior exposure to WIN compared with controls. In contrast, a previous study reported a trend toward an increase in cocaine self-administration during the short (7-day) acquisition phase (30-min session) in female rats with prior exposure to the cannabinoid receptor agonist CP55,940 but not in male rats39. However, this study did not discriminate between inactive and active levers, and no difference in cocaine self-administration was observed during the 14-day maintenance phase (2-h session) in either sex39. A recent study showed that adolescent WIN exposure caused impairments in an attentional set-shifting task, a measure of cognitive flexibility, in adulthood (PND > 85)62. An alternative hypothesis is that the slower acquisition of cocaine self-administration in adulthood that was observed in the present study may be attributable to cognitive impairment that slows the acquisition of operant responding. In humans, several studies have indicated that the adolescent use of cannabis can lead to long-term cognitive deficits, including problems with attention and memory65. During escalation, no differences were observed between the rats that were exposed to vehicle in adolescence and the rats that were exposed to WIN in adolescence. This suggests that if cognitive impairments affected the initial acquisition of self-administration, then they did not produce long-term deficits.
The model of long-access to cocaine self-administration is one of the most validated animal models of cocaine use disorder and drug addiction in general21,22,66. This model has been shown to result in all seven of the diagnostic criteria of the Diagnostic and Statistical Manual of Mental Disorders, 4th edition (DSM-IV), and seven of the 11 DSM-5 criteria, including most of the criteria that are required for severe use disorder: (i) tolerance67, (ii) withdrawal68,69, (iii) substance taken in larger amount than intended22, (iv) unsuccessful efforts to quit70,71, (v) considerable time spent to obtain the drug72, (vi) important social, work, or recreational activities given up because of use26,69,73, and (vii) continued use despite adverse consequences23–25,69,71,74,75. The present study found no effect of adolescent cannabinoid exposure in the escalation model, suggesting that adolescent WIN exposure may not facilitate the acquisition, maintenance, or escalation of cocaine use in adulthood. An alternative hypothesis is that the effect of cannabinoid use may not be observed on cocaine intake per se; instead, cannabinoid exposure may produce an increase in the motivation for cocaine, leading to an increase in compulsive cocaine seeking. Indeed, prior exposure to another potential gateway drug, alcohol, was found to have no effect on subsequent cocaine self-administration per se but produced greater motivation and compulsive-like cocaine seeking under a PR schedule of reinforcement76. However, we observed no differences between the WIN-exposed and control groups in adulthood when we used a PR schedule of reinforcement to examine whether rats with prior exposure to WIN express alterations of the motivation to self-administer cocaine.
One limitation of long-term behavioral studies in adolescent rats, including the present study, is that puberty in rats is relatively short (~PND38-60 in males)27. Compared with adults, rats that are allowed to self-administer cocaine during adolescence (PND42-54) have been shown to be more vulnerable to cocaine addiction77. Unfortunately, in the model of cannabinoid exposure during adolescence (PND42-52), cocaine self-administration can only be studied starting in late adolescence and continuing into adulthood because rats exit puberty by PND6027. Because of this limitation, one possibility is that cannabinoid exposure during adolescence may affect cocaine intake in adolescence.
The present results demonstrate that chronic exposure to cannabinoids does not facilitate the acquisition of cocaine self-administration or compulsive-like cocaine intake in adulthood, measured by the escalation of cocaine self-administration and PR responding in a relevant model of cocaine use disorder. These results suggest that cannabinoid exposure per se is unlikely to be causally responsible for the association between prior cannabis use and future cocaine use in adulthood as purported by the gateway hypothesis. However, we found that cannabinoid exposure produced long-lasting increases in irritability-like behavior, which may indirectly facilitate the emergence of social conflicts and other mental disorders that may contribute to the abuse of drugs other than cocaine. Additionally, the cross-sensitization between WIN and cocaine in adolescence—which was not observed in adulthood—may highlight a short-term increase in the vulnerability to cocaine-induced behaviors.
In summary, the present results showed that cannabinoid exposure during adolescence in rats produced cross-sensitization to cocaine in adolescence and a long-lasting increase in irritability-like behavior in adulthood. However, it did not facilitate the acquisition or escalation of cocaine self-administration or compulsive-like responding for cocaine in adulthood.
Acknowledgements
We thank Michael Arends for proofreading the manuscript. This study was funded by National Institutes of Health grants U01 DA043799 and AA006420 (Animal Core), the Pearson Center for Alcoholism and Addiction Research, the Sigrid Juselius Foundation (JK), and the Emil Aaltonen Foundation (JK).
Author Contributions
J.K. and O.G. wrote the manuscript. J.K., M.K. and G.d.G. analyzed the data. J.K. and A.K. conducted the experiments. J.K., P.M., M.S., P.F., D.B.K., E.R.K. and O.G. designed the experiments. All of the authors reviewed and edited the manuscript.
Data Availability
The datasets that were generated and analyzed in the present study are available from the corresponding author upon reasonable request.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.UNODC. United Nations Office on Drugs and Crime. World Drug Reports, http://www.unodc.org/doc/wdr2016/WORLD_DRUG_REPORT_2016_web.pdf (2016).
- 2.Kandel D. Stages in adolescent involvement in drug use. Science. 1975;190:912–914. doi: 10.1126/science.1188374. [DOI] [PubMed] [Google Scholar]
- 3.Kandel DB. Does marijuana use cause the use of other drugs? JAMA. 2003;289:482–483. doi: 10.1001/jama.289.4.482. [DOI] [PubMed] [Google Scholar]
- 4.Agrawal A, Prescott CA, Kendler KS. Forms of cannabis and cocaine: a twin study. Am J Med Genet B Neuropsychiatr Genet. 2004;129B:125–128. doi: 10.1002/ajmg.b.30046. [DOI] [PubMed] [Google Scholar]
- 5.Lopez-Quintero C, et al. Probability and predictors of transition from first use to dependence on nicotine, alcohol, cannabis, and cocaine: results of the National Epidemiologic Survey on Alcohol and Related Conditions (NESARC) Drug Alcohol Depend. 2011;115:120–130. doi: 10.1016/j.drugalcdep.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kandel D, Kandel E. The Gateway Hypothesis of substance abuse: developmental, biological and societal perspectives. Acta Paediatr. 2015;104:130–137. doi: 10.1111/apa.12851. [DOI] [PubMed] [Google Scholar]
- 7.Malone DT, Hill MN, Rubino T. Adolescent cannabis use and psychosis: epidemiology and neurodevelopmental models. Br J Pharmacol. 2010;160:511–522. doi: 10.1111/j.1476-5381.2010.00721.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Meier MH, et al. Persistent cannabis users show neuropsychological decline from childhood to midlife. Proc Natl Acad Sci USA. 2012;109:E2657–2664. doi: 10.1073/pnas.1206820109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Busquets-Garcia A, et al. Pregnenolone blocks cannabinoid-induced acute psychotic-like states in mice. Mol Psychiatry. 2017;22:1594–1603. doi: 10.1038/mp.2017.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.D’Amico EJ, Tucker JS, Pedersen ER, Shih RA. Understanding Rates of Marijuana Use and Consequences Among Adolescents in a Changing Legal Landscape. Curr Addict Rep. 2017;4:343–349. doi: 10.1007/s40429-017-0170-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miech, R. A. et al. National Adolescent Drug Trends in 2017: Findings Released. Monitoring the Future. http://www.monitoringthefuture.org/ (2017).
- 12.Levine A, Clemenza K, Rynn M, Lieberman J. Evidence for the Risks and Consequences of Adolescent Cannabis Exposure. J Am Acad Child Adolesc Psychiatry. 2017;56:214–225. doi: 10.1016/j.jaac.2016.12.014. [DOI] [PubMed] [Google Scholar]
- 13.Morral AR, McCaffrey DF, Paddock SM. Reassessing the marijuana gateway effect. Addiction. 2002;97:1493–1504. doi: 10.1046/j.1360-0443.2002.00280.x. [DOI] [PubMed] [Google Scholar]
- 14.Pistis M, et al. Adolescent exposure to cannabinoids induces long-lasting changes in the response to drugs of abuse of rat midbrain dopamine neurons. Biol Psychiatry. 2004;56:86–94. doi: 10.1016/j.biopsych.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 15.Melas PA, et al. Cannabinoid Modulation of Eukaryotic Initiation Factors (eIF2alpha and eIF2B1) and Behavioral Cross-Sensitization to Cocaine in Adolescent Rats. Cell Rep. 2018;22:2909–2923. doi: 10.1016/j.celrep.2018.02.065. [DOI] [PubMed] [Google Scholar]
- 16.Kandel DB, Yamaguchi K, Chen K. Stages of progression in drug involvement from adolescence to adulthood: further evidence for the gateway theory. J Stud Alcohol. 1992;53:447–457. doi: 10.15288/jsa.1992.53.447. [DOI] [PubMed] [Google Scholar]
- 17.Ellgren M, Hurd YL, Franck J. Amphetamine effects on dopamine levels and behavior following cannabinoid exposure during adolescence. Eur J Pharmacol. 2004;497:205–213. doi: 10.1016/j.ejphar.2004.06.048. [DOI] [PubMed] [Google Scholar]
- 18.Somkuwar SS, et al. Abstinence from prolonged ethanol exposure affects plasma corticosterone, glucocorticoid receptor signaling and stress-related behaviors. Psychoneuroendocrinology. 2017;84:17–31. doi: 10.1016/j.psyneuen.2017.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kimbrough, A. et al. CRF1 Receptor-Dependent Increases in Irritability-Like Behavior During Abstinence from Chronic Intermittent Ethanol Vapor Exposure. Alcohol Clin Exp Res, 10.1111/acer.13484 (2017). [DOI] [PMC free article] [PubMed]
- 20.Xue S, et al. An enzymatic advance in nicotine cessation therapy. Chem Commun (Camb) 2018;54:1686–1689. doi: 10.1039/C7CC09134F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Edwards S, Koob GF. Escalation of drug self-administration as a hallmark of persistent addiction liability. Behav Pharmacol. 2013;24:356–362. doi: 10.1097/FBP.0b013e3283644d15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ahmed SH, Koob GF. Transition from moderate to excessive drug intake: change in hedonic set point. Science. 1998;282:298–300. doi: 10.1126/science.282.5387.298. [DOI] [PubMed] [Google Scholar]
- 23.Vanderschuren LJ, Everitt BJ. Drug seeking becomes compulsive after prolonged cocaine self-administration. Science. 2004;305:1017–1019. doi: 10.1126/science.1098975. [DOI] [PubMed] [Google Scholar]
- 24.Ahmed SH. The science of making drug-addicted animals. Neuroscience. 2012;211:107–125. doi: 10.1016/j.neuroscience.2011.08.014. [DOI] [PubMed] [Google Scholar]
- 25.Xue Y, Steketee JD, Sun W. Inactivation of the central nucleus of the amygdala reduces the effect of punishment on cocaine self-administration in rats. Eur J Neurosci. 2012;35:775–783. doi: 10.1111/j.1460-9568.2012.08000.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lenoir, M., Augier, E., Vouillac, C. & Ahmed, S. H. A choice-based screening method for compulsive drug users in rats. Curr Protoc Neurosci Chapter 9, Unit9 44, 10.1002/0471142301.ns0944s64 (2013). [DOI] [PubMed]
- 27.Schneider M. Adolescence as a vulnerable period to alter rodent behavior. Cell Tissue Res. 2013;354:99–106. doi: 10.1007/s00441-013-1581-2. [DOI] [PubMed] [Google Scholar]
- 28.Rubino T, et al. Chronic delta 9-tetrahydrocannabinol during adolescence provokes sex-dependent changes in the emotional profile in adult rats: behavioral and biochemical correlates. Neuropsychopharmacology. 2008;33:2760–2771. doi: 10.1038/sj.npp.1301664. [DOI] [PubMed] [Google Scholar]
- 29.Scherma M, et al. Adolescent Delta(9)-Tetrahydrocannabinol Exposure Alters WIN55,212-2 Self-Administration in Adult Rats. Neuropsychopharmacology. 2016;41:1416–1426. doi: 10.1038/npp.2015.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aguilar, M. A. et al. Adolescent Exposure to the Synthetic Cannabinoid WIN 55212-2 Modifies Cocaine Withdrawal Symptoms in Adult Mice. Int J Mol Sci18, 10.3390/ijms18061326 (2017). [DOI] [PMC free article] [PubMed]
- 31.Riittinen ML, et al. Impoverished rearing conditions increase stress-induced irritability in mice. Dev Psychobiol. 1986;19:105–111. doi: 10.1002/dev.420190203. [DOI] [PubMed] [Google Scholar]
- 32.Lagerspetz K, Portin R. Simulation of cues eliciting aggressive responses in mice at two age levels. J Genet Psychol. 1968;113:53–63. doi: 10.1080/00221325.1968.10533808. [DOI] [PubMed] [Google Scholar]
- 33.Caine SB, Koob GF. Modulation of cocaine self-administration in the rat through D-3 dopamine receptors. Science. 1993;260:1814–1816. doi: 10.1126/science.8099761. [DOI] [PubMed] [Google Scholar]
- 34.de Guglielmo G, et al. Cebranopadol Blocks the Escalation of Cocaine Intake and Conditioned Reinstatement of Cocaine Seeking in Rats. J Pharmacol Exp Ther. 2017;362:378–384. doi: 10.1124/jpet.117.241042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kallupi M, et al. Kappa opioid receptor-mediated dysregulation of gamma-aminobutyric acidergic transmission in the central amygdala in cocaine addiction. Biol Psychiatry. 2013;74:520–528. doi: 10.1016/j.biopsych.2013.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Carroll ME, Lac ST. Acquisition of i.v. amphetamine and cocaine self-administration in rats as a function of dose. Psychopharmacology (Berl) 1997;129:206–214. doi: 10.1007/s002130050182. [DOI] [PubMed] [Google Scholar]
- 37.Mantsch JR, Ho A, Schlussman SD, Kreek MJ. Predictable individual differences in the initiation of cocaine self-administration by rats under extended-access conditions are dose-dependent. Psychopharmacology (Berl) 2001;157:31–39. doi: 10.1007/s002130100744. [DOI] [PubMed] [Google Scholar]
- 38.Richardson NR, Roberts DC. Progressive ratio schedules in drug self-administration studies in rats: a method to evaluate reinforcing efficacy. J Neurosci Methods. 1996;66:1–11. doi: 10.1016/0165-0270(95)00153-0. [DOI] [PubMed] [Google Scholar]
- 39.Higuera-Matas A, et al. Augmented acquisition of cocaine self-administration and altered brain glucose metabolism in adult female but not male rats exposed to a cannabinoid agonist during adolescence. Neuropsychopharmacology. 2008;33:806–813. doi: 10.1038/sj.npp.1301467. [DOI] [PubMed] [Google Scholar]
- 40.Schroeder JP, Epps SA, Grice TW, Weinshenker D. The selective dopamine beta-hydroxylase inhibitor nepicastat attenuates multiple aspects of cocaine-seeking behavior. Neuropsychopharmacology. 2013;38:1032–1038. doi: 10.1038/npp.2012.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schlosburg JE, et al. Long-term antagonism of kappa opioid receptors prevents escalation of and increased motivation for heroin intake. J Neurosci. 2013;33:19384–19392. doi: 10.1523/JNEUROSCI.1979-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wee S, Specio SE, Koob GF. Effects of dose and session duration on cocaine self-administration in rats. J Pharmacol Exp Ther. 2007;320:1134–1143. doi: 10.1124/jpet.106.113340. [DOI] [PubMed] [Google Scholar]
- 43.Siciliano CA, Fordahl SC, Jones SR. Cocaine Self-Administration Produces Long-Lasting Alterations in Dopamine Transporter Responses to Cocaine. J Neurosci. 2016;36:7807–7816. doi: 10.1523/JNEUROSCI.4652-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Siciliano CA, et al. Amphetamine Reverses Escalated Cocaine Intake via Restoration of Dopamine Transporter Conformation. J Neurosci. 2018;38:484–497. doi: 10.1523/JNEUROSCI.2604-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Matzeu A, Kallupi M, George O, Schweitzer P, Martin-Fardon R. Dynorphin Counteracts Orexin in the Paraventricular Nucleus of the Thalamus: Cellular and Behavioral Evidence. Neuropsychopharmacology. 2018;43:1010–1020. doi: 10.1038/npp.2017.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kirkham TC. Cannabinoids and appetite: food craving and food pleasure. Int Rev Psychiatry. 2009;21:163–171. doi: 10.1080/09540260902782810. [DOI] [PubMed] [Google Scholar]
- 47.Stopponi S, et al. Chronic THC during adolescence increases the vulnerability to stress-induced relapse to heroin seeking in adult rats. Eur Neuropsychopharmacol. 2014;24:1037–1045. doi: 10.1016/j.euroneuro.2013.12.012. [DOI] [PubMed] [Google Scholar]
- 48.Ruginsk SG, Vechiato FM, Uchoa ET, Elias LL, Antunes-Rodrigues J. Type 1 cannabinoid receptor modulates water deprivation-induced homeostatic responses. Am J Physiol Regul Integr Comp Physiol. 2015;309:R1358–1368. doi: 10.1152/ajpregu.00536.2014. [DOI] [PubMed] [Google Scholar]
- 49.Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2010;35:217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tarter RE, Blackson T, Brigham J, Moss H, Caprara GV. The association between childhood irritability and liability to substance use in early adolescence: a 2-year follow-up study of boys at risk for substance abuse. Drug Alcohol Depend. 1995;39:253–261. doi: 10.1016/0376-8716(95)01175-6. [DOI] [PubMed] [Google Scholar]
- 51.Chadwick B, Miller ML, Hurd YL. Cannabis Use during Adolescent Development: Susceptibility to Psychiatric Illness. Front Psychiatry. 2013;4:129. doi: 10.3389/fpsyt.2013.00129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schneider M, et al. Enhanced Functional Activity of the Cannabinoid Type-1 Receptor Mediates Adolescent Behavior. J Neurosci. 2015;35:13975–13988. doi: 10.1523/JNEUROSCI.1937-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bambico FR, Nguyen NT, Katz N, Gobbi G. Chronic exposure to cannabinoids during adolescence but not during adulthood impairs emotional behaviour and monoaminergic neurotransmission. Neurobiol Dis. 2010;37:641–655. doi: 10.1016/j.nbd.2009.11.020. [DOI] [PubMed] [Google Scholar]
- 54.Keeley RJ, Trow J, McDonald RJ. Strain and sex differences in puberty onset and the effects of THC administration on weight gain and brain volumes. Neuroscience. 2015;305:328–342. doi: 10.1016/j.neuroscience.2015.07.024. [DOI] [PubMed] [Google Scholar]
- 55.Wegener N, Koch M. Behavioural disturbances and altered Fos protein expression in adult rats after chronic pubertal cannabinoid treatment. Brain Res. 2009;1253:81–91. doi: 10.1016/j.brainres.2008.11.081. [DOI] [PubMed] [Google Scholar]
- 56.Bruijnzeel AW, et al. Behavioral Characterization of the Effects of Cannabis Smoke and Anandamide in Rats. PLoS One. 2016;11:e0153327. doi: 10.1371/journal.pone.0153327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Murphy M, et al. Chronic Adolescent Delta(9)-Tetrahydrocannabinol Treatment of Male Mice Leads to Long-Term Cognitive and Behavioral Dysfunction, Which Are Prevented by Concurrent Cannabidiol Treatment. Cannabis Cannabinoid Res. 2017;2:235–246. doi: 10.1089/can.2017.0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lamarque S, Taghzouti K, Simon H. Chronic treatment with Delta(9)-tetrahydrocannabinol enhances the locomotor response to amphetamine and heroin. Implications for vulnerability to drug addiction. Neuropharmacology. 2001;41:118–129. doi: 10.1016/S0028-3908(01)00039-9. [DOI] [PubMed] [Google Scholar]
- 59.Muschamp JW, Siviy SM. Behavioral sensitization to amphetamine follows chronic administration of the CB1 agonist WIN 55,212-2 in Lewis rats. Pharmacol Biochem Behav. 2002;73:835–842. doi: 10.1016/S0091-3057(02)00910-3. [DOI] [PubMed] [Google Scholar]
- 60.Landa L, Sulcova A, Slais K. Involvement of cannabinoid CB1 and CB2 receptor activity in the development of behavioural sensitization to methamphetamine effects in mice. Neuro Endocrinol Lett. 2006;27:63–69. [PubMed] [Google Scholar]
- 61.Dow-Edwards D, Izenwasser S. Pretreatment with Delta9-tetrahydrocannabinol (THC) increases cocaine-stimulated activity in adolescent but not adult male rats. Pharmacol Biochem Behav. 2012;100:587–591. doi: 10.1016/j.pbb.2011.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gomes, F. V., Guimaraes, F. S. & Grace, A. A. Effects of pubertal cannabinoid administration on attentional set-shifting and dopaminergic hyper-responsivity in a developmental disruption model of schizophrenia. Int J Neuropsychopharmacol18, 10.1093/ijnp/pyu018 (2014). [DOI] [PMC free article] [PubMed]
- 63.Rodriguez-Arias M, et al. Effect of adolescent exposure to WIN 55212-2 on the acquisition and reinstatement of MDMA-induced conditioned place preference. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34:166–171. doi: 10.1016/j.pnpbp.2009.10.019. [DOI] [PubMed] [Google Scholar]
- 64.Rodriguez-Arias M, et al. Effects of Cannabinoid Exposure during Adolescence on the Conditioned Rewarding Effects of WIN 55212-2 and Cocaine in Mice: Influence of the Novelty-Seeking Trait. Neural Plast. 2016;2016:6481862. doi: 10.1155/2016/6481862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Squeglia LM, Jacobus J, Tapert SF. The influence of substance use on adolescent brain development. Clin EEG Neurosci. 2009;40:31–38. doi: 10.1177/155005940904000110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Koob G, Kreek MJ. Stress, dysregulation of drug reward pathways, and the transition to drug dependence. Am J Psychiatry. 2007;164:1149–1159. doi: 10.1176/appi.ajp.2007.05030503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ben-Shahar O, Moscarello JM, Jacob B, Roarty MP, Ettenberg A. Prolonged daily exposure to i.v. cocaine results in tolerance to its stimulant effects. Pharmacol Biochem Behav. 2005;82:411–416. doi: 10.1016/j.pbb.2005.09.013. [DOI] [PubMed] [Google Scholar]
- 68.Ahmed SH, Kenny PJ, Koob GF, Markou A. Neurobiological evidence for hedonic allostasis associated with escalating cocaine use. Nat Neurosci. 2002;5:625–626. doi: 10.1038/nn872. [DOI] [PubMed] [Google Scholar]
- 69.Vendruscolo LF, et al. Escalation patterns of varying periods of heroin access. Pharmacol Biochem Behav. 2011;98:570–574. doi: 10.1016/j.pbb.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ahmed SH, Cador M. Dissociation of psychomotor sensitization from compulsive cocaine consumption. Neuropsychopharmacology. 2006;31:563–571. doi: 10.1038/sj.npp.1300834. [DOI] [PubMed] [Google Scholar]
- 71.Lenoir M, Ahmed SH. Heroin-induced reinstatement is specific to compulsive heroin use and dissociable from heroin reward and sensitization. Neuropsychopharmacology. 2007;32:616–624. doi: 10.1038/sj.npp.1301083. [DOI] [PubMed] [Google Scholar]
- 72.Wee S, Mandyam CD, Lekic DM, Koob GF. Alpha 1-noradrenergic system role in increased motivation for cocaine intake in rats with prolonged access. Eur Neuropsychopharmacol. 2008;18:303–311. doi: 10.1016/j.euroneuro.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.George O, Mandyam CD, Wee S, Koob GF. Extended access to cocaine self-administration produces long-lasting prefrontal cortex-dependent working memory impairments. Neuropsychopharmacology. 2008;33:2474–2482. doi: 10.1038/sj.npp.1301626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Vendruscolo LF, et al. Corticosteroid-dependent plasticity mediates compulsive alcohol drinking in rats. J Neurosci. 2012;32:7563–7571. doi: 10.1523/JNEUROSCI.0069-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Seif T, et al. Cortical activation of accumbens hyperpolarization-active NMDARs mediates aversion-resistant alcohol intake. Nature neuroscience. 2013;16:1094–1100. doi: 10.1038/nn.3445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Griffin EA, Jr, et al. Prior alcohol use enhances vulnerability to compulsive cocaine self-administration by promoting degradation of HDAC4 and HDAC5. Science advances. 2017;3:e1701682. doi: 10.1126/sciadv.1701682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wong WC, Ford KA, Pagels NE, McCutcheon JE, Marinelli M. Adolescents are more vulnerable to cocaine addiction: behavioral and electrophysiological evidence. J Neurosci. 2013;33:4913–4922. doi: 10.1523/JNEUROSCI.1371-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets that were generated and analyzed in the present study are available from the corresponding author upon reasonable request.