Abstract
The silica, alumina, ceria, and titania supports were modified with uranyl ions (5 wt%) and investigated using X-ray photoelectron spectroscopy. The data show the U4f photoelectron spectra and charge state of uranium for uranyl ions deposited on different supports. The additional in situ XPS experiments with simultaneous irradiation of the sample using a 450 nm light-emitting diode were performed, and the XPS spectra, revealing a partial reduction of uranium under visible irradiation, are presented. The data show the effect of support material on the chemical states of uranium and oxygen on the surface of uranyl-modified oxides under visible light.
Specifications Table
| Subject area | Surface chemistry |
| More specific subject area | Photochemistry |
| Type of data | Figure, table |
| How data was acquired | XPS analysis: SPECS (Germany) spectrometer equipped with hemispherical PHOIBOS-150-MCD-9 analyzer and FOCUS-500 (AlKα radiation, hν = 1486.74 eV, 200 W radiation power) monochromator |
| Data format | Analyzed |
| Experimental factors | Uranyl-modified oxides (5 wt%) were prepared via the impregnation of a support with UO2(NO3)2 aqueous solution followed by drying at 100 °C and grinding using an agate mortar and pestle. The sample preparation for XPS analysis was performed under room irradiation from luminescent lamps |
| Experimental features | XPS experiments were performed in in situ mode when the sample in a spectrometer chamber was simultaneously irradiated using a high-power light-emitting diode (LED) with a maximum at 450 nm. The survey spectra were taken at analyzer pass energy of 50 eV. The detailed U4f and O1s spectral regions were registered at 20 eV |
| Data source location | Boreskov Institute of Catalysis, Lavrentieva 5, Novosibirsk 630090, Russian Federation |
| Data accessibility | Data are accessible within this article |
| Related research article | T.N. Filippov, D.A. Svintsitskiy, I.A. Chetyrin, I.P. Prosvirin, D.S. Selishchev, D. V Kozlov, Photocatalytic and photochemical processes on the surface of uranyl-modified oxides: An in situ XPS study, Appl. Catal. A, Gen. 558 (2018) 81–90. doi:10.1016/j.apcata.2018.03.015 |
Value of the data
-
•
Data show the photoelectron U4f and O1s spectral regions for different oxides supported with uranyl ions.
-
•
New data about the uranium transformations on the surface of silica, alumina, ceria, and titania under the visible light are obtained using in situ XPS technique.
-
•
Data show the effect of support material on the changing of uranium charge state under long-term visible irradiation.
-
•
Data are useful for further studies on the elucidation of mechanisms for photochemical and photocatalytic reactions on the surface of uranyl-modified oxides.
-
•
In situ XPS technique has great promise for the investigation of photochemical processes on the surface of different materials.
1. Data
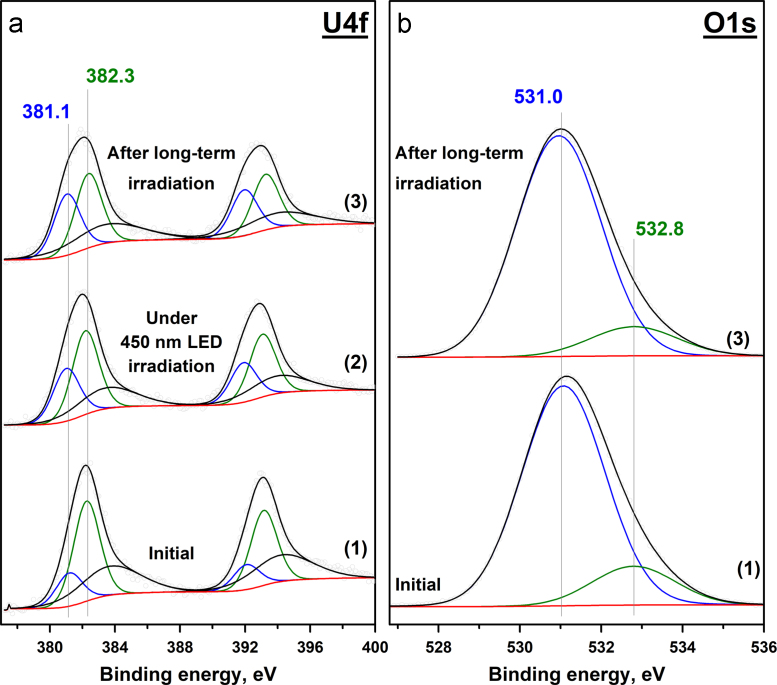
In this study, in situ XPS experiments when the sample in a spectrometer chamber was simultaneously irradiated using 450 nm LED were performed to understand the behavior of uranyl species under the visible light on the surface of different oxides. Fig. 1, Fig. 2, Fig. 3, Fig. 4 show the photoelectron U4f and O1s spectral regions for the 5 wt% uranyl-loaded samples based on SiO2 (5US), Al2O3 (5UA), CeO2 (5UC), and TiO2 (5UT), respectively, before and after irradiation using 450 nm LED. According to literature data [1], [2], [3], [4], [5], [6], different positions of the U4f7/2 peaks (Fig. 1, Fig. 2, Fig. 3, Fig. 4a) can be attributed for different charge states of uranium. The relationship between the binding energy and corresponding charge state of uranium is shown in Table 1.
Fig. 1.
Photoelectron U4f (a) and O1s (b) spectral regions for the 5US sample. The plots correspond to: the initial sample (1); after 450 nm LED irradiation for 60 min (2); the final state after long-term irradiation (3).
Fig. 2.
Photoelectron U4f (a) and O1s (b) spectral regions for the 5UA sample. The plots correspond to: the initial sample (1); after 450 nm LED irradiation for 30 min (2); the final state after long-term irradiation.
Fig. 3.
Photoelectron U4f (a) and O1s (b) spectral regions for the 5UC sample. The plots correspond to: the initial sample (1); after 450 nm LED irradiation for 10 min (2); the final state after long-term irradiation (3).
Fig. 4.
Photoelectron U4f (a) and O1s (b) spectral regions for the 5UT sample. The plots correspond to: the initial sample (1); after 450 nm LED irradiation for 100 min (2); the final state after long-term irradiation (3).
Table 1.
Relationship between the position of U4f7/2 peak and the charge state of uranium.
| Charge state | Binding energy (U4f7/2), eV |
|---|---|
| U6+ | 382.0–382.4 |
| U5+ | 381.1 |
| U4+ | 380.1 |
The peaks observed in the O1s spectral region (Fig. 1, Fig. 2, Fig. 3, Fig. 4b) can be attributed to the lattice oxygen, surface oxygen, oxygen from water and OH groups. The relationship between the position of the O1s peaks and the chemical state of oxygen was suggested on the basis of previously published papers [7], [8], [9]. The results for each sample are shown in Table 2.
Table 2.
Relationship between the position of O1s peak and the chemical state of oxygen in the samples.
| Chemical state of oxygen | Binding energy (O1s), eV |
|||
|---|---|---|---|---|
| 5US | 5UA | 5UC | 5UT | |
| Lattice (Olat) | 532.2 eV | 531.0 eV | 529.2 eV | 530.0 eV |
| Surface | – | – | 530.5 eV | – |
| ОН | – | – | 531.6 eV | 531.5 eV |
| H2O | – | 532.8 eV | 532.8 eV | 532.8 eV |
The collected photoelectron spectra were used for the calculation of the ratio between the different chemical states of uranium and oxygen. The data for each sample before and after long-term 450 nm LED irradiation are shown in Table 3. In the table, lattice oxygen is referred to as Olat, and the combination of the other oxygen forms is marked as Orest.
Table 3.
Ratio between the different chemical states of uranium and oxygen in the absence and presence of 450 nm LED irradiation.
|
5US |
5UA |
5UC |
5UT |
|||||
|---|---|---|---|---|---|---|---|---|
| U5+/U6+ | Orest/Olat | U5+/U6+ | Orest/Olat | U5+/U6+ | Orest/Olat | U4+/U6+ | Orest/Olat | |
| Initial | 0.15 | 0 | 0.33 | 0.17 | 0.28 | 0.92 | 0 | 0.24 |
| Under irradiation | 0.53 | 0 | 0.63 | – | 0.36 | – | 3.33 | 0.19 |
| After long-term irradiation | 0.63 | 0 | 0.83 | 0.14 | 0.45 | 0.21 | 5 | 0.17 |
2. Experimental design, materials, and methods
2.1. Materials
The uranyl nitrate hexahydrate (UO2(NO3)2 × 6H2O) from Izotop LLC (Russia) was employed for the modification of silica, alumina, ceria, and titania supports. SiO2 Type H (amorphous, as,BET = 440 m2/g) from Sigma-Aldrich (USA), Al2O3 (γ-phase, as,BET = 180 m2/g) from AO AZKiOS (Russia), CeO2 (fluorite-type, as,BET = 110 m2/g) prepared by the precipitation from a cerium nitrate solution with an aqueous ammonia solution [10], and TiO2 Hombifine N (100% anatase, as,BET = 350 m2/g) from Sachtleben Chemie GmbH (Germany) were used as supports.
2.2. Sample preparation
The silica, alumina, ceria, and titania supports were modified with uranyl ions via the impregnation method using UO2(NO3)2 aqueous solution [11]. Typically, 0.5 g of a support was suspended in 10 mL of deionized water, and 1 mL of 0.063 M aqueous solution of UO2(NO3)2 was added to the suspension. The theoretically calculated content of UO2(NO3)2 in the samples was 5 wt%. After vigorous stirring for 30 min, the suspension was evaporated. The precipitate was dried at 100 °C in air followed by the final grinding using an agate mortar and pestle.
2.3. X-ray photoelectron spectroscopy
A SPECS (Germany) spectrometer equipped with a hemispherical PHOIBOS-150-MCD-9 analyzer and FOCUS-500 (AlKα radiation, hλ = 1486.74 eV, 200 W radiation power) monochromator was employed for in situ XPS experiments. In addition to a typical packaging, the presence of a special window in the analyzer chamber allowed for simultaneous irradiation of the investigated sample with visible light during the spectra collecting (Fig. 5). The sample preparation for the XPS analysis was performed under room irradiation from luminescent lamps. The position of the oxide-forming element for support was used as an internal standard for the calibration of photoelectron spectra. The analysis of each sample was performed as follows:
-
(1)
Spectra collecting for the initial sample (i.e., without 450 nm LED irradiation);
-
(2)
Spectra collecting for the sample 450 nm LED irradiated for a certain period;
-
(3)
Spectra collecting after long-term irradiation (i.e., 1–2 h) and turning LED off.
Fig. 5.
Photograph of the experimental setup used for in situ XPS measurements.
The ratio between different charge states of a particular element was calculated using the areas of corresponding spectral lines or curve-fitted peaks, respectively.
Acknowledgments
This work was conducted within the framework of the budget project #АААА-А17-117041710087-3 for Boreskov Institute of Catalysis.
Footnotes
Transparency document associated with this article can be found in the online version at https://doi.org/10.1016/j.dib.2018.06.121.
Transparency document. Supplementary material
Transparency document
.
References
- 1.Ilton E.S., Bagus P.S. XPS determination of uranium oxidation states. Surf. Interface Anal. 2011;43:1549–1560. [Google Scholar]
- 2.Ilton E.S., Boily J.-F., Bagus P.S. Beam induced reduction of U(VI) during X-ray photoelectron spectroscopy: the utility of the U4f satellite structure for identifying uranium oxidation states in mixed valence uranium oxides. Surf. Sci. 2007;601:908–916. [Google Scholar]
- 3.Schindler M., Hawthorne F., Freund M., Burns P. XPS spectra of uranyl minerals and synthetic uranyl compounds. I: the U4f spectrum. Geochim. Cosmochim. Acta. 2009;73:2471–2487. [Google Scholar]
- 4.Taylor S.H., O’Leary S.R. A study of uranium oxide based catalysts for the oxidative destruction of short chain alkanes. Appl. Catal. B Environ. 2000;25:137–149. [Google Scholar]
- 5.Mercier-Bion F., Drot R., Ehrhardt J.J., Lambert J., Roques J., Simoni E. X-ray photoreduction of U(VI)-bearing compounds. Surf. Interface Anal. 2011;43:777–783. [Google Scholar]
- 6.Filippov T.N., Svintsitskiy D.A., Chetyrin I.A., Prosvirin I.P., Selishchev D.S., Kozlov D.V. Photocatalytic and photochemical processes on the surface of uranyl- modified oxides: an in situ XPS study. Appl. Catal. A Gen. 2018;558:81–90. [Google Scholar]
- 7.Liang F., Yu Y., Zhou W., Xu X., Zhu Z. Highly defective CeO2 as a promoter for efficient and stable water oxidation. J. Mater. Chem. A. 2015;3:634–640. [Google Scholar]
- 8.J.F. Moulder, W.F. Stickle, P.E. Sobol, K.D. Bomben, Handbook of X-ray photoelectron spectroscopy. A Reference Book of Standard Spectra for Identification and Interpretation of XPS Data, Perkin-Elmer Corporation Physical Electronics Division, 1992. 〈https://www.cnyn.unam.mx/~wencel/XPS/MANXPS.pdf〉 (Accessed 12 February 2018).
- 9.Svintsitskiy D.A., Slavinskaya E.M., Kardash T.Y., Avdeev V.I., Senkovskiy B.V., Koscheev S.V., Boronin A.I. Low-temperature catalytic CO oxidation over mixed silver–copper oxide Ag2Cu2O3. Appl. Catal. A Gen. 2016;510:64–73. [Google Scholar]
- 10.Slavinskaya E.M., Gulyaev R.V., Stonkus O.A., Zadesenets A.V., Plyusnin P.E., Shubin Y.V., Korenev S.V., Ivanova A.S., Zaikovskii V.I., Danilova I.G., Boronin A.I. Low-temperature oxidation of carbon monoxide on Pd(Pt)/CeO2 catalysts prepared from complex salts. Kinet. Catal. 2011;52:282–295. [Google Scholar]
- 11.Kolinko P.A., Filippov T.N., Kozlov D.V., Parmon V.N. Ethanol vapor photocatalytic oxidation with uranyl modified titania under visible light: comparison with silica and alumina. J. Photochem. Photobiol. A Chem. 2012;250:72–77. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Transparency document